Abstract
Optimization of protein production from methanol-induced Pichia pastoris cultures is necessary to ensure high productivity rates and high yields of recombinant proteins. We investigated the effects of temperature and different linear or exponential methanol-feeding rates on the production of recombinant Fusarium graminearum galactose oxidase (EC 1.1.3.9) in a P. pastoris Mut+ strain, under regulation of the AOX1 promoter. We found that low exponential methanol feeding led to 1.5-fold higher volumetric productivity compared to high exponential feeding rates. The duration of glycerol feeding did not affect the subsequent product yield, but longer glycerol feeding led to higher initial biomass concentration, which would reduce the oxygen demand and generate less heat during induction. A linear and a low exponential feeding profile led to productivities in the same range, but the latter was characterized by intense fluctuations in the titers of galactose oxidase and total protein. An exponential feeding profile that has been adapted to the apparent biomass concentration results in more stable cultures, but the concentration of recombinant protein is in the same range as when constant methanol feeding is employed. © 2014 The Authors Biotechnology Progress published by Wiley Periodicals, Inc. on behalf of American Institute of Chemical Engineers Biotechnol. Prog., 30:728–735, 2014
Keywords: galactose oxidase, Pichia pastoris, methanol feeding, optimization, Fusarium graminearum
Introduction
Galactose 6-oxidase1 (GalOx; D-galactose:oxygen 6-oxidoreductase; EC 1.1.3.9) is a monomeric free-radical copper oxidase produced and secreted by various fungal species,2 including species of the genus Fusarium.3,4 This enzyme catalyzes the oxidation of the C6 hydroxyl group of galactose, and also other primary alcohols to aldehydes, producing hydrogen peroxide.5 The reaction involves a transfer of two electrons, despite the single copper in the active site.6 Being among the simplest copper-containing oxidases, GalOx produced by the genus Fusarium –and especially Fusarium graminearum– has been studied extensively.5,7–9
GalOx is currently used in several biotechnological applications, due to its selectivity and unique mode of action of producing a reactive aldehyde functionality and hydrogen peroxide. It has been employed, for example, as a possible dental anti-plaque system10,11 and in various analytical methods for the determination of lactose and other galactosides,12–14 in glycoprotein detection and bioconjugation,15,16 and in disease diagnostics.17,18 Likewise, GalOx has been used for chemo-enzymatic polysaccharide functionalization in the production of novel biopolymers and cellulosic materials.19–24 GalOx has also been the subject of significant protein engineering efforts to improve production and stability, and to diversify substrate specificity in order to expand the range of application of the enzyme.2,9,25–28
The numerous industrial and medical applications of GalOx require scalable production strategies. Thus, this enzyme has been produced heterologously in large amounts in Escherichia coli2,26 and Pichia pastoris.29 A more recent comparative study from our laboratories indicated that expression of GalOx-encoding genes in P. pastoris resulted in higher volumetric productivity in shaking-flask cultures than with common E. coli systems. Coupled with the ability to secrete protein directly into the medium, in the presence of the α-factor signal peptide and the simplicity of subsequent downstream processing, this suggests that the yeast system would be advantageous for scale-up of GalOx production.30 In addition, P. pastoris remains a popular platform for the production of recombinant enzymes due to its high specific growth rate, its strong preference for respiratory growth compared to fermentative yeasts, its ability to grow on simple growth medium, and its convenience regarding the genetic manipulations required. Also, it has the ability to perform eukaryotic protein modifications, such as proteolytic processing, folding, disulfide bond formation and glycosylation, and exhibits high levels of intra- and extracellular protein expression compared to cell culture systems of higher eukaryotes.
One very efficient expression system of P. pastoris is regulated by the alcohol oxidase 1 (AOX1) promoter. The native alcohol oxidase is the first enzyme in the catabolic pathway of methanol, and it is strongly inducible by methanol but highly repressed by other carbon sources such as sugars or ethanol.31 Integration of a recombinant protein in the P. pastoris genome under the regulation of the AOX1 promoter can lead to high protein production.32 Over 600 recombinant proteins have been successfully produced heterologously in P. pastoris,33 reaching high production yields commonly in the range of milligrams to grams per liter of culture. Methanol can be toxic at high concentrations, however, due to the accumulation of formaldehyde and hydrogen peroxide, both of which are products of the assimilation of methanol.34 Thus, the methanol-feeding rate is of utmost importance for efficient protein production, and several different methods have been examined.35,36
The methanol-feeding strategy generally suggested by the manufacturer of a widely-used P. pastoris expression system (Invitrogen Co., San Diego, CA, USA; “Pichia Fermentation Process Guidelines”) is based on monitoring of the methanol concentration with the dissolved oxygen tension (DOT) spike method. Methanol feeding is suspended regularly and the time required for the DOT to increase 10% is determined. If it is less than 1 min, methanol is considered the limiting factor and an increase in the feeding is proposed. During this process, the methanol fed is expected to be consumed instantly; its concentration in the cultivation broth is practically zero. This, however, may not be the optimal process design for the methanol fed-batch phase, and therefore alternative methanol-feeding strategies have been applied in the literature (i.e., providing methanol at constant, linear or exponential rates), which have indicated that constant feeding is the most efficient for recombinant protein production.36 When constant feeding rates are applied, methanol is continuously diluted in the growth medium and the amount that is being fed to each cell decreases, as the biomass increases.37
To our knowledge, the present work describes the first-ever attempt to optimize the recombinant F. graminearum GalOx production using a P. pastoris Mut+ SMD1168H strain under controlled fermentation conditions. Our aim was to evaluate the effect of different feeding profiles of glycerol and methanol, and also culture temperature, during the methanol-induction phase on the titre of active GalOx. Although this study describes a rather product-specific approach, our results provide insights into the level of tolerance of P. pastoris under these conditions, which will probably apply to similar production strategies employing SMD1168H and other Mut+ strains as well.
