Abstract
Background
The objective of this systematic review and meta-analysis was to assess the relationship between the chloride content of intravenous resuscitation fluids and patient outcomes in the perioperative or intensive care setting.
Methods
Systematic searches were performed of PubMed/MEDLINE, Embase and Cochrane Library (CENTRAL) databases in accordance with PRISMA guidelines. Randomized clinical trials, controlled clinical trials and observational studies were included if they compared outcomes in acutely ill or surgical patients receiving either high-chloride (ion concentration greater than 111 mmol/l up to and including 154 mmol/l) or lower-chloride (concentration 111 mmol/l or less) crystalloids for resuscitation. Endpoints examined were mortality, measures of kidney function, serum chloride, hyperchloraemia/metabolic acidosis, blood transfusion volume, mechanical ventilation time, and length of hospital and intensive care unit stay. Risk ratios (RRs), mean differences (MDs) or standardized mean differences (SMDs) and confidence intervals were calculated using fixed-effect modelling.
Results
The search identified 21 studies involving 6253 patients. High-chloride fluids did not affect mortality but were associated with a significantly higher risk of acute kidney injury (RR 1·64, 95 per cent c.i. 1·27 to 2·13; P < 0·001) and hyperchloraemia/metabolic acidosis (RR 2·87, 1·95 to 4·21; P < 0·001). High-chloride fluids were also associated with greater serum chloride (MD 3·70 (95 per cent c.i. 3·36 to 4·04) mmol/l; P < 0·001), blood transfusion volume (SMD 0·35, 0·07 to 0·63; P = 0·014) and mechanical ventilation time (SMD 0·15, 0·08 to 0·23; P < 0·001). Sensitivity analyses excluding heavily weighted studies resulted in non-statistically significant effects for acute kidney injury and mechanical ventilation time.
Conclusion
A weak but significant association between higher chloride content fluids and unfavourable outcomes was found, but mortality was unaffected by chloride content.
Introduction
The administration of intravenous fluids for resuscitation occurs routinely in the perioperative setting and in the management of critically ill patients. There has been considerable interest in defining optimal fluid resuscitation strategies1, yet practice patterns and fluid selection vary considerably2. While much attention has been directed at the ‘colloid versus crystalloid’ debate, increasing evidence suggests clinically important differences related to intravenous fluid chloride content3–7.
Often referred to as ‘normal saline’, 0·9 per cent saline contains sodium and chloride in supraphysiological concentrations. Balanced solutions, in contrast, contain significantly lower concentrations of sodium and chloride, making them closer in composition to plasma than 0·9 per cent saline6. Despite a lack of evidence supporting the superiority of 0·9 per cent saline7, it is commonly used as a resuscitation fluid and has generally served as the ‘control fluid’ in large trials8,9. Administration of 0·9 per cent saline causes hyperchloraemic metabolic acidosis10–15, and consequently some guidelines recommend the use of balanced solutions as a default during resuscitation16. Hyperchloraemia has also been associated with decreased renal perfusion17–21, impaired immune function22–24 and mortality25, suggesting that hyperchloraemia may have clinically relevant effects.
Studies26,27 have examined differences between groups treated with high-chloride versus low-chloride solutions, and a Cochrane systematic review28 of randomized controlled trials (RCTs) examined clinical outcomes following the perioperative use of buffered versus non-buffered fluids. A recent systematic review29 of prospective RCTs evaluated the impact of near-isotonic or isotonic crystalloids on acid–base status and other physiological, haemodynamic and clinical outcomes. However, no analyses have focused specifically on the chloride content of crystalloids administered for resuscitation in the broader context of both perioperative and critical care medicine. Therefore, a systematic review and meta-analysis was conducted to determine whether the chloride content of resuscitation fluids used in the operating theatre or intensive care unit (ICU) setting is associated with differences in outcomes.
Methods
Study selection
Approval for the study was obtained from the Duke University Institutional Review Board on 7 August 2013. In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement30, systematic searches of the PubMed/MEDLINE, Embase and Cochrane Central Register of Controlled Trials Library (CENTRAL) databases were carried out using predefined search terms that addressed study design, intervention and intravenous fluid type (Appendix S1, supporting information). These were supplemented by manual searches by the investigators in order to capture as much of the literature as possible. Searches were limited to published English-language studies in human subjects, covering all dates up to and including 22 August 2013. Unique articles were screened based on their title/abstract, and studies requiring full review were identified.
Inclusion in the meta-analysis required that a given study meet the following criteria: comparison of an isotonic crystalloid fluid characterized by a supraphysiological chloride concentration (ion concentration greater than 111 mmol/l up to and including 154 mmol/l, for example 0·9 per cent saline) with a near-isotonic or isotonic crystalloid fluid characterized by a near-physiological chloride concentration (ion concentration 111 mmol/l or less, for example Ringer's lactate), given intravenously for the purpose of fluid resuscitation or replacement; comparison in either acutely ill patients in the ICU or surgical patients in the perioperative period; and evaluation of at least one of the following endpoints: mortality, acute kidney injury (AKI)/renal failure (including use of dialysis), hospital length of stay (LOS), ICU LOS, hyperchloraemia/metabolic acidosis, serum creatinine, serum chloride, urine output, mechanical ventilation time and transfusion. For the purposes of this meta-analysis, isotonic crystalloids with supraphysiological chloride concentrations are referred to as high-chloride fluids, whereas those with near-physiological chloride concentrations are referred to as low-chloride fluids. RCTs, controlled clinical trials (CCTs) and observational studies were included in the analysis.
Studies that compared a hypertonic crystalloid with another solution were excluded from the analysis, as were studies comparing a crystalloid with a colloid, or comparing two low-chloride crystalloids. Studies were also excluded if the intravenous fluids compared were given for maintenance purposes, ‘preloading/volume optimization’ before surgery, priming of the cardiopulmonary bypass circuit, or the treatment of ischaemic stroke or subarachnoid haemorrhage. Study eligibility for inclusion in the meta-analysis was assessed and confirmed by two reviewers, and differences were resolved by discussion between reviewers.
