ABSTRACT
The cytokinetic furrow is organized by the RhoA GTPase, which recruits actin and myosin II to the furrow and drives contractility. Here, we show that the RhoA GTPase-activting protein (GAP) p190RhoGAP-A (also known as ARHGAP35) has a role in cytokinesis and is involved in regulating levels of RhoA-GTP and contractility. Cells depleted of p190RhoGAP-A accumulate high levels of RhoA-GTP and markers of high RhoA activity in the furrow, resulting in failure of the cytokinetic furrow to progress to abscission. The loss of p190RhoGAP-A can be rescued by a low dose of the myosin II inhibitor blebbistatin, suggesting that cells fail cytokinesis because they have too much myosin activity. p190RhoGAP-A binds the cytokinetic organizer anillin, and mutants of p190RhoGAP-A that are unable to bind anillin or unable to inactivate RhoA fail to rescue cytokinesis defects in p190RhoGAP-A-depleted cells. Taken together, these data demonstrate that a complex of p190RhoGAP-A and anillin modulates RhoA-GTP levels in the cytokinetic furrow to ensure progression of cytokinesis.
KEY WORDS: p190RhoGAP, Anillin, Myosin II, Cytokinesis, Rho
INTRODUCTION
Cytokinesis is the final step in cell division where formation and ingression of the cytokinetic furrow results in separation of two daughter cells. The process initiates in anaphase, continues throughout telophase when membrane invagination at the equatorial cell cortex occurs and is completed upon membrane abscission (Glotzer, 2001). The contractile forces required for furrow ingression are provided by a ring of filamentous actin and myosin II that are juxtaposed to the cell membrane at the equator of the dividing cell. Assembly and regulation of this contractile ring is crucial for achieving proper cell division and is under the control of the small GTPase RhoA (Piekny et al., 2005).
Early in cytokinesis, RhoA localizes to the site of the nascent cytokinetic furrow (Nishimura et al., 1998; Takaishi et al., 1995), where its activity is required for cytokinetic furrow formation and contraction, as well as progression through cytokinesis (Bement et al., 2006). During early anaphase, RhoA-GTP levels increase at the contractile ring (Kimura et al., 2000; Maddox and Burridge, 2003; Yoshizaki et al., 2004), and active RhoA mediates contractile ring assembly through its downstream effectors, mDia2 and ROCK I/II kinases (Piekny et al., 2005). mDia2 functions as an actin nucleator that induces the polymerization of long unbranched actin filaments (Watanabe et al., 2008), along with microtubule alignment and stabilization (Narumiya and Yasuda, 2006). ROCK kinases activates the contractile mechanism of myosin II by phosphorylating Thr18 and Ser19 of the regulatory light chain of myosin II (MYL2, hereafter called MLC II) (Matsumura, 2005). Phosphorylation of these sites on MLC II triggers the motor (ATPase) activity of myosin II, which in turn promotes crosslinking with newly created actin filaments to form a fully functional contractile ring (Vavylonis et al., 2008; Wu et al., 2006) that provides the mechanical force required for furrow contraction and ingression (Bresnick, 1999).
Actomyosin filaments are assembled on a network of cytoskeletal proteins at the cell cortex, which connect the filaments to the plasma membrane. Anillin, an actin-binding protein, is a crucial component of this scaffold that is required for cytokinesis (Oegema et al., 2000; Piekny and Glotzer, 2008; Liu et al., 2012). Anillin has been shown to interact with actin, myosin, microtubules, septin, mDia Ect2, MgcRacGAP (also known as Cyk4 and RacGAP1), citron kinase and RhoA (Gai et al., 2011; Piekny and Maddox, 2010). In addition, overexpression of anillin increases RhoA activation, suggesting that anillin functions in the regulation of RhoA during cytokinesis (Suzuki et al., 2005). Taken together, these findings support the model that anillin plays a vital role in linking the structural components of the contractile ring to RhoA-regulated signaling proteins, which govern cytokinesis.
RhoA is a molecular switch that cycles between active (GTP-bound) and inactive (GDP-bound) states. Transitions between activation states are facilitated by guanine-nucleotide-exchange factors (GEFs) (activators), GTPase-activating proteins (GAPs) (inactivators) and guanine-nucleotide-dissociation inhibitors (GDIs) (inactivators) (Jaffe and Hall, 2005). MgcRacGAP is a major GAP known to function in cytokinesis (Zhao and Fang, 2005; Loria et al., 2012). It binds with the mitotic kinesin-like protein MKLP1 (also known as Zen4 and KIF23), to form the heterotetrameric centralspindlin complex, a key structural component of the spindle midzone (Mishima et al., 2002). Centralspindlin carries out several crucial functions during cell division, including bundling of antiparallel microtubules to form the central spindle (Jantsch-Plunger et al., 2000), positioning of the cell division plane, and promoting cycles of RhoA GTPase activity (D'Avino et al., 2005). The GAP domain of MgcRacGAP limits the amount of RhoA-GTP at the furrow and is required for cytokinesis to occur correctly (Miller and Bement 2009). In early cytokinesis, MgcRacGAP also activates RhoA through complex formation with the GEF Ect2 (Somers and Saint, 2003, Yüce et al., 2005, Glotzer, 2009). Recently it has been shown that MgcRacGAP can bind the plasma membrane through its C1 domain and promotes cortical contractility and the anchoring of the cytokinetic furrow to the midbody (Lekomtsev et al., 2012). Although it is undisputed that MgcRacGAP is a crucial cytokinesis regulator, it is not clear whether it acts as a RhoGAP or a RacGAP or both because there is also evidence that MgcRacGAP inhibits Rac activity during cytokinesis (D'Avino et al., 2004, Canman et al., 2008, Bastos et al., 2012). A second RhoGAP, M phase GAP (MP-GAP, also known as ARHGAP11A), has been shown to repress ‘blebbing’ in mitotic cells, and cells depleted of MP-GAP fail in cytokinesis ∼18% of the time (Zanin et al., 2013).
Our previous findings suggest that mammalian cells have an additional level of RhoA regulation during cytokinetic furrow progression that is mediated by p190RhoGAP-A (also known as ARHGAP35, and hereafter called p190) (Su et al., 2003; Mikawa et al., 2008). p190 localizes to the cytokinetic furrow (Su et al., 2003), and cells ectopically expressing the protein exhibit reduced RhoA-GTP levels in the furrow and become multinucleated, supporting the idea that proper cycling of RhoA between active and inactive states is required for completion of cytokinesis (Su et al., 2009). Moreover, in the absence of ectopic expression, it has been demonstrated that a transient, proteasome-mediated partial reduction (50%) of endogenous p190 levels during anaphase is required for completion of cytokinesis (Manchinelly et al., 2010; Su et al., 2003), suggesting that p190 levels and activities are also crucial for cytokinesis. During the current study, we overcame a technical challenge that has allowed us to characterize the loss-of-function phenotypes of p190 in cytokinesis. We report that cells depleted of p190 have increased levels of RhoA-GTP in the cytokinetic furrow and often fail to divide. Low concentrations of the myosin II inhibitor blebbistatin can rescue loss of p190, suggesting that cells fail cytokinesis because they have increased contractility. Additionally, p190 physically associates with anillin, and mutants of p190 that are unable to interact with anillin fail in cytokinesis. Evidence is presented to indicate that the interaction between p190 and anillin is regulated by contractile forces, as the binding is disrupted by the addition of the myosin II inhibitor blebbistatin. Taken together, these data demonstrate that p190 acts as a RhoGAP during cytokinesis in mammalian cells and provide evidence that MLC II, anillin and p190 act in a feedback loop that modulates RhoA GTPase activity in the cytokinetic furrow.
RESULTS
p190 is required for proper cytokinesis in HeLa cells
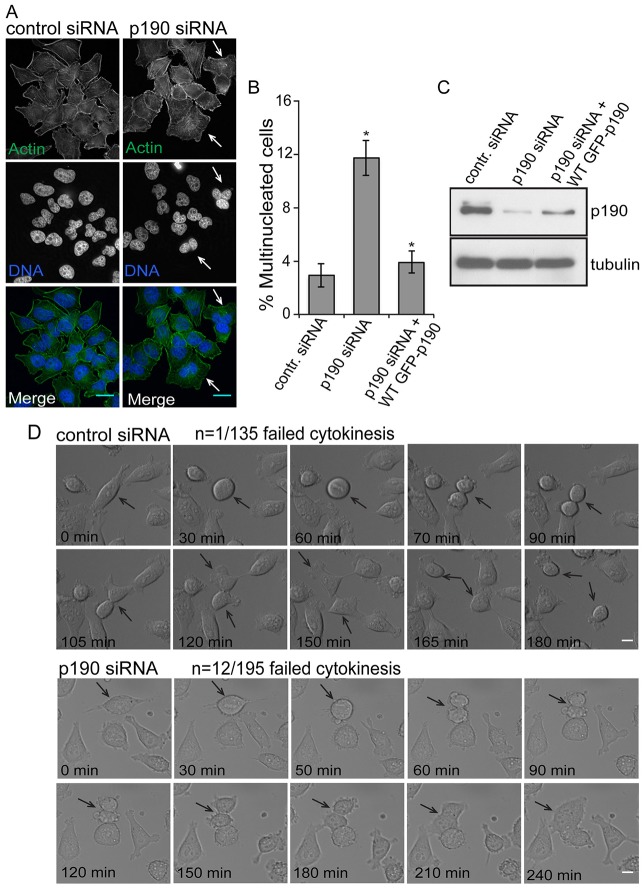
To determine whether depletion of p190 causes failure of cytokinesis, HeLa cells were transfected with p190-specific small interfering RNAs (siRNAs) for 48 hours, and the number of multinucleated cells were quantified (Fig. 1A,B). A total of 12–15% of p190-depleted cells were multinucleated, which is significantly higher compared to control-siRNA-treated cells (n>200). Fig. 1C shows the level of p190 depletion after 48 hours of siRNA treatment. To determine whether exogenous p190 expression could restore normal cell division, we generated a stable, wild-type p190 doxycycline-inducible HeLa cell line that is resistant to siRNA depletion. Replacement of endogenous p190 with the doxycycline-inducible wild-type p190 rescued the multinucleation phenotype, thereby addressing concerns about off-target siRNA effects and demonstrating the requirement of p190 for productive cytokinesis (Fig. 1B,C; supplementary material Fig. S1A).
