Abstract
Bovine primary uterine endometrial epithelial cells (EECs) are not ideal for long-term studies, because primary EECs lose hormone responsiveness quickly, and/or they tend to have a short life span. The aims of this study were to establish immortalized bovine EECs and to characterize these cells following long-term cultures. Immortalized bovine EECs were established by transfecting retroviral vectors encoding human papillomavirus (HPV) E6 and E7, and human telomerase reverse transcriptase (hTERT) genes. Established bovine immortalized EECs (imEECs) showed the same morphology as primary EECs, and could be grown without any apparent changes for over 60 passages. In addition, imEECs have maintained the features as EECs, exhibiting oxytocin (OT) and interferon tau (IFNT) responsiveness. Therefore, these imEECs, even after numbers of passages, could be used as an in vitro model to investigate cellular and molecular mechanisms, by which the uterine epithelium responds to IFNT stimulation, the event required for the maternal recognition of pregnancy in the bovine species.
Keywords: bovine, endometrial epithelial cell, immortalization
Introduction
In most mammals, conceptus implantation to the uterine endometrium consists of blastocyst hatching, migration, apposition/attachment, invasion and subsequent placental formation. It is known that close to 50% of fertilized pre-implantation embryos in mammals, including humans, fail to implant (Wilcox et al. 1988). The 40–50% of embryonic losses that occur between days 8 and 17 of pregnancy in cattle are thought to result from insufficient communication between the conceptus and the maternal environment (Wolf et al. 2003). To reduce embryonic losses in the bovine species, biochemical communication between the conceptus and endometrium needs to be characterized. However, in the bovine species continuous sampling of uterine and conceptus tissues is difficult to achieve. Therefore, development of in vitro models to study implantation processes is required.
Recently, we have established an in vitro co-culture system with bovine trophoblast CT-1 cells and primary uterine endometrial epithelial cells (EECs) that mimic the in vivo attachment process (Sakurai et al. 2012). However, primary EECs are not ideal for long-term studies, because these cells undergo some de-differentiation in culture, for example, loss of hormone and growth factor/cytokine responsiveness, and have a short life span before senescence. In addition, consistent supply of primary EECs is required, if the in vitro culture system is continued to be executed. In sheep, immortalized cell lines of luminal and glandular epithelial cells and stromal cells have been established and characterized (Johnson et al. 1999). In the bovine, a spontaneously derived bovine endometrial epithelial cell line, bovine endometrial cell (BEND), is used as a model to investigate the mechanisms regulating prostaglandin (PG) production (Binelli et al. 2001). Furthermore, bovine immortalized EECs (named bEEL) were established using lentiviral vector expressing human telomerase (Krishnaswamy et al. 2009). The bEEL is a good in vitro model to investigate the mechanism associated with the inhibition of oxytocin (OT)-induced PGF2α production by conceptus interferon tau (IFNT).
In days 8 and 17 of bovine pregnancy, during which the process of maternal recognition of pregnancy must occur (Bazer et al. 1991), attenuation of OT-induced PGF2α production is undoubtedly important in determining success or failure of pregnancy (Spencer et al. 1999). However, the subsequent events, such as conceptus attachment and limited invasion to the maternal endometrium, are also critical for the implantation processes to proceed. If the immortalized EECs, of which characteristics of primary EECs could be maintained for longer periods, were established, the in vitro co-culture system (Sakurai et al. 2012) would become a more valuable tool to study interaction as well as events associated with conceptus attachment and limited invasion to the maternal endometrium. Unfortunately, these events are often overlooked or are not able to be studied, even though early embryonic losses occur during this time period. The aims of this study were to establish immortalized bovine EECs from an early passage stage of primary EECs, and to characterize cell responsiveness to OT and/or IFNT following long-term cultures.
Materials and Methods
Culture and immortalization of bovine EECs
Primary bovine EECs were isolated and cultured as previously described (Skarzynski et al. 2000). In brief, uteri of Holstein cows were obtained from a local abattoir in accordance with protocols approved by the local Institutional Animal Care and Use Committee. Healthy uteri without a visible conceptus were obtained within 10–20 min after exsanguination and immediately transported to the laboratory on ice. The stages of the estrous cycle were determined macroscopically. The cells were maintained at 37°C in air with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12, 1:1 (v:v) (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% (v/v) newborn calf serum (Invitrogen, Carlsbad, CA, USA) and antibiotic/antimycotic solution (Invitrogen).
