Abstract
Objective
To examine the effect of penile vibratory stimulation (PVS) in the preservation and restoration of erectile function and urinary continence in conjunction with nerve-sparing radical prostatectomy (RP).
Patients and Methods
The present study was conducted between July 2010 and March 2013 as a randomized prospective trial at two university hospitals. Eligible participants were continent men with an International Index of Erectile Function-5 (IIEF-5) score of at least 18, scheduled to undergo nerve-sparing RP.
Patients were randomized to a PVS group or a control group. Patients in the PVS group were instructed in using a PVS device (FERTI CARE® vibrator).
Stimulation was performed at the frenulum once daily by the patients in their own homes for at least 1 week before surgery. After catheter removal, daily PVS was re-initiated for a period of 6 weeks.
Participants were evaluated at 3, 6 and 12 months after surgery with the IIEF-5 questionnaire and questions regarding urinary bother. Patients using up to one pad daily for security reasons only were considered continent. The study was registered at http://clinicaltrials.gov/ (NCT01067261).
Results
Data from 68 patients were available for analyses (30 patients randomized to PVS and 38 patients randomized to the control group).
The IIEF-5 score was highest in the PVS group at all time points after surgery with a median score of 18 vs 7.5 in the control group at 12 months (P = 0.09), but the difference only reached borderline significance.
At 12 months, 16/30 (53%) patients in the PVS group had reached an IIEF-5 score of at least 18, while this was the case for 12/38 (32%) patients in the control group (P = 0.07).
There were no significant differences in the proportions of continent patients between groups at 3, 6 or 12 months. At 12 months 90% of the PVS patients were continent, while 94.7% of the control patients were continent (P = 0.46).
Conclusion
The present study did not document a significant effect of PVS. However, the method proved to be acceptable for most patients and there was a trend towards better erectile function with PVS. More studies are needed to explore this possible effect further.
Keywords: erectile dysfunction, penile rehabilitation, penile vibratory stimulation, prostate cancer, radical prostatectomy, urinary incontinence
Introduction
Radical prostatectomy (RP) is a commonly employed treatment for localized prostate cancer. Unfortunately, a substantial proportion of patients will experience adverse effects in the form of urinary incontinence and erectile dysfunction (ED) after the surgery 1. The cavernous nerves are responsible for inducing the physiological erection, and as these nerves run in close proximity to the prostate gland, they are in danger of being damaged during RP. Thus, it is well accepted that the main pathophysiological mechanism behind post-prostatectomy ED is damage to the cavernous nerves. To improve erectile function after surgery, nerve-sparing procedures have therefore been developed, and whenever tumour characteristics allow it, these are routinely employed 2. However, even when the cavernous nerves are left anatomically intact, it is likely that they are affected by mechanical manipulation, heating, ischaemic effects and local inflammation 3,4. This is believed to cause neuropraxia, defined as a temporary block of nerve transmission despite an anatomically intact nerve fibre. Postoperative incontinence can be caused by damage to the urinary sphincter and changes in the course of the urethra after surgery. However incontinence can also occur if these structures are not compromised, which could be connected to changes in the closing pressure of the urinary sphincter and sometimes reduced bladder capacity 5. In these cases, it is likely that nerve damage plays a pathophysiological role. Rehabilitation of patients' sexual function is often attempted with various regimens of phosphodiesterase type 5 (PDE5) inhibitors, vacuum erection devices and/or injection therapy 6–8. Meanwhile, rehabilitation regarding urinary continence is routinely performed by instructing patients in pelvic floor exercises before or after their surgery. Unfortunately, these rehabilitation attempts are often unsuccessful and new methods are needed 9,10. One possible reason that current methods have generally shown disappointing results in preserving erectile function and continence is that they do not target the pelvic nerves.
It has previously been shown that one can stimulate the nerves of the pelvic floor by means of penile vibratory stimulation (PVS). Thus PVS is capable of inducing ejaculations in ≈90% of men with spinal cord injuries 11 and the treatment is known to increase the pressure in the external urethral sphincter as well as the bladder capacity in this patient group 12,13. In addition, mechanical nerve stimulation through vibration applied at the perineum has shown promise in treating urinary incontinence in women, with 24/33 (74%) women experiencing complete resolution of symptoms after 6 weeks of stimulation 14. Therefore it is feasible that this method can improve nerve function and thereby prevent or minimize the occurrence of incontinence and ED after pelvic surgery. The purpose of the present study is to examine the effect of PVS in the preservation and restoration of erectile function and urinary continence in conjunction with nerve-sparing RP.