Risk of bias assessment
Risk of bias was assessed using one of two approaches, depending on study design. For RCTs, the seven-category Review Manager risk of bias tool was used (RevMan version 5.2; The Cochrane Collaboration, Oxford, UK), with risk assessed as either high, unclear or low according to criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions31. For non-RCTs, the Newcastle–Ottawa Scale (NOS)32 was used, in accordance with previously published methodology33. Briefly, the NOS allots a maximum of nine points based on the representativeness of the intervention and control groups, ascertainment of intervention, absence of outcomes at study start, comparability of groups based on study design and analysis, blinded assessment/record linkage to confirm outcomes, sufficient length of follow-up and sufficiently low withdrawal rate.
Data extraction and outcomes
Data extracted, in duplicate using a standard form, included general study characteristics (authors, design), as well as information about the study population (age, setting, condition/diagnosis), intervention (fluids compared, intervention timing and volume) and outcomes (author definition of outcome, point estimates, summary statistics, author conclusions).
For continuous variables, the mean(s.d.) value was extracted or derived from the reported data. When a study report did not provide variability data for a point estimate, the data were requested from the study's corresponding author. Data were included in the meta-analysis only when: mean(s.d.) values could be extracted or derived from the reported data; or study authors could provide clarification. Where ventilator-free days were reported, ventilation time was derived using the period over which ventilator-free time was measured.
Statistical analysis
For dichotomous variables, RR and 95 per cent c.i. were calculated using a fixed-effect model and the Mantel–Haenszel statistical method. Studies that included a count of zero for both intervention groups for a given endpoint were not included in the analysis for that endpoint34. For continuous variables, the effect measure and 95 per cent c.i. were calculated using a fixed-effect model and the inverse-variance statistical method. The mean difference (MD) was used as the effect measure where all studies reported the endpoint using the same units or scale. Alternatively, the standardized mean difference (SMD), which assumes that differences in the standard deviation reflect differences in measurement scales, was used as the effect measure when different studies reported the same endpoint using different scales or units.
For continuous variables not reported as mean(s.d.) values, these statistics were derived from the reported data using published methodologies or approaches outlined in the Cochrane Handbook31. For continuous variables reported as median (range), the mean(s.d.) values were calculated according to methods published by Hozo and colleagues35. Data presented as the geometric mean (95 per cent c.i.) of log-transformed data were converted to mean(s.d.) on the raw scale using the methodology of Higgins et al.36. For further details, see Appendix S1 (supporting information).
Forest plot generation and statistical analyses were performed using RevMan version 5.2. P < 0·050 was considered to be statistically significant. The RevMan program reports P values to one or two significant digits; where requested, a standard normal (Z) table was consulted to report P values to three decimal places37. For each endpoint analysed, statistical heterogeneity was examined using the I2 statistic, which provides an estimate of the percentage of variation across studies arising from study heterogeneity rather than chance38. When substantial heterogeneity (I2 greater than 60 per cent) was detected for an endpoint showing a statistically significant effect of high- versus low-chloride fluids, this heterogeneity was further investigated using a random-effects analysis model or subgroup analysis, as appropriate. When visual inspection of a forest plot suggested that the overall effect was driven by a single study (weight greater than 50 per cent), sensitivity analysis excluding this study was performed39.
Results
Included studies
In total, the database search yielded 7330 unique articles, of which 492 passed the initial screen and were reviewed for study inclusion (Fig. 1). Of these, 470 articles were excluded and 21 studies (in 22 articles)10,12,14,26,27,40–56 met the inclusion criteria (Table 1). Studies comprised 15 RCTs and six non-RCTs, and involved 6253 patients. The study by Yunos and colleagues reported data in two unique articles, with biochemical55 and clinical26 outcomes published separately. Eleven studies examined critically ill patients requiring volume resuscitation in the ICU setting, and ten examined patients receiving these interventions in the perioperative period. In all included studies, the high-chloride intravenous crystalloid was 0·9 per cent saline (chloride concentration approximately 154 mmol/l) and the low-chloride intravenous crystalloid was either Ringer's lactate (chloride concentration about 109 mmol/l), Hartmann's solution (chloride concentration about 111 mmol/l) or Plasma-Lyte® (Baxter Healthcare, Deerfield, Illinois, USA) (chloride concentration 98 mmol/l). Two studies12,40 compared three different intravenous fluids (0·9 per cent saline, Ringer's lactate and Plasma-Lyte®). For these studies, patients receiving Ringer's lactate and Plasma-Lyte® were combined into a single low-chloride fluids group by deriving pooled mean(s.d.) values from the individual group data.
Fig 1.
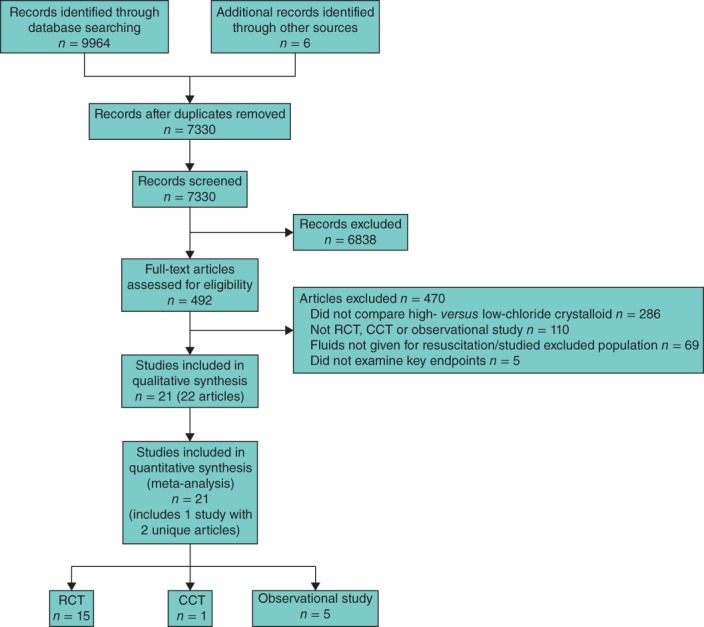