Retroviral vectors were kindly provided by Dr. T. Kiyono, National Cancer Center Research Institute, Tokyo, Japan. Production of recombinant retroviruses was performed according to the methods described by Kyo et al. (2003). Briefly, retroviral vectors, human papillomavirus (HPV)-16 E6, HPV-16 E7, human telomerase reverse transcriptase (hTERT) and packaging construct, pCL-10A1 (IMGENEX., San Diego, CA, USA), were co-transfected into Platinum-GP (Plat-GP) cells (Cell Biolabs, Inc., San Diego, CA, USA) using FuGene6 (Roche Diagnostics, Tokyo, Japan) according to the manufacturer's instructions. The culture fluid was harvested 48 h after transfection, and viral supernatant was collected through the filtration with a 0.45-μm low protein binding filter (Millipore, Bedford, MA, USA). Viral supernatant was then combined with 1 mL of serum-free DMEM/Ham's F-12 medium containing protamine sulfate (Sigma-Aldrich) and overlaid onto third-passage primary EECs that had been grown to 70% confluence in six-well dishes.
Cell proliferation assays
Cell proliferation assays were conducted using the Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) according to the manufacturer's protocol. Primary or immortalized EECs were seeded onto a six-well plate coated with or without collagen type IA and cultured as described above. After attachment (0, 24 or 48 h), seeded cells were treated with 1 mL of DMEM/Ham's F-12 with CCK-8 reagent (1: 10, v/v), and the cells were incubated for 1 h. To estimate proliferated cell numbers, absorbance was measured for each well at a wavelength of 450 nm using an auto-microplate reader (Wallac 1420; Perkin Elmer Japan, Tokyo, Japan).
RNA extraction and analysis
Total RNA was extracted from EECs with ISOGEN (Nippon Gene, Tokyo, Japan) according to the protocol provided by the manufacturer. For PCR, isolated RNA (total 1 μg) was reverse-transcribed to complementary DNA (cDNA) using ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan) including 1 × RT buffer, enzyme mix, and primer mix in a 10 μL reaction volume, and the resulting cDNA (RT template) was stored at 4°C until use. The cDNA reaction mixture was diluted 1:10 using deoxyribonuclease (DNase)- and ribonuclease (RNase)-free molecular biology-grade water and 3 μL were taken for each amplification reaction. PCR was carried out with 0.5 units of ExTaq HS polymerase (Takara Bio, Tokyo, Japan), 1 × ExTaq HS buffer, 0.2 μmol/L of the oligonucleotide primers described in Table 1 and 0.2 mmol/L of dNTP in a final volume 20 μL. The thermal profile for PCR was at 95°C for 2 min, followed by 30 cycles of 95°C for 30 s, 60°C for 30 s and 72°C for 30 s. The PCR products were separated on a 1.5% agarose gel containing ethidium bromide and were visualized under UV light.
Table 1.
Primers for RT-PCR analyses
| Name (GenBank accession no.) | Sequence | Length (bp) |
|---|---|---|
| E6 | F: GCAACAGTTACTGCGACGTG | |
| (FJ237042.1) | R: GGACACAGTGGCTTTTGACA | 234 |
| E7 | F: CATGCATGGAGATACACCTACAT | |
| (FJ237041.1) | R: TTATGGTTTCTGAGAACAGAT | 298 |
| hTERT | F: CGGAAGAGTGTCTGGAGCAA | |
| (BC172541.1) | R: GGATGAAGCGGAGTCTGGA | 145 |
| IRF1 | F: GCTGGGACATCAACAAGGAT | |
| (XM_001787207.1) | R: CTGCTCTGGTCCTTCACCTC | 163 |
| IRF2 | F: AAACTGGGCCATCCATACAG | |
| (XM_592435.3) | R: TTAGAAGGCCGCTCAGACAT | 192 |
| IRF9 | F: GAGAACTGCTGTCCCTGGAG | |
| (NM_001024506.1) | R: GGGTTTGGGAAAGATCACCT | 179 |
| MX1 | F: GTCCCTGCTAACGTGGACAT | |
| (NM_173940) | R: ACCAGGTTTCTCACCACGTC | 155 |
| MX2 | F: GCAGATCAAGGCACTCATCA | |
| (NM_173941.2) | R: ACCAGGTCTGGTTTGGTCAG | 168 |
| STAT1 | F: GCCTCAAGTTTGCCAGTGGC | |
| (BC151378.1) | R: GGCTCCCTTGATAGAACTGT | 314 |
| STAT2 | F: CAGCCAGTACCAGATGCTCA | |
| (XM_588270) | R: CCAGCAGTGGCTCTAAGTCC | 296 |
| ACTB | F: CTCTTCCAGCCTTCCTTCCT | |
| (BC102948) | R: GGGCAGTGATCTCTTTCTGC | 178 |
F, forward; R, reverse.