Patients and Methods
The study was conducted between July 2010 and March 2013 as a randomized controlled trial at two university hospitals. Eligible participants were men scheduled to undergo nerve-sparing RP. Only men who were sexually active with an International Index of Erectile Function-5 (IIEF-5) 15 score of at least 18 without erectogenic aids, and fully continent before surgery (as assessed by the validated Danish Prostate Symptom Score [DAN-PSS], were included in the study. The DAN-PSS is a patient-administered questionnaire based on 12 symptoms related to bladder storage and voiding function and describes both the severity and the perceived bother related to each symptom 16. To maintain a uniform patient group, exclusion criteria included any condition that would prevent the participant from attempting postoperative treatment with a PDE5-inhibitor.
Data regarding preoperative erectile function (assessed by the IIEF-5 questionnaire), and preoperative LUTS (assessed by the DAN-PSS questionnaire) were collected at inclusion. Eligible patients were then randomized by a draw using opaque envelopes to either a PVS group or a control group. In both groups, the patients received one preoperative session with pelvic floor muscle training instruction. In addition, patients in the PVS group were instructed in using a PVS device (FERTI CARE® vibrator, Multicept A/S, Frederiksberg, Denmark) during the same session (Fig. 1). The device was set to an amplitude of 2 mm and a vibration frequency of 100 Hz. Patients were instructed in stimulating the frenulum once daily with a sequence consisting of 10 s of stimulation followed by a 10-s pause repeated 10 times (for a total of 100 s of stimulation every day). The patients were given a FERTI CARE vibrator to use daily in their homes for at least 1 week before surgery. In conjunction with surgery, the laterality of nerve-sparing (unilateral/bilateral) was noted and patients who underwent a non-nerve-sparing procedure were excluded at this point. After the surgery, the remaining participants in the PVS group were instructed to re-initiate the stimulation at catheter removal and continue daily stimulation for a period of 6 weeks. All participants in both groups were contacted by phone to ensure compliance with pelvic floor exercises and PVS at 2 and 6 weeks after surgery. At these contacts patients in the PVS group were asked systematically about side-effects to the treatment. In both groups the patients were offered on-demand or daily PDE5 inhibitor treatment at 1 month after surgery.
Fig 1.

The FERTI CARE® vibrator. The vibratory stimulation of the device is delivered through a reusable but disposable black plastic disc as seen on the right side of the picture. The device was set to an amplitude of 2 mm and a vibration frequency of 100 Hz. Patients were instructed in stimulating the frenulum once daily with a sequence consisting of 10 s of stimulation followed by a 10-s pause repeated 10 times (for a total of 100 s of stimulation every day).
Participants were evaluated at 3, 6 and 12 months after surgery with the IIEF-5 questionnaire for erectile function and DAN-PSS for urinary bother. In addition, patients were asked to rate their continence and to report their use of pads/diapers at each visit. Patients reporting use of up to one pad daily for security reasons only were considered continent.
The primary endpoint regarding erectile function was the difference in median IIEF-5 score between the groups. The primary endpoint regarding continence was time to continence after surgery. Secondary outcome measures included the number of patients who had achieved an IIEF-5 score of at least 18 with or without PDE5-inhibitors at 3, 6 and 12 months after surgery as well as the overall difference in reported pad use and the difference in postoperative DAN-PSS. In addition, we conducted post hoc multivariate analyses to assess the influence of nerve-sparing and use of PDE5 inhibitors on erectile function and to assess the influence of nerve-sparing on continence.
The sample size was calculated based on the IIEF-5 questionnaire. With a two-sided significance level set at 0.05, it was calculated that 64 patients would be needed to detect a minimally clinically meaningful difference of 5 with an sd of 6 and a power of 80%. To account for subsequent exclusion, dropout and anticipated non-compliance, we aimed to include 80 patients in the preoperative phase.