Materials and Methods
Strains
In this study we used the recombinant strain PCE of P. pastoris, which overproduces the galactose oxidase of Fusarium sp. that has been His-tagged at the C-terminus.30 It is derived from the SMD1168H strain, which is deficient in protease activity,38 and it was maintained at −80 °C in 20 % glycerol.
Growth media
The composition of the fed-batch cultivation medium for P. pastoris was as follows (per L): 40 g glycerol, 0.93 g CaSO4, 18.2 g K2SO4, 14.9 g MgSO4.7H2O, 4.13 g KOH, 7 g K2HPO4, 22.7 mL H3PO4 (85 %), 0.01 % v/v Breox FMT30 antifoam, and 12 mL of trace-elements solution. The composition of the trace-elements solution was (per L): 6 g CuSO4.5H2O, 0.08 g NaI, 3 g MnSO4.H2O, 0.2 g Na2MoO4.2H2O, 0.02 g H3BO3, 0.5 g CoCl2, 20 g ZnCl2, 65 g FeSO4.7H2O and 0.2 g biotin. NH4OH (28 % v/v) was used as a nitrogen source and for pH adjustment.39
Precultures were prepared in Buffered Glycerol Complex Medium (BMGY) in 500-mL shake flasks, with the following composition (per L): 10 g glycerol, 10 g yeast extract, 20 g peptone, 1.34 % (v/v) yeast nitrogen base (YNB), and 0.004 g biotin in 100 mM potassium phosphate buffer, pH 6.0.
Preparation of inoculum
A fresh culture with the recombinant P. pastoris PCE strain was grown for 18 to 20 h at 30 °C and 250 rpm in 500-mL baffled flasks containing 50 mL of BMGY. The cells were centrifuged and resuspended in fresh BMGY medium to a final optical density (OD) of approximately 0.3 for inoculation of bioreactors.
Cultivation conditions
To determine the physiological characteristics of the strain and enhance extracellular galactose oxidase production, fed-batch fermentations were performed, using 2.7 L DasGip bioreactors (DasGip AG, Jülich, Germany) equipped with two Rushton four-blade disc turbines and pH and temperature control. Temperature was maintained at 25 or 30 °C and pH was maintained at 6.0 by automatic addition of NH4OH, and both were kept constant throughout the cultivation. The initial stirring speed was set to 1,000 rpm and the aeration rate to 1.5 vvm; these were both controlled automatically to prevent conditions of oxygen limitation conditions, by restricting DOT to a minimum of 20 %.
The fermentations were initiated with a batch phase with 40 g L−1 glycerol as the sole carbon source. After its exhaustion, a high concentration (50 % v/v) of glycerol feed was applied, at a rate of 36 mL h−1 Linitial culture−1 for different time periods. When the glycerol concentration became the limiting factor for growth, determined by the increase in DOT, the methanol-feeding phase was initiated. Methanol was provided at a rate of 3 mL h−1 for adaptation. When DOT became stable, the cells were adapted to methanol as the sole carbon source, and different methanol-feeding rates were used for the production phase.
Quantification of biomass, enzymatic activity, and protein
Cell dry weight was determined using nitrocellulose filters (pore size 0.45 μm; Gelman Sciences) from weight difference before and after biomass filtration, after drying in an oven at 100 °C for 24 h. GalOx was activated with the addition of CuSO4 as previously described,30 and its activity was assayed by using a spectrophotometric method with 2,2′-azino-bis-(3-benzthiazoline-6-sulfonic acid) (ABTS) as substrate.5 The enzyme-substrate reaction produced a soluble blue-green product that was measurable at 405 nm (Southern Biotech, Birmingham, AL, USA). Protein was quantified using the Bio-Rad protein assay with bovine serum albumin as standard (Bio-Rad Laboratories).
Results and Discussion
To optimize the production of recombinant GalOx in a P. pastoris Mut+ strain, the effects of different induction temperatures and of various methanol- and glycerol- feeding conditions were investigated.
Effect of temperature
There have been several reports on the effect of induction temperature on recombinant protein production by P. pastoris. It is generally suggested that a decrease in temperature to below 30 °C has a positive effect on protein production.40–42 More specifically, Batra et al. observed a 2.5 to 10-fold increase in the yield of a recombinant protein when the induction phase was sustained at 20 °C rather than 30 °C.42 Also, Dragosits et al. reported an increase in the yield of the antibody fragment Fab 3H6 from 5.4 mg L−1 to double and triple values, and an increase in the specific productivity from 21 to 45 and 65 µg Fab g-1cell biomass h−1 when the temperature was reduced from 30 to 25 and 20 °C, respectively, in order to achieve a specific growth rate (µ) of 80 % and 60 % of µmax in chemostat experiments.41
In the present study, two different temperatures were used for both the growth phase and the induction phase (25 °C and 30 °C). The detectable total protein production occurred sooner when P. pastoris was grown at 30 °C (most probably due to a higher biomass concentration), but the volumetric GalOx productivity and the biomass concentration became rather constant after 85 h (Figure 1). We believe that after 85 h, carbon limitation occurred due to the higher temperature, under which the cells grow faster, and due to the applied constant methanol feeding profile. On the other hand, at 25 °C the volumetric GalOx activity in the fermentation broth continued to increase and reached 600 kU L−1 after 110 h, when the cultivation was terminated. Also, the maximum specific activity was almost doubled at 25 °C (Table1), indicating that the production of correctly folded recombinant protein was favored at this temperature relative to 30 °C. Although the total extracellular protein concentration was higher at 30 °C, the volumetric productivity of active GalOx was 66 % higher when the production was performed at 25 °C (Table1). Again, this agrees with a previous report that at 30 °C the enzyme produced can be partially misfolded and therefore more inactive than when produced at the lower temperature.30
Figure 1.