PRISMA flow diagram showing study selection. RCT, randomized controlled trial; CCT, controlled clinical trial
Characteristics of included studies
| Reference | Year | Design | Country | Study population | Total study population size | Interventions compared | Key endpoints |
|---|---|---|---|---|---|---|---|
| Berger et al.41 | 2000 | Retrospective | Switzerland | Adults with thermal burns | 40 | Bicarbonated 0·9% saline versus Ringer's lactate | Mortality, acute renal injury, ICU LOS, mechanical ventilation time, hyperchloraemia/metabolic acidosis, urine output |
| Cho et al.42 | 2007 | RCT | Korea | Adults with rhabdomyolysis | 28 | 0·9% saline versus Ringer's lactate | Serum chloride |
| Chua et al.43 | 2012 | Retrospective | Australia | Adults with severe DKA | 23 | 0·9% saline versus Plasma-Lyte® 148 | ICU LOS, urine output |
| Cieza et al.44 | 2013 | Observational | Peru | Adults with severe dehydration | 40 | 0·9% saline versus Ringer's lactate | Serum creatinine, serum chloride |
| Hadimioglu et al.12 | 2008 | RCT | Turkey | Adults undergoing kidney transplantation | 90* | 0·9% saline versus Plasma-Lyte® and Ringer's lactate | Acute renal injury, serum creatinine, serum chloride, urine output |
| Hasman et al.40 | 2012 | RCT | Turkey | Adults with moderate or severe dehydration | 90* | 0·9% saline versus Plasma-Lyte® and Ringer's lactate | Serum chloride |
| Khajavi et al.45 | 2008 | RCT | Iran | Adults undergoing kidney transplantation | 52 | 0·9% saline versus Ringer's lactate | Serum creatinine, urine output |
| Kim et al.46 | 2013 | RCT | Korea | Adults undergoing kidney transplantation | 60 | 0·9% saline versus Plasma-Lyte® A | Serum creatinine, serum chloride, urine output, transfusion volume |
| Mahajan et al.47 | 2012 | RCT | India | Children with severe dehydration | 22 | 0·9% saline versus Ringer's lactate | Mortality, hospital LOS, serum chloride |
| Mahler et al.48 | 2011 | RCT | USA | Adults with DKA | 45 | 0·9% saline versus Plasma-Lyte® A | Serum chloride |
| Modi et al.49 | 2012 | RCT | Saudi Arabia | Adults undergoing kidney transplantation | 74 | 0·9% saline versus Ringer's lactate | Serum chloride, serum creatinine |
| O'Malley et al.50 | 2005 | RCT | USA | Adults undergoing renal transplantation | 51 | 0·9% saline versus Ringer's lactate | Acute renal injury, hospital LOS, hyperchloraemia/metabolic acidosis, serum creatinine, serum chloride, urine output |
| Scheingraber et al.10 | 1999 | RCT | Germany | Adults undergoing elective abdominal gynaecological surgery | 24 | 0·9% saline versus Ringer's lactate | Urine output |
| Shaw et al.27 | 2012 | Retrospective | USA | Adult surgical patients | 3704† | 0·9% saline versus Plasma-Lyte® 148 or Plasma-Lyte® A | Mortality, acute kidney injury, hospital LOS, mechanical ventilation time |
| Takil et al.51 | 2002 | RCT | Turkey | Adult spinal surgery patients | 30 | 0·9% saline versus Ringer's lactate | Hospital LOS, ICU LOS, serum chloride, urine output, transfusion volume |
| Van Zyl et al.52 | 2012 | RCT | South Africa | Adults with DKA | 54 | 0·9% saline versus Ringer's lactate | Hospital LOS, serum creatinine, serum chloride |
| Waters et al.14 | 2001 | RCT | USA | Adult patients undergoing aortic reconstructive surgery | 66 | 0·9% saline versus Ringer's lactate | Mortality, acute renal injury, hospital LOS, ICU LOS, mechanical ventilation time, serum creatinine, serum chloride, urine output, transfusion volume |
| Wu et al.53 | 2011 | RCT | USA | Adults with acute pancreatitis | 40 | 0·9% saline versus Ringer's lactate | Acute renal injury, hospital LOS |
| Young et al.54 | 2014 | RCT | USA | Adults with traumatic injury | 65 | 0·9% saline versus Plasma-Lyte® A | Mortality, acute renal injury, hospital LOS, ICU LOS, mechanical ventilation time, serum creatinine, serum chloride, urine output, transfusion volume |
| Yunos et al.26,55 | 2011, 2012 | CCT | Australia | Adult ICU patients | 1533 | Chloride-rich fluids (0·9% saline, 4% succinylated gelatin solution, 4% albumin) versus balanced solutions (Hartmann's, Plasma-Lyte® 148, chloride-poor 20% albumin) | Mortality, acute renal injury, hospital LOS, ICU LOS, serum chloride, serum creatinine, urine output |
| Zunini et al.56 | 2011 | Retrospective | Uruguay | Children undergoing craniofacial surgery | 122 | 0·9% saline versus Ringer's lactate | Hyperchloraemia/metabolic acidosis |
Studies compared three different intravenous fluids: 0·9 per cent saline, Ringer's lactate and Plasma-Lyte® (Baxter Healthcare, Deerfield, Illinois, USA); pooled means and variances were derived for the Ringer's lactate and Plasma-Lyte® groups in both cases.
Propensity-matched population. ICU, intensive care unit; LOS, length of stay; RCT, randomized controlled trial; DKA, diabetic ketoacidosis; CCT, controlled clinical trial.
Risk of bias
The overall risk of bias of RCTs meeting study inclusion criteria was acceptable (Fig. S1, supporting information). None of the included RCTs was judged to have a high risk of bias with respect to randomization or allocation concealment, although two studies presented unclear risks of bias, as specifics were not discussed. Blinding of participants and study personnel was ascertained for most studies; however, two reports indicated that study personnel were not blinded to the experimental intravenous fluid administered, and two additional reports did not adequately address blinding for an assessment to be made (Table S1, supporting information). All included non-RCTs were allotted at least six of nine possible points using the NOS, suggesting an overall acceptable risk of bias (Fig. S2 and Table S2, supporting information).