The Wilcoxon–Mann–Whitney test was used to assess differences in continuous variables, while Fisher's exact test or the chi-squared test was used to compare groups with regard to categorical variables. For the multivariate analyses, we used logistic regression. Outcome measures are presented as percentages or as medians and range. All statistical tests were performed with the SAS version 9.2 statistical software package for windows (SAS Institute Inc., Cary, NC, USA). The study was approved by the Danish ethical counsel and the Danish Data Protection Agency. It was registered at http://www.clinicaltrials.org (NCT01067261).
Results
A total of 91 eligible patients were identified and asked to participate in the study and 83 patients were included before surgery (42 randomized to the PVS group and 41 randomized to the control group). In total, data from 68 patients were available for analysis (30 patients randomized to vibration therapy and 38 patients randomized to the control group). Reasons for exclusion included non-nerve-sparing surgery (n = 5), withdrawn consent (n = 3), loss of partner (n = 1) and non-compliance with the PVS protocol (n = 6). Of the six non-compliant patients, four could not use the device after surgery because they had a catheter in place for an extended period of time, while one felt pain on vibration. The last patient stated that he did not feel comfortable with PVS. The flow of patients is illustrated in Fig. 2.
Fig 2.
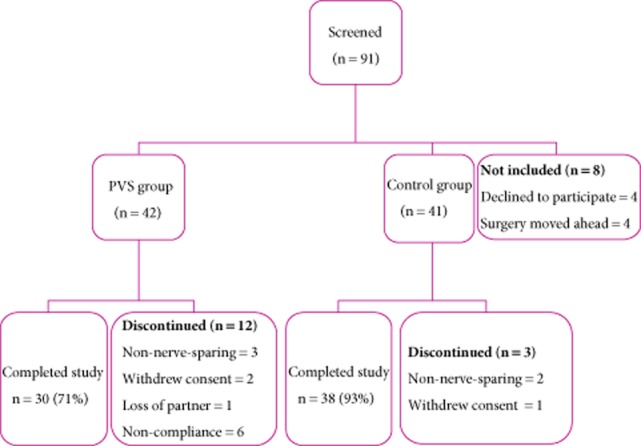
The flow of patients throughout the study.
There were no statistically significant differences in age, degree of nerve-sparing, robotic/open surgery, preoperative IIEF-5 score or postoperative use of PDE5 inhibitors between the groups; however, patients randomized to the PVS group had significantly more LUTS before surgery (P = 0.048) (Table 1). There were no significant differences in tumour stage (P = 0.7), Gleason score (P = 0.19) or preoperative PSA level (P = 0.66) between groups. Likewise, there were no statistically significant differences regarding any of the mentioned variables between the final 68 patients and the 10 patients who were excluded for reasons other than a lack of nerve-sparing. Follow-up data were available for 64/68 patients at 3 months, 67/68 patients at 6 months and 68/68 patients at 12 months.
Table 1.
Patient characteristics
| Variables | PVS | Control | P value |
|---|---|---|---|
| Median age, years | 62 (46–73) | 65 (49–76) | 0.095 |
| Nerve-sparing, n | 0.23 | ||
| Bilateral | 19 | 18 | |
| Unilateral | 11 | 20 | |
| Robot-assisted surgery, n | 0.99 | ||
| Yes | 27 | 34 | |
| No | 3 | 4 | |
| Median (range) preoperative IIEF-5 score | 25 (19–25) | 25 (18–25) | 0.68 |
| Median (range) preoperative DAN-PSS score | 3.5 (0–27) | 2 (0–20) | 0.048 |
| Proportion of patients using postoperative PDE5 inhibitors, n/N | |||
| 3 Months | 9/30 | 17/38 | 0.16 |
| 6 Months | 19/30 | 25/38 | 0.72 |
| 12 Months | 17/30 | 19/38 | 0.58 |
The IIEF-5 score was higher in the PVS group at all time points after surgery, but the difference between groups only reached borderline significance with a median (range) score of 18 (0–25) in the PVS group vs 7.5 (0–25) in the control group at 12 months (Table 2). At 12 months after surgery, 16/30 (53%) patients in the PVS group had reached an IIEF-5 score of at least 18, compared with 12/38 (32%) patients in the control group (P = 0.07). There was also a non-significant trend towards more patients returning to an IIEF-5 score of at least 18 at 6 months (P = 0.09) while there was no difference in potency rates at 3 months after surgery (P = 0.46).
Table 2.
