Abstract
Objective
The increased risk of progressive multifocal leukoencephalopathy (PML) with natalizumab treatment is associated with the presence of anti–JC virus (JCV) antibodies. We analyzed whether anti-JCV antibody levels, measured as index, may further define PML risk in seropositive patients.
Methods
The association between serum or plasma anti-JCV antibody levels and PML risk was examined in anti-JCV antibody–positive multiple sclerosis (MS) patients from natalizumab clinical studies and postmarketing sources. For PML and non-PML patients, the probabilities of having an index below and above a range of anti-JCV antibody index thresholds were calculated using all available data and applied to the PML risk stratification algorithm. Longitudinal stability of anti-JCV antibody index was also evaluated.
Results
Anti-JCV antibody index data were available for serum/plasma samples collected >6 months prior to PML diagnosis from 71 natalizumab-treated PML patients and 2,522 non-PML anti-JCV antibody–positive patients. In patients with no prior immunosuppressant use, anti-JCV antibody index distribution was significantly higher in PML patients than in non-PML patients (p < 0.0001). Among patients who were anti-JCV antibody negative at baseline in the AFFIRM and STRATIFY-1 trials, 97% remained consistently negative or below an index threshold of 1.5 over 18 months. Retrospective analyses of pre-PML samples collected longitudinally from PML patients displayed sustained higher anti-JCV antibody index over time.
Interpretation
Anti-JCV antibody levels in serum/plasma, measured as index, may differentiate PML risk in anti-JCV antibody–positive MS patients with no prior immunosuppressant use. Continued evaluation of anti-JCV antibody index and PML risk is warranted. Ann Neurol 2014;76:802–812
Natalizumab is a highly efficacious therapy for patients with relapsing multiple sclerosis (MS) based on significant reductions in annualized relapse rate and the risk of sustained disability progression in a 2-year phase 3 study1 and in 4- to 5-year open-label observational studies.2,3 Natalizumab treatment is associated with an increased risk of progressive multifocal leukoencephalopathy (PML), and understanding this risk is necessary for informed benefit–risk evaluation and treatment decisions.
The presence of anti–JC virus (JCV) antibodies in serum or plasma is a risk factor for PML development, and in anti-JCV antibody–positive patients, the use of immunosuppressants prior to natalizumab treatment and extended duration of natalizumab treatment (especially beyond 2 years) increase PML risk.4 Detection of anti-JCV antibodies using a 2-step enzyme-linked immunosorbent assay (ELISA) has been proven to reliably stratify the risk of PML. Using this assay, anti-JCV antibodies were detected in 232 of 234 natalizumab-treated patients (99%) who had a blood sample available for anti-JCV antibody analysis >6 months prior to PML diagnosis (data as of August 5, 2014).5 The reported prevalence of anti-JCV antibodies using the 2-step ELISA ranges from approximately 50% to 70% in natalizumab-treated MS patients.6–10 The risk of PML in anti-JCV antibody–negative patients is 1 per 10,000 patients, as demonstrated by data from postmarketing sources as well as from a large prospective study, STRATIFY-2.5,6 A small proportion of patients demonstrate fluctuating anti-JCV antibody status over time, and the risk of PML in these patients is unclear.11,12 The overall incidence of PML in anti-JCV antibody–positive patients with no prior immunosuppressant use remains <1 per 1,000 patients during the first 2 years of natalizumab treatment.4,5
A recent exploratory analysis of anti-JCV antibody data from natalizumab-treated MS patients showed higher anti-JCV antibody levels in 9 patients who developed PML compared with non-PML anti-JCV antibody–positive patients.10 Higher antibody levels to other polyomaviruses, BK virus (BKV) and Merkel cell virus (MCV), in patients with increased viral burden also have been observed.13–15 We therefore performed an extensive retrospective analysis to determine whether anti-JCV antibody levels may further define PML risk in anti-JCV antibody–positive patients. Data from this analysis have been presented at 2013 meetings of the Consortium of Multiple Sclerosis Centers–Americas Committee for Treatment and Research in Multiple Sclerosis and the European Neurological Society.16,17
Patients and Methods
Anti-JCV antibody serological status and index were determined by the 2-step second-generation anti-JCV antibody assay (STRATIFY JCV DxSelect; Focus Diagnostics, Cypress, CA).18 Focus Diagnostics has received 510(k) clearance from the Centers for Diagnostics and Radiological Health for this device. The device label18 includes detailed information on the test procedure and index results reporting methodology, analytical validation parameters, test reproducibility, and clinical agreement as evaluated between 3 testing sites, including Focus Diagnostics and 2 external testing sites. The anti-JCV antibody assay is currently performed in 3 laboratories: Focus Diagnostics (Cypress, CA), Unilabs (Copenhagen, Denmark), and Cirion Central Laboratory (Laval, Canada). The second-generation assay has improved sensitivity to detect anti-JCV antibodies compared with the first-generation STRATIFY JCV assay (limit of detection = 60ng/ml and 350ng/ml, respectively) and was shown by Focus Diagnostics to have positive agreement