Measurement of PGF2α
To evaluate the OT responsiveness, primary and immortalized EECs that had been cultured for 48 h were treated with a range (1 to 1000 ng/mL) of OT (Teikoku Hormone MFG Co., Tokyo, Japan) for 24 h. After incubation, the conditioned media were collected, from which PGF2α concentrations were determined by enzyme immunoassay (EIA) as previously described (Woclawek-Potocka et al. 2004). The remaining cells were subjected to cell number analysis using the CCK-8 method. The final PGF2α concentrations were obtained following the cell number correction from the value of CCK-8.
Immunocytochemistry
EECs were plated onto non-coated six-well dishes and analyzed for expression of epithelial-specific marker cytokeratin or stromal-specific marker vimentin. The cells were grown to 70% confluence and fixed in 4% paraformaldehyde at room temperature for 15 min. Non-specific binding was inhibited by blocking the cells with 10% normal goat serum (Invitrogen) for 30 min. The cells were incubated with primary antibodies, cytokeratin (Dako, Glostrup, Denmark; diluted 1:500 in phosphate-buffered saline (PBS)) or vimentin (Dako, diluted 1:50 in PBS), for 2 h, and with biotin-conjugated secondary antibody for 30 min. Cells were then incubated with Avidin Alexa 568 Streptavidin Conjugates (Invitrogen) for 30 min. The cells were then examined under light microscope (BX-51; Olympus, Tokyo, Japan).
Statistical analysis
The concentrations of PGF2α in the culture media of OT-treated primary and immortalized EECs, measured by the enzyme immunoassay, represent the results of triplicate samples within an assay, and were expressed as the mean ± SEM. These data were analyzed by one-way analysis of variance (ANOVA), followed by Dunnett's test for multiple comparisons with the StatView statistical analysis software (version 5; SAS Institute Inc., Cary, NC, USA). Differences of P < 0.05 were considered to be significant.
Results
Establishment and characterization of immortalized EECs
The primary bovine EECs were obtained as previously described (Skarzynski et al. 2000). These cells proliferated for a limited number of passages. To extend the life span of EECs, attempts were made to establish immortalized EECs through the transfection of E6, E7 and hTERT genes into primary EECs. Most transfected EECs exhibited various degree of transfected gene expression, inconsistent growth rates or OT responsiveness. However, one line named imEECs exhibited the similar expression of three transfected genes, of which characteristics were subsequently analyzed. Under the microscope, the slight morphological changes in imEECs were observed. Soon after initial plating, imEECs were generally flat to cuboidal in shape, namely ‘cobblestone’ appearance (Fig. 1A). As the cells grew to confluence, these cells appeared as epithelial whorls, characteristics of the primary EECs. Expression of E6, E7 and hTERT, examined by RT-PCR, showed the expression of E6, E7 and hTERT messenger RNAs (mRNAs) in a similar manner (Fig. 1B). More importantly, transcripts of ovarian steroid hormone receptors (ER and PR) and type I interferon receptors (IFNAR1 and 2) were also found in imEECs even after 60 passages (Fig. 1C).
Figure 1.