Erectile function outcomes in the two groups after RP
| Erectile function outcomes | PVS | Control | P value |
|---|---|---|---|
| Median (range) IIEF-5 | |||
| At 3 months | 5 (0–25) | 5 (0–25) | 0.25 |
| At 6 months | 10.5 (0–25) | 5 (0–25) | 0.08 |
| At 12 months | 18 (0–25) | 7.5 (0–25) | 0.09 |
| IIEF ≥18, n/N (%) | |||
| At 3 months | 5/30 (17) | 4/38 (11) | 0.46 |
| At 6 months | 13/30 (43) | 9/38 (24) | 0.09 |
| At 12 months | 16/30 (53) | 12/38 (32) | 0.07 |
There were no significant differences in the proportions of continent patients between groups at either 3, 6 or 12 months after surgery (Table 3). At 12 months, 90% of the PVS patients were continent, compared with 94.7% of the control patients (P = 0.46). There was a nonsignificant trend towards a higher number of pads in the PVS group at 3 months, but there were no differences at 6 and 12 months (Table 3). Likewise, there were no significant differences in total DAN-PSS between groups at any point after the surgery (Table 4).
Table 3.
Continence rates and pad use after surgery
| PVS | Control | P value | |
|---|---|---|---|
| Continence rate | |||
| At 3 months | 65.5% | 62.9% | 0.83 |
| At 6 months | 83.3% | 91.9% | 0.28 |
| At 12 months | 90% | 94.7% | 0.46 |
| Median (range) pad use | |||
| At 3 months | 1 (0–6) | 1 (0–4) | 0.09 |
| At 6 months | 0 (0–3) | 1/3 (0–6)* | 0.14 |
| At 12 months | 0 (0–2) | 0 (0–3) | 0.56 |
One patient reported using a third of a pad daily. As there was no pre-specified decision on how to deal with such reporting, it was taken at face value when analysing the results.
Table 4.
Median (range) DAN-PSS after surgery
| PVS | Control | P value | |
|---|---|---|---|
| Median (range) DAN-PSS | |||
| 3 Months | 1 (0–34) | 5 (0–34) | 0.74 |
| 6 Months | 2 (0–41) | 1 (0–48) | 0.74 |
| 12 Months | 3 (0–36) | 0.5 (0–21) | 0.13 |
Due to the skewed preoperative DAN-PSS between the two groups, post hoc analyses were conducted to assess whether the preoperative DAN-PSS was associated with urinary outcomes. These analyses showed that a high preoperative score was associated with incontinence at 12 months (P = 0.035) and with postoperative DAN-PSS at all time points during follow-up.
The post hoc analysis regarding the influence of nerve-sparing and the use of PDE5 inhibitors on erectile function showed a similar picture to the univariate analysis, with an odds ratio of regaining satisfactory erectile function at 12 months of 2.3 (95% CI: 0.8–6.4, P = 0.12) in the PVS group compared with the control group. Likewise, nerve-sparing status did not significantly alter the effect of PVS on continence outcomes, as the odds ratio of regaining continence at 12 months was 0.44 (95% CI 0.07 – 2.9; P = 0.39) in the PVS group compared with the control group on multivariate analysis. The laterality of nerve-sparing was a significant predictor of postoperative erectile function, with an odds ratio of 3.5 (95% CI: 1.2 – 10.3) for regaining satisfactory function with bilateral nerve-sparing compared with unilateral nerve-sparing (P = 0.026). Meanwhile, the use of PDE5 inhibitors did not influence recovery of erectile function at 12 months (P = 0.4) and nerve-sparing did not influence continence at 12 months (P = 0.43).
Out of the original 42 patients in the PVS group, five experienced side-effects related to the PVS. One described red spots on the glans penis, while one patient had a small laceration with minimal bleeding. In addition, two patients described that they had become sore and another patient experienced frank pain in the early postoperative period. All side-effects were self-limiting and no medical treatment was required. However, as stated earlier, the patient who experienced pain seized PVS because of this.