of >97% and a negative agreement of >90% when compared with the first-generation assay.18 The improved sensitivity and reproducibility of the second-generation assay was enabled by lyophilization and stabilization of the well-characterized JC virus like particle (VLP) to microwell strips and by optimizing the coating concentration, surface chemistry, and conjugate dilution.12 Quality control samples used in the test include the cutoff calibrator (CO), positive control (PC), and negative control (NC). The CO is used to calculate sample index values, and the PC and NC are used to evaluate assay performance and inter-test variability over time. The CO and PC controls are prepared from pooled sera collected from anti-JCV antibody–positive healthy human volunteers, and the NC is prepared from pooled sera with undetectable level of anti-JCV antibodies as determined in the test. The CO has been formulated to give the optimum differentiation between negative and positive specimens. Each new lot of quality control samples is rigorously qualified using predefined acceptance criteria to closely match (within 10%) the mean optical density (OD) values of the new lot to that of the previous lot. The following acceptance criteria are defined for the CO, PC, and NC in the STRATIFY JCV Dx SELECT device label (PI.EL1950 rev. C; date written: January 18, 2013; Focus Diagnostics). Acceptance criterion for the CO is based on OD values, the primary assay data output. Inter- and intra-assay performance is confirmed using index-based acceptance criteria for the PC and NC samples on the plate (OD of PC or NC divided by OD of CO on the same plate). The index value for the patient sample is derived in a manner similar to that for the PC and NC by normalizing the OD value of the patient sample to the OD derived for the CO on the same plate. Although the absorbance value may vary between runs and between laboratories, the mean value for the CO must be within 0.600 to 1.700 absorbance OD units. At least 3 of the 4 replicates of the CO ODs must be within 0.100 absorbance OD units from the mean value. One replicate may be excluded; if a replicate is excluded, the mean OD must be recalculated using the 3 acceptable values. The PC index value must be between 0.90 and 1.70 absorbance OD units, and the inhibition by PC in the inhibition test must be >70%. The NC index value must be <0.20 absorbance OD units.
Briefly, patient serum or plasma and quality control samples are diluted 1:101 and added to JC VLP precoated microwells and incubated. Patient samples and 2 dilutions each of the quality control samples are tested in duplicate. The plates are then washed, and antihuman immunoglobulin (H+L) conjugated with horseradish peroxidase is added to plates and incubated. After a subsequent wash, the tetramethylbenzidine substrate solution is added for color development. The reaction is stopped with sulfuric acid solution, and OD is measured at 450 nm using a plate reader. Index is calculated by dividing the OD value for the samples by the OD of the CO to normalize results across plates. An index >0.40 denotes anti-JCV antibody positivity, index <0.20 denotes anti-JCV antibody negativity, and index ≥0.20 but ≤0.40 denotes indeterminate response requiring further evaluation in the confirmation test. In the confirmation test, 1 aliquot of patient sample diluted 1:101 is preinhibited with JC VLP in solution. Then the preinhibited patient sample and the noninhibited aliquot of patient sample are tested in the anti-JCV antibody test following the procedure described above. Results from the confirmation test are reported as percentage inhibition, calculated as 100 × (1 − [mean OD450 preinhibited sample/mean OD450 noninhibited sample]). Samples in the confirmation test are scored positive when the index is ≥0.20 but ≤0.40 and the inhibition is >45%.12,18 Unlike antibody titer values, which are obtained by serial sample dilution to determine the greatest dilution that gives a positive result, index is calculated by testing patient samples at a single dilution. However, the proportionality between the index and anti-JCV antibody titers derived from serial sample dilution has been experimentally demonstrated for index ≤3.0 (unpublished data).12
Anti-JCV antibody index (titer equivalent) was also evaluated in 100 normal healthy volunteers (samples diluted 1:101). The mean index (with 95% confidence interval [CI]) in this population was found to be 1.332 (1.111–1.553) absorbance OD units. The median index value in the healthy volunteers was 1.039 absorbance OD units, with a 25th percentile index value of 0.246 and 75th percentile index value of 2.397.
Samples
Anti-JCV antibody index data were collected from anti-JCV antibody–positive MS patients who did not develop PML from 3 natalizumab clinical studies, AFFIRM (n = 359),1 STRATIFY-1 (n = 680),7 and STRATIFY-2 (n = 1,483)6; the total number of samples was 5,834. Because PML is an uncommon adverse event in natalizumab-treated patients, PML patient samples were obtained from clinical studies and postmarketing sources (n = 71 as of September 2012); only pre-PML samples available >6 months prior to PML diagnosis were included (total number of samples = 316, collected at a median duration of 4 years prior to PML diagnosis). Patients enrolled in clinical studies provided informed consent for participation. For all other patients, data were collected through standard pharmacovigilance practices.