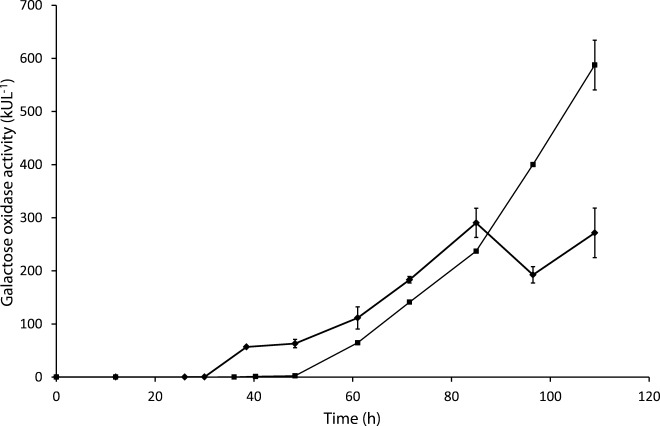
Volumetric GalOx activity in the fermentation broth at 25 °C (▪) and 30 °C (♦). Time corresponds to the total time of cultivation. Methanol feeding was initiated at 30 h. The detectable production of active and correctly folded GalOx sets off earlier at 30 °C, apparently due to the higher biomass concentration (data not shown). It stabilizes after 85 h of culture. At 25 °C, the volumetric GalOx activity increases continuously to 600 kU L−1.
Table 1.
Galactose Oxidase Activity and Productivity at Different Induction Temperatures
| Fermentation | Protein concentration (g L−1) | Volumetric activity (kU L−1) | Specific activity (U mg−1) | Volumetric productivity (kU L−1 h−1) |
|---|---|---|---|---|
| Constant methanol feed (3 mL h−1 Linitial culture volume) at 25 °C | 0.76 | 588 | 768 | 5.39 |
| Constant methanol feed (3 mL h−1 Linitial culture volume) at 30 °C | 0.93 | 354 | 396 | 3.24 |
High vs. low exponential methanol-feeding profiles
After the glycerol batch and fed-batch phase was over and glycerol became the limiting factor for growth, methanol was provided in the culture medium at a rate of 3 mL h−1 Linitial culture−1. When the DOT level in the bioreactor stabilized, the cells were regarded as being adapted to methanol. To optimize the volumetric productivity of active enzyme, which is still the key target parameter for bioprocess engineers, different exponential methanol-feeding profiles were tested at 25 °C (Table2). We designed our experiments in order to determine the most efficient way of avoiding methanol intoxication in the fermentation broth. Thus, there would be no need to follow the fermentation with a methanol detector, which can be affected by other compounds such as ammonia. Furthermore, accumulation of methanol negatively affects the viability of the cells and it also warrants safety considerations, especially in large-scale production processes.
Table 2.
Methanol-Feeding Profiles, Total Protein Concentration, and GalOx Productivity at Low and High Exponential Feeding Profiles
| Experiment | Methanol feeding profile* | Protein concentration (g L−1) | Volumetric activity (kU L−1) | Specific activity (U mg−1) | Volumetric productivity (kU L−1h−1) | |
|---|---|---|---|---|---|---|
| A | F(t) = e0.28t | High exponential | 0.45 | 66.4 | 148 | 1.3 |
| B | F(t) = e0.28EXP(0.28t) | 0.35 | 46.1 | 132 | 1.4 | |
| C | F(t) = 6e0.1t | Low exponential | 0.8 | 501.3 | 627 | 9.1 |
| D | F(t) = 4.76e0.1t | 0.97 | 564.5 | 582 | 10.3 | |
Low feeding profiles resulted in higher total GalOx activity and also higher volumetric productivity.
F(t) corresponds to the feeding flow rate in mL h−1 and t refers to the time in h after the start of the induction phase.
The first two experiments (experiments A and B; Table2) were performed with high exponential feeding rates (Figure 2A): experiment A at feeding rate that was double the maximum specific growth rate of P. pastoris on methanol which was reported to be 0.14 h−1,43 and experiment B with a double exponential feeding rate with the purpose to increase the stress for the cells. The volumetric productivity was basically the same in these two experiments, i.e., 1.3 and 1.4 kU L−1 h−1, respectively. However, production of active GalOx was found to be 148 U mg−1 in fermentation A, where a lower feeding profile was used, and 132 U mg−1 in fermentation B. These two experiments already indicated that there was a positive effect of a lower methanol-feeding rate on the production of active GalOx. To confirm this hypothesis, we performed two similar fed-batch experiments with only small differences in the initial flow and the same exponential feeding profiles, which were lower than the maximum specific growth rate (experiments C and D; Table2). In fact, the volumetric productivity was almost 8-fold higher when low methanol-feeding rates were applied rather than high feeding rates. Not only was the overall amount of extracellular protein significantly higher, but also the amount of active, correctly folded GalOx increased almost 5-fold, as shown from the values of the specific GalOx activity (Table2). The small differences in the initial flow did not see to affect the yield, although experiment D, with the lower initial flow, resulted in slightly better volumetric productivity and volumetric activity.
Figure 2.