Discussion
Nerve-sparing and nerve regeneration are believed to be key components with regard to post-prostatectomy functional outcomes and the present study represents the first attempt to utilize nerve stimulation in a rehabilitation programme. The neuroanatomical background is that afferent nerve fibres from the glans penis run through the dorsal penile nerve to join with fibres from the pudendal nerve 17,18. Through this they reach the spinal cord at the spinal levels S2–S4 17,18. Conversely, parasympathetic fibres from S2–S4 in the spinal cord constitute the efferent limb of the erectile response via the cavernous nerve, while somatic fibres running through the pudendal nerve innervate the pelvic floor muscles and the external urinary sphincter. Meanwhile, afferent nerves from the penis also reach the sympathetic centre in the thoracolumbar part of the spinal cord where they might affect bladder contractility 19–21. Possibly working through these pathways, studies have identified several potential benefits from genital and perineal PVS, including ejaculation and reduction of bladder overactivity in spinal cord-injured men and an improvement in stress incontinence in women 12,14,20. The first hypothesis of the current study was that PVS in the early postoperative period after RP can stimulate the cavernous nerves through the described reflex arch and help in the restitution from neuropraxia. This, in turn, could improve long-term erectile function. The second hypothesis was that PVS could improve urinary control through improved ability to contract the pelvic floor muscles and the external urinary sphincter. Unfortunately we were unable to identify a significant effect of 6 weeks of PVS after RP as assessed by our primary outcome measures. The results regarding urinary continence were particularly disappointing. However, the study does show that the method is acceptable to patients and that side-effects are limited. In addition, the trends towards better erectile function in the treatment group imply that PVS might have some effect on long-term erectile function. Two post hoc analyses showed that results were not altered significantly when stratifying for laterality of nerve-sparing and use of PDE5 inhibitors, as there was still a trend towards better erectile function in the PVS group while there was no effect on urinary incontinence. These analyses also confirmed previous knowledge that nerve-sparing is crucial for the return of erectile function. Surprisingly the multivariate analyses did not show a significant effect of PDE5 inhibitors on erectile function. This is probably because most patients tried PDE5 inhibitors, thus watering down the statistical effect.
The stimulation variables in the present study were chosen based on previous research regarding urinary incontinence in women 14 and on clinical data from treating post-prostatectomy incontinence with PVS at our centre (unpublished data). As described, PVS was reinitiated at catheter removal ≈ 2 weeks after RP and the stimulation period lasted 6 weeks. This means that the PVS treatment was continued until about 2 months after the surgery. Previous studies on recovery of urinary continence using electrical stimulation or magnetic innervation after RP have generally used similar short-term treatment algorithms 9,22. Meanwhile, studies on penile rehabilitation have used treatments lasting between 2 and 9 months after surgery 23–30. Interestingly, the study that used the shortest rehabilitation regimen (50 mg of sildenafil three times a week for 2 months) found an apparent effect on maintaining the integrity of the penile tissue 24, while a study using penile prostaglandin E1 injections three times a week for 3 months found an apparent clinical effect of the treatment 30. Meanwhile, several studies using longer protocols showed no clinical effects 26–29. This is indicative of the fact that we do not yet know the optimal length of penile rehabilitation programmes, and it could suggest that rehabilitation is most important in the early postoperative period. In support of this, animal studies indicate that penile changes are most pronounced in the early postoperative period 31,32. Taking this knowledge into consideration in conjunction with the fact that patient compliance with long-term penile rehabilitation programmes is known to be poor 33, we decided to maintain the relatively short treatment duration of 6 weeks after catheter removal. The only adjustment to the previous PVS protocol was that participants were instructed to begin treatment at least 1 week before surgery. This was a pragmatic decision, which was intended partly to optimize nerve function before surgery and partly to get the patients used to the PVS device.
Admittedly, the wisdom of stopping PVS ≈ 2 months after RP can be questioned in retrospect, as a major drawback of the present study is the lack of data regarding effects of long-term PVS after RP. Such knowledge would have been especially interesting, as nerve regeneration after RP is known to be slow. However, the focus of the present study was to investigate a rehabilitation modality with broad clinical applicability and, in this connection, it seemed prudent to start by investigating the effects of a relatively short period of treatment. Considering the patient acceptance, it would now be obvious to initiate studies of PVS extended for a period of more than 6 weeks in post-prostatectomy sexual rehabilitation and to experiment with longer daily treatment sessions.