Association between Anti-JCV Antibody Index and PML
The association of index and PML risk was initially explored using a test set of samples and then confirmed using a verification set.
The test data set consisted of 1,039 non-PML anti-JCV antibody–positive MS patients from AFFIRM and STRATIFY-1 and 45 MS patients who developed PML from clinical studies (excluding STRATIFY-2) and postmarketing sources. Because only 9 of the 45 PML cases came from the AFFIRM and STRATIFY-1 populations (STRATA PML patients previously in AFFIRM [n = 7] + STRATIFY-1 [n = 2]), the PML cases in the test set could have arisen from a population not captured in the clinical studies. Thus, we verified the results in a population giving rise to PML and non-PML patients—STRATIFY-2, in which patients either were receiving natalizumab or were interested in or considering natalizumab treatment. The verification data set consisted of 1,483 non-PML anti-JCV antibody–positive MS patients (baseline samples) and 26 MS patients who developed PML from STRATIFY-2. Integrated analyses of the combined data sets were performed on 2,522 non-PML anti-JCV antibody–positive MS patients and 71 MS patients who developed PML. For cross-sectional analyses, the lowest index was used for patients with >1 available index sample; p values were calculated using a Wilcoxon rank sum test. The median exposure to natalizumab at the time when the lowest index was obtained in the non-PML population was 19 infusions for the test data set, 23 infusions for the verification data set, and 21 infusions for the combined data set. Association analyses also were performed on each data set to assess the potential relationships between anti-JCV antibody index and other PML risk factors (prior immunosuppressant use and natalizumab treatment duration ≤24 vs >24 months).
Distribution of PML and Non-PML Anti-JCV Antibody–Positive Patients across Different Index Thresholds
Due to the serious nature of PML, the analysis of PML risk in reference to index thresholds focused primarily on clinical criteria (aiming to keep the false-negative rate ≤10%) rather than on the traditional statistical measures of sensitivity and specificity. Because physicians and patients have their own personal appreciation of risk tolerance and make conscious risk/benefit decisions based on various factors, the selection of 1 optimal index threshold was considered not as clinically useful. Thus, data from all anti-JCV antibody–positive patients from the test and verification sets were stratified over a range of index thresholds from 0.7 to 1.5 into lower-index (at or below threshold) and higher-index (above threshold) cohorts. The predicted probabilities to have an anti-JCV antibody index below and above the thresholds for PML and non-PML patients were calculated using all available longitudinal samples. A repeated-measures analysis was used with predicted probabilities, odds ratios, and p values estimated from generalized estimating equations with a logit link. An exchangeable correlation structure was assumed. A Bonferroni correction was applied to p values and CIs to correct for multiplicity of analyses with 5 thresholds.
Calculation of PML Risk Estimates across a Range of Index Thresholds
The predicted probabilities of having an anti-JCV antibody index below and above the thresholds for PML and non-PML patients were then applied to the numerators and denominators of anti-JCV antibody–positive patients in the PML risk stratification algorithm (based on data as of September 2012, with 285 confirmed PML cases).5 Ninety-nine percent CIs were calculated using the bootstrap percentile method with 2,000 bootstrap samples. A cluster bootstrap was used for sampling PML and non-PML patients with replacement. The predicted probabilities were calculated for each bootstrap sample.
Assessment of Longitudinal Stability of Anti-JCV Antibody Status and Index
Longitudinal analyses of index were performed on samples collected every 6 months from AFFIRM and STRATIFY-1 over a period of 18 months.1,11 The stability of index values was assessed over time in patients who maintained or changed serostatus from anti-JCV antibody negative at baseline to positive at subsequent time points using the following categories: (1) consistently lower, with all positive samples consistently at or below index threshold; (2) higher at any point, with 1 or more samples above index threshold; and (3) consistently higher, with 2 or more consecutive samples above index threshold. Longitudinal stability of index was also examined in natalizumab-treated patients who developed PML and had 2 or more pre-PML samples.
Results
Association between Anti-JCV Antibody Index and PML
The initial exploratory analysis of the association between index and PML risk using the test data set showed that the distribution of anti-JCV antibody index was significantly higher in pre-PML samples from natalizumab-treated patients who developed PML than in samples from non-PML anti-JCV antibody–positive patients (median = 2.4 vs 1.4; p < 0.0001; Fig 1A). Subsequent analysis using the verification data set confirmed the association between index and PML, with a significantly higher index distribution for pre-PML samples from PML patients than for samples from non-PML patients (median = 2.3 vs 1.9; p = 0.0199; see Fig 1B). In cross-sectional analysis of the combined PML and non-PML population for both data sets, there was no association detected between index and natalizumab treatment duration or prior immunosuppressant use (Fig 2).