Fig. 1.
p190 is required for cytokinesis. (A) Depletion of p190 results in multinucleation because of cytokinesis failure. 48 hours after LacZ (control) or p190 siRNA treatment HeLa cells were processed for immunofluorescence for actin staining with phalloidin (green) and DNA staining with DAPI (blue). Arrows indicate multinucleated cells. Scale bars: 10 µm. (B) Quantification of multinucleation in HeLa cells depleted of p190 by siRNA. Cells were depleted of p190 and, as indicated, simultaneously also treated with 1 µg/ml of doxycycline to induce expression of wild-type p190. 48 hours post-transfection and doxycycline treatment, cells were fixed and stained with phalloidin (actin) or DAPI (DNA) and scored blindly for binucleation or multinucleation (n≥300). Results are mean±s.d. from three independent experiments. *P<0.001 (Student's t-test ) for both p190 siRNA-treated cells compared to control, and p190 siRNA-treated cells compared to cells rescued by wild-type p190. (C) Immunoblot to measure relative p190 levels. (D) DIC live-cell imaging of LacZ- or p190-siRNA-treated HeLa cells. Recording started after 24 hours transfection of either siRNA. The top two panels represent LacZ-siRNA-treated cells, and the bottom two panels represent p190-siRNA-treated cells. Arrows indicate single cell divisions after treatment with either siRNA. 195 cells treated with p190 siRNA and 135 cells treated with control siRNA were scored, and the number of multinucleated cells/total number of cells was quantified. Scale bars: 10 µm.
To further characterize the cytokinetic defect caused by p190, we followed the behavior of p190-depleted HeLa cells by live-cell differential interference contrast (DIC) microscopy (Fig. 1D; supplementary material Movies 1, 2). p190 depletion did not affect cytokinetic furrow formation and initial ingression, processes that are regulated by MgcRacGAP (Zhao and Fang, 2005). However, p190-depleted cells often failed in abscission, and cells became multinucleated. The most striking phenotype of p190-depleted cells in telophase was an inability to complete furrow contraction. In addition, telophase control cells usually migrated in opposite directions and this movement was lost in p190-depleted cells. Quantification of the live-cell imaging showed that 7% of p190-siRNA-treated cells (12/195) failed cytokinesis compared to 1% of control-siRNA-treated cells (1/135), which is similar to the failure rate measured in fixed cells. We conclude that p190 is required for optimal cytokinesis.
p190 regulates RhoA-GTP during cytokinesis in HeLa cells
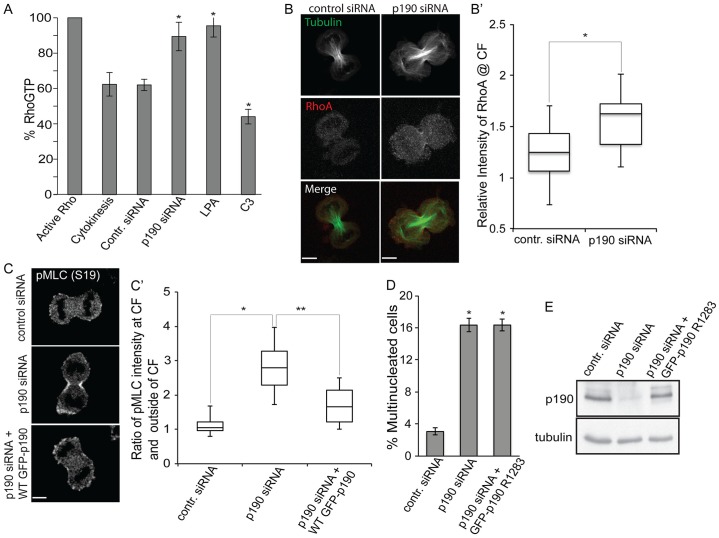
p190 is a RhoGAP that downregulates RhoA-GTP levels in interphase cells (Ridley et al., 1993); however, the effect of loss of p190 on RhoA-GTP levels in cells undergoing cytokinesis had not been measured. To address this question, p190-depleted HeLa cells were synchronized in nocodazole for 14–16 hours and released into drug-free medium for 40 minutes to enrich for cells in late mitosis and undergoing cytokinesis. Lysates were subjected to a RhoA G-LISA assay, a highly quantitative colorometric assay to measure the amount of RhoA-GTP. Fig. 2A shows that p190-depleted cells in cytokinesis exhibited significantly elevated RhoA-GTP levels compared to those of untreated (cytokinetic) cells or cells treated with a control Luciferase siRNA or with C3, a toxin that efficiently inhibits RhoA activity. Moreover, RhoA-GTP levels in p190-depleted cytokinetic cells were similar to those of cells treated with lysophosphatidic acid (LPA), a potent activator of RhoA. To determine whether RhoA levels are increased at the cytokinetic furrow in p190-depleted cells, we fixed cells with TCA and immunostained for RhoA. TCA precipitation is a validated method to preserve RhoA bound to membranes for immunofluorescence (Yonemura et al., 2004). We found significantly higher concentrations of RhoA at the cytokinetic furrow in cells late in cytokinesis after p190 depletion (Fig. 2B,B′). Thus, silencing of p190 affected both localization of RhoA and the levels of RhoA-GTP in cells undergoing cytokinesis. This increase is similar to the level of RhoA-GTP measured in the furrow by FRET following ectopic expression of a dominant negative form of p190 in HeLa cells (Su et al., 2009).
Fig. 2.
p190 acts as a Rho GAP in the cytokinesis furrow. (A) p190-depleted cells have increased RhoA-GTP levels in the cytokinetic furrow. RhoA-GTP levels were measured with the colorimetric RhoA G-LISA assay. Active RhoA-GTP protein was used as a positive control and set at 100%. Results are expressed as the mean±s.e.m. percentage of RhoA-GTP, n = 4. *P<0.05 (Student's t-test) as compared to cytokinetic cells. (B) Confocal images of HeLa cells during cytokinesis stained for RhoA and tubulin 48 hours after transfection of siRNA targeted against LacZ (control siRNA) or p190. Images shown are representative of n>20. Scale bars: 7 µm. (B′) Quantification of RhoA intensities at the cytokinetic furrow (CF). n>20, *P = 0.0002 (Student's t-test). The box represents the 25–75th percentiles, and indicates the median. The whiskers show the range. (C) Confocal images of pMLC II in wild-type p190 doxycycline-inducible HeLa cells during cytokinesis and at 48 hours after treatment with control siRNA (LacZ), p190 siRNA and p190 siRNA with doxycycline treatment. Fixed cells were stained with anti-pSer19 MLC II antibody. Images shown are representative of n>20. Scale bars: 4.5 µm. (C′) Quantification of pMLC at the cytokinetic furrow. Box-and-whisker plot quantifying the ratio of intensity of pMLC II at the cytokinetic furrow and outside region of cytokinetic furrow. n>2 with >20 cells/experiment. *, **P<0.005 as compared to LacZ-siRNA-treated cells. (D) A GAP mutant of p190 does not rescue p190-silencing-induced multinucleation. Cells were depleted of p190 and simultaneously were treated with 1 µg/ml of doxycycline to re-express the doxycycline-inducible GAP mutant of p190. Failed cytokinesis was scored as the percentage of binucleated or multinucleated interphase cells (n≥300). Results are mean±s.d. from three independent experiments. *P<0.0001 for both p190 siRNA-treated cells and cells rescued by p190 GAP mutant (p190 R1283A) compared to control. Note that the experiment in Fig. 1B was performed under identical conditions and at the same time, and acts as a control for rescue with wild-type protein. (E) Immunoblot to mesure relative p190 levels. HeLa cells were depleted of endogenous p190 and a GAP mutant of p190 (p190 R1283A) was expressed by the addition of doxycyclin where indicated. Cell lysates were prepared for immunoblot analysis 48 hours post-transfection.