With regard to the clinical applicability, the inclusion of both unilaterally nerve-spared and bilaterally nerve-spared patients and the broad use of PDE5 inhibitors warrant discussion. We allowed for these possible confounders in the design of the study as we wanted to explore PVS in the actual clinical setting after RP. Furthermore, ethical considerations prevented us from denying participants from receiving PDE5 inhibitors after surgery, as these drugs have been shown to be effective in this setting while effects of PVS were unknown 34. The lack of significant differences in the two variables between the two groups suggests that it did not influence the results of the present study. This is supported by our multivariate post hoc analyses in which we stratify for these variables. However, it is possible that there could, in fact, be a greater effect of PVS with more rigorous nerve-sparing and that the effect could be modified with daily administration of PDE5 inhibitors. Unfortunately, the sample size does not allow for meaningful post hoc analyses to explore these issues further.
Regarding the costs of PVS with the FERTI CARE vibrator, each machine is priced at ≈ €500 while the disposable plastic discs (see Fig. 1) are about €5 apiece. The device can be cleaned and sterilized between patients and can thus be reused several times. In the authors' experience there is no set maximum number of times a device can be reused. However, the battery life goes down over time and patients have been known to break the device (e.g. by dropping it or by taking it into the shower). One disc is sufficient for each patient as there is no significant wear on these. However, they are disposed of between patients for hygienic reasons.
The present study is the first of its kind and while this must be considered a significant strength, the study is not without weaknesses. Patients randomized to the PVS group had significantly more LUTS before surgery than the no-treatment group. Subsequent analyses revealed that this probably had an impact on postoperative urinary function, calling the results of the present study into question with regard to this outcome. It is also worthy of consideration that the sd was larger than expected. This means that the study population might have been too small to show a significant difference between the groups regarding erectile function, even if such a difference was present. The trends toward more potent patients and higher IIEF-5 scores at both 6 and 12 months in the PVS group (Table 2) imply that this could be the case. In general, the statistical effect of small numbers is that there is an increased risk of type 2 errors, meaning that an actual effect of a specific treatment could be missed. In this regard, one should be careful not to interpret any P value >0.05 as evidence that the null hypothesis is true in studies that might not be adequately powered. Another limitation in the study is the lack of a placebo treatment in the control group. However, due to the nature of the intervention, it was not possible to create a believable sham device, which could maintain blinding of the study subjects. For the same reason, previous randomized studies with electrical stimulation for post-prostatectomy incontinence and vacuum erection devices or injection therapy in penile rehabilitation have been conducted without placebo controls. In addition, it can be speculated that potential placebo effects of PVS applied until 2 months after RP would have faded over time, resulting in limited significance 12 months after surgery.
While post-prostatectomy PVS cannot be recommended based on the results of the present study, the borderline significant results of the study certainly justify further research in this area. In this regard, it is obvious that alternative treatment regimens are possible. In addition to an increase in the duration of the treatment, modifications to the treatment protocol should be considered. Studies have shown that stimulation of the frenulum only is sufficient to activate the dorsal penile nerve and induce ejaculation and urinary sphincter contractions in most spinal cord-injured men 11,12. However, a mode of stimulation where both the ventral and dorsal sides of the glans are stimulated could potentially increase this effect, as it has also been seen with spinal cord-injured men 35. Other modifications could include adjustments of the vibratory amplitude and frequency. However, it must be cautioned that experience from other patient groups suggests that the relatively high amplitude employed in the present study is required to induce a physiological response.
We report the first experience with PVS in the recovery of urinary continence and erectile function after nerve-sparing RP. The present study did not document a significant effect of 6 weeks of postoperative PVS. However, the method proved to be acceptable for most patients and there was a trend towards better erectile function in patients who had undergone PVS. More studies are needed to explore this possible effect further. In this regard, future research should attempt to make adjustments to the PVS protocol.
Acknowledgments
The authors would like to thank Per Rathenborg, Henrik Jakobsen, Linda Meyhoff and Tina Kiærulf Nielsen, from Herlev Hospital, and Birgit Kaa Bach from Skejby Hospital for their help with recruiting participants for the study. We would also like to thank physiotherapist Jette Steffensen form Skejby Hospital for her participation with pelvic floor muscle exercise instructions for participants at this centre. Finally, we thank Mette Schmidt at Herlev Hospital for providing invaluable help in practical aspects of the study.