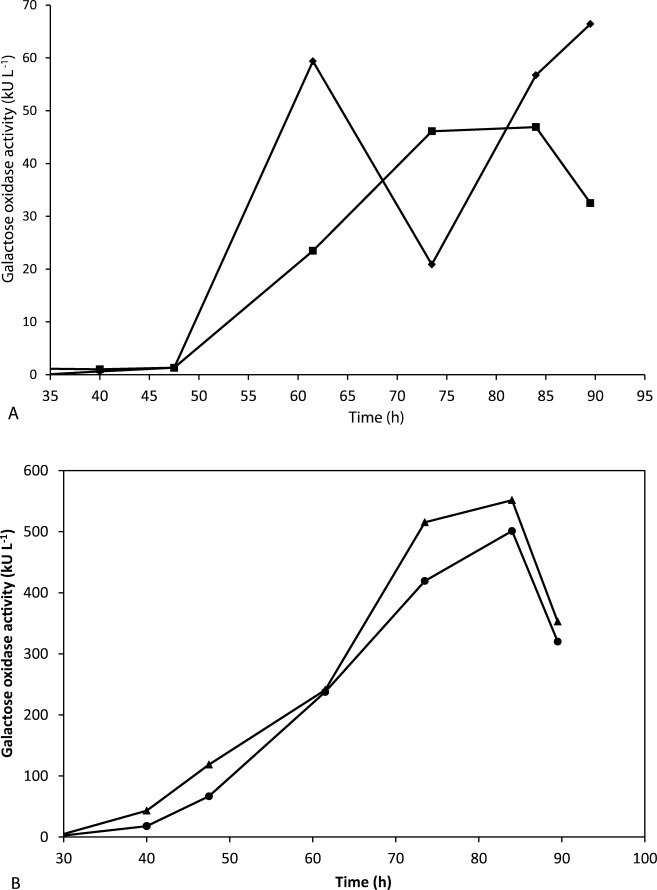
Volumetric GalOx activity as a function of time in culture using (A) high methanol-feeding rates, following the function F(t) = e0.28t (♦) and F(t) = e0.28EXP(0.28t) (▪), and (B) low methanol-feeding rates following the function F(t) = 6e0.1t (•) and F(t) = 4.76e0.1t (▴). Under mild conditions, the volumetric GalOx activity in the fermentation broth was almost 10 times higher than in the former case. Time refers to the total time in culture. The exponential phase started at 40 h.
Optimization of the methanol-feeding profile
Considering that low exponential methanol-feeding rates proved to be favorable in terms of GalOx productivity, the effect of different low exponential feeding rates was further evaluated in order to fine-tune the feeding profile (Table3). For this purpose, the conclusions from the model developed by Zhang et al.37 were used to correlate the specific growth rate (μ), the product formation, and the methanol concentration. According to this model and their subsequent experimental results, the maximum μ was 0.08 h−1 and 0.0709 h−1 respectively, when the methanol concentration in the growth medium was kept constant at 3.65 g L−1, while lower or higher concentrations resulted in methanol limitation or inhibition. In this respect, we designed four different fed-batch experiments, where the feeding profiles were designed in such a way as to obtain certain specific growth rates, as shown in Table3. We wanted to cover different μ-ranges, from the predetermined maximum of around 0.0709 h−1 to only a third of that. The initial flow (α, Table3) was adjusted, so that the range of the pumps would fit the range of flows required in the duration of the culture.
Table 3.
Methanol-Feeding Rate, Exponential Feeding Rate in Relation to the Optimum μmax According to Zhang et al.,37 Protein Concentration, and GalOx Productivity in Experiments E-H
| Experiment | Exponential growth rate | Methanol feeding profile* F(t) = α eβt | Exponential feeding rate relative to optimum µmax (%)† | Protein concentration (g L−1) | Volumetric activity (kU L−1) | Specific activity (U mg−1) | Volumetric productivity (kU L−1h−1) | |
|---|---|---|---|---|---|---|---|---|
| α | β | |||||||
| E | High | 26.96 | 0.071 | 100 | 0.57 | 346 | 608 | 9.9 |
| F | Medium | 20.89 | 0.0497 | 70 | 0.81 | 559 | 694 | 16.0 |
| G | Low | 9.64 | 0.0213 | 30 | 0.61 | 491 | 811 | 14.0 |
| H | Low with low initial methanol concentration | 2.22 | 0.0213 | 30 | 0.38 | 239 | 630 | 10.7 |
F(t) corresponds to the feeding flow rate in mL h−1 and t refers to the time in h after the start of the induction phase.
Predicted optimum μmax, according to the results by Zhang et al.,37 is 0.071 h−1, when methanol concentration in the medium remains constant at 3.65 g L−1.
As shown in Table3, the highest volumetric productivity of 16 kU L−1 h−1 was obtained at a medium specific growth rate of 0.05 h−1 (experiment F), compared to the 14 kU L−1 h−1 at a low specific growth rate and 9.9 kU L−1 h−1 at a high specific growth rate. Volumetric activity and protein concentration were similarly higher at the medium specific growth rate. However, the specific activity of extracellular GalOx in the fermentation broth was higher at a lower specific growth rate of 0.02 h−1 (experiment G). At the lower specific growth rate, more correctly folded and active GalOx was apparently secreted into the fermentation broth than at higher specific growth rates.
In order to investigate the potentially toxic effect of methanol, even at the low specific growth rate, we performed experiment H (Table3), where the methanol-feeding was provided at the same exponential rate but with a lower starting concentration. As can be seen from Table3, the lower initial methanol concentration did not result in the same volumetric productivity, but in a 25% reduction (14.0 and 10.7 kU L−1h−1, respectively). Not only was less total protein secreted by the cells, but also less active GalOx was produced, as seen by the reduced specific activity (Table3). It appears that induction efficiency was restricted under these conditions by the low methanol concentration and also by the slightly lower biomass concentration. Indeed, the latter approximated 82 g L−1 at medium specific growth rate, but was lower in all other cases.
Influence of the glycerol-feeding phase on the methanol-induction phase
Considering that higher biomass concentrations have been reported,44 pinpointing the relationship between recombinant protein productivity and biomass concentration at the start of the induction phase, we performed four additional fed-batch experiments to investigate a possible influence of the glycerol fed-batch phase, and thus the biomass concentration, on the subsequent methanol-induction phase and the concomitant production of recombinant GalOx, and to further determine whether constant methanol feeding is more favorable for the production of active GalOx than an exponential feeding profile (experiments I-L, Table4).
Table 4.