An independent measure of RhoA-GTP levels is the phosphorylation of myosin II light chain (MLC II) on Thr18 and Ser19, which we assessed by using antibodies against MLC II phosphorylated on Ser19 (anti-pSer19 MLC II antibody) and antibodies against MLC II phosphorylated on both Thr18 and Ser19 (anti-pThr18/Ser19 MLC II antibody). Both antibodies recognize cytokinetic furrows, and have different staining patterns as anti-pSer19 MLC II antibody stains phosphorylated MLC II (pMLC II) localized only to the contracting membrane, whereas anti-pThr18/Ser19 antibody also stains pMLC localized to the midzone (Kondo et al., 2012). Cells were stained with anti-pSer19 MLC II antibody, the intensity of staining at cytokinetic furrows was quantified (areas quantified are outlined in supplementary material Fig. S1C), and the ratio of pMLC II at the cytokinetic furrow and outside of the furrow area was determined. We observed an approximate twofold increase in specific MLC II Ser19 phosphorylation in the furrows of p190-depleted cells as compared to controls (Fig. 2C,C′). To confirm that the increase in pMLC at the cytokinetic furrow is not due to an increase in total myosin, cells were co-stained with antibodies to anti-pThr18/Ser19 MLC II and total MLC II, the intensity of staining of both antibodies at the cytokinetic furrow was quantified and the ratio of pMLC II:MLC II was determined (supplementary material Fig. S1D,D′). We see an increase in RhoA activity measured in this manner using pMLC II antibodies against MLC II pSer19 or MLC II pThr18 and pSer19 (Fig. 2C,C′; supplementary material Fig. S1D). Moreover, the increase in phosphorylation of MLC II at the cytokinetic furrow due to p190 depletion could be rescued by exogenously expressing siRNA-resistant wild-type p190 (Fig. 2C,C′), ruling out siRNA off-target effects.
To further investigate whether p190 downregulates RhoA-GTP levels through its GAP activity, we generated a stable doxycycline-inducible HeLa cell line that expressed a p190 (R1283A) point mutant that is defective in GAP activity (Li et al., 1997) (supplementary material Fig. S1B). Cells expressing this mutant were analyzed for their ability to rescue the cytokinesis defect associated with depletion of endogenous p190. The number of multinucleated cells was significantly decreased after rescue by exogenous wild-type (Fig. 1B) but not the GAP mutant p190 (Fig. 2D). The expression level of p190 (R1283A) was similar to that of the endogenous protein (Fig. 2E). We conclude that p190 acts as a RhoGAP to downregulate RhoA-GTP in the cytokinetic furrow.
Overexpression of p190 decreases RhoA-GTP at the furrow
Overexpression of p190 had the opposite effect to that of depletion on RhoA-GTP levels in the furrow. HeLa cells were transiently transfected with GFP–p190 or vector control plasmid, immunostained with anti-pThr18/Ser19 MLC II and anti-MLC II antibodies 24 hours later, and examined by fluorescent confocal microscopy. Approximately 90% of cells showed reduced phosphorylation of MLC II in their cytokinetic furrows as compared to vector-transfected controls (Fig. 3A,B; supplementary material Fig. S1E). Moreover, the ratio of pMLC II:MLC II was ∼twofold lower (Fig. 3C,D). These effects were similar to those seen after depletion of anillin or addition of C3 toxin, both of which have been previously shown to affect RhoA activation at the cytokinetic furrow (Aktories et al., 1989; Piekny and Glotzer, 2008). Neither p190 overexpression nor C3 treatment affected the levels of total RhoA protein (supplementary material Fig. S1F), nor did p190 overexpression affect the localization of RhoA, actin, Aurora B or microtubules (supplementary material Fig. S1G), suggesting that p190 specifically affected RhoA GTPase activity during cytokinesis.
Fig. 3.
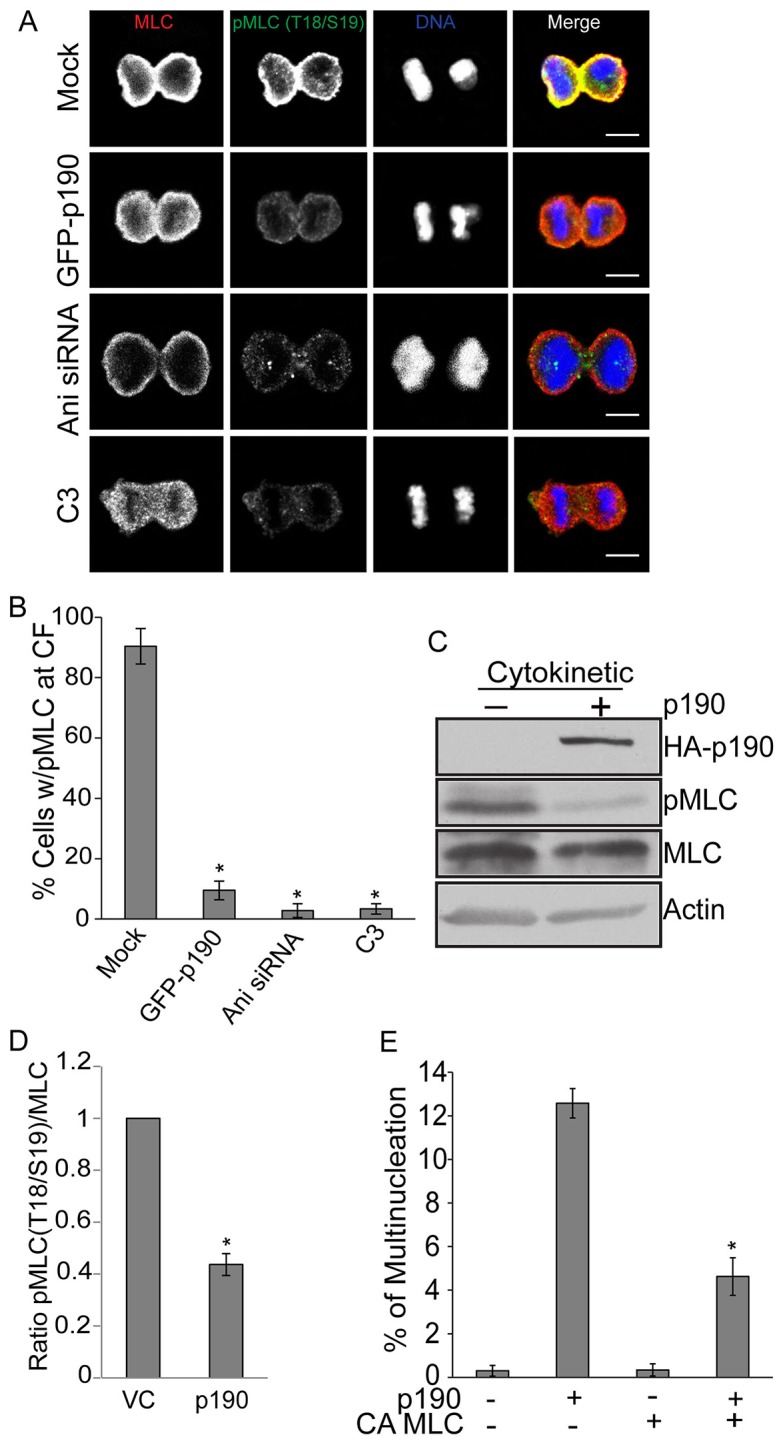
Overexpression of p190 decreases RhoA-GTP levels. (A) Confocal images of total MLC II and pMLC II in HeLa cells during cytokinesis. HeLa cells were mock-treated or transiently transfected with empty plasmid or p190 plasmid. Separate cultures were treated with anillin siRNA or C3 toxin. Cells were then fixed and stained for total MLC II (red), pThr18/Ser19 MLC II (green) and DNA (blue). Images shown are representative of n>30. Scale bars: 5 µm. (B) Quantification of the number of HeLa cells with enriched pMLC II at the cytokinetic furrow during cytokinesis. Results are expressed as the mean±s.e.m. percentage of total cells in cytokinesis or cytokinetic cells positive for GFP–p190 that exhibited localization of pMLC II at the cytokinetic furrow (CF). n>3, with >15 cells/experiment. *P<0.005 (Student's t-test) as compared to mock. (C) HeLa cells were either mock-treated (–) or transiently transfected with HA–p190 plasmid (+). Cells were synchronized by nocodazole block and release and the levels of total MLC II, pMLC II, and HA–p190 were assessed by western blot analysis. (D) Quantification of C. Overexpression of p190 decreases RhoA activity as measured by pMLC II. Levels were normalized to actin, and the ratio of pMLC II:total MLC II was then determined and normalized to the ratio in vector-transfected (VC) cells, which was set to 1. Results are expressed as the mean±s.e.m. fold ratio over that of VC cells, n>3. *P<0.05 as compared to VC. (E) Constitutively active (CA) MLC II rescues multinucleation induced by p190 overexpression. HeLa cells were mock-treated or transiently transfected with HA–p190 or CA MLC II plasmids singly or in combination, and scored for multinucleation. Results are expressed as the mean±s.e.m. percentage of total cells (Mock) or cells positive for HA–p190 and or CA MLC II that were multinucleate, n>3, with >90 cells/experiment. *P<0.005 as compared to mock.
We tested whether constitutively active MLC II [MLCII (T18D, S19D); CA-MLC-II] could rescue the multinucleation phenotype generated by p190 overexpression to determine whether reduced activation of myosin II caused the failure in cytokinesis. Co-transfection of p190 and CA MLC II significantly decreased the amount of p190-induced multinucleation, whereas transfection of CA-MLC II alone had little effect (Fig. 3E). Taken together, these data demonstrate that p190 is required for the completion of cytokinesis in HeLa cells, where it acts as a RhoGAP to down-modulate RhoA-GTP and pMLC II levels to modulate proper cytokinetic furrow contraction.