Glossary
- DAN-PSS
Danish Prostate Symptom Score
- ED
erectile dysfunction
- IIEF-5
International Index of Erectile Function-5
- PVS
penile vibratory stimulation
- RP
radical prostatectomy
Conflict of Interest
The study was funded by unrestricted grants from the Velux Foundation and Grosserer L.F. Foghts Foundation. M.F. is a consultant for Eli Lilly. D.O. is a consultant for Pfizer and a speaker for Eli Lilly. J.S. is also a consultant and speaker for Eli Lilly as well as a speaker for Pfizer. J.S. is a shareholder in Multicept, Denmark.
References
- 1.Penson DF, McLerran D, Feng Z, et al. 5-Year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study. J Urol. 2008;179(5 Suppl):S40–44. doi: 10.1016/j.juro.2008.03.136. [DOI] [PubMed] [Google Scholar]
- 2.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 3.Masterson TA, Serio AM, Mulhall JP, Vickers AJ, Eastham JA. Modified technique for neurovascular bundle preservation during radical prostatectomy: association between technique and recovery of erectile function. BJU Int. 2008;101:1217–1222. doi: 10.1111/j.1464-410X.2008.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett AL. Rationale for cavernous nerve restorative therapy to preserve erectile function after radical prostatectomy. Urology. 2003;61:491–497. doi: 10.1016/s0090-4295(02)02271-9. [DOI] [PubMed] [Google Scholar]
- 5.Hammerer P, Huland H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. J Urol. 1997;157:233–236. [PubMed] [Google Scholar]
- 6.Tal R, Teloken P, Mulhall JP. Erectile function rehabilitation after radical prostatectomy: practice patterns among AUA members. J Sex Med. 2011;8:2370–2376. doi: 10.1111/j.1743-6109.2011.02355.x. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano F, Amar E, Chevallier D, Montaigne O, Joubert JM, Chartier-Kastler E. How urologists manage erectile dysfunction after radical prostatectomy: a national survey (REPAIR) by the French urological association. J Sex Med. 2008;5:448–457. doi: 10.1111/j.1743-6109.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 8.Teloken P, Mesquita G, Montorsi F, Mulhall J. Post-radical prostatectomy pharmacological penile rehabilitation: practice patterns among the international society for sexual medicine practitioners. J Sex Med. 2009;6:2032–2038. doi: 10.1111/j.1743-6109.2009.01269.x. [DOI] [PubMed] [Google Scholar]
- 9.Campbell SE, Glazener CM, Hunter KF, Cody JD, Moore KN. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev. 2012;(1) doi: 10.1002/14651858.CD001843.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Fode M, Ohl D, Ralph D, Sønksen J. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int. 112:998–1008. doi: 10.1111/bju.12228. [DOI] [PubMed] [Google Scholar]
- 11.Sonksen J, Biering-Sorensen F, Kristensen JK. Ejaculation induced by penile vibratory stimulation in men with spinal cord injuries. The importance of the vibratory amplitude. Paraplegia 2013. 1994;32:651–660. doi: 10.1038/sc.1994.105. [DOI] [PubMed] [Google Scholar]
- 12.Sonksen J, Ohl DA, Wedemeyer G. Sphincteric events during penile vibratory ejaculation and electroejaculation in men with spinal cord injuries. J Urol. 2001;165:426–429. doi: 10.1097/00005392-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Laessoe L, Sonksen J, Bagi P, et al. Effects of ejaculation by penile vibratory stimulation on bladder capacity in men with spinal cord lesions. J Urol. 2003;169:2216–2219. doi: 10.1097/01.ju.0000058770.15127.d6. [DOI] [PubMed] [Google Scholar]
- 14.Sonksen J, Ohl DA, Bonde B, Laessoe L, McGuire EJ. Transcutaneous mechanical nerve stimulation using perineal vibration: a novel method for the treatment of female stress urinary incontinence. J Urol. 2007;178:2025–2028. doi: 10.1016/j.juro.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 16.Schou J, Poulsen AL, Nordling J. The value of a new symptom score (DAN-PSS) in diagnosing uro-dynamic infravesical obstruction in BPH. Scand J Urol Nephrol. 1993;27:489–492. doi: 10.3109/00365599309182282. [DOI] [PubMed] [Google Scholar]