Figure 1.
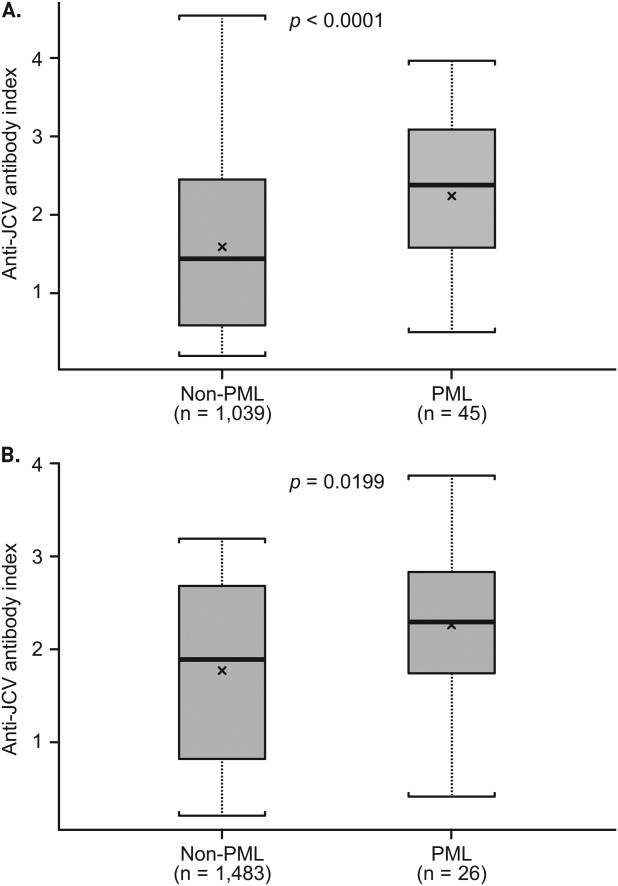
Anti–JC virus (JCV) antibody index in non–progressive multifocal leukoencephalopathy (PML) and PML patients. (A) Test data set includes lowest index for 1,039 non-PML patients who tested anti-JCV antibody–positive and 45 PML patients with pre-PML samples >6 months prior to PML diagnosis, as of September 2012. (B) Verification data set includes samples from 1,483 non-PML patients who tested anti-JCV antibody–positive at baseline of STRATIFY-2 and pre-PML samples (>6 months prior to PML diagnosis) from 26 patients who developed PML in STRATIFY-2. For the non-PML group in the verification data set, optical densities >3.0 used to calculate anti-JCV antibody index were reported as 3.0 by the testing laboratory. The lowest index value was used for patients who had multiple samples available. Box = interquartile range; thick black horizontal line = median; horizontal bars = range; x = mean.
Figure 2.
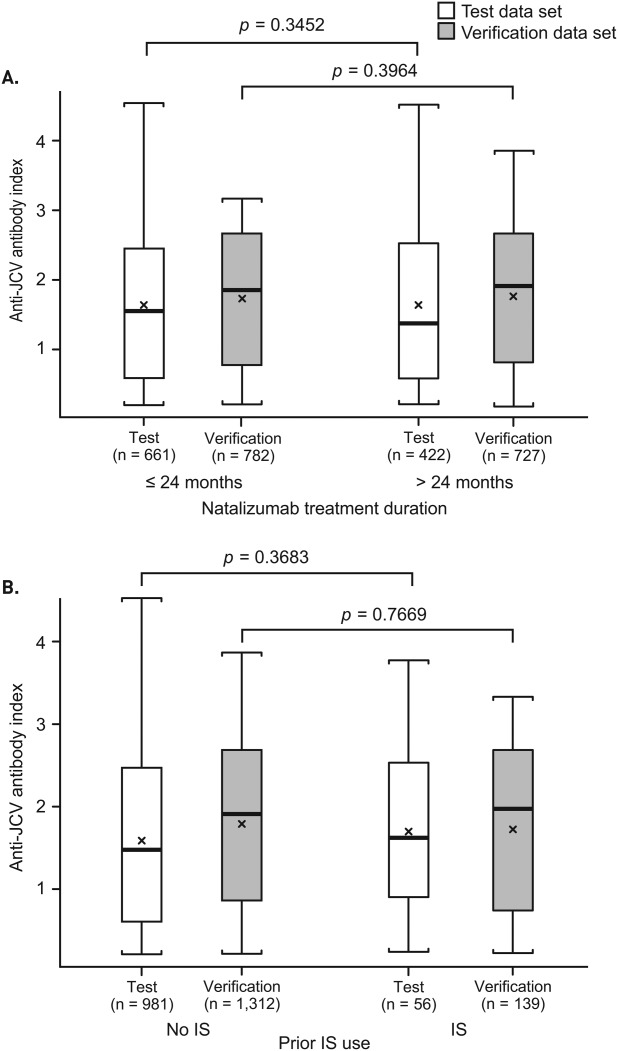
Association between (A) natalizumab treatment duration or (B) prior immunosuppressant (IS) use and index in the combined non–progressive multifocal leukoencephalopathy (PML) and PML population for the test and verification data sets. The test data set includes the combined population of 1,039 non-PML patients who tested anti–JC virus (JCV) antibody–positive and 45 PML patients with samples available >6 months prior to PML diagnosis, as of September 2012. The verification data set includes the combined population of 1,483 non-PML patients who tested anti-JCV antibody–positive and 26 PML patients with samples available >6 months prior to PML diagnosis, as of September 2012. The lowest index value was used for patients who had multiple samples available. Box = interquartile range; thick black horizontal line = median; horizontal bars = range; x = mean.