Experimental Design to Investigate Any Possible Influence of the Glycerol-Feeding Phase and Different Methanol-Feeding Profiles on the Production of Active GalOx
| Experiment | Glycerol feeding phase (h) | Methanol feeding profile* | Protein concentration (g L−1) | Volumetric activity (kU L−1) | Specific activity (U mg−1) | Volumetric productivity (kU L−1h−1) | |
|---|---|---|---|---|---|---|---|
| I | 4 | 3 mL h−1 Linitial culture volume | 1.38 | 1447 | 1045 | 10.56 | |
| J | 8 | 3 mL h−1 Linitial culture volume | 1.12 | 1508 | 1343 | 11.01 | |
| K† | 8 | F(t) = 3.6 e0.0213t | 0.9 | 909 | 1005 | 6.63 | |
| 1.61 (131 h) | 2016 (131 h) | 1250 (131 h) | 15.39 (131 h) | ||||
| L | 8 | constant according to wet cell weight | 1.05 | 1588 | 1509 | 11.59 |
The protein concentration, volumetric activity, specific activity, and volumetric productivity at the final time point (137 h) are presented.
F(t) corresponds to the feeding flow rate in mL h−1 and t refers to the time in h after the start of the induction phase.
For experiment K, the respective values at 131 h are also shown, to demonstrate their fluctuation.
After 140 h of cultivation there was no difference in the volumetric productivity between experiment I (4 h of glycerol feeding) and J (8 h of glycerol feeding) (Figure 3). Of course, the production of active GalOx started sooner when glycerol was only fed in for 4 h, but already after around 80 h the amount of active GalOx in the fermentation broth in experiments I and J were the same. Also, the cell concentration seemed to reach its maximum density at the end of the induction phase, yielding comparable final total protein titres. There is apparently no significant effect of the glycerol fed-batch phase on the subsequent production of active GalOx in the induction phase. Due to the fact that yeasts consume more oxygen and produce more heat when metabolizing methanol rather than glycerol,43 a prolonged glycerol-feeding phase should be favorable. To investigate this, we used a glycerol-feeding phase of 8 h in the remaining fed-batch experiments (K and L; Table4) where we wanted to test whether an exponential feeding rate with a predefined feeding function gives a higher titre of product than an exponential feeding rate, which we adjusted according to the current wet cell weight. The latter strategy involving an exponential feeding rate is where the cells are fed at a constant specific substrate uptake rate (qs), which is similar to that of recently published studies.45,46 As shown in Figure 3, the methanol-feeding strategy based on constant qs was superior to an exponential feeding profile with a predefined feeding function regarding volumetric productivity. Furthermore, the latter feeding profile resulted in strong fluctuations in the production of active GalOx, especially in the later phases of the induction period (Figure 3). Also, the total extracellular protein fluctuated similarly (data not shown), which suggests that methanol becomes inhibitory at certain concentrations and cell densities. By applying an exponential feeding profile with a predefined feeding function, the culture apparently cannot be controlled reliably: potential changes in yields cannot be predicted, there is a risk of over-feeding or starvation periods for the cells, and the uncontrolled conditions could result in the production of undesirable metabolites. Even so, although exponential methanol-feeding rates appear to be detrimental for the productivity, during prolonged cultivation times the population manages to adapt to the high methanol concentration.
Figure 3.
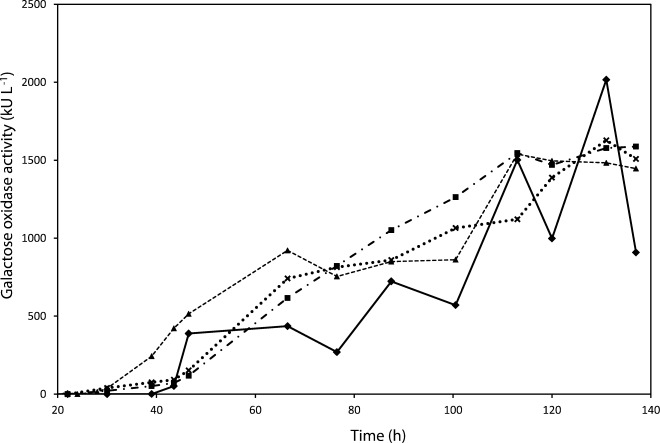
Volumetric GalOx activity in the fermentation broth under different fed-batch conditions; (I) 4-h glycerol-feeding phase and constant methanol-feeding rate (3 mL h−1 Linitial culture volume−1) (▴), (J) 8-h glycerol feeding phase with constant methanol feeding rate (3 mL h−1 Linitial culture volume−1) (x), (K) 8-h glycerol-feeding phase and exponential methanol-feeding rate [F(t) = 3.6e0.0213t] (♦), (L) 8-h glycerol feeding phase with constant methanol feeding rate to wet cell weight ratio (▪). Time corresponds to the total time of the cultivation. Methanol adaptation started at 30 h and lasted for 2 h.