Clinical endpoints
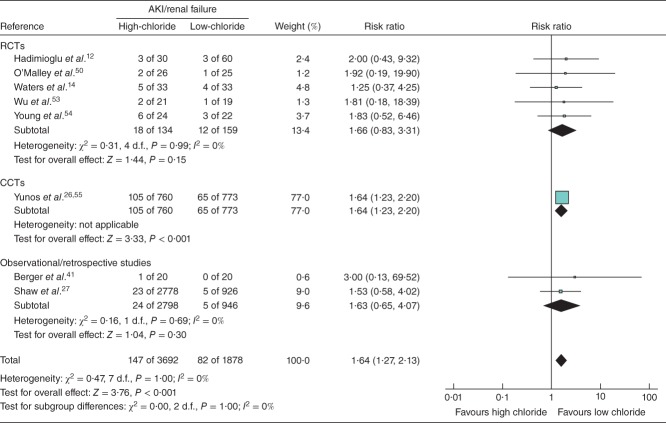
Meta-analysis results for clinical endpoints are summarized in Figs 4. No statistically significant impact on mortality was found in the six studies that included this endpoint (RR 1·13, 95 per cent c.i. 0·92, 1·39; P = 0·230) (Fig. 2). High-chloride crystalloids were associated with a significantly increased risk of AKI/renal failure (RR 1·64, 1·27 to 2·13; P < 0·001) (Fig. 3) and hyperchloraemia/metabolic acidosis (RR 2·87, 1·95 to 4·21; P < 0·001) (Fig. 4). AKI/renal failure outcomes were defined by criteria set forth by the individual studies and were not uniform. For both mortality and AKI/renal failure, heterogeneity, estimated by the I2 statistic, was 0 per cent, suggesting a consistent direction and magnitude of effect across studies. For hyperchloraemia/metabolic acidosis, each study indicated a RR greater than 1 (range from 2·12 to 16·37), but the overall heterogeneity (I2 = 61 per cent) led to a re-examination of the association using a random-effects model. This re-examination did not meaningfully change the RR (4·07, 95 per cent c.i. 1·23 to 13·53; P = 0·022), suggesting that the observed heterogeneity was probably due to differences in effect magnitude rather than effect direction.
Fig 4.
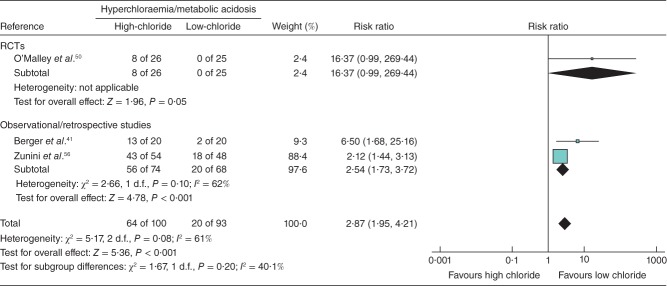
Forest plot illustrating hyperchloraemia/metabolic acidosis risk following volume resuscitation with high-chloride versus low-chloride intravenous fluids. A Mantel–Haenszel fixed-effect model was used for meta-analysis. Risk ratios are shown with 95 per cent c.i. RCT, randomized controlled trial; CCT, controlled clinical trial
Fig 2.
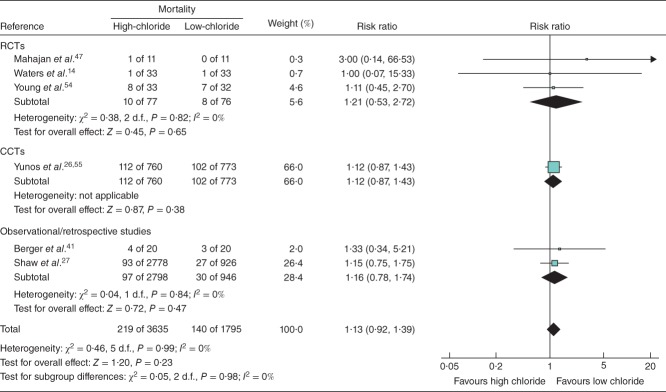
Forest plot illustrating mortality risk following volume resuscitation with high-chloride versus low-chloride intravenous fluids. Where necessary, mortality incidence was derived from reported survival. A Mantel–Haenszel fixed-effect model was used for meta-analysis. Risk ratios are shown with 95 per cent c.i. RCT, randomized controlled trial; CCT, controlled clinical trial
Fig 3.
Forest plot illustrating acute kidney injury (AKI)/renal failure risk following volume resuscitation with high-chloride versus low-chloride intravenous fluids. A Mantel–Haenszel fixed-effect model was used for meta-analysis. Risk ratios are shown with 95 per cent c.i. RCT, randomized controlled trial; CCT, controlled clinical trial
Examination of ICU and/or hospital LOS revealed no significant association between chloride content and ICU LOS (SMD −0·01, 95 per cent c.i. −0·10 to 0·09; P = 0·897; I2 = 0 per cent) (Fig. S3, supporting information). However, hospital LOS appeared to favour high-chloride fluids (SMD −0·07, −0·13 to −0·01; P = 0·017; I2 = 0 per cent) (Fig. S4, supporting information).
Surrogate endpoints
Analysis of surrogate endpoints revealed that high-chloride intravenous fluids were associated with significantly higher serum chloride levels (MD 3·70 (95 per cent c.i. 3·36 to 4·04) mmol/l; P < 0·001; I2 = 98 per cent) (Fig. S5, supporting information), greater blood transfusion volume (SMD 0.35, 0·07 to 0·63; P = 0·014; I2 = 0 per cent) (Fig. 5) and longer mechanical ventilation time (SMD 0·15, 0·08 to 0·23; P < 0·001; I2 = 17 per cent) (Fig. S6, supporting information). There was no significant difference between high- versus low-chloride intravenous fluids with respect to serum creatinine levels (SMD 0·10, −0·02 to 0·23; P = 0·095; I2 = 18 per cent) (Fig. S7, supporting information) and a small effect on urine output (SMD 0·17, 0·02 to 0·32; P = 0·030; I2 = 70 per cent) (Fig. S8, supporting information). Given the observed heterogeneity, urine output was re-evaluated using a random-effects model, which demonstrated a non-significant effect of high- versus low-chloride intravenous fluids (SMD 0·08, −0·22 to 0·38; P = 0·589).
Fig 5.
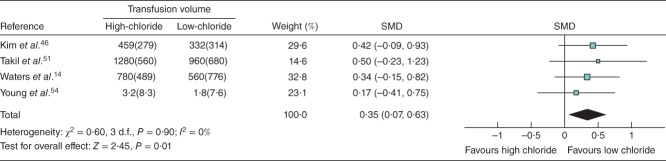
Forest plot illustrating mean(s.d.) blood transfusion volume following volume resuscitation with high-chloride versus low-chloride intravenous fluids. All included studies reporting this endpoint were randomized controlled trials. An inverse-variance fixed-effect model was used for meta-analysis. Standardized mean differences (SMDs) are shown with 95 per cent c.i.
