Abstract
Background
In chronic spontaneous urticaria (CSU) mast cell activation together with inflammatory changes in the skin are well documented and may play an important role in mechanisms of tissue oedema.
Objectives
To confirm and extend these observations by measuring microvascular markers, leucocytes and mast cell numbers in lesional and uninvolved skin and to compare findings with a control group.
Methods
Paired biopsies (one from 4–8-h spontaneous weals and one from uninvolved skin) were taken from eight patients with CSU and nine control subjects and studied using immunohistochemistry and confocal microscopy using the lectin Ulex europaeus agglutinin 1 (UEA-1).
Results
Lesional skin in CSU contained significantly more CD31+ endothelial cells; CD31+ blood vessels, neutrophils, eosinophils, basophils and macrophages; and CD3+ T cells than nonlesional skin. Increased vascularity was confirmed by confocal imaging using the lectin UEA-1. Uninvolved skin from CSU contained significantly more CD31+ endothelial cells, CD31+ blood vessels and eosinophils compared with the control subjects. There was a threefold increase in mast cell numbers when CSU was compared with controls but no difference was observed between lesional and uninvolved skin.
Conclusions
Increased vascular markers together with eosinophil and neutrophil infiltration are features of lesional skin in CSU and might contribute to tissue oedema. Eosinophils and microvascular changes persist in uninvolved skin, which, together with increased mast cells, suggests that nonlesional skin is primed for further wealing.
What's already known about this topic?
Earlier studies, mainly using conventional histology, showed increases in eosinophils and neutrophils in chronic urticaria weals.
There are conflicting reports on mast cell numbers.
What does this study add?
Increases in vascularity, as well as eosinophil, neutrophil and basophil infiltration are features of lesional skin in chronic spontaneous urticaria.
Uninvolved skin may be primed as there were persisting vascular markers and eosinophils, as well as increases in mast cells.
Chronic spontaneous (‘idiopathic’) urticaria (CSU) is a common disease associated with recurrent weals and/or angio-oedema believed to result from intermittent activation of skin mast cells by unknown mechanisms.1 Wealing in patients with CSU affects the upper and mid-dermis and is characterized by a local or generalized red, raised, itchy rash with vasodilatation, increased blood flow and vascular permeability. Although functional autoantibodies against the high-affinity IgE receptor (FcεR1) have been demonstrated in about one-third of patients with CSU, suggesting an autoimmune basis, the clinical significance of this finding still remains unclear.2,3
Characteristically the lesions of CSU arise spontaneously, peak between 4 and 8 h, and generally resolve by 24 h. It is doubtful whether histamine alone can account for persistent wealing especially as antihistamines, even in high doses, are often ineffective, particularly in the severe form of the disease. In previous immunohistochemical studies, the presence of neutrophils and eosinophils in biopsies from urticarial weals was demonstrated.4,5 This raised the possibility that both neutrophil-dependent oedema, originally described by Wedmore and Williams,6 and eosinophil-mediated mast cell activation7 may be important mechanisms in vascular leakage in CSU. For example, mast cell-mediated neutrophil recruitment with vascular leakage through the NLRP3 inflammasome was reported by Nakamura et al.8 in a mouse model of urticaria. Earlier studies, using conventional histology, showed eosinophil and neutrophil infiltration in lesional, but not in uninvolved, skin in chronic urticaria.9 For all these reasons it seemed important to confirm and extend these observations using immunohistochemical methods. In addition we have used two approaches to study changes in the microvasculature, i.e. CD31 staining and confocal microscopy using the lectin Ulex europaeus agglutinin 1 (UEA-1). Thus, although urticaria is by definition a vascular reaction, with dilatation of postcapillary venules, as far as we are aware new blood vessel formation in both involved and uninvolved CSU skin has not previously been reported.
There are conflicting reports in the literature regarding the numbers of cutaneous mast cells in CSU. Early studies using morphological criteria alone reported that skin mast cells in CSU are increased up to tenfold,9,10 whereas subsequent studies using a specific mast cell antitryptase antibody did not show this difference.11 For this reason we have also re-evaluated mast cell numbers in involved and uninvolved CSU skin as well as healthy control skin.