Effect of Prior Immunosuppressant Use on Association between Index and PML Risk
Further analysis of the test data set identified a different relationship between index and PML for patients based on prior immunosuppressant use (Fig 3A). In patients with no prior immunosuppressant use, the index distribution was significantly higher for PML patients than for non-PML patients (median = 2.4 vs 1.4; p < 0.0001). In contrast, index distribution was similar for non-PML and PML patients with prior immunosuppressant use (median = 1.6 for both groups; p = 0.82). A similar relationship was observed for the verification data set (see Fig 3B) and the combined data set (interaction p = 0.0158; Fig 4A). Thus, subsequent analyses of index and PML risk were limited to patients with no immunosuppressant use prior to natalizumab treatment. Scatterplot representation of anti-JCV antibody index data for the combined data sets of patients with no prior immunosuppressant treatment highlights the significantly higher index distribution for pre-PML samples of PML patients compared with non-PML patients, with only 1 of 51 PML cases having index < 0.9 and 6 of 51 PML cases having index < 1.5 (see Fig 4B). Results remained consistent after removing 239 patients from the non-PML group who were not treated with natalizumab at the time of sampling (n = 2,003; p < 0.0001).
Figure 3.
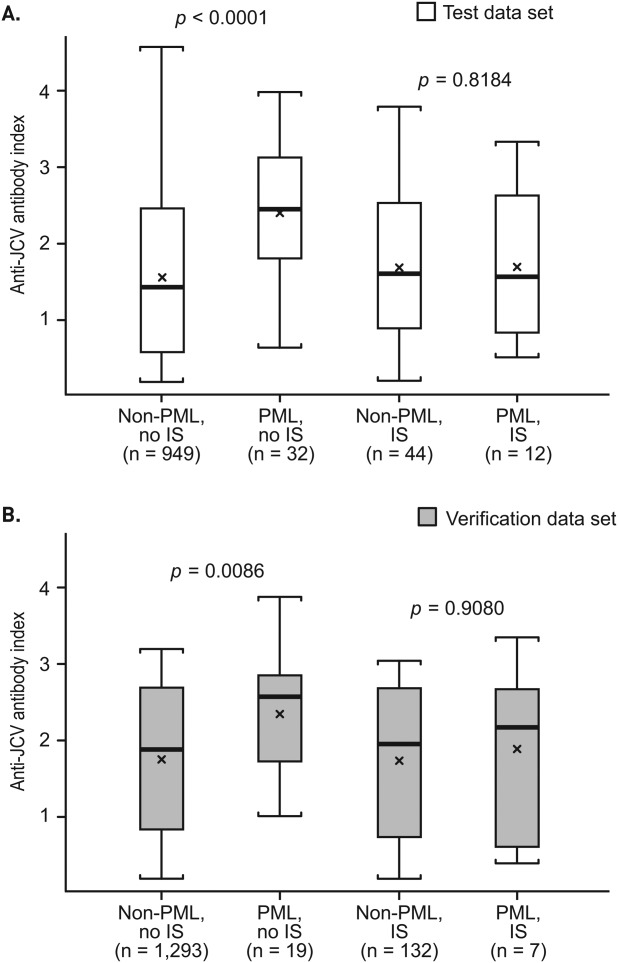
Anti–JC virus (JCV) antibody index in non–progressive multifocal leukoencephalopathy (PML) and PML patients by prior immunosuppressant (IS) use for the (A) test and (B) verification data sets. The test data set includes 993 non-PML patients who tested anti-JCV antibody–positive and 44 PML patients (with samples available >6 months prior to PML diagnosis) who had information available on prior IS use, as of September 2012. The verification data set includes 1,425 non-PML patients who tested anti-JCV antibody–positive and 26 PML patients (with samples available >6 months prior to PML diagnosis) who had information available on prior IS use, as of September 2012. The lowest index value was used for patients who had multiple samples available. Box = interquartile range; thick black horizontal line = median; horizontal bars = range; x = mean.