Sensitivity analyses
For some endpoints, forest plot inspection indicated that overall effect measures were driven largely by a single heavily weighted study (in all cases, either Yunos et al.26,55, Zunini et al.56 or Shaw et al.27). To understand how these individual studies might influence effect estimates, sensitivity analyses were repeated after excluding them. Exclusion of the Yunos et al.26,55 and Zunini et al.56 studies from the analyses of AKI/renal failure, hyperchloraemia/metabolic acidosis and serum chloride did not affect the direction of effect, but did convert to a non-statistically significant effect for the AKI/renal failure endpoint (Table 2). Exclusion of the Shaw et al.27 study from the pooled analysis of mechanical ventilation time also resulted in a shift to an effect that was not statistically significant. Similarly, when the Shaw et al.27 study was excluded from the LOS analyses, no statistically significant effect was observed. Exclusion of the Yunos et al.26 study from the mortality analysis had no impact.
Table 2.
Summary of sensitivity analyses
| Overall analysis | Sensitivity analysis | |||||
|---|---|---|---|---|---|---|
| Effect size | P | Excluded study | Effect size | P | ||
| Reference | Weight (%) | |||||
| Clinical endpoints | ||||||
| Acute kidney injury | RR 1·64 (1·27, 2·13) | < 0·001 | Yunos et al.26 | 77·0 | RR 1·65 (0·95, 2·87) | 0·078 |
| Hyperchloraemia/metabolic acidosis | RR 2·87 (1·95, 4·21) | < 0·001 | Zunini et al.56 | 65·5 | RR 8·50 (2·49, 29·08) | 0·001 |
| Mortality | RR 1·13 (0·92, 1·39) | 0·230 | Yunos et al.26 | 65·0 | RR 1·17 (0·81, 1·68) | 0·401 |
| ICU LOS | SMD −0·01 (−0·10, 0·09) | 0·897 | Yunos et al.26 | 88·7 | SMD −0·07 (−0·35, 0·21) | 0·624 |
| Hospital LOS | SMD −0·07 (−0·13, −0·01) | 0·017 | Shaw et al.27 | 60·1 | SMD −0·01 (−0·10, 0·08) | 0·849 |
| Surrogate endpoints | ||||||
| Serum chloride | MD 3·70 (3·36, 4·04) | < 0·001 | Yunos et al.55 | 55·3 | MD 5·31 (4·79, 5·82) | < 0·001 |
| Mechanical ventilation time | SMD 0·15 (0·08, 0·23) | < 0·001 | Shaw et al.27 | 95·1 | SMD −0·06 (−0·38, 0·27) | 0·734 |
Values in parentheses are 95 per cent c.i. RR, risk ratio; ICU, intensive care unit; LOS, length of stay; SMD, standardized mean difference; MD, mean difference.
Significant heterogeneity with respect to reported serum chloride levels (I2 = 98 per cent) was investigated by subgroup analysis based on timing of chloride measurement and by using a random-effects model. Although high-chloride fluids were associated with higher serum chloride levels in both RCT subgroups (Fig. S5, supporting information), this analysis suggests that subgroup differences do exist based on time point of measurement (MD 11·18 (95 per cent c.i. 10·29 to 12·06) mmol/l; P < 0·001 for studies reporting intraoperative or postoperative levels, versus MD 2·24 (1·60 to 2·89) mmol/l; P < 0·001 for studies reporting levels at other time points). Analysis of serum chloride using a random-effects model revealed no significant change in effect measure or significance (MD 5·06 (2·30 to 7·82) mmol/l; P < 0·001).
Two studies47,56 included in the present analysis involved paediatric patients. Although both studies were small (22 and 122 patients respectively) and therefore unlikely to impact significantly on the effect estimates, sensitivity analysis excluding these two trials was performed in order to account for potential differences in fluid physiology in paediatric versus adult patients. There was no impact on either effect direction or significance for any of the measured endpoints when these studies were excluded: mortality (RR 1·13, 95 per cent c.i. 0·92 to 1·39; P = 0·250), hospital LOS (SMD −0·07, −0·13 to −0·02; P = 0·013), hyperchloraemia/metabolic acidosis (RR 8·50, 2·49 to 29·0; P < 0·001) or serum chloride (MD 4·02 (3·66 to 4·37) mmol/l; P < 0·001).
Discussion
Meta-analysis of all available studies demonstrated a significantly higher risk of AKI, metabolic acidosis, blood transfusion and time on mechanical ventilation with high-chloride fluid resuscitation. The association between high-chloride fluids, metabolic acidosis and higher serum chloride levels is supported by several studies10,11,54 that did not meet the inclusion criteria for the present meta-analysis, and provides face validity for a conceptual mechanism of deleterious effects of hyperchloraemia. No increased risk for mortality was found.
High-chloride fluids were significantly associated with an increased risk of AKI/renal failure. This endpoint was driven heavily by one study26, and sensitivity analysis excluding this study showed the same direction of effect, but loss of statistical significance. Each remaining study demonstrated a directionally similar association between increased AKI/renal failure and high-chloride fluids. One limitation in interpreting these data is the lack of uniformity among definitions of AKI. Ideally a standardized definition would be applied for the analysis; however, this was not possible with the available data. Moreover, serum creatinine concentration, a surrogate marker of renal function, did not demonstrate an association with the fluid chloride content. Failure to detect such an association may be related to reporting of serum creatinine levels at variable time points, and the possibility that the magnitude of differences in serum creatinine concentration may vary with time.
Greater urine output was significantly associated with high-chloride fluids, which may appear inconsistent with greater risk of AKI. However, the effect of fluid chloride content on urinary output may be largely time-dependent57,58, and in this context urine output may not be a useful surrogate measure of renal function. Variability in the timing of urine output measurements and diuretic use may also confound these results. In light of the variability in AKI/renal failure definitions, timing of creatinine measurement and effects on urine output, this AKI harm signal should be interpreted cautiously.