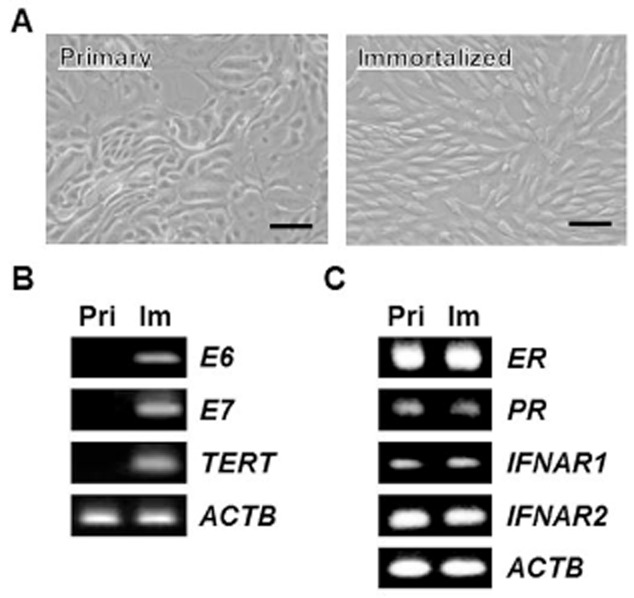
The morphology and characterization of immortalized bovine uterine endometrial epithelial cells (EECs). (A) Immortalized bovine uterine EECs were obtained by transfection of retroviral vectors, containing HPV-16 E6, E7 and hTERT genes to extend their life span. The representative photographs of EECs are shown (left: primary, right: immortalized). Bar = 200 μm. (B) The expression of HPV-16 E6, E7 and hTERT mRNAs in transfected EECs. Transcripts were examined by RT-PCR in primary and immortalized EECs. ACTB was used as an internal control. (C) The expression of steroid and interferon A (IFNA) receptor gene transcripts in primary and immortalized EECs. RNAs, extracted from primary or immortalized EECs at 60th passage, were subjected to PCR analysis. ACTB was used as an internal control.
Proliferation of primary and immortalized EECs
ImEECs were grown without apparent signs of senescence for at least 60 passages. Primary EECs at third passages and imEECs at 60th passages were plated onto collagen-coated or non-coated plates and cultured for 24–48 h. Proliferation of primary and immortalized EECs was assessed with the CCK-8 method. As shown in Figure 2, imEECs maintained the proliferation ability equivalent to primary ECCs even after 60 passages (0 h in the figure). Moreover, differences in imEECs' proliferation were seen after 24 h culture and further increase in proliferation was observed at 48 h.
Figure 2.
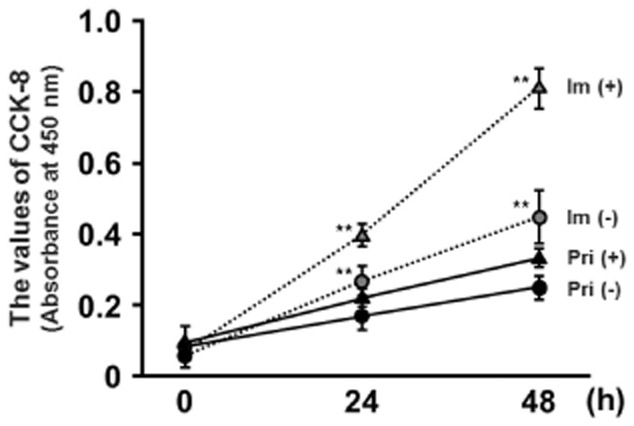
Proliferation of primary and immortalized endometrial epithelial cells (EECs). Primary (Pri) and immortalized at 60th passage (Im, broken line) EECs were cultured on collagen type IA-coated (+, triangle) or non-coated (−, circle) six-well plates for up to 48 h. The values of CCK-8 (MTT absorbance at 450 nm) were measured at 450 nm at 0, 24, or 48 h (n = 3). Duplicate samples were examined for each treatment, and three independent experiments were performed. Values represent means ± SEM. Statistical significance: **P –; 0.01 vs. Pri (−) within a time period.