Finally, we felt it important to determine whether uninvolved skin in CSU was ‘normal’ by determining whether inflammatory changes persisted in areas where there was no wealing. For this purpose we have compared inflammatory cell infiltration and cutaneous microvasculature in nonlesional CSU skin with healthy control skin.
Materials and methods
Patients and controls
Eight patients (five female, three male) with chronic spontaneous urticaria (age range 32–71 years; mean 51 years) as defined by the EAACI/GA2LEN/EDF/WAO guidelines12 were recruited from the urticaria clinic of the Department of Dermatology and Allergy at Charité–Universitätsmedizin Berlin. The duration of disease ranged from 9 months to 14 years. On the screening day, subjects were assessed for their suitability to enter the study, and informed consent was obtained. On the study day, patients notified the clinical investigator as soon as they observed an urticarial lesion on the thigh, trunk or upper arm. Biopsies (either spindle-shaped or 4-mm punch) were then taken from the lesions and a comparable area of nonaffected skin (i.e. skin that was normal to the naked eye, had no evidence of wealing and was not pruritic). Biopsies were not taken from linear or asymmetrical weals. Skin biopsies were also taken from nine healthy subjects (five female and four male), aged between 56 and 76 years (mean 59 years) who were recruited as controls.
None of the patients or controls had a history of concurrent autoimmune, inflammatory (including osteoarthritis and asthma) or infectious disease. None had had anaphylactic episodes. Patients with physical urticarias or urticarial vasculitis were excluded. None had taken H1 or H2 selective antihistamines for the previous 3 days. Additional exclusion criteria included the current or recent (< 4 weeks) use of oral corticosteroids or a leucotriene receptor antagonist, or the current or recent (< 6 weeks) use of doxepin or other tricyclic antidepressants, or the current or recent (< 8 weeks) use of immunosuppressants (e.g. ciclosporin, azathioprine). One patient took a single dose of prednisolone 50 mg as a rescue medication 3 days before the biopsy. At the time of the study there was no presence of, or prior history of, malignancy. Patients with acute illness, which, in the opinion of the investigator, could pose a threat or harm to the volunteer or obscure interpretation of data, were excluded. Also excluded were drug and alcohol abuse, pregnancy and lactation. The study was approved by the ethics committee of the Charite–Universitätsmedizin Berlin and the NHS Health Research Authority, U.K. (ref. 07/Q0411/41).
Tissue preparation and immunohistochemistry
Tissue biopsies were immediately fixed in 4% paraformaldehyde (BDH Chemicals Ltd, Dagenham, U.K.) in 0·1 mol L−1 phosphate-buffered saline (PBS), pH 7·4, and washed in 15% PBS-buffered sucrose, embedded in optimum cutting temperature compound and snap-frozen in isopentane precooled in liquid nitrogen. Samples were air-freighted to London on dry ice and kept at −80 °C until use. Cryostat sections (6 μm) were cut from the biopsies, mounted on 0·1% poly-l-lysine-coated slides, dried overnight at 37 °C, then stored with silica gel (VWR International Ltd, Lutterworth, U.K.) at −80 °C until used.