Figure 4.
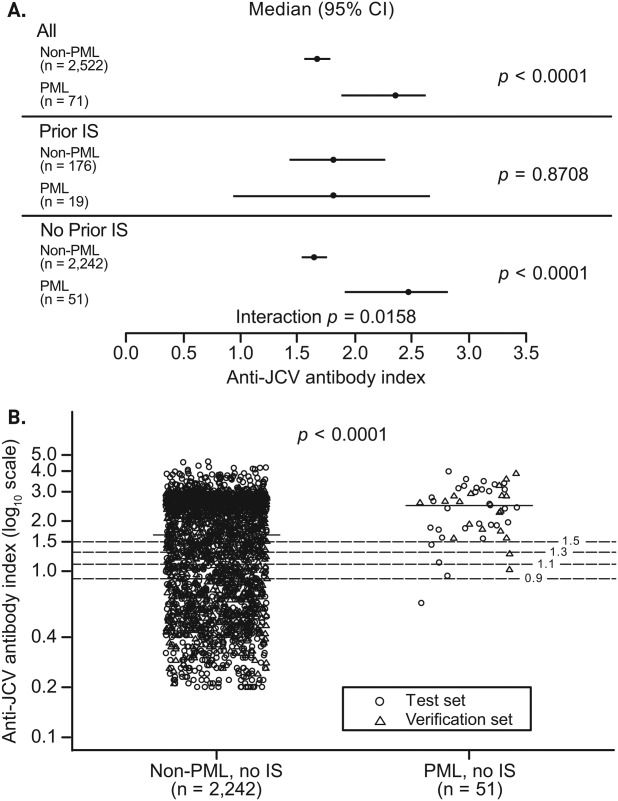
Anti–JC virus (JCV) antibody index in anti-JCV antibody–positive non–progressive multifocal leukoencephalopathy (PML) and PML patients with or without prior immunosuppressant (IS) use. (A) Results based on combined test and verification data sets including 2,522 non-PML and 71 PML patients, stratified based on prior IS use. Interaction p value tests difference in association between anti-JCV antibody index and PML risk by prior IS use. (B) Results based on data for 2,242 non-PML and 51 PML patients who had no prior IS use and who tested anti-JCV antibody–positive as of September 2012; 104 non-PML patients and 1 PML patient were missing prior IS use information and were excluded from analyses. Optical densities >3.0 used to calculate anti-JCV antibody index for the non-PML group in the verification data set were reported as 3.0 by the testing laboratory. The lowest index value was used for patients who had multiple samples available. Horizontal line = median; horizontal dashed lines = index at 0.9, 1.1, 1.3, and 1.5. CI = confidence interval.
Anti-JCV Antibody Index Threshold and PML Risk
The estimated proportions of anti-JCV antibody–positive non-PML and PML patients with no prior immunosuppressant use having an anti-JCV antibody index at or below thresholds ranging from 0.7 to 1.5 are shown in Table1 for the combined data sets. Across the range of index thresholds, results were statistically significant, with odds ratios ranging from approximately 7 to 45.
Table 1.
Estimated Proportions of Anti-JCV Antibody–Positive Non-PML and PML Patients with No Prior Immunosuppressant Use by Index Thresholds
| Anti-JCV Antibody Index | % Non-PML Below | 99% CI | % PML Below | 99% CI | OR | 99% CI | pa |
|---|---|---|---|---|---|---|---|
| ≤0.7 | 21.1 | 19.0–23.3 | 0.6 | 0–7.0 | 45.6 | 3.5–589.6 | <0.001 |
| ≤0.9 | 28.2 | 25.9–30.6 | 1.7 | 0.1–18.6 | 22.9 | 1.7–305.4 | 0.009 |
| ≤1.1 | 33.6 | 31.2–36.2 | 4.4 | 1.0–17.7 | 11.1 | 2.4–52.4 | <0.001 |
| ≤1.3 | 37.9 | 35.4–40.5 | 7.5 | 2.2–22.5 | 7.5 | 2.1–26.8 | <0.001 |
| ≤1.5 | 42.9 | 40.4–45.6 | 10.1 | 3.5–26.1 | 6.7 | 2.1–21.1 | <0.001 |
Data for patients with no prior immunosuppressant use from the combined test and validation data sets; n = 2,242 for non-PML anti-JCV antibody–positive patients and n = 51 for PML patients with available anti-JCV antibody index data >6 months prior to PML diagnosis. A total of 5,547 samples were analyzed by repeated measures, with predicted probabilities, odds ratios, and p values estimated from generalized estimating equations with a logit link. An exchangeable correlation structure was assumed.