- 17.Shafik A, el-Sherif M, Youssef A, Olfat ES. Surgical anatomy of the pudendal nerve and its clinical implications. Clin Anat. 1995;8:110–115. doi: 10.1002/ca.980080205. [DOI] [PubMed] [Google Scholar]
- 18.Schraffordt SE, Tjandra JJ, Eizenberg N, Dwyer PL. Anatomy of the pudendal nerve and its terminal branches: a cadaver study. ANZ J Surg. 2004;74:23–26. doi: 10.1046/j.1445-1433.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- 19.Reitz A, Schmid DM, Curt A, Knapp PA, Schurch B. Afferent fibers of the pudendal nerve modulate sympathetic neurons controlling the bladder neck. Neurourol Urodyn. 2003;22:597–601. doi: 10.1002/nau.10134. [DOI] [PubMed] [Google Scholar]
- 20.Laessoe L, Sonksen J, Bagi P, et al. Effects of ejaculation by penile vibratory stimulation on bladder reflex activity in a spinal cord injured man. J Urol. 2001;166:627. doi: 10.1016/s0022-5347(05)66011-9. [DOI] [PubMed] [Google Scholar]
- 21.Farag FF, Martens FM, Rijkhoff NJ, Heesakkers JP. Dorsal genital nerve stimulation in patients with detrusor overactivity: a systematic review. Curr Urol Rep. 2012;13:385–388. doi: 10.1007/s11934-012-0273-x. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama T, Nishiguchi J, Watanabe T, et al. Comparative study of effects of extracorporeal magnetic innervation versus electrical stimulation for urinary incontinence after radical prostatectomy. Urology. 2004;63:264–267. doi: 10.1016/j.urology.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz EJ, Wong P, Graydon RJ. Sildenafil preserves intracorporeal smooth muscle after radical retropubic prostatectomy. J Urol. 2004;171(2 Pt 1):771–774. doi: 10.1097/01.ju.0000106970.97082.61. [DOI] [PubMed] [Google Scholar]
- 24.Iacono F, Prezioso D, Somma P, Chierchia S, Galasso R, Micheli P. Histopathologically proven prevention of post-prostatectomy cavernosal fibrosis with sildenafil. Urol Int. 2008;80:249–252. doi: 10.1159/000127335. [DOI] [PubMed] [Google Scholar]
- 25.Padma-Nathan H, McCullough AR, Levine LA, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20:479–486. doi: 10.1038/ijir.2008.33. [DOI] [PubMed] [Google Scholar]
- 26.Montorsi F, Brock G, Lee J, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–931. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 27.Aydogdu O, Gokce MI, Burgu B, Baltaci S, Yaman O. Tadalafil rehabilitation therapy preserves penile size after bilateral nerve sparing radical retropubic prostatectomy. Int Braz J Urol. 2011;37:336–344. doi: 10.1590/s1677-55382011000300007. [DOI] [PubMed] [Google Scholar]
- 28.Kohler TS, Pedro R, Hendlin K, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int. 2007;100:858–862. doi: 10.1111/j.1464-410X.2007.07161.x. [DOI] [PubMed] [Google Scholar]
- 29.Raina R, Agarwal A, Ausmundson S, et al. Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. Int J Impot Res. 2006;18:77–81. doi: 10.1038/sj.ijir.3901380. [DOI] [PubMed] [Google Scholar]
- 30.Montorsi F, Guazzoni G, Strambi LF, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol. 1997;158:1408–1410. [PubMed] [Google Scholar]
- 31.Klein LT, Miller MI, Buttyan R, et al. Apoptosis in the rat penis after penile denervation. J Urol. 1997;158:626–630. [PubMed] [Google Scholar]
- 32.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–1179. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 33.Salonia A, Gallina A, Zanni G, et al. Acceptance of and discontinuation rate from erectile dysfunction oral treatment in patients following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;53:564–570. doi: 10.1016/j.eururo.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Miles CL, Candy B, Jones L, Williams R, Tookman A, King M. Interventions for sexual dysfunction following treatments for cancer. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD005540.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Brackett NL, Kafetsoulis A, Ibrahim E, Aballa TC, Lynne CM. Application of 2 vibrators salvages ejaculatory failures to 1 vibrator during penile vibratory stimulation in men with spinal cord injuries. J Urol. 2007;177:660–663. doi: 10.1016/j.juro.2006.09.044. [DOI] [PubMed] [Google Scholar]