Immunohistochemistry
Antibody staining was detected by the alkaline phosphatase–antialkaline phosphatase (APAAP) method, as previously described.13,14 Normal human serum (10%) was used to prevent nonspecific binding of the second- and third-layer antibodies. A mouse IgG1 myeloma protein and a rabbit IgG protein were used as negative controls. Monoclonal antibodies against CD31, CD68, mast cell tryptase and neutrophil elastase were purchased from Dako Ltd (Ely, U.K.) and anti-CD3 from Becton Dickinson (Oxford, U.K.). Primary antibodies were detected with a secondary donkey antimouse antibody obtained from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA, U.S.A.). APAAP was obtained from Dako Ltd. Positively stained cells were detected with Fast Red (Sigma, Poole, U.K.). Affinity-purified polyclonal antimajor basic protein (MBP) was obtained from Dr H. Kita (Mayo Clinic, Rochester, MN, U.S.A.) and antibasogranulin monoclonal (BB1) was a gift from Dr Andrew F. Walls (University of Southampton, Southampton, U.K.). Cells were counted in a blind fashion at ×400 magnification on an Olympus BX40 microscope (Olympus Imaging & Audio Ltd, Southend-on-Sea, U.K.) connected with a Zeiss Vision KS300 imaging system (Carl Zeiss, Göttingen, Germany) and total cells expressing phenotypic markers enumerated in two entire biopsy sections. CD31+ endothelial cells were counted as described.13 For CD31+ blood vessels, we used the methods of Hashimoto et al.,14 modified by Corrigan et al.15 Microvessels were identified as CD31+ vascular endothelium forming a round or spherical structure, which was considered a CD31+ blood vessel. The total number of vessels of entire sections were counted and divided by the total areas measured by the imaging system. Data were expressed as the total numbers of cells per unit area. The mean + SD entire cross-sectional area of the cutaneous biopsy sections was 4·2 + 0·4 mm2. The between-observer coefficients of variation for duplicate counts of all markers examined for cutaneous biopsies were 4·4–6·8%.
Immunofluorescence staining for confocal microscopy
Labelling of sections with the endothelial marker, UEA-1 lectin, was performed using a modification of a protocol used previously.16 Briefly, frozen sections were thawed at room temperature for 30 min, then permeabilized and blocked in 0·1% saponin/5% human serum in PBS for 30 min at room temperature. Fluorescein isothiocyanate-conjugated UEA-1 lectin (Vector Laboratories, Peterborough, U.K.) was added to the sections for 2 h at room temperature in the dark. Slides were washed in PBS 3 × 5 min, then coverslips were mounted on sections using VectaShield Hardset mounting medium (Vector Laboratories), and stored at 4 °C. Sections were examined using a Leica SP2 confocal microscope system (Leica, Milton Keynes, U.K.) fitted with argon ion and 561-nm diode lasers, and images were acquired using the Leica confocal software.
Statistics
Data were analysed with the aid of a commercially available statistical package (Minitab for Windows release 9.2; Minitab Inc., Coventry, U.K.). Significant differences between paired data obtained from lesional and nonlesional biopsies were calculated using the Wilcoxon signed-rank test. The Mann–Whitney U–test (with Bonferroni's correction) was used to compare variance between groups. For all tests P < 0·05 was considered significant.
Results
Chronic spontaneous urticaria weals showed significant increases in eosinophils, neutrophils, basophils, macrophages, T cells, endothelial cells and blood vessels
There were significantly more MBP+ eosinophils (P = 0·01, approximate sixfold increase), elastase-positive neutrophils (P = 0·001, approximate 14-fold increase), BB1+ basophils (P = 0·04, approximate twofold increase) and CD68+ macrophages (P = 0·01, approximate 30% increase) in lesional compared with nonlesional skin of patients with CSU (Fig.1). Similar results were obtained for CD3+ T cells (P = 0·011, approximate threefold increase, data not shown). Photomicrographic examples of MBP+, elastase-positive, BB1+ cells and CD68+ macrophages in involved and uninvolved skin of patients with CSU are shown in Figure2. There were small but significant increases in CD31+ endothelial cells (P = 0·04, approximate 50% increase) and CD31+ blood vessels (P = 0·02, approximate 50% increase) when lesional was compared with nonlesional skin (Fig.3). Confocal microscopy using UEA-1 staining confirmed the increase in lesional vs. nonlesional blood vessels detected by CD31 staining (Fig.4).
Fig 1.