Low doses of blebbistatin can rescue loss of p190
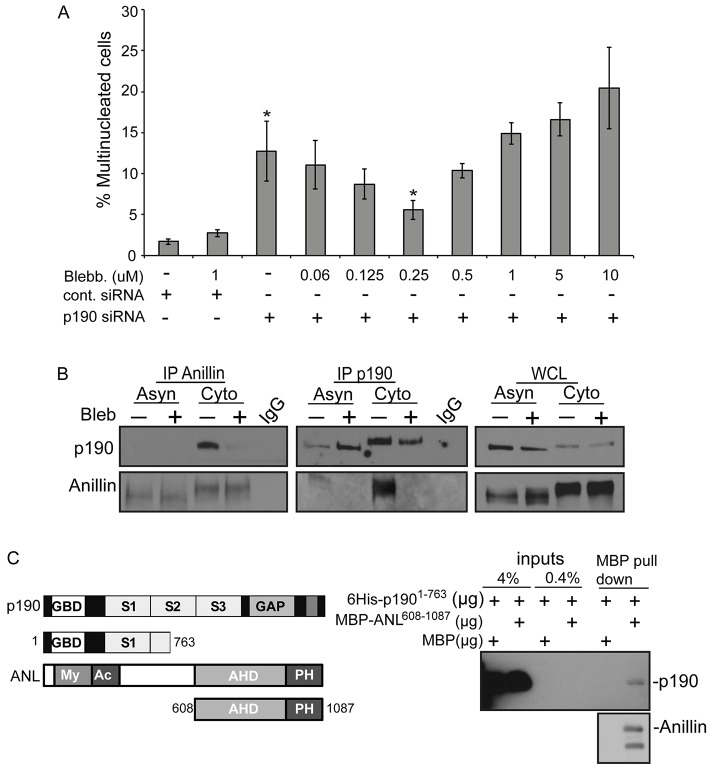
Given that we find higher levels of RhoA activity at the cytokinetic furrow, we tested whether high contractility caused the cytokinesis failure associated with p190 depletion. Specifically, we asked whether low concentrations of the myosin II inhibitor blebbistatin, which weakens contractility, could rescue the multinucleation phenotype generated by p190 depletion. HeLa cells were synchronized using a double-thymidine block, and endogenous p190 was depleted by siRNA for 48 hours. At 8 hours after release from thymidine, HeLa cells were treated for 6 hours with concentrations of blebbistatin below the amount used to fully inhibit myosin activity. Cells were then fixed and prepared for confocal microscopy analysis and scored for multinucleation (Fig. 4A). Low concentrations of blebbistatin rescued p190 depletion in a concentration-dependent manner with 0.25 µM giving the best rescue. Higher concentrations of blebbistatin generated multinucleated cells, as one would expect for full inhibition of myosin. To complement this experiment, we also inhibited ROCK I/II kinases, which is a RhoA-dependent activator of myosin, with the inhibitor Y27632. A dose of Y27632 that was below the concentration used to fully inhibit ROCK activity also partially rescued the generation of multinucleated cells after p190 depletion (supplementary material Fig. S2A). The fact that low doses of blebbistatin and Y27632 both rescue p190 depletion argues that cells depleted of p190 fail cytokinesis because of too much RhoA-stimulated myosin activity.
Fig. 4.
p190 and anillin interact during cytokinesis. (A) p190-dependent multinucleation phenotype was rescued by the myosin II inhibitor blebbistatin. Failed cytokinesis events were measured by assessing the percentage of binucleated or multinucleated interphase cells relative to the total population of cells (n≥300). Results are mean±s.d. from three independent experiments. *P<0.005 for p190-siRNA-treated cells compare to Luc-siRNA-treated cells, and *P<0.03 for p190 siRNA compared to p190-siRNA-treated cells in 0.25 µM blebbistatin. (B) Co-immunoprecipitation of anillin and p190 in asynchronous or cytokinetic HeLa cells. HeLa cells were either untreated (Asyn) or synchronized with nocodazole (Cyto). Cells were then washed out of nocodazole and either mock-treated or treated with 50 µM blebbistatin for 40 minutes and cell lysates generated for immunoprecipitations (IPs). Western blots shown are representative of n>3. WCL, whole cell lysate. (C) Diagrams of p190 and anillin constructs used for recombinant protein expression. MBP and MBP-tagged C-terminal anillin (MBP-ANL) were bound to amylose beads, which were incubated with equal amount of 6×His tagged p190(1-763). Interaction was detected by western blotting with anti-His and anti-MBP antibodies.
p190 binds with anillin during cytokinesis
Anillin is a key regulator of cytokinesis in metazoans, and is thought to function as a scaffold through its ability to bind Rho, Ect2, MgcRacGAP, actin and MLC II (Piekny and Maddox, 2010; Kechad et al., 2012). Anillin localizes at the cytokinetic furrow during cytokinesis. It has been shown that p190 also binds RhoA and Ect2 (Ludwig et al., 2009; Mikawa et al., 2008) and localizes to the cytokinetic furrow during all stages of cytokinesis (Su et al., 2003). Therefore, we tested whether these two proteins interact with each other. Immunoprecipitation of endogenous p190 co-precipitated endogenous anillin, and vice versa. The interaction was enriched in lysates from cells undergoing cytokinesis (Fig. 4B, –bleb).
It has been shown that the anillin homology domain (AHD) interacts with RhoA (Piekny and Glotzer, 2008; Zhao and Fang, 2005) and MgcRacGAP (D'Avino et al., 2008; Gregory et al., 2008). We determined whether this same region of anillin could directly interact with p190. We expressed and purified a His-tagged N-terminal region of p190 (amino acids 1–763) (which contains the anillin-binding region that we will identify in the next section) and a maltose-binding protein (MBP)-tagged C-terminal region of anillin (amino acids 608–1087) from bacteria (Fig. 4C). The purified proteins migrated as monomers after gel filtration, suggesting they are properly folded (supplementary material Fig. S2B). We bound either MBP or MBP–anillin(608–1087) protein to amylose beads, which were incubated with p190(1–763). The beads were washed and the bound proteins detected by immunoblotting. p190(1–763) was specifically pulled down by beads containing MBP–anillin(608–1087) (Fig. 4C). We estimate that between 4 and 0.4% of the input p190(1–763) protein was pulled down suggesting that the direct interaction is weak and that additional factors or protein modifications are required for the strong interaction that we detect in immunoprecipitations.
Anillin localizes p190 to the cleavage furrow
The interaction of p190 with anillin raised the question of whether anillin was required to localize p190 to the cleavage furrow during cytokinesis. To test this hypothesis, HeLa cells were depleted of anillin by siRNA, and the subcellular location of p190 was assessed by confocal microscopy. p190 was no longer enriched at the cytokinetic furrow following anillin depletion, whereas localization of MLC II and actin were unaffected (supplementary material Fig. S3A,B). However, MLC II phosphorylation was markedly decreased and RhoA was mislocalized (supplementary material Fig. S3C), consistent with published reports (Piekny and Glotzer, 2008). Taken together, these data suggest that p190 and anillin interact during cytokinesis and that this interaction enriches p190 at the cytokinetic furrow.
The interaction between p190 and anillin is required for efficient cytokinesis
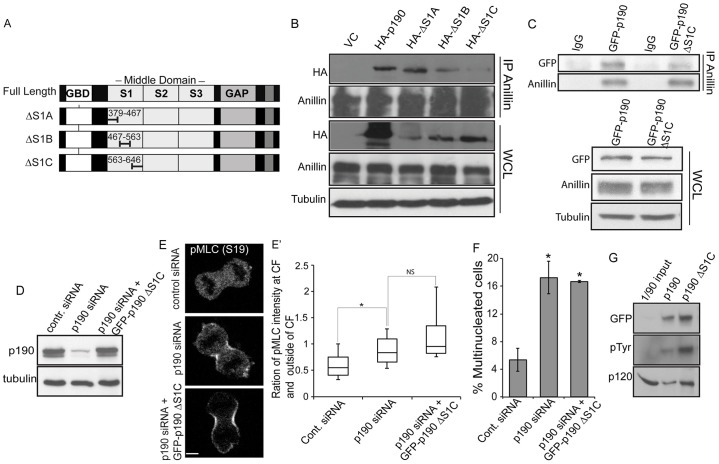
We transfected a series of plasmid constructs expressing different regions of p190 into HeLa cells to map the anillin-interacting domain on p190 (Fig. 5A; supplementary material Fig. S4A). Constructs expressing either the N-terminal region of p190 [GTP binding domain (GBD) and section 1 (S1) of the middle domain] or the entire middle domain (MD) of p190, bound endogenous anillin (supplementary material Fig. S4B). The common region of these two constructs contains S1. An isolated S1 domain of p190 also bound anillin after transient transfection, defining this domain as an interacting region (supplementary material Fig. S4C). We then generated constructs of full-length p190 that contained three similarly sized deletions of sequences within section 1 (Fig. 5A). We assayed their ability to interact with endogenous anillin by transient transfection. p190 lacking the C-terminal third of S1 (ΔS1C) was poorly precipitated by anillin (Fig. 5B). p190 mutant proteins lacking the middle section (ΔS1B) also did not bind as well as wild-type p190 or protein lacking the first section of section 1 (ΔS1A), suggesting that the C-terminal portion of the domain, as well as some of S1B, mediated anillin binding.
Fig. 5.