No reported difference in mortality may reflect either no true difference, variability in the time periods over which mortality was reported, inadequate sample size to detect a difference, or variability in the risk factors in the included patient populations. Because this meta-analysis included studies in both perioperative and ICU settings, lower-risk patients were included than if the analysis had been limited solely to critical illness, lowering the overall mortality rate and thus decreasing the likelihood of detecting a possible mortality signal. It is also conceivable that an increased risk of AKI may occur without an increase in short-term mortality. These factors may help to explain why an increase in AKI, but not mortality, was observed with high-chloride crystalloid use in this meta-analysis.
Two recent studies ineligible for this meta-analysis have examined the relationship between mortality and either hyperchloraemia25 or crystalloid fluid choice59. A retrospective, propensity-matched, cohort study25 of surgical patients demonstrated a significant association between hyperchloraemia and mortality, as well as AKI. Notably, the relationship between serum chloride and fluid administered was not assessed. In a propensity-matched cohort study59 of non-operative patients with vasopressor-dependent sepsis, use of balanced fluids (low-chloride by the present definition) was associated with lower in-hospital mortality, but no effect was found for AKI. One could infer that, because high-chloride fluids lead to hyperchloraemia and hyperchloraemia is associated with mortality, high-chloride fluids must be associated with mortality. However, the relationship is not clearly true in all populations studied and may vary based on risk.
Among the four eligible studies14,46,51,54 reporting transfusion outcomes, all reported at least a small or modest31,60 increase in transfusion volume with high-chloride fluid administration. Shaw and co-workers27 also demonstrated an association between high-chloride fluids and greater transfusion volume, but the study was not included in the analysis owing to lack of variability data. Although the total number of patients included in the present meta-analysis of blood transfusion volume was low (high-chloride, 102; low-chloride, 100), the potential for a true difference raises relevant questions. Clinicians may consider any difference meaningful given the expense and risks associated with red cell transfusions61,62. Although causality has not been established between high-chloride fluids and increased transfusion, the potential clinical impact of a true effect is hypothesis-generating.
When administered in large volumes, 0·9 per cent saline has been shown to cause coagulopathy63,64. Total volumes of fluid administered did not vary substantially between the groups (Table S3, supporting information), suggesting that dilutional coagulopathy does not account for the observed differences in transfusion. Mechanisms of saline-induced coagulopathy are not fully known, and the data lead us to consider whether hyperchloraemia/acidosis has an independent effect on coagulation. Alternatively, a reverse association cannot be excluded, in that fewer transfusions may have been administered in the low-chloride groups owing to the possibility of incompatibility with citrated blood. However, in two46,54 of the four studies, the low-chloride solution did not contain calcium, and in one14 of the other studies the providers were blinded to the fluid that was being administered.
Findings for additional endpoints investigated should not be considered definitive, owing to the low event rate and small sample size. Further studies including these endpoints are needed to arrive at firm conclusions.
Combining RCTs, CCTs and observational studies may provide more generalizable results and help to offset the limitations that accompany the often low event rates in smaller RCTs33,65–68. In addition, a recent meta-analysis68 demonstrated no significant differences between the risks of adverse events between RCTs and observational studies, leading the authors to advocate the inclusion of a broad range of studies when analysing harm signals. The present authors believe that the addition of observational data strengthens this study and the influence of observable confounding variables is specifically reduced by propensity-matching.
Combining RCTs and non-RCTs could alternatively be viewed as a limitation of this study, as some advocate that data from RCTs and observational studies should not be pooled for analysis; rather, if the RCTs and observational studies warrant equal confidence, both types of evidence should be presented separately69. As such, in addition to presenting the overall effect in the forest plots, the studies are grouped by type so that plots may be visualized accordingly. Despite inclusion of observational studies, the total number of patients remains low and several outcomes were examined in only a small number of studies. Further limitations include variability in the populations studied, the potential for significant bias in observational studies, and variability in outcome assessment. There is also potential for bias due to the exclusion of abstracts and non-English-language manuscripts. Assessment for publication bias was not possible owing to the small number of studies that met the inclusion criteria.
The present findings argue against the intravenous administration of supraphysiological concentrations of chloride (above 111 mmol/l), yet most studies were small in size, thereby preventing firm conclusions from being drawn. These findings underscore the need for large, well designed RCTs that are adequately powered to detect differences in outcomes such as mortality and morbidity, including AKI. This provides an opportunity for international collaboration70 in surgical and critical care research; already, the Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group has begun enrolment in the SPLIT study71 comparing 0·9 per cent saline with Plasma-Lyte® 148 for fluid therapy in the ICU. Future considerations could include a priori subgroup distinctions such as comparison of medical versus surgical patients, or low-risk versus high-risk surgical patients.
Acknowledgments
The authors thank the authors of reviewed studies, who kindly provided unpublished data for incorporation into the meta-analysis, as well as F. Peyerl of Boston Strategic Partners, for managerial support.
This study was designed and conducted at Boston Strategic Partners, Boston, Massachusetts, USA, in collaboration with Duke University Medical Center, Durham, North Carolina, USA, with remote management by Baxter Healthcare Corporation, Deerfield, Illinois, USA, which funded the study. S.M.P. is an employee of Boston Strategic Partners, Inc., funded by Baxter Healthcare Corporation to perform the analysis. C.R.S. is an employee of Baxter Healthcare Corporation. A.D.S. has received payment for consulting services provided to Baxter Healthcare Corporation.