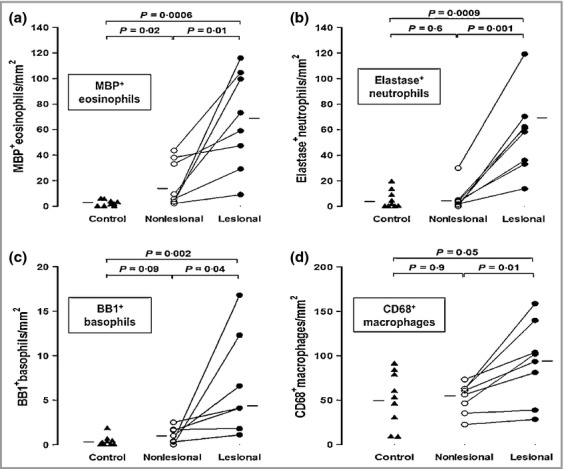
The number of (a) major basic protein (MBP)+ eosinophils, (b) elastase-positive neutrophils, (c) BB1+ basophils and (d) CD68+ macrophages in paired lesional and nonlesional skin biopsies compared with controls. The Wilcoxon signed-rank test was used to compare the differences between lesional and nonlesional skin biopsies and the Mann–Whitney U–test to compare the differences between groups.
Fig 2.
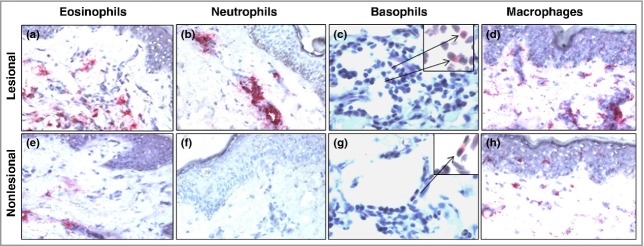
Photomicrographs of (a, e) major basic protein (MBP)+ eosinophils, (b, f) elastase-positive neutrophils, (c, g) BB1+ basophils and (d, h) CD68+ macrophages in lesional and nonlesional skin from patients with chronic spontaneous urticaria. Original magnification: ×200; inset (c) and (g): ×1000.
Fig 3.
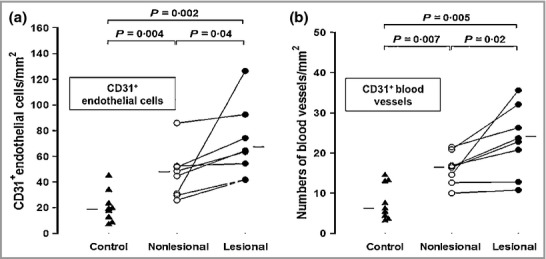
The number of (a) CD31+ endothelial cells and (b) CD31+ blood vessels in lesional and nonlesional skin compared with controls; statistics were performed as in Fig.1.
Fig 4.
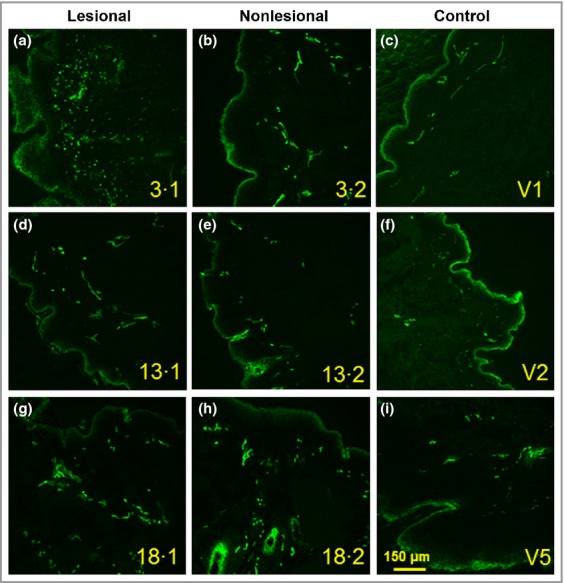
(a, b, d, e, g, h) Examples of lectin Ulex europaeus agglutinin 1 staining of blood vessels from three paired (3·1 and 3·2; 13·1 and 13·2; 18·1 and 18·2) lesional and nonlesional chronic spontaneous urticaria skin biopsies; (c, f, i) biopsies from three control subjects (V1, V2 and V5).