The interaction between p190 and anillin is necessary for proper cytokinesis. (A) Diagram of the p190 constructs: full-length, ΔS1A (Δ379-467), ΔS1B (Δ467-563) and ΔS1C (Δ563-646). (B) Mapping of the anillin-binding site in p190. HeLa cells were transiently transfected with HA-tagged variants of p190 (as described in A) or empty vector (VC), synchronized with nocodazole, and released into mitosis. Extracts were immunoprecipitated (IP) as described in the Materials and Methods and subjected to western blotting analysis to detect association of exogenous HA–p190 with anillin. (C) Cells expressing p190ΔS1C poorly interact with anillin. Cell lines were treated with doxycycline to express either GFP–p190 or GFP–p190ΔS1C, lysates were immunoprecipitated with anti-anillin antibody and blotted for GFP to detect the interaction between anillin and p190. (D–G) Cytokinesis is not rescued by expression of the anillin-binding mutant of p190. (D) p190 protein levels in cells depleted of p190 or after doxycycline expression of p190ΔS1C. (E) RhoA-GTP activity was indirectly measured by anti-pSer19 MLC II antibody. Images shown are representative of n>30. Scale bars: 4 µm. (E′) Quantification of pMLC II at the cleavage furrow. Box and whisker plot quantifying the ratio of pMLC II intensity at cytokinetic furrow and outside region of cytokinetic furrow. The box represents the 25–75th percentiles, and indicates the median. The whiskers show the range. n>15 with >20 cells/experiment. *P<0.005 as compared to Luc-siRNA-treated cells (Student's t-test), NS, not significant. (F) p190ΔS1C does not rescue the multinucletion induced by depletion of p190 (n ≥300). Results are mean±s.d. from three independent experiments. *P<0.005 (Student's t-test) for both p190-siRNA-treated cells and cells rescued by anillin-binding mutant of p190 (p190 ΔS1C) compared to LacZ-siRNA-treated cells. (G) The p190ΔS1C protein retains two traditional p190 functions. Cell lines were treated with doxycycline to express GFP tagged p190 or the anillin-binding mutant of p190 (p190 ΔS1C), lysates were immunoprecipitated with anti-GFP and blotted for the p190-interacting protein p120RasGAP (p120) and phosphorylated tyrosine residues (pTyr).
To examine whether the interaction between p190 and anillin is crucial for proper cytokinesis, we generated a stable doxycycline-inducible HeLa cell line that expressed the p190 mutant ΔS1C (supplementary material Fig. S2C), which is defective in binding anillin (Fig. 5C). This mutant was then analyzed for its ability to rescue the increase in phosphorylation of MLC II at the cytokinetic furrow and the cytokinesis defect associated with depletion of endogenous p190. Mutant p190 Dox-inducible HeLa cell lines were treated with p190 siRNA for 48 hours to deplete endogenous protein, and the siRNA-resistant anillin-binding mutant of p190 was induced to endogenous levels. Cells were then prepared for confocal microscopy analysis for measuring the intensity of pMLC II at the cytokinetic furrow and scoring multinucleation. Replacement of endogenous p190 with similar levels of the anillin-binding mutant of p190 (Fig. 5D) did not reduce MLC II phosphorylation (Fig. 5E,E′) at the cytokinetic furrow and did not rescue the multinucleation phenotype generated by p190 depletion (Fig. 5F), whereas wild-type p190 did (Fig. 1C; Fig. 2B′). Taken together, these results demonstrate that p190 must interact with anillin to regulate proper RhoA levels at the cytokinetic furrow to allow correct cytokinesis in HeLa cells.
To exclude the possibility that p190ΔS1C was a null mutant, we determined whether it retained two other identified functions of p190. The cell lines expressing the p190 or p190ΔS1C were treated with doxycycline to express GFP-tagged p190 proteins, lysates were immunoprecipitated with anti-GFP and immunoblotted for the p190-interacting protein p120RasGAP (also known as RASA1) (Bryant et al., 1995). p190 is also an important substrate of the Src tyrosine kinase, so we also immunoblotted the immunoprecipitations with anti-phosphotyrosine antibodies (Bouton et al., 1991). Fig. 5G shows that immunoprecipitated p190ΔS1C was tyrosine phosphorylated and bound p120RasGAP to a similar extent as wild-type p190. These results indicate that p190ΔS1C protein retains two traditional p190 functions and is not a null mutant.
p190 and anillin associate during cytokinesis in a contractility-dependent manner
HeLa cells in cytokinesis were treated for 40 minutes with 50 µM blebbistatin, a non-muscle myosin II inhibitor, which produces a state of weak contractility and blocks cytokinesis (Straight et al., 2003). The association between p190 and anillin was lost after blebbistatin treatment (Fig. 4B). This result suggests that the interaction between p190 and anillin is regulated. The finding that blebbistatin blocks cytokinetic furrow progression suggests that the p190–anillin interaction either requires contractile forces or is a late cytokinetic furrow event. To determine whether high levels of blebbistatin affected RhoA-GTP levels in a manner similar to p190 depletion, we visualized MLC II phosphorylation by using the anti-pThr18/Ser19 MLC II antibody and confocal microscopy. Blebbistatin treatment resulted in an almost twofold increase in the pMLC II:MLC II ratio, indicating an increase in RhoA-GTP levels (Fig. 6A,A′). These changes in RhoA activity are similar to those seen after depletion of p190 (Fig. 2A–B′).
Fig. 6.
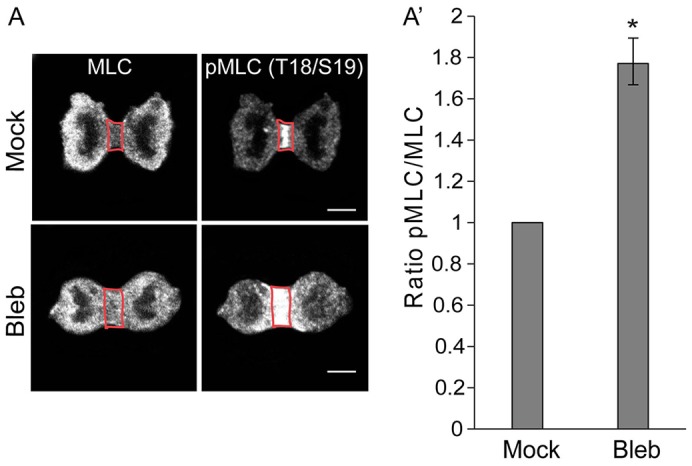
p190 and anillin associate during cytokinesis in a contractility-dependent manner to regulate MLC II activation. (A) Confocal images of total and pMLC II in HeLa cells during cytokinesis. HeLa cells were either mock-treated or treated with 50 µM blebbistatin for 40 minutes and stained for MLC or pMLC (Thr18/Ser19). The area outlined in red defines the cytokinetic furrow (region of interest, ROI), which was analyzed for pixel intensity. Images shown are representative of n>30. Scale bars: 5 µm. (A′) Quantification of specific MLC II activity. The ratio of pMLC II to MLC II intensities was calculated, and results are expressed as the mean±s.e.m. pixel intensity ratio, n>3, with >15 cells/experiment. *P<0.005 (Student's t-test) as compared to mock, which was set to 1.
DISCUSSION
The small GTPase RhoA is a crucial regulator of cellular contractility. We suggest that p190 is required to maintain the proper amount of RhoA-GTP at the cytokinetic furrow in HeLa cells. Three independent experiments support this conclusions: in vivo silencing of p190 during cytokinesis (1) increases the amount of RhoA-GTP, (Fig. 2A; Fig. 2B-B′); (2) increases the phosphorylation of MLC II at furrows (Fig. 2C-C′); and (3) causes failure of cells to progress to abscission, ultimately terminating in multinucleation (Fig. 1). We were also able to rescue the loss of p190 by adding low doses of blebbistatin, which suggests that cells fail in cytokinesis because they have too much myosin II activity. Moreover, a p190 GAP point mutant (p190 R1283A) failed to rescue the multinucleation phenotype.
p190 is one of a growing number of Rho GAPs required for cytokinesis. MgcRacGAP is required at an early stage to establish a cytokinetic furrow (Minoshima et al., 2003; Zhao and Fang, 2005). MgcRacGAP has additional roles including the localization of the Rho GEF Ect2 to furrow, and it is a matter of debate whether MgcRacGAP acts as a RhoGAP or RacGAP during cytokinetic furrow formation (Bastos et al., 2012; Maddox and Oegema, 2003; Glotzer, 2009; Davies and Canman, 2012). In contrast, p190 action does not appear to be crucial for the formation of the furrow, suggesting possible temporal separation of these RhoGAPs. It has also been proposed that MgcRacGAP functions in late stages of cytokinesis by linking midzone microtubules to the plasma membrane (Lekomtsev et al., 2012). MP-GAP also limits RhoA activity throughout mitosis to stabilize the cortex and limit the RhoA zone during cytokinesis (Zanin et al., 2013). Similar to p190 depletion, Zanin et al. found that depletion of MP-GAP results in partial cytokinesis failure (15–18% of cells). Thus, multiple RhoGAPs are required to assure completion of cytokinesis, and further defining the roles of the three cytokinetic GAPs is an important line of future experimentation.