Despite the difference in the initial biomass concentration between the 4 h and the 8 h glycerol-feeding phases, the final active GalOx productivity was similar. This result is surprising and contradicts the report of Wang et al.,44 who also examined the growth and protein production of a recombinant P. pastoris Mut+ strain. Although they found that the cell concentration reached similar final levels (122 g L−1) regardless of its value at the start of the induction stage (62.5, 90 or 122 g L−1), at the end of the induction the enzyme titre was higher, proportionally to the initial biomass concentration (102, 168 and 207 U mL−1 respectively), with similarly affected volumetric productivities (1.14, 1.87 and 2.39 U mL−1 h−1). They also tested different constant methanol-feeding rates, following the highest biomass levels they achieved, and showed that in agreement (to some extent) with our results, the high methanol-feeding rates stress the cell machinery and negatively affect the process performance. In a relevant study, Cunha et al. demonstrated a positive effect of the initial biomass on the protein production during the induction phase.47
Several studies to date have concentrated on the feeding strategies with glycerol and methanol regarding recombinant protein production in P. pastoris. Zhou et al. and Trinh et al., for example, investigated the effects of co-feeding methanol and glycerol during the induction phase. Zhou et al. reported a higher protein yield when co-feeding with glycerol and methanol.48 Trinh et al. investigated the effect of three different strategies on protein production, in addition to the co-feeding. They reported that feeding with methanol at a predefined exponential rate to a μ of 0.02 h−1 was superior for endostatin production to cultivations controlled by a methanol sensor or controlled by the oxygen consumption, in terms of specific productivity (0.72 mg endostatin g−1DCW compared to 0.32 mg endostatin g−1DCW in both other cases).35 On the other hand, Li et al. reported higher productivity when methanol was provided at a constant rate of 3, 6, or 10 g L−1 than with exponential feeding profiles.36 Results are apparently inconsistent and thus recommendations are different. In our study, we tested and compared both feeding strategies (experiments J and K; Table4). As shown in Figure 3, both feeding strategies basically resulted in the same range of final volumetric productivities, although the exponential feeding profile resulted in higher fluctuations in GalOx activity. These fluctuations were also evident in the protein concentrations (data not shown), indicating the uncontrollable reaction of the population to cycles of cell intoxication due to over-feeding and subsequent adaptation.
Conclusion
In this study, we tested different induction temperatures and different glycerol-and methanol-feeding strategies to optimize the production of GalOx with a recombinant P. pastoris Mut+ strain. Our results indicate that an induction temperature of 25 °C is superior to 30 °C, as both the production of active enzyme and the volumetric productivity increased up to 8-fold. Also, a low exponential methanol-feeding profile gives more active GalOx and almost 1.5-fold higher volumetric productivity than a high exponential feeding strategy. Although these findings correspond to previous reports in literature, they still might be product or strain-specific. The duration of the glycerol-feeding phase has no direct effect on the subsequent methanol-induction phase for the investigated Mut+ strain. In terms of oxygen consumption and heat production, we would recommend prolonged glycerol fed-batch phases leading to a higher initial biomass concentration for induction. A linear methanol-feeding profile and an exponential methanol-feeding profile resulted in basically the same volumetric productivities, while one adapted to the apparent biomass concentration in the bioreactor gives more stable cultures. By applying this method, the risk of under-feeding or over-feeding the cultures is omitted (e.g., when yields change during cultivation due to recombinant protein production). This finding is not product-specific and can be of great relevance for other recombinant P. pastoris Mut+ strains.
To our knowledge this is the first time that it is highlighted that despite the differences in the pre-induction biomass concentrations and the non-toxic methanol feeding profiles, there is a plateau in the volumetric activity values where all processes converge. A conservative methanol-feeding profile would actually contribute to the stability of the culture and avoid uncontrollable fluctuations in the concentrations of recombinant proteins.
Acknowledgments
This work was supported by the Knut and Alice Wallenberg Foundation through the Wallenberg Wood Science Center (http://www.wwsc.se).
Literature Cited
- 1.Avigad G, Amaral D, Asensio C, Horecker BL. The D-galactose oxidase of Polyporus circinatus. J Biol Chem. 1962;237:2736–2743. [PubMed] [Google Scholar]
- 2.Sun L, Petrounia IP, Yagasaki M, Bandara G, Arnold FH. Expression and stabilization of galactose oxidase in Escherichia coli by directed evolution. Protein Eng. 2001;14:699–704. doi: 10.1093/protein/14.9.699. [DOI] [PubMed] [Google Scholar]
- 3.Whittaker JW. Free radical catalysis by galactose oxidase. Chem Rev. 2003;103:2347–2363. doi: 10.1021/cr020425z. [DOI] [PubMed] [Google Scholar]
- 4.Whittaker JW. The radical chemistry of galactose oxidase. Arch Biochem Biophys. 2005;433:227–239. doi: 10.1016/j.abb.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Baron AJ, Stevens C, Wilmot C, Seneviratne KD, Blakeley V, Dooley DM, Phillips SE, Knowles PF, McPherson MJ. Structure and mechanism of galactose oxidase. The free radical site. J Biol Chem. 1994;269:25095–25105. [PubMed] [Google Scholar]