Disclosure: The authors declare no further conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article
Electronic health database search terms (Word document)
Risk of bias graph for randomized controlled trials meeting meta-analysis inclusion criteria (Word document)
Risk of bias graph for non-randomized studies meeting meta-analysis inclusion criteria (Word document)
Analysis of intensive care unit (ICU) length of stay following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Analysis of hospital length of stay following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Serum chloride concentration following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Analysis of mechanical ventilation time following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Analysis of serum creatinine concentration following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Analysis of urine output following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Risk of bias of included randomized controlled trials (Word document)
Risk of bias of included non-randomized studies (Word document)
Study fluid volumes received (Word document)
References
- 1.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–1251. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 2.Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, et al. SAFE TRIPS Investigators. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veech RL. The toxic impact of parenteral solutions on the metabolism of cells: a hypothesis for physiological parenteral therapy. Am J Clin Nutr. 1986;44:519–551. doi: 10.1093/ajcn/44.4.519. [DOI] [PubMed] [Google Scholar]
- 4.Ho AM, Karmakar MK, Contardi LH, Ng SS, Hewson JR. Excessive use of normal saline in managing traumatized patients in shock: a preventable contributor to acidosis. J Trauma. 2001;51:173–177. doi: 10.1097/00005373-200107000-00033. [DOI] [PubMed] [Google Scholar]
- 5.Awad S, Allison SP, Lobo DN. The history of 0·9% saline. Clin Nutr. 2008;27:179–188. doi: 10.1016/j.clnu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Guidet B, Soni N, Della Rocca G, Kozek S, Vallet B, Annane D, et al. A balanced view of balanced solutions. Crit Care. 2010;14:325. doi: 10.1186/cc9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent ‘pre-renal’ acute kidney injury?: con. Kidney Int. 2014 doi: 10.1038/ki.2014.105. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, et al. SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 9.Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 10.Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 11.McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0·9% saline for intra-operative fluid replacement. Anaesthesia. 1994;49:779–781. doi: 10.1111/j.1365-2044.1994.tb04450.x. [DOI] [PubMed] [Google Scholar]
- 12.Hadimioglu N, Saadawy I, Saglam T, Ertug Z, Dinckan A. The effect of different crystalloid solutions on acid–base balance and early kidney function after kidney transplantation. Anesth Analg. 2008;107:264–269. doi: 10.1213/ane.0b013e3181732d64. [DOI] [PubMed] [Google Scholar]
- 13.Morgan TJ, Venkatesh B, Hall J. Crystalloid strong ion difference determines metabolic acid–base change during in vitro hemodilution. Crit Care Med. 2002;30:157–160. doi: 10.1097/00003246-200201000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Handy JM, Soni N. Physiological effects of hyperchloraemia and acidosis. Br J Anaesth. 2008;101:141–150. doi: 10.1093/bja/aen148. [DOI] [PubMed] [Google Scholar]
- 16.Powell-Tuck J, Gosling P, Lobo DN, Allison SP, Carlson GL, Gore M, et al. British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients (GIFTASUP) 2013. 2011. http://www.bapen.org.uk/pdfs/bapen_pubs/giftasup.pdf [accessed 20 October ] [Google Scholar]
- 17.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0·9% saline and Plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 18.Hansen PB, Jensen BL, Skott O. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension. 1998;32:1066–1070. doi: 10.1161/01.hyp.32.6.1066. [DOI] [PubMed] [Google Scholar]
- 19.Bullivant EM, Wilcox CS, Welch WJ. Intrarenal vasoconstriction during hyperchloremia: role of thromboxane. Am J Physiol. 1989;256:F152–F157. doi: 10.1152/ajprenal.1989.256.1.F152. [DOI] [PubMed] [Google Scholar]
- 20.Imig JD, Passmore JC, Anderson GL, Jimenez AE. Chloride alters renal blood flow autoregulation in deoxycorticosterone-treated rats. J Lab Clin Med. 1993;121:608–613. [PubMed] [Google Scholar]
- 21.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125:243–248. doi: 10.1378/chest.125.1.243. [DOI] [PubMed] [Google Scholar]
- 23.Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130:962–967. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]
- 24.Kellum JA, Song M, Li J. Science review: extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care. 2004;8:331–336. doi: 10.1186/cc2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;117:412–421. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- 26.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 27.Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0·9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 28.Burdett E, Dushianthan A, Bennett-Guerrero E, Cro S, Gan TJ, Grocott MP, et al. Perioperative buffered versus non-buffered fluid administration for surgery in adults. Cochrane Database Syst Rev. 2012;12:CD004089. doi: 10.1002/14651858.CD004089.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Orbegozo Cortes D, Rayo Bonor A, Vincent JL. Isotonic crystalloid solutions: a structured review of the literature. Br J Anaesth. 2014;112:968–981. doi: 10.1093/bja/aeu047. [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W264. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Oxford: The Cochrane Collaboration; 2011. [updated March ]., 2011. [Google Scholar]
- 32.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta Analyses. 2012. http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf [accessed 10 July 2013] [Google Scholar]
- 33.Hutton B, Joseph L, Fergusson D, Mazer CD, Shapiro S, Tinmouth A. Risks of harms using antifibrinolytics in cardiac surgery: systematic review and network meta-analysis of randomised and observational studies. BMJ. 2012;345:e5798. doi: 10.1136/bmj.e5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med. 2008;27:6072–6092. doi: 10.1002/sim.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zar JH. Biostatistical Analysis. Upper Saddle River: Prentice Hall; 1999. 4th edn. [Google Scholar]
- 38.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailar JC, Hoaglin DC. Medical Uses of Statistics. Hoboken: John Wiley & Sons; 2009. 3rd edn. [Google Scholar]
- 40.Hasman H, Cinar O, Uzun A, Cevik E, Jay L, Comert B. A randomized clinical trial comparing the effect of rapidly infused crystalloids on acid–base status in dehydrated patients in the emergency department. Int J Med Sci. 2012;9:59–64. doi: 10.7150/ijms.9.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger MM, Pictet A, Revelly JP, Frascarolo P, Chioléro RL. Impact of a bicarbonated saline solution on early resuscitation after major burns. Intensive Care Med. 2000;26:1382–1385. doi: 10.1007/s001340000615. [DOI] [PubMed] [Google Scholar]