We favor models where MgcRacGAP establishes furrows and p190 functions to maintain proper forces during contraction. Consistent with this model are our observations that all cells initiate furrow formation after p190 depletion, that higher levels of MLC phosphorylation are seen at furrows in cells depleted of p190 and that these elevated levels could be rescued by exogenous expression of wild-type p190 or low levels of blebbistatin. We also show that an interaction between p190 and anillin is required for cytokinesis. The crucial experiment is the replacement of endogenous p190 with a mutant p190 that does not bind anillin. These cells were not able to decrease MLC phosphorylation at the cytokinetic furrow and failed cytokinesis, whereas wild-type p190 was able to rescue these phenotypes. In addition, the interaction between p190 and anillin is inhibited by blebbistatin, which suggests that contractile forces regulate the action of p190 at the furrow. Our current experiments could not measure a significant change in the contraction rate, and thus we cannot rule out that p190 has roles in abscission. However, we believe it is more likely that p190 has a role in the furrow, because we measure higher levels of pMLC II at furrows in p190-depleted cells.
That RhoA-GTP levels need to be exquisitely controlled during cytokinesis is underscored not only by the identification of three RhoGAPs involved in the process [(MgcRacGAP – (Zhao and Fang, 2005), MP-GAP (Zanin et al., 2013), and p190 (Su et al., 2003)] but also by our previous finding that levels of p190 are reduced by ∼50% in late cytokinesis (Su et al., 2003) and our current findings that contraction appears to regulate the proper positioning of p190 (through association with anillin) to reduce levels of activated RhoA at the appropriate intervals. RhoA function, like other small GTPases, is highly dynamic, switching between activated and inactivated states to maintain the proper tension on the myosin-actin network (Fidyk et al., 2006 Biochemistry 45: 7750-62; Vavylonis et al., 2008 Science 319:97–100). How the three identified RhoGAPs coordinate with one another, and how reduced levels of p190 and its regulated association with anillin accomplish this, in conjunction with Ect2, are major unanswered questions requiring further investigation.
Our finding that the interaction between p190 and anillin is inhibited by treatment of the cells with blebbistatin is an exciting result, but one whose importance is still unclear. Blebbistatin inhibits the action of class II myosins by inhibiting actin-activated ATPase activity (Straight et al., 2003). The simplest interpretation of our result is that the interaction between p190 and anillin is initiated by actin- and myosin-dependent contractility, although we cannot rule out more complicated interpretations at this time. Our ability to rescue p190 depletion by partial inhibition of myosin II activity by blebbistatin or Y27632 argues that cells fail cytokinesis because contractile forces are too high (Fig. 4A; supplementary material Fig. S2A). Consistent with this interpretation, we see an increase in RhoA activity after treatment with higher doses of blebbistatin as measured by MLC II phosphorylation (Fig. 6A,A′). That MLC II is downstream of p190 is further emphasized by the fact that the inability to complete cytokinesis due to p190 overexpression (and decreased RhoA-GTP levels) (Mikawa et al., 2008; Su et al., 2003; Su et al., 2009) can be rescued with a constitutively active MLC II.
Fig. 7 depicts a speculative model that is consistent with our data. Optimal RhoA-GTP levels are ensured by a negative-feedback loop induced by the contractility-sensing nature of the p190–anillin complex. During cytokinesis, RhoA-GTP is enriched at the cytokinetic furrow by specific localization of Ect2 and MgcRacGAP resulting in activation of ROCK and other effectors of Rho. This in turn leads to myosin II activation and actin polymerization, resulting in cytokinetic furrow contraction. Once contractility forces reach a threshold, p190 productively associates with anillin at the cytokinetic furrow and lowers RhoA-GTP levels. This event in turn results in a decrease in myosin II activation, reduced actin polymerization and p190 release, completing the cycle. Given that anillin also binds the RhoGEF Ect2, this model can accommodate reactivation of RhoA-GTP after release of p190. Overall, this feedback mechanism would maintain contractility forces at an appropriate level for completion of cytokinesis. To test this model, it will be important to determine whether and how contractile forces drive a functional interaction between anillin and p190.
Fig. 7.
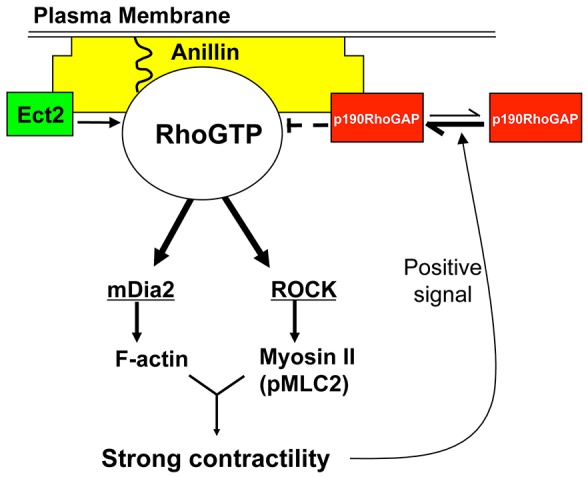
Model for regulation of RhoA-GTP through the p190–anillin complex. See text for details.
MATERIALS AND METHODS
Cell culture and synchronization
HeLa cells were obtained from American Tissue Culture Collection (Manassas, VA) and maintained by serial passage in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Carlsbad, CA) containing 10% fetal calf serum (Gibco) and 1% penicillin-streptomycin (Gibco) at 37°C in a 5% CO2, humidified environment. Cells were synchronized for arrest in mitosis by treatment with 50 ng/ml nocodazole (cat. number, M1404, Sigma, St Louis, MO) for 14–16 hours, released from the nocodazole block for 40 minutes, and harvested by mitotic shake off. Where indicated, 50 µM blebbistatin (cat. number 203390, Calbiochem, San Diego, CA) was added at the time of nocodazole release. Cells were also synchronized by treatment with 2 mM thymidine for 24 hours, released into fresh medium for 12 hours, arrested again in 2 mM thymidine for 24 hours, and released for 8–16 hours. At 8 hours after release from thymidine, either blebbistatin or the ROCK inhibitor Y27632 was added at the indicated concentrations for an additional 6 hours at which time cells were fixed for immunofluorescence analysis.
Mutant constructs
The p190 deletion mutants were constructed using primers purchased from Integrated DNA Technologies, Inc. The pkH3 triple-HA-tagged full-length p190 plasmid served as the template for PCR amplification. Fragments of p190 were amplified and the desired portion of protein was deleted. Deletion fragments of p190 were replaced using BamH1 and Bmt1 restrictions sites. Amplified deletion mutants were ligated into the pkH3 vector using the T4 DNA Ligase I following standard ligation protocols. All mutants obtained by PCR were confirmed by sequencing prior to use. Verified plasmids were purified from competent DH5α E.coli using Maxi-prep (Qiagen) according to manufacturer's protocol.
Transient transfection
HeLa cells were transfected with Polyfect Transfection Reagent (Qiagen Inc., Valencia, CA) according to the manufacturer's protocol. Plasmids used included the pKH3 plasmid encoding triple HA-tagged, wild-type p190 (a gift from Ian Macara, Vanderbilt University, Nashville, TN), all mutants of p190 derived from this plasmid, and the GFP plasmid expressing GFP–p190 or GFP-tagged constiutively active (CA) MLC II (phosphomimetic T18D, S19D) (gift from Rick Horwitz, University of Virginia, Charlottesville, VA). Cells were plated in either 100-mm or six-well dishes (Corning). Mock-transfected cells were treated with transfection reagents alone. When two or more plasmids were co-transfected, total plasmid levels were equalized with empty vector. Vector control experiments were performed with empty pKH3 HA-vectors or empty GFP vectors with equivalent amounts of vector per sample.
siRNA transfection
Human p190RhoGAP-A was silenced using double-stranded p190 RNAi oligonucleotides custom-made by Dharmacon that targeted a unique sequence at the N-terminus of the protein. The sequence of the double stranded oligonucleotides was 5′-AAGAUGCACAUUGUGGAGCAG-3′. Human anillin was silenced with the Dharmacon ANLN ON-TARGETplus SMARTpool (5′-GCAAACAACUAGAAACCAA-3′, 5′-GGCGAUGCCUCUUUGAAUA-3′, 5′-GAUCAAGCAUUAGCAGAAA-3′ and 5′-ACGCAACACUUUUGAAUUA-3′). Glass coverslips were coated with 1 mg/ml poly-L-lysine (Sigma), and cells were treated with p190 oligonucleotides (50 pmol) using the Lipofectamine RNAiMax reagent (Invitrogen), as per the manufacturer's instructions. After 24 hours, cells were synchronized in mitosis by treatment with nocodazole as described above.
Generation of HeLa Tet-on p190-inducible cell lines and knockdown rescue experiments
The Tet-on expression system obtained from CLONTECH Laboratories, Inc. was used according to the manufacturer's directions. pcDNA5/FRT/TO-p190, pcDNA5/FRT/TO-p190 (R1283A), pcDNA5/FRT/TO-p190ΔS1C Flp-InTM expression vectors and the Flp recombinase vector, pOG44, were co-transfected into Flp-InTM HeLa T-REx cells using Lipofectamine 2000 (Invitrogen). Cells were selected in the presence of 0.2 mg/ml Hygromycin B and screened for p190 expression after Dox treatmant. Dox-inducible HeLa cell lines were plated at 30% density and after 16 hours, cells were transfected with 75 nM p190 siRNA. 1 µg/ml doxycycline was used to induce expression of full-length p190, the GAP mutant p190 (R1283A) or the anillin-binding mutant of p190. Culture medium was changed after 24 hours, and the cells were allowed to incubate for 48 hours post-transfection in the presence of 1 µg/ml doxycycline.