Mast cell and eosinophil numbers, as well as blood vessels, are increased in nonlesional chronic spontaneous urticaria skin compared with the skin of control subjects
There was an approximate threefold increase in the numbers of mast cells in nonlesional compared with control skin (P = 0·0006) (Fig.5a). Photomicrographic examples of skin mast cells are shown in Figure5b–d. There was also a significant increase in the numbers of MBP+ eosinophils (P = 0·02), but not neutrophils, basophils, T cells or macrophages, in nonlesional CSU skin compared with controls (Fig.1, and data not shown). In addition CD31+ endothelial cells and CD31+ blood vessels were significantly elevated (P = 0·004 and P = 0·007, respectively) when uninvolved skin was compared with controls (Fig.3).
Fig 5.
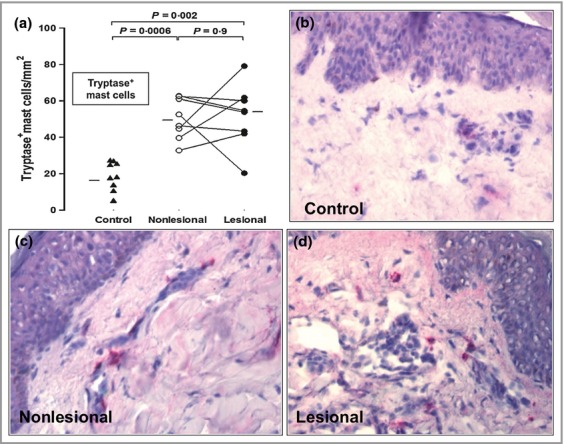
(a) The number of tryptase-positive mast cells in lesional and nonlesional skin compared with controls; statistics were performed as in Fig.1. (b–d) Photomicrographs of antitryptase staining in control (b), nonlesional (c) and lesional (d) skin; original magnification ×400.
There were no significant relationships between the duration of disease and the expression of vascular and inflammatory markers.
Discussion
The novel findings in this study are firstly that in CSU, lesional skin has significantly more CD31+ endothelial cells and CD31+ blood vessels, as well as eosinophils and neutrophils, than uninvolved skin, supporting the view that inflammatory cell infiltration contributes to vascular leakage. Basophils, macrophages and T cells were also increased in lesional compared with nonlesional skin but these differences were less marked than with eosinophils and neutrophils. The second novel finding was the observation that uninvolved skin, unlike skin from the control group, appeared to have persisting eosinophils and CD31+ endothelial cells and CD31+ blood vessels on a background of raised mast cell counts. The latter findings suggest that nonlesional skin, unlike ‘normal’ skin, may be primed for further wealing. Although the number of subjects in the study group was small, the differences in the various markers measured were statistically significant and often substantial.