- 6.Kosman DJ, Ettinger MJ, Weiner RE, Massaro EJ. The molecular properties of the copper enzyme galactose oxidase. Arch Biochem Biophys. 1974;165:456–467. doi: 10.1016/0003-9861(74)90271-9. [DOI] [PubMed] [Google Scholar]
- 7.Rogers MS, Tyler EM, Akyumani N, Kurtis CR, Spooner RK, Deacon SE, Tamber S, Firbank SJ, Mahmoud K, Knowles PF, Phillips SE, McPherson MJ, Dooley DM. The stacking tryptophan of galactose oxidase: A second-coordination sphere residue that has profound effects on tyrosyl radical behavior and enzyme catalysis. Biochemistry. 2007;46:4606–4618. doi: 10.1021/bi062139d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito N, Phillips SE, Stevens C, Ogel ZB, McPherson MJ, Keen JN, Yadav KD, Knowles PF. Novel thioether bond revealed by a 1.7 A crystal structure of galactose oxidase. Nature. 1991;350:87–90. doi: 10.1038/350087a0. [DOI] [PubMed] [Google Scholar]
- 9.Deacon SE, Mahmoud K, Spooner RK, Firbank SJ, Knowles PF, Phillips SE, McPherson MJ. Enhanced fructose oxidase activity in a galactose oxidase variant. ChemBioChem. 2004;5:972–979. doi: 10.1002/cbic.200300810. [DOI] [PubMed] [Google Scholar]
- 10.McFaul SJ, Lin H, Everse J. The mechanism of peroxidase-mediated cytotoxicity. I. Comparison of horseradish peroxidase and lactoperoxidase. Proc Soc Exp Biol Med. 1986;183:244–249. doi: 10.3181/00379727-183-42413. [DOI] [PubMed] [Google Scholar]
- 11.Majerus PM, Courtois PA. Susceptibility of Candida albicans to peroxidase-catalyzed oxidation products of thiocyanate, iodide and bromide. J Biol Buccale. 1992;20:241–245. [PubMed] [Google Scholar]
- 12.Szabo EE, Adanyi N, Varadi M. Application of biosensor for monitoring galactose content. Biosens Bioelectronics. 1996;11:1051–1058. doi: 10.1016/0956-5663(96)87664-0. [DOI] [PubMed] [Google Scholar]
- 13.Adányi N, Szabó EE, Váradi M. Multi-enzyme biosensors with amperometric detection for determination of lactose in milk and dairy products. Eur Food Res Technol. 1999;209:220–226. [Google Scholar]
- 14.Amárita Vega F, Núñez CG, Weigel B, Hitzmann B, Diaz Ricci JC. On-line monitoring of galactoside conjugates and glycerol by flow injection analysis. Anal Chim Acta. 1998;373:57–62. [Google Scholar]
- 15.Kinoshita M, Inagake K, Kawabata A, Kuroda R, Oda Y, Kakehi K. Fluorometric determination of mucin-type glycoproteins by the galactose oxidase-peroxidase method. Anal Biochem. 2000;284:87–92. doi: 10.1006/abio.2000.4689. [DOI] [PubMed] [Google Scholar]
- 16.Henderson GE, Isett KD, Gerngross TU. Site-specific modification of recombinant proteins: a novel platform for modifying glycoproteins expressed in E. coli. Bioconjug Chem. 2011;22:903–912. doi: 10.1021/bc100510g. [DOI] [PubMed] [Google Scholar]
- 17.Said IT, Shamsuddin AM, Sherief MA, Taleb SG, Aref WF, Kumar D. Comparison of different techniques for detection of Gal-GalNAc, an early marker of colonic neoplasia. Histology Histopathol. 1999;14:351–357. doi: 10.14670/HH-14.351. [DOI] [PubMed] [Google Scholar]
- 18.Carter JH, Deddens JA, Pullman JL, Colligan BM, Whiteley LO, Carter HW. Validation of the galactose oxidase-Schiff's reagent sequence for early detection and prognosis in human colorectal adenocarcinoma. Clin Cancer Res. 1997;3:1479–1489. [PubMed] [Google Scholar]
- 19.Leppänen A-S, Xu C, Parikka K, Eklund P, Sjöholm R, Brumer H, Tenkanen M, Willför S. Targeted allylation and propargylation of galactose-containing polysaccharides in water. Carbohydr Polym. 2014;100:46–54. doi: 10.1016/j.carbpol.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 20.Parikka K, Leppanen AS, Xu C, Pitkanen L, Eronen P, Osterberg M, Brumer H, Willfor S, Tenkanen M. Functional and anionic cellulose-interacting polymers by selective chemo-enzymatic carboxylation of galactose-containing polysaccharides. Biomacromolecules. 2012;13:2418–2428. doi: 10.1021/bm300679a. [DOI] [PubMed] [Google Scholar]
- 21.Xu C, Spadiut O, Araujo AC, Nakhai A, Brumer H. Chemo-enzymatic assembly of clickable cellulose surfaces via multivalent polysaccharides. ChemSusChem. 2012;5:661–665. doi: 10.1002/cssc.201100522. [DOI] [PubMed] [Google Scholar]
- 22.Lang P, Masci G, Dentini M, Crescenzi V, Cooke D, Gidley MJ, Fanutti C, Reid JSG. Tamarind seed polysaccharide: preparation, characterisation and solution properties of carboxylated, sulphated and alkylaminated derivatives. Carbohydr Polym. 1992;17:185–198. [Google Scholar]
- 23.Yalpani M, Hall LD. Some chemical and analytical aspects of polysaccharide modifications. II. A high-yielding, specific method for the chemical derivatization of galactose-containing polysaccharides: Oxidation with galactose oxidase followed by reductive amination. J Polym Sci: Polym Chem Ed. 1982;20:3399–3420. [Google Scholar]
- 24.Kupper CE, Rosencrantz RR, Henssen B, Pelantova H, Thones S, Drozdova A, Kren V, Elling L. Chemo-enzymatic modification of poly-N-acetyllactosamine (LacNAc) oligomers and N,N-diacetyllactosamine (LacDiNAc) based on galactose oxidase treatment. Beilstein J Org Chem. 2012;8:712–725. doi: 10.3762/bjoc.8.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escalettes F, Turner NJ. Directed evolution of galactose oxidase: Generation of enantioselective secondary alcohol oxidases. ChemBioChem. 2008;9:857–860. doi: 10.1002/cbic.200700689. [DOI] [PubMed] [Google Scholar]