Effect of OT or IFNT treatment on primary and immortalized EECs
Effects of OT or IFNT treatment were tested for primary and immortalized EECs. OT treatment induced a concentration-dependent increase in PGF2α accumulation in both cells (Fig. 3A). More importantly, IFNT treatment induced the expression of IFN stimulated gene transcripts; interferon-inducible Mx proteins (Mx1 and Mx2), interferon regulatory factors (IRFs), and signal transducers and activator of transcriptions (STATs). Thus, imEECs have maintained the ability to respond to OT and IFNT stimulation even after 60 passages and the degree of response at this stage was similar to those of primary EECs (Fig. 3).
Figure 3.
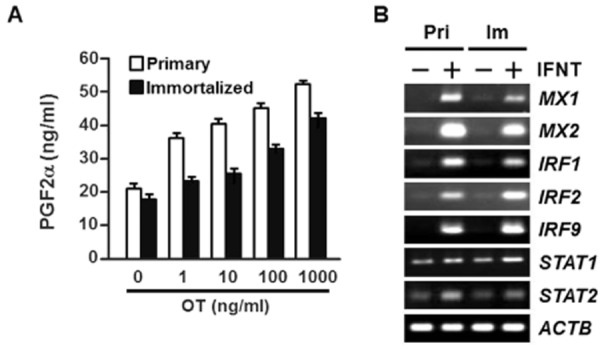
Effect of OT on PGF2α accumulation and the expression of interferon (IFN) stimulated genes following recombinant ovine IFNT treatment in immortalized endometrial epithelial cells (imEECs). (A) The primary (white bar) and immortalized at 60th passage (black bar) EECs were cultured on 48-well plates coated with collagen type IA. The cells were treated with increasing concentrations of OT (0, 1, 10, 100 or 1000 ng/mL) and PGF2α accumulation in conditioned media after 24 h incubation were measured using an enzyme immunoassay. The concentrations were corrected for cell numbers as determined by Cell Counting Kit-8 (CCK-8) (see Materials and Methods). The mean concentration of prostaglandin F2α (PGF2α) was derived from an average of three wells within a single plate experiment. Values represent means ± SEM. (B) The expression of IFN-stimulated gene transcripts in primary and immortalized EECs. The primary (Pri) and immortalized (Im) EECs were seeded on six-well plates coated with collagen type IA. The cells were treated with IFNT (1 μg/mL) for 24 h and the expression of IFN stimulated gene transcripts was analyzed by RT-PCR. IFNT stimulated genes include interferon-inducible Mx proteins (Mx1 and Mx2), interferon regulatory factors (IRF1, 2 and 9) and signal transducers and activator of transcriptions (STAT1 and 2). ACTB was used as an internal control.
Expression of vimentin and cytokeratin in immortalized EECs
Immunofluorescence studies were then performed. Primary EECs exhibited positive signals for cytokeratin and negative for vimentin, while imEECs cells stained positive for both cytokeratin and vimentin (Fig. 4).
Figure 4.
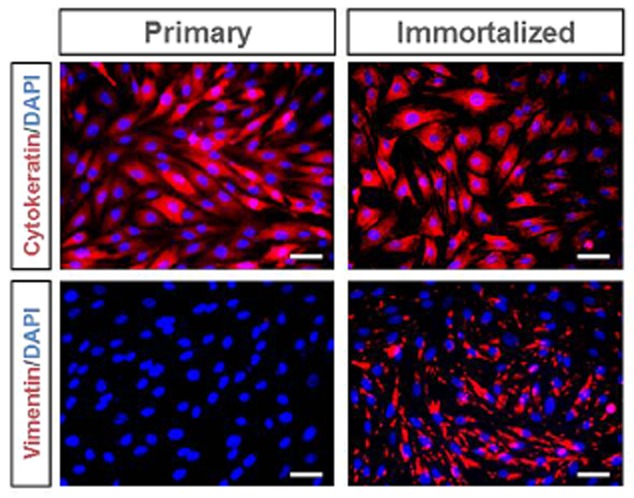
Immunofluorescence characterization of the immortalized endometrial epithelial cell (imEECs). Immunofluorescence staining analysis on primary (left panels) or immortalized at 60th passage (right panels) EECs was carried out using antibodies against cytokeratin (upper panels) as epithelial marker, or vimentin (lower panels) as stromal marker. Bar = 200 μm.