Although histamine is clearly a key mediator in CSU it is unlikely to account solely for the entire wealing process characteristically observed over a 24-h period. Biopsies from CSU weals have several pathological features in common with the allergen-induced late-phase allergic skin reaction (LPR). In order to support the view that the LPR can serve as a model for CSU we considered it important to take biopsies from weals of > 4 h duration (when the LPR is established and the early histamine reaction is resolved) and < 8 h (when the LPR begins to decline). Sabroe et al.5 observed more neutrophils and eosinophils in lesional compared with uninvolved skin. However, they took biopsies from weals at < 4 h and > 12 h. The differences they reported, although significant, were not as marked as those observed in the 4–8-h biopsies used in the present study. In contrast to our own findings, these same investigators did not find an increase in eosinophils in uninvolved skin. However, the affinity-purified polyclonal anti-MBP used in the present study17 appeared to be more stringent in detecting tissue eosinophils. Polyclonal anti-MBP antibody detects multiple epitopes on MBP molecules and thus may allow us to identify eosinophils with greater sensitivity than with a monoclonal antibody. Furthermore, close examination of the photomicrograph reveals diffuse extracellular staining of eosinophil MBP, particularly in lesional skin, suggesting that some component(s) of eosinophils are released into the tissues. Elevated serum concentrations of eotaxin, a potent eosinophil chemotactic factor, have previously been identified in CSU and presumably reflect ongoing mast cell activation.18 Whether eosinophils and their products contribute directly to wealing in CSU, or act more as repair cells, is presently unclear. Eosinophil-derived MBP does have direct, nonspecific, mast cell degranulating properties that may be relevant to mechanisms.7
We propose that in CSU mast cell activation leads to wealing by at least two mechanisms, i.e. histamine release and inflammatory cell infiltration producing neutrophil-dependent oedema. At the same time angiogenic factors, particularly vascular endothelial growth factor (VEGF), are expressed. VEGF is known to be released by activated mast cells and has a rapid effect on endothelial cells.19 We (article submitted for publication), and others,18 have shown VEGF expression in the skin of CSU. Thus increased VEGF brings about new blood vessel formation as shown in our present study by increases in CD31+ endothelial cells and CD31+ blood vessels. It is assumed that when urticarial weals resolve, either spontaneously or as the result of treatment, so do the numbers of inflammatory cells and CD31+ endothelial cells and blood vessels. However, as shown in Figure3, the number of CD31+ endothelial cells and CD31+ blood vessels did not return to baseline levels, i.e. to those of the control group.
We have confirmed our previous findings of low numbers of basophils in biopsies from CSU4 and here extend this to demonstrate slightly higher numbers in lesional compared with nonlesional skin. The significance of this observation is unclear, as the overall numbers are small compared with neutrophils and eosinophils. However, we cannot exclude a role for basophil products in vascular leakage events. The recruitment of basophils to skin lesions in patients with CSU may explain the basopenia commonly seen in these patients.20
Although urticaria is by definition a vascular reaction, with dilatation of postcapillary venules, as far as we are aware this is the first demonstration of new blood vessel formation in both involved and uninvolved CSU skin. We were surprised that new blood vessel formation appeared to occur quite rapidly, i.e. in 4–8-h weals. However, in an earlier study using human endothelial cell cultures, VEGF-induced early endothelial cell activation, as shown by enhanced inositol phosphate accumulation, occurred within 5 min, and increased P-selectin expression was demonstrated within 15 min.19 Previous studies identified elevations of P- and E-selectin, and intercellular adhesion molecule-1 in both lesional and nonlesional skin, indicating that in this disease small vessels are ‘primed’ even at baseline.21 The present study supports these findings in that there was greater expression of CD31+ endothelial cells and CD31+ blood vessels in lesional compared with nonlesional skin, and nonlesional skin compared with controls, observations confirmed by confocal imaging with the lectin UEA-1. Therefore, uninvolved skin is not normal as there are persisting eosinophils, increased mast cell numbers and a greater number of blood vessels compared with controls. We found no association between the duration of disease and the expression of vascular markers, indicating that these changes occur within months rather than years following the onset of symptoms.
In conclusion, we have shown that vascular markers, together with eosinophil and neutrophil infiltration, are the dominant features of lesional skin in CSU and may contribute to tissue oedema. On the other hand, eosinophils and microvascular changes persist in uninvolved sites and might, together with increased mast cells, prime skin for further wealing.
Acknowledgments
We are grateful to Dr Qiu Meng for technical assistance, Dr Lars Brunken and Dr Joachim Fluhr for help in collecting skin biopsies and Dr Anna Chapman for input with the protocol design.