- 26.Deacon SE, McPherson MJ. Enhanced expression and purification of fungal galactose oxidase in Escherichia coli and use for analysis of a saturation mutagenesis library. ChemBioChem. 2011;12:593–601. doi: 10.1002/cbic.201000634. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, Bulter T, Alcalde M, Petrounia IP, Arnold FH. Modification of galactose oxidase to introduce glucose 6-oxidase activity. ChemBioChem. 2002;3:781–783. doi: 10.1002/1439-7633(20020802)3:8<781::AID-CBIC781>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Rannes JB, Ioannou A, Willies SC, Grogan G, Behrens C, Flitsch SL, Turner NJ. Glycoprotein labeling using engineered variants of galactose oxidase obtained by directed evolution. J Am Chem Soc. 2011;133:8436–8439. doi: 10.1021/ja2018477. [DOI] [PubMed] [Google Scholar]
- 29.Whittaker MM, Whittaker JW. Expression of recombinant galactose oxidase by Pichia pastoris. Protein Expr Purif. 2000;20:105–111. doi: 10.1006/prep.2000.1287. [DOI] [PubMed] [Google Scholar]
- 30.Spadiut O, Olsson L, Brumer H., III A comparative summary of expression systems for the recombinant production of galactose oxidase. Microb Cell Fact. 2010;9:68. doi: 10.1186/1475-2859-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins D, Cregg J. Pichia Protocols. Totowa, New Jersey: Humana Press; 1998. p. 103. 270. [Google Scholar]
- 32.Cregg JM, Cereghino JL, Shi J, Higgins DR. Recombinant protein expression in Pichia pastoris. Mol Biotechnol. 2000;16:23–52. doi: 10.1385/MB:16:1:23. [DOI] [PubMed] [Google Scholar]
- 33.Zhang AL, Luo JX, Zhang TY, Pan YW, Tan YH, Fu CY, Tu FZ. Recent advances on the GAP promoter derived expression system of Pichia pastoris. Mol Biol Rep. 2009;36:1611–1619. doi: 10.1007/s11033-008-9359-4. [DOI] [PubMed] [Google Scholar]
- 34.Couderc R, Baratti J. Oxidation of Methanol by the Yeast, Pichia pastoris. Purification and properties of the alcohol oxidase. Agric Biol Chem. 1980;44:2279–2289. [Google Scholar]
- 35.Trinh LB, Phue JN, Shiloach J. Effect of methanol feeding strategies on production and yield of recombinant mouse endostatin from Pichia pastoris. Biotechnol Bioeng. 2003;82:438–444. doi: 10.1002/bit.10587. [DOI] [PubMed] [Google Scholar]
- 36.Li ZM, Ping XB, Ye Q, Huang XD, Cao ZF. Production and optimization of recombinant human augmenter of liver regeneration by Pichia pastoris. Enzyme Microb Technol. 2010;47:222–227. [Google Scholar]
- 37.Zhang W, Bevins MA, Plantz BA, Smith LA, Meagher MM. Modeling Pichia pastoris growth on methanol and optimizing the production of a recombinant protein, the heavy-chain fragment C of botulinum neurotoxin, serotype A. Biotechnol Bioeng. 2000;70:1–8. doi: 10.1002/1097-0290(20001005)70:1<1::aid-bit1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Inan M, Meagher MM. Rational design and optimization of fed-batch and continuous fermentations. In: Cregg JM, editor. Pichia Protocols. Vol. 389. Humana Press; 2007. pp. 43–63. [DOI] [PubMed] [Google Scholar]
- 39.Bushell ME, Rowe M, Avignone-Rossa CA, Wardell JN. Cyclic fed-batch culture for production of human serum albumin in Pichia pastoris. Biotechnol Bioeng. 2003;82:678–683. doi: 10.1002/bit.10616. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Tang C, Shi H, Wu M. Cloning and optimized expression of a neutral endoglucanase gene (ncel5A) from Volvariella volvacea WX32 in Pichia pastoris. J Biosci Bioeng. 2011;111:537–540. doi: 10.1016/j.jbiosc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Dragosits M, Frascotti G, Bernard-Granger L, Vazquez F, Giuliani M, Baumann K, Rodriguez-Carmona E, Tokkanen J, Parrilli E, Wiebe MG, Kunert R, Maurer M, Gasser B, Sauer M, Branduardi P, Pakula T, Saloheimo M, Penttila M, Ferrer P, Luisa Tutino M, Villaverde A, Porro D, Mattanovich D. Influence of growth temperature on the production of antibody Fab fragments in different microbes: A host comparative analysis. Biotechnol Prog. 2011;27:38–46. doi: 10.1002/btpr.524. [DOI] [PubMed] [Google Scholar]
- 42.Batra G, Gurramkonda C, Nemani SK, Jain SK, Swaminathan S, Khanna N. Optimization of conditions for secretion of dengue virus type 2 envelope domain III using Pichia pastoris. J Biosci Bioeng. 2010;110:408–414. doi: 10.1016/j.jbiosc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Jungo C, Marison I, von Stockar U. Mixed feeds of glycerol and methanol can improve the performance of Pichia pastoris cultures: A quantitative study based on concentration gradients in transient continuous cultures. J Biotechnol. 2007;128:824–837. doi: 10.1016/j.jbiotec.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Wang Z, Du G, Hua Z, Liu L, Li J, Chen J. Enhancement of alkaline polygalacturonate lyase production in recombinant Pichia pastoris according to the ratio of methanol to cell concentration. Bioresour Technol. 2009;100:1343–1349. doi: 10.1016/j.biortech.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 45.Dietzsch C, Spadiut O, Herwig C. A dynamic method based on the specific substrate uptake rate to set up a feeding strategy for Pichia pastoris. Microb Cell Fact. 2011;10:14. doi: 10.1186/1475-2859-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dietzsch C, Spadiut O, Herwig C. A fast approach to determine a fed batch feeding profile for recombinant Pichia pastoris strains. Microb Cell Fact. 2011;10:85. doi: 10.1186/1475-2859-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunha AE, Clemente JJ, Gomes R, Pinto F, Thomaz M, Miranda S, Pinto R, Moosmayer D, Donner P, Carrondo MJ. Methanol induction optimization for scFv antibody fragment production in Pichia pastoris. Biotechnol Bioeng. 2004;86:458–467. doi: 10.1002/bit.20051. [DOI] [PubMed] [Google Scholar]
- 48.Zhou XS, Fan WM, Zhang YX. Effects of different methanol feeding strategy on hirudin production in high-density fermentation by recombinant Pichia pastoris. Chin J Biotechnol. 2002;18:348–351. [PubMed] [Google Scholar]