Bonferroni adjusted.
CI = confidence interval; JCV = JC virus; OR= odds ratio; PML = progressive multifocal leukoencephalopathy.
Using the predicted probabilities from the combined data sets, PML risk estimates were generated for anti-JCV antibody index thresholds of 0.9 to 1.5 (Table2). For anti-JCV antibody–positive patients with no prior immunosuppressant use and an anti-JCV antibody index at or below thresholds of 0.9 to 1.5, the risk of PML was approximately 0.1 per 1,000 patients during the first 2 years of natalizumab treatment, and it ranged from 0.3 to 1.3 per 1,000 patients from month 25 to 48 and from month 49 to 72. For patients with no prior immunosuppressant use and an anti-JCV antibody index > 1.5, the risk of PML was approximately 1 per 1,000 patients during the first 2 years of natalizumab treatment, and ranged from 8.1 to 8.5 per 1,000 patients from month 25 to 48 and from month 49 to 72.
Table 2.
PML Risk Estimates by Index Threshold in Anti-JCV Antibody–Positive Patients with No Prior IS Use
| Anti-JCV Antibody Index | PML Risk Estimates per 1,000 Anti-JCV Antibody–Positive Patients by Natalizumab Treatment Duration, No Prior IS Use | ||
|---|---|---|---|
| 1–24 Months (99% CI) | 25–48 Months (99% CI) | 49–72 Months (99% CI) | |
| ≤0.9 | 0.1 (0–0.15) | 0.3 (0–1.28) | 0.4 (0–1.25) |
| ≤1.1 | 0.1 (0–0.23) | 0.7 (0–1.85) | 0.7 (0–1.98) |
| ≤1.3 | 0.1 (0–0.28) | 1.0 (0–2.38) | 1.2 (0–2.56) |
| ≤1.5 | 0.1 (0–0.30) | 1.2 (0.20–2.61) | 1.3 (0.24–2.78) |
| >1.5 | 1.0 (0.84–1.07) | 8.1 (7.06–8.98) | 8.5 (7.41–9.46) |
| No indexa | 0.6 (0.42–0.88) | 5.2 (4.28–6.19) | 5.4 (4.03–7.14) |
PML risk estimates across anti-JCV antibody index thresholds were calculated based on the PML risk stratification algorithm (from September 2012) and predicted probabilities shown in Table1 for the anti-JCV antibody–positive population at or below respective index thresholds from 0.9 to 1.5. For index thresholds at or below 0.7, PML patient numbers were insufficient to allow for calculation of risk estimates.
Based on existing PML risk stratification algorithm using September 2012 data for the anti-JCV antibody positive group with no prior IS use.
CI = confidence interval; IS = immunosuppressant; JCV = JC virus; PML = progressive multifocal leukoencephalopathy.
Longitudinal Assessment of Index Stability
Longitudinal data were available every 6 months over a period of 18 months for 553 anti-JCV antibody–negative patients at baseline who had no prior immunosuppressant use from the AFFIRM and STRATIFY-1 studies. Of these 553 patients, the majority (87%; 479 of 553) remained negative at all subsequent testing over 18 months (Table3). Of the 74 patients (13%) who changed serostatus from negative to positive at 1 or more time points over 18 months, 29 (39%) reverted back to negative at least once during the 18 months; 69% (51 of 74) remained consistently below the anti-JCV antibody index threshold of 0.9, and 74% (55 of 74) remained below 1.5. Thus, 96% (530 of 553) to 97% (534 of 553) of patients with an anti-JCV antibody–negative result at baseline remained either negative or consistently below anti-JCV antibody index thresholds of 0.9 to 1.5 over 18 months (see Table3).
Table 3.
Anti-JCV Antibody Index over 18 Months for Patients Who Were Anti-JCV Antibody Negative at Baseline
| Index Threshold | |||
|---|---|---|---|
| 0.9 | 1.2 | 1.5 | |
| ≥1 positive test consistently below index threshold, % | 9.2 | 9.4 | 9.9 |
| Consistently lower risk,a % | 95.8 | 96.0 | 96.6 |
| Higher at any point (≥1 sample above index threshold), % | 4.2 | 4.0 | 3.4 |
| Consistently higher (≥2 consecutive samples above index threshold), % | 2.2 | 2.0 | 1.6 |
Consistently negative test = 86.6%.
Includes longitudinal samples collected every 6 months from 553 anti-JCV antibody–negative patients at baseline who had no prior immunosuppressant use and were followed for a period of 18 months in AFFIRM and STRATIFY-1.