References
- 1.Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664–72. doi: 10.1067/mai.2000.105706. [DOI] [PubMed] [Google Scholar]
- 2.Hide M, Francis DM, Grattan CE, et al. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599–604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 3.Eckman JA, Hamilton RG, Gober LM, et al. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol. 2008;128:1956–63. doi: 10.1038/jid.2008.55. [DOI] [PubMed] [Google Scholar]
- 4.Ying S, Kikuchi Y, Meng Q, et al. Th1/Th2 cytokines and inflammatory cells in skin biopsies from chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002;109:694–700. doi: 10.1067/mai.2002.123236. [DOI] [PubMed] [Google Scholar]
- 5.Sabroe RA, Poon E, Orchard GE, et al. Cutaneous inflammatory cell infiltrate in chronic idiopathic urticaria: comparison of patients with and without anti-FcεRI or anti-IgE autoantibodies. J Allergy Clin Immunol. 1999;103:484–93. doi: 10.1016/s0091-6749(99)70475-6. [DOI] [PubMed] [Google Scholar]
- 6.Wedmore CV, Williams TJ. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981;289:646–50. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- 7.Piliponsky AM, Gleich GJ, Bar I, Levi-Schaffer F. Effects of eosinophils on mast cells: a new pathway for the perpetuation of allergic inflammation. Mol Immunol. 2002;38:1369. doi: 10.1016/s0161-5890(02)00090-1. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Kambe N, Saito M, et al. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J Exp Med. 2009;206:1037–46. doi: 10.1084/jem.20082179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas N, Toppe E, Henz BM. Microscopic morphology of different types of urticaria. Arch Dermatol. 1998;134:41–6. doi: 10.1001/archderm.134.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Natbony SF, Phillips ME, Elias JM, et al. Histologic studies of chronic idiopathic urticaria. J Allergy Clin Immunol. 1983;71:177–83. doi: 10.1016/0091-6749(83)90096-9. [DOI] [PubMed] [Google Scholar]
- 11.Smith CH, Kepley C, Schwartz LB, Lee TH. Mast cell number and phenotype in chronic idiopathic urticaria. J Allergy Clin Immunol. 1995;96:360–4. doi: 10.1016/s0091-6749(95)70055-2. [DOI] [PubMed] [Google Scholar]
- 12.Zuberbier T, Asero R, Bindslev-Jensen C, et al. Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA2LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–43. [Google Scholar]
- 13.Ying S, Robinson DS, Meng Q, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma and their association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–16. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Tanaka H, Abe H. Quantitative analysis of bronchial wall vascularity in the medium and small airways of patients with asthma and COPD. Chest. 2005;127:965–72. doi: 10.1378/chest.127.3.965. [DOI] [PubMed] [Google Scholar]
- 15.Corrigan CJ, Wang W, Meng Q, et al. T-helper cell type 2 (Th2) memory T cell-potentiating cytokine 25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci USA. 2011;108:1579–84. doi: 10.1073/pnas.1014241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams MJ, Lowrie MB, Bennett JP, et al. Cadherin-10 is a novel blood-brain barrier adhesion molecule in human and mouse. Brain Res. 2005;1058:62–72. doi: 10.1016/j.brainres.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 17.Leiferman KM, Ackerman SJ, Sampson HA, et al. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med. 1985;313:282–5. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- 18.Tedeschi A, Asero R, Lorini M, et al. Serum eotaxin levels in patients with chronic spontaneous urticaria. Eur Ann Allergy Clin Immunol. 2012;44:188–92. [PubMed] [Google Scholar]
- 19.Hippenstiel S, Krüll M, Ikemann A, et al. VEGF induces hyperpermeability by a direct action on endothelial cells. Am J Physiol. 1998;274:L678–84. doi: 10.1152/ajplung.1998.274.5.L678. [DOI] [PubMed] [Google Scholar]
- 20.Grattan CE, Walpole D, Francis DM, et al. Flow cytometric analysis of basophil numbers in chronic urticaria: basopenia is related to serum histamine releasing activity. Clin Exp Allergy. 1997;27:1417–24. doi: 10.1046/j.1365-2222.1997.1630972.x. [DOI] [PubMed] [Google Scholar]
- 21.Zuberbier T, Schadendorf D, Haas N, et al. Enhanced P-selectin expression in chronic and dermographic urticaria. Int Arch Allergy Immunol. 1997;114:86–9. doi: 10.1159/000237648. [DOI] [PubMed] [Google Scholar]