Rho GTPase assay
HeLa cells were treated with control (Luciferase) or p190 siRNA for 24 hours as described above, then serum-starved for an additional 24 hours. Remaining plates were serum-starved for 24 hours, and all plates were then synchronized with nocodazole and released from the block as described above to capture cells in cytokinesis. Individual plates were then treated with either 10 µM LPA (cat. number, L7260, Sigma) for 40 minutes or 0.5 ug/ml C3 toxin (Cat. Number: CT04-A Cytoskeleton Inc., Denver, CO) for 2 hours before harvest. RhoA-GTP levels were measured at 4°C using the RhoA G-LISA assay from Cytoskeleton Inc. according to manufacturer's protocol.
Live-cell imaging and confocal microscopy
To perform live-cell imaging, HeLa cells were plated onto poly-L-lysine-coated 1.5 Borosilicate two-well chambered coverglass dishes (LabTex), and images were acquired using a 20× dry objective lens (NA 0.75; Olympus) on a deconvolution microscope (DeltaVision). Single-plane multipoint acquisitions were captured on a CoolSNAP HQ2 camera (Photometrics) every 5 minutes within a 37°C chamber. Images of cells treated with control or p190 siRNAs were collected with identical exposure times and scaled equally. The acquisition software used was SoftWoRX from Applied Precision.
Cell fixation, Immunofluorescence microscopy and quantification
HeLa cells were grown on poly-L-lysine-coated coverslips, depleted of p190 or anillin, and synchronized in mitosis as described above for 48 hours. Cells were fixed for 20 minutes with 4% paraformaldehyde and processed for immunofluorescence. For RhoA immunostaning, cells were fixed with 10% ice cold trichloroacetic acid (TCA) on ice for 15 minutes, than were rinsed three times with 1×PBS containing 30 mM glycine (G-PBS). (Hayashi et al., 1999; Yonemura et al., 2004).
Cells were analyzed on a Zeiss Axiovert 200 wide-field fluorescence microscope, fitted with a confocal scanner using a krypton-argon laser (Perkin Elmer), Hamamatsu EMCCD camera, a NanoScanZ motor (Prior) and a 63× oil Plan-Apochromat objective. An acousto-optic tunable filter (AOTF) was used for detection of light at 488, 568 and 647 nm. Photographs were taken as z-series with 0.4-µm z-steps. Exposure times were consistent for each channel throughout individual experiments, and no images were altered after capture. Quantification was performed using Volocity®5.5 (Perkin Elmer) or ImageJ (NIH) softwares. The areas [regions of interest (ROI)] around the cytokinetic furrow at two sites were outlined using a lasso tool (mask) on a single z-section to determine the average pixel intensity per square micron, which was then averaged per cell. This was background corrected by subtracting the average pixel intensity per square micron measured using the same mask. We measured pixel intensity at regions outside of the cytokinetic furrow similarly. The ratio between these two values was then determined to define the specific phosphorylation of myosin II light chain using antibodies again pSer19 MLC II, pThr18/Ser19 MLC II and total MLC II (Cell Signaling) (supplementary material Fig. S1B). 53 cells per treatment were analyzed for each experiment.
Protein purification, gel filtration and in vitro pulldown assay
6×His-tagged p190(1–763) and MBP-tagged anillin(608–1087), a gift from the Alisa Piekny lab, Concordia University, Montreal, QC, Canada were bacterially expressed and purified using amylose beads (NEB) for MBP fusion anillin and Ni-NTA agarose beads (Qiagen) for His-tagged p190. The beads were washed, and the 6×His-tagged p190 was eluted from Ni-NTA agarose with 300 mM imidazole and MBP-tagged anillin was eluted with 100 mM maltose at 4°C. Proteins were concentrated and fractionated on the Superdex 200 gel filtration column in buffer (50 mM Tris-HCl pH 7.6, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT). Protein concentration was determined by a Bradford protein assay. To investigate the direct interaction of the p190 and anillin, an in vitro binding assay was performed using recombinant 6His–p190(1–763) and MBP–anillin(608-1087) proteins in 200 ml buffer (50 mM Tris-HCl pH = 7.6, 150 mM NaCl, 5 mM MgCl2, 0.5% Triton X-100). 5 µg of p190(1–763) was added to the buffer containing 5 µg of MBP or MBP–anillin, and proteins were incubated with amylose beads by rotating at room temperature for 1 hour. Beads where washed four or five times with buffer before adding SDS sample buffer, and bound proteins were detected by western blotting. Blots were probed by 1∶250 mouse anti-His (Santa Cruz) and 1∶10,000 mouse anti-MBP (NEB) antibodies.
Immunoprecipitation
Cell lysates were prepared as described for western blotting and incubated with 5 µg of the indicated immunoprecipitating antibody overnight. After primary antibody incubation, immunocomplexes were precipitated by using protein-G–agarose (Invitrogen) and separated by centrifugation at 2300 g for 1 minute. After washes with cold PBS, SDS sample buffer was added to each sample, boiled for 5 minutes, and subjected to western blotting analysis, as described above.
Supplementary Material
Acknowledgments
We are grateful to J. Pritchard and M. Skalski for technical help, and the Cytokinesis Group for helpful discussions.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
S.S.M and S.J.P. made the original observation that p190 interacts with anillin and provide Fig. 4B. K.L. and S.J.P. generated Fig. 3 and Fig. 6. A.M. and P.T.S. provided the rest of the data and wrote the paper with critical input from K.L. and S.J.P.
Funding
This work was supported by the National Cancer Institute, National Institutes of Health [grant numbers CA39438 to S.J.P., CA009109 (Institutional T32), GM063045 to A.K.M. and P.T.S.]; and University of Virginia Cancer Training grant [grant number 5 T32 CA9109 to A.M.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.151647/-/DC1
References
- Aktories K., Braun U., Rösener S., Just I., Hall A. (1989). The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem. Biophys. Res. Commun. 158, 209–213 10.1016/S0006-291X(89)80199-8 [DOI] [PubMed] [Google Scholar]
- Bastos R. N., Penate X., Bates M., Hammond D., Barr F. A. (2012). CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. J. Cell Biol. 198, 865–880 10.1083/jcb.201204107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement W. M., Miller A. L., von Dassow G. (2006). Rho GTPase activity zones and transient contractile arrays. BioEssays 28, 983–993 10.1002/bies.20477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton A. H., Kanner S. B., Vines R. R., Wang H. C., Gibbs J. B., Parsons J. T. (1991). Transformation by pp60src or stimulation of cells with epidermal growth factor induces the stable association of tyrosine-phosphorylated cellular proteins with GTPase-activating protein. Mol. Cell. Biol. 11, 945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick A. R. (1999). Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 11, 26–33 10.1016/S0955-0674(99)80004-0 [DOI] [PubMed] [Google Scholar]
- Bryant S. S., Briggs S., Smithgall T. E., Martin G. A., McCormick F., Chang J. H., Parsons S. J., Jove R. (1995). Two SH2 domains of p120 Ras GTPase-activating protein bind synergistically to tyrosine phosphorylated p190 Rho GTPase-activating protein. J. Biol. Chem. 270, 17947–17952 10.1074/jbc.270.30.17947 [DOI] [PubMed] [Google Scholar]
- Canman J. C., Lewellyn L., Laband K., Smerdon S. J., Desai A., Bowerman B., Oegema K. (2008). Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science 322, 1543–1546 10.1126/science.1163086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Avino P. P., Savoian M. S., Glover D. M. (2004). Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J. Cell Biol. 166, 61–71 10.1083/jcb.200402157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Avino P. P., Savoian M. S., Glover D. M. (2005). Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy. J. Cell Sci. 118, 1549–1558 10.1242/jcs.02335 [DOI] [PubMed] [Google Scholar]
- D'Avino P. P., Takeda T., Capalbo L., Zhang W., Lilley K. S., Laue E. D., Glover D. M. (2008). Interaction between Anillin and RacGAP50C connects the actomyosin contractile ring with spindle microtubules at the cell division site. J. Cell Sci. 121, 1151–1158 10.1242/jcs.026716 [DOI] [PubMed] [Google Scholar]
- Davies T., Canman J. C. (2012). Stuck in the middle: Rac, adhesion, and cytokinesis. J. Cell Biol. 198, 769–771 10.1083/jcb.201207197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidyk N., Wang J. B., Cerione R. A. (2006). Influencing cellular transformation by modulating the rates of GTP hydrolysis by Cdc42. Biochemistry 45, 7750–7762 10.1021/bi060365h [DOI] [PubMed] [Google Scholar]
- Gai M., Camera P., Dema A., Bianchi F., Berto G., Scarpa E., Germena G., Di Cunto F. (2011). Citron kinase controls abscission through RhoA and anillin. Mol. Biol. Cell 22, 3768–3778 10.1091/mbc.E10-12-0952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. (2001). Animal cell cytokinesis. Annu. Rev. Cell Dev. Biol. 17, 351–386 10.1146/annurev.cellbio.17.1.351 [DOI] [PubMed] [Google Scholar]