Discussion
Early embryonic loss is undoubtedly one of the major causes for not being able to improve pregnancy rates, particularly multiparous cows in Japan and throughout the world. It has been recognized numerous times that biochemical communications between conceptus and uterine endometrium during the peri-implantation period must be elucidated, if the pregnancy rate could be improved. However, a lack of experimental systems in studying such communication has limited investigators to elucidate the conceptus-endometrial dialogue. Stable EECs, which could overcome such limitations, are ideal to study trophoblast-endometrial epithelial cell interactions. Here, we established immortalized bovine EECs (imEECs), which have been maintained without any apparent morphological change in continuous cultures for more than 60 passages (Figs 2).
The established imEECs responded to OT treatment, resulting in a concentration-dependent PGF2α production (Fig. 3A). These cells also responded to IFNT treatment. These results show that established imEECs maintained the characteristics of uterine EECs during long-term culture periods. Thus, the imEECs could be a useful experimental model for studying biochemical-molecular communication between the conceptus and uterine epithelium, the events prerequisite for the implantation process to proceed. Since we established an in vitro co-culture system with bovine trophoblast CT-1 cells and EECs that mimic the in vivo attachment process (Sakurai et al. 2012), a next step forward is to examine if the imEECs could be used to mimic trophoblast CT-1 cell attachment processes, similar to those seen when primary EECs were used in our co-culture system.
Although the established imEECs maintained the characteristics of uterine EECs following numerous passages, these cells were positive for both epithelial marker, cytokeratin and stromal marker, vimentin. It has been reported numerous times that cytokeratin-positive epithelial cells acquire vimentin during in vitro culture periods (Bergh et al. 1984; Wang et al. 2000; Zeiler et al. 2007). The co-expression of both epithelial and mesenchymal markers in vitro has been explained by a limited epithelial to mesenchymal transition (EMT) (Pagan et al. 1996). It was also thought that this transition correlates with a loss of cell-to-cell contacts that result from cell disaggregation during the period of epithelial cell culture preparation (Zeiler et al. 2007). In vivo, the expression of vimentin, while trophoblasts maintained epithelial nature, has recently been reported (Yamakoshi et al. 2012). However, this was at the trophoblast side, not at the uterine epithelium, which was predicted two decades ago (Denker 1993). In addition to these observations, a previous study (Krishnaswamy et al. 2009) also indicated that the development of conceptus and endometrium requires biochemical communications as well as cell-to-cell interactions, if a pregnancy is to proceed, and suggest that the communication between the conceptus and uterine epithelium must be studied in not only co-culture systems but also those in flexible and dynamic cell environments. For these reasons, imEECs could be a more valuable tool than primary EECs in studying such changes as well as gene expression as the implantation process proceeds.
Conclusion
Our established imEECs, even after a number of passages, offer an in vitro model to elucidate molecular mechanisms associated with proper conceptus-endometrial dialogue in the bovine species, in which in vivo experiments are difficult to conduct.
Acknowledgments
Authors would like to thank Dr. T. Kiyono, National Cancer Center Research Institute, Tokyo, Japan, for kindly providing us with lentiviral vectors. This study was supported by the program for Promotion of Basic Research Activities for Innovative Bioscience (BRAIN) to K.I. This work was also supported by a Grant-in-Aid for Scientific Research (23780002) to T.S and H.B. was supported by Research Fellow of JSPS.