- 42.Cho YS, Lim H, Kim SH. Comparison of lactated Ringer's solution and 0·9% saline in the treatment of rhabdomyolysis induced by doxylamine intoxication. Emerg Med J. 2007;24:276–280. doi: 10.1136/emj.2006.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chua HR, Venkatesh B, Stachowski E, Schneider AG, Perkins K, Ladanyi S, et al. Plasma-Lyte 148 vs 0·9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27:138–145. doi: 10.1016/j.jcrc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Cieza JA, Hinostroza J, Huapaya JA, Leon CP. Sodium chloride 0·9% versus lactated Ringer in the management of severely dehydrated patients with choleriform diarrhoea. J Infect Dev Ctries. 2013;7:528–532. doi: 10.3855/jidc.2531. [DOI] [PubMed] [Google Scholar]
- 45.Khajavi MR, Etezadi F, Moharari RS, Imani F, Meysamie AP, Khashayar P, et al. Effects of normal saline vs. lactated ringer's during renal transplantation. Ren Fail. 2008;30:535–539. doi: 10.1080/08860220802064770. [DOI] [PubMed] [Google Scholar]
- 46.Kim SY, Huh KH, Lee JR, Kim SH, Jeong SH, Choi YS. Comparison of the effects of normal saline versus Plasmalyte on acid–base balance during living donor kidney transplantation using the stewart and base excess methods. Transplant Proc. 2013;45:2191–2196. doi: 10.1016/j.transproceed.2013.02.124. [DOI] [PubMed] [Google Scholar]
- 47.Mahajan V, Sajan SS, Sharma A, Kaur J. Ringers lactate vs normal saline for children with acute diarrhea and severe dehydration – a double blind randomized controlled trial. Indian Pediatr. 2012;49:963–968. doi: 10.1007/s13312-012-0251-x. [DOI] [PubMed] [Google Scholar]
- 48.Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29:670–674. doi: 10.1016/j.ajem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Modi MP, Vora KS, Parikh GP, Shah VR. A comparative study of impact of infusion of Ringer's lactate solution versus normal saline on acid–base balance and serum electrolytes during live related renal transplantation. Saudi J Kidney Dis Transpl. 2012;23:135–137. [PubMed] [Google Scholar]
- 50.O'Malley CM, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, et al. A randomized, double-blind comparison of lactated Ringer's solution and 0·9% NaCl during renal transplantation. Anesth Analg. 2005;100:1518–1524. doi: 10.1213/01.ANE.0000150939.28904.81. [DOI] [PubMed] [Google Scholar]
- 51.Takil A, Eti Z, Irmak P, Yilmaz GöğüşF. Early postoperative respiratory acidosis after large intravascular volume infusion of lactated Ringer's solution during major spine surgery. Anesth Analg. 2002;95:294–298. doi: 10.1097/00000539-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis – Ringer's lactate versus normal saline: a randomized controlled trial. QJM. 2012;105:337–343. doi: 10.1093/qjmed/hcr226. [DOI] [PubMed] [Google Scholar]
- 53.Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, et al. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710–717. doi: 10.1016/j.cgh.2011.04.026. e711. [DOI] [PubMed] [Google Scholar]
- 54.Young JB, Utter GH, Schermer CR, Galante JM, Phan HH, Yang Y, et al. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: a randomized trial. Ann Surg. 2014;259:255–262. doi: 10.1097/SLA.0b013e318295feba. [DOI] [PubMed] [Google Scholar]
- 55.Yunos NM, Kim IB, Bellomo R, Bailey M, Ho L, Story D, et al. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39:2419–2424. doi: 10.1097/CCM.0b013e31822571e5. [DOI] [PubMed] [Google Scholar]
- 56.Zunini GS, Rando KA, Cox RG. Fluid replacement in craniofacial pediatric surgery: normal saline or Ringer's lactate? J Craniofac Surg. 2011;22:1370–1374. doi: 10.1097/SCS.0b013e31821c94db. [DOI] [PubMed] [Google Scholar]
- 57.Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer's solution versus 0·9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999–1003. doi: 10.1097/00000539-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann's solution: a randomized double-blind crossover study. Clin Sci (Lond) 2003;104:17–24. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 59.Raghunathan K, Shaw A, Nathanson B, Sturmer T, Brookhart A, Stefan MS, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–1591. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 60.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 61.Hofmann A, Ozawa S, Farrugia A, Farmer SL, Shander A. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol. 2013;27:59–68. doi: 10.1016/j.bpa.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 63.Todd SR, Malinoski D, Muller PJ, Schreiber MA. Lactated Ringer's is superior to normal saline in the resuscitation of uncontrolled hemorrhagic shock. J Trauma. 2007;62:636–639. doi: 10.1097/TA.0b013e31802ee521. [DOI] [PubMed] [Google Scholar]
- 64.Ahn HJ, Yang M, Gwak MS, Koo MS, Bang SR, Kim GS, et al. Coagulation and biochemical effects of balanced salt-based high molecular weight vs saline-based low molecular weight hydroxyethyl starch solutions during the anhepatic period of liver transplantation. Anaesthesia. 2008;63:235–242. doi: 10.1111/j.1365-2044.2007.05345.x. [DOI] [PubMed] [Google Scholar]
- 65.Bradburn MJ, Deeks JJ, Berlin JA, Russell LocalioA. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 66.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 67.Sutton AJ, Cooper NJ, Lambert PC, Jones DR, Abrams KR, Sweeting MJ. Meta-analysis of rare and adverse event data. Expert Rev Pharmacoecon Outcomes Res. 2002;2:367–379. doi: 10.1586/14737167.2.4.367. [DOI] [PubMed] [Google Scholar]
- 68.Golder S, Loke YK, Bland M. Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med. 2011;8:e1001026. doi: 10.1371/journal.pmed.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66:158–172. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 70.Soreide K, Alderson D, Bergenfelz A, Beynon J, Connor S, Deckelbaum DL, et al. International Research Collaboration in Surgery (IRIS) ad-hoc working group. Strategies to improve clinical research in surgery through international collaboration. Lancet. 2013;382:1140–1151. doi: 10.1016/S0140-6736(13)61455-5. [DOI] [PubMed] [Google Scholar]
- 71.Australian New Zealand Clinical Trials Registry (ANZCTR) 0·9% Saline vs. Plasma-Lyte 148 for Intensive Care Fluid Therapy (The SPLIT Study) 2013. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365460 [accessed 22 May 2014] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic health database search terms (Word document)
Risk of bias graph for randomized controlled trials meeting meta-analysis inclusion criteria (Word document)
Risk of bias graph for non-randomized studies meeting meta-analysis inclusion criteria (Word document)
Analysis of intensive care unit (ICU) length of stay following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Analysis of hospital length of stay following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Serum chloride concentration following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Analysis of mechanical ventilation time following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Analysis of serum creatinine concentration following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Analysis of urine output following volume resuscitation with high-chloride versus low-chloride intravenous fluids (Word document)
Risk of bias of included randomized controlled trials (Word document)
Risk of bias of included non-randomized studies (Word document)
Study fluid volumes received (Word document)