- Glotzer M. (2009). Cytokinesis: GAP gap. Curr. Biol. 19, R162–R165 10.1016/j.cub.2008.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S. L., Ebrahimi S., Milverton J., Jones W. M., Bejsovec A., Saint R. (2008). Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr. Biol. 18, 25–29 10.1016/j.cub.2007.11.050 [DOI] [PubMed] [Google Scholar]
- Hayashi K., Yonemura S., Matsui T., Tsukita S., Tsukita S. (1999). Immunofluorescence detection of ezrin/radixin/moesin (ERM) proteins with their carboxy-terminal threonine phosphorylated in cultured cells and tissues: application of a novel fixation protocol using trichloroacetic acid (TCA) as a fixative. J. Cell Sci. 112, 1149–1158. [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. (2005). Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 10.1146/annurev.cellbio.21.020604.150721 [DOI] [PubMed] [Google Scholar]
- Jantsch-Plunger V., Gönczy P., Romano A., Schnabel H., Hamill D., Schnabel R., Hyman A. A., Glotzer M. (2000). CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J. Cell Biol. 149, 1391–1404 10.1083/jcb.149.7.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechad A., Jananji S., Ruella Y., Hickson G. R. (2012). Anillin acts as a bifunctional linker coordinating midbody ring biogenesis during cytokinesis. Curr. Biol. 22, 197–203 10.1016/j.cub.2011.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Tsuji T., Takada Y., Miki T., Narumiya S. (2000). Accumulation of GTP-bound RhoA during cytokinesis and a critical role of ECT2 in this accumulation. J. Biol. Chem. 275, 17233–17236 10.1074/jbc.C000212200 [DOI] [PubMed] [Google Scholar]
- Kondo T., Isoda R., Uchimura T., Sugiyama M., Hamao K., Hosoya H. (2012). Diphosphorylated but not monophosphorylated myosin II regulatory light chain localizes to the midzone without its heavy chain during cytokinesis. Biochem. Biophys. Res. Commun. 417, 686–691 10.1016/j.bbrc.2011.11.151 [DOI] [PubMed] [Google Scholar]
- Lekomtsev S., Su K. C., Pye V. E., Blight K., Sundaramoorthy S., Takaki T., Collinson L. M., Cherepanov P., Divecha N., Petronczki M. (2012). Centralspindlin links the mitotic spindle to the plasma membrane during cytokinesis. Nature 492, 276–279 10.1038/nature11773 [DOI] [PubMed] [Google Scholar]
- Li R., Zhang B. and Zheng Y. (1997). Structural determinants required for the interaction between Rho GTPase and the GTPase-activating domain of p190. J. Biol. Chem. 272, 32830–32835 10.1074/jbc.272.52.32830 [DOI] [PubMed] [Google Scholar]
- Liu J., Fairn G. D., Ceccarelli D. F., Sicheri F., Wilde A. (2012). Cleavage furrow organization requires PIP(2)-mediated recruitment of anillin. Curr. Biol. 22, 64–69 10.1016/j.cub.2011.11.040 [DOI] [PubMed] [Google Scholar]
- Loria A., Longhini K. M., Glotzer M. (2012). The RhoGAP domain of CYK-4 has an essential role in RhoA activation. Curr. Biol. 22, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig K., Manchinelly S. A., Su L., Mikawa M., Parsons S. J. (2009). p190RhoGAP-A. UCSD Nature Molecule Pages a001712.01 10.1038/mp.a001712.01 [DOI] [Google Scholar]
- Maddox A. S., Burridge K. (2003). RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J. Cell Biol. 160, 255–265 10.1083/jcb.200207130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox A. S., Oegema K. (2003). Closing the GAP: a role for a RhoA GAP in cytokinesis. Mol. Cell 11, 846–848 10.1016/S1097-2765(03)00151-5 [DOI] [PubMed] [Google Scholar]
- Manchinelly S. A., Miller J. A., Su L., Miyake T., Palmer L., Mikawa M., Parsons S. J. (2010). Mitotic down-regulation of p190RhoGAP is required for the successful completion of cytokinesis. J. Biol. Chem. 285, 26923–26932 10.1074/jbc.M110.103804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F. (2005). Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 15, 371–377 10.1016/j.tcb.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Mikawa M., Su L., Parsons S. J. (2008). Opposing roles of p190RhoGAP and Ect2 RhoGEF in regulating cytokinesis. Cell Cycle 7, 2003–2012 10.4161/cc.7.13.6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. L., Bement W. M. (2009). Regulation of cytokinesis by Rho GTPase flux. Nat. Cell Biol. 11, 71–77 10.1038/ncb1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima Y., Kawashima T., Hirose K., Tonozuka Y., Kawajiri A., Bao Y. C., Deng X., Tatsuka M., Narumiya S., May W. S., Jr et al. (2003). Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev. Cell 4, 549–560 10.1016/S1534-5807(03)00089-3 [DOI] [PubMed] [Google Scholar]
- Mishima M., Kaitna S., Glotzer M. (2002). Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell 2, 41–54 10.1016/S1534-5807(01)00110-1 [DOI] [PubMed] [Google Scholar]
- Narumiya S., Yasuda S. (2006). Rho GTPases in animal cell mitosis. Curr. Opin. Cell Biol. 18, 199–205 10.1016/j.ceb.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Nakano K., Mabuchi I. (1998). Localization of Rho GTPase in sea urchin eggs. FEBS Lett. 441, 121–126 10.1016/S0014-5793(98)01531-2 [DOI] [PubMed] [Google Scholar]
- Oegema K., Savoian M. S., Mitchison T. J., Field C. M. (2000). Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J. Cell Biol. 150, 539–552 10.1083/jcb.150.3.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekny A. J., Glotzer M. (2008). Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr. Biol. 18, 30–36 10.1016/j.cub.2007.11.068 [DOI] [PubMed] [Google Scholar]
- Piekny A. J., Maddox A. S. (2010). The myriad roles of Anillin during cytokinesis. Semin. Cell Dev. Biol. 21, 881–891 10.1016/j.semcdb.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Piekny A., Werner M., Glotzer M. (2005). Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 15, 651–658 10.1016/j.tcb.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Self A. J., Kasmi F., Paterson H. F., Hall A., Marshall C. J., Ellis C. (1993). rho family GTPase activating proteins p190, bcr and rhoGAP show distinct specificities in vitro and in vivo. EMBO J. 12, 5151–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers W. G., Saint R. (2003). A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4, 29–39 10.1016/S1534-5807(02)00402-1 [DOI] [PubMed] [Google Scholar]
- Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299, 1743–1747 10.1126/science.1081412 [DOI] [PubMed] [Google Scholar]
- Su L., Agati J. M., Parsons S. J. (2003). p190RhoGAP is cell cycle regulated and affects cytokinesis. J. Cell Biol. 163, 571–582 10.1083/jcb.200308007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Pertz O., Mikawa M., Hahn K., Parsons S. J. (2009). p190RhoGAP negatively regulates Rho activity at the cleavage furrow of mitotic cells. Exp. Cell Res. 315, 1347–1359 10.1016/j.yexcr.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki C., Daigo Y., Ishikawa N., Kato T., Hayama S., Ito T., Tsuchiya E., Nakamura Y. (2005). ANLN plays a critical role in human lung carcinogenesis through the activation of RHOA and by involvement in the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 65, 11314–11325 10.1158/0008-5472.CAN-05-1507 [DOI] [PubMed] [Google Scholar]
- Takaishi K., Sasaki T., Kameyama T., Tsukita S., Tsukita S., Takai Y. (1995). Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene 11, 39–48. [PubMed] [Google Scholar]
- Vavylonis D., Wu J. Q., Hao S., O'Shaughnessy B., Pollard T. D. (2008). Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science 319, 97–100 10.1126/science.1151086 [DOI] [PubMed] [Google Scholar]
- Watanabe S., Ando Y., Yasuda S., Hosoya H., Watanabe N., Ishizaki T., Narumiya S. (2008). mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol. Biol. Cell 19, 2328–2338 10.1091/mbc.E07-10-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Sakamoto T., Zhang F., Sellers J. R., Hammer J. A., III (2006). In vitro reconstitution of a transport complex containing Rab27a, melanophilin and myosin Va. FEBS Lett. 580, 5863–5868 10.1016/j.febslet.2006.09.047 [DOI] [PubMed] [Google Scholar]
- Yonemura S., Hirao-Minakuchi K., Nishimura Y. (2004). Rho localization in cells and tissues. Exp. Cell Res. 295, 300–314 10.1016/j.yexcr.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Yoshizaki H., Ohba Y., Parrini M. C., Dulyaninova N. G., Bresnick A. R., Mochizuki N., Matsuda M. (2004). Cell type-specific regulation of RhoA activity during cytokinesis. J. Biol. Chem. 279, 44756–44762 10.1074/jbc.M402292200 [DOI] [PubMed] [Google Scholar]
- Yüce O., Piekny A., Glotzer M. (2005). An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170, 571–582 10.1083/jcb.200501097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin E., Desai A., Poser I., Toyoda Y., Andree C., Moebius C., Bickle M., Conradt B., Piekny A., Oegema K. (2013). A conserved RhoGAP limits M phase contractility and coordinates with microtubule asters to confine RhoA during cytokinesis. Dev. Cell 26, 496–510 10.1016/j.devcel.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W. M., Fang G. (2005). MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis. Proc. Natl. Acad. Sci. USA 102, 13158–13163 10.1073/pnas.0504145102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.