References
- Bazer FW, Thatcher WW, Hansen PJ, Mirando MA, Ott TL, Plante C. Physiological mechanisms of pregnancy recognition in ruminants. Journal of Reproduction and Fertility Supplement. 1991;43:39–47. [PubMed] [Google Scholar]
- Bergh J, Nilsson K, Dahl D, Andersson L, Virtanen I, Lehto VP. Expression of intermediate filaments in established human lung cancer cell lines. An indicator of differentiation and derivation. Laboratory Investigation. 1984;51:307–316. [PubMed] [Google Scholar]
- Binelli M, Subramaniam P, Diaz T, Johnson JA, Hansen TR, Badinga L, Thatcher WW. Bovine interferon-tau stimulates the Janus kinase-signal transducer and activator of transcription pathway in bovine endometrial epithelial cells. Biology of Reproduction. 2001;64:654–665. doi: 10.1095/biolreprod64.2.654. [DOI] [PubMed] [Google Scholar]
- Denker HW. Implantation: a cell biological paradox. American Society of Zoologists. 1993;266:541–558. doi: 10.1002/jez.1402660606. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Burghardt RC, Newton GR, Bazer FW, Spencer TE. Development and characterization of immortalized ovine endometrial cell lines. Biology of Reproduction. 1999;61:1324–1330. doi: 10.1095/biolreprod61.5.1324. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy N, Danyod G, Chapdelaine P, Fortier MA. Oxytocin receptor down-regulation is not necessary for reducing oxytocin-induced prostaglandin F2α accumulation by interferon-tau in a bovine endometrial epithelial cell line. Endocrinology. 2009;150:897–905. doi: 10.1210/en.2008-0704. [DOI] [PubMed] [Google Scholar]
- Kyo S, Nakamura M, Kiyono T, Maida Y, Kanaya T, Tanaka M, et al. Successful immortalization of endometrial glandular cells with normal structural and functional characteristics. American Journal of Pathology. 2003;163:2259–2269. doi: 10.1016/S0002-9440(10)63583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan R, Martín I, Alonso A, Llobera M, Vilaró S. Vimentin filaments follow the preexisting cytokeratin network during epithelial-mesenchymal transition of cultured neonatal rat hepatocytes. Experimental Cell Research. 1996;222:333–344. doi: 10.1006/excr.1996.0043. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Bai H, Bai R, Arai M, Iwazawa M, Zhang J, et al. Coculture system that mimics in vivo attachment processes in bovine trophoblast cells. Biology of Reproduction. 2012;87:60. doi: 10.1095/biolreprod.112.100180. [DOI] [PubMed] [Google Scholar]
- Skarzynski DJ, Miyamoto Y, Okuda K. Production of prostaglandin F2α by cultured bovine endometrial cells in response to tumor necrosis factor alpha: cell type specificity and intracellular mechanisms. Biology of Reproduction. 2000;62:1116–1120. doi: 10.1095/biolreprod62.5.1116. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Stagg AG, Ott TL, Johnson GA, Ramsey WS, Bazer FW. Differential effects of intrauterine and subcutaneous administration of recombinant ovine interferon tau on the endometrium of cyclic ewes. Biology of Reproduction. 1999;61:464–470. doi: 10.1095/biolreprod61.2.464. [DOI] [PubMed] [Google Scholar]
- Wang G, Johnson GA, Spencer TE, Bazer FW. Isolation, immortalization, and initial characterization of uterine cell lines: an in vitro model system for the porcine uterus. In Vitro Cellular & Developmental Biology – Animal. 2000;36:650–656. doi: 10.1290/1071-2690(2000)036<0650:iiaico>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. The New England Journal of Medicine. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Woclawek-Potocka I, Deptula K, Bah MM, Lee HY, Okuda K, Skarzynski DJ. Effects of nitric oxide and tumor necrosis factor-alpha on production of prostaglandin F2alpha and E2 in bovine endometrial cells. Journal of Reproduction and Development. 2004;50:333–340. doi: 10.1262/jrd.50.333. [DOI] [PubMed] [Google Scholar]
- Wolf E, Arnold GJ, Bauersachs S, Beier HM, Blum H, Einspanier R, et al. Embryo-maternal communication in bovine – strategies for deciphering a complex cross-talk. Reproduction in Domestic Animals. 2003;38:276–289. doi: 10.1046/j.1439-0531.2003.00435.x. [DOI] [PubMed] [Google Scholar]
- Yamakoshi S, Bai R, Chaen T, Ideta A, Aoyagi Y, Sakurai T, et al. Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction. 2012;143:377–387. doi: 10.1530/REP-11-0364. [DOI] [PubMed] [Google Scholar]
- Zeiler M, Leiser R, Johnson GA, Tinneberg HR, Pfarrer C. Development of an in vitro model for bovine placentation: a comparison of the in vivo and in vitro expression of integrins and components of extracellular matrix in bovine placental cells. Cells, Tissues, Organs. 2007;186:229–242. doi: 10.1159/000107947. [DOI] [PubMed] [Google Scholar]