Consistently negative test + ≥1 positive test consistently below index threshold.
JCV = JC virus.
Twenty-five natalizumab-treated MS patients with no prior immunosuppressant use who developed PML had at least 2 pre-PML samples. For 24 of these patients (96%), all samples had an anti-JCV antibody index > 0.9, and for 21 of 25 patients (84%), all samples had an anti-JCV antibody index > 1.5 (Fig 5). In 1 patient, 3 of 4 available samples had an anti-JCV antibody index ≤ 0.9, 2 of which were collected within 12 months of PML diagnosis.
Figure 5.
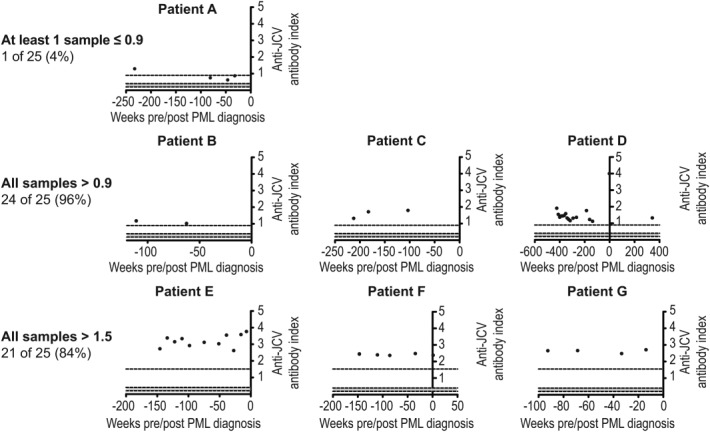
Longitudinal pre–progressive multifocal leukoencephalopathy (PML) samples generally demonstrate consistently high anti–JC virus (JCV) antibody index over time: examples of individual cases. Longitudinal scatter JC plot data are given from 7 individual case examples (A–G) out of 25 PML patients with no prior immunosuppressant use who had at least 2 pre-PML samples available >6 months prior to PML diagnosis.
Discussion
The current analysis has extended our prior observations to show that in anti-JCV antibody–positive natalizumab-treated patients, higher levels of serum or plasma anti-JCV antibodies, as measured by anti-JCV antibody index, are associated with higher PML risk. Persistently higher antibody levels have previously been shown in subjects with increased viral burden to other viruses. Higher anti-BKV VP1 antibody levels have been reported in individuals with higher BKV replication and in those with virus reactivation from latency.13,19 Similarly, an association between MC polyomavirus load and capsid-specific MCV antibody titers in MC carcinoma patients, between Epstein–Barr virus load and antiviral capsid or anti-ZEBRA immunoglobulin G titer in Hodgkin lymphoma patients, and between anti–viral capsid antigen titers and viral load in human immunodeficiency virus–infected subjects has been demonstrated.14,15,20–22 Furthermore, increased viral replication has been linked to the frequent emergence of polyomavirus variants with rearrangement of the archetype noncoding control region,23–26 and human leukocyte antigen genotypes and host genetics have been implicated along with immune modulation in the resultant increase in viral burden.27,28
Our results suggest that it is possible for MS patients who test anti-JCV antibody–positive and have no prior immunosuppressant use to be further delineated into lower and higher PML risk groups based on serum or plasma anti-JCV antibody levels, as measured by index. Interestingly, in our study, difference in median index level was observed between non-PML patients in the test (AFFIRM and STRATIFY-1) and verification (STRATIFY-2) cohorts (see Fig 1). It is possible that differences in median age, gender, duration of natalizumab treatment at study entry, and/or other geographic factors and disease characteristics may have contributed to this finding. Regardless, the association of higher index with higher PML risk was observed in both patient cohorts. Patients with no prior immunosuppressant use who were anti-JCV antibody–positive with index of from ≤0.9 to ≤1.5 in the first 24 months of natalizumab treatment had an estimated PML risk of 0.1 per 1,000 patients, whereas those with index > 1.5 had an estimated PML risk of 1.0 per 1,000 patients. The PML risk of 0.1 per 1,000 patients in the lower-index group is similar to that for anti-JCV antibody–negative patients and is approximately 6-fold lower than the level generated by the existing PML risk stratification algorithm using September 2012 data for the anti-JCV antibody–positive group with no prior immunosuppressant use in the first 24 months of natalizumab treatment (0.6 per 1,000).5 The estimated PML risk over 25 to 48 months of natalizumab exposure was 17-fold lower for patients with index ≤ 0.9 (0.3 vs 5.2 per 1,000) and 4-fold lower for patients with index ≤ 1.5 (1.2 vs 5.2 per 1,000) compared with all anti-JCV antibody–positive patients.5 The estimated PML risk for patients with 49 to 72 months of natalizumab exposure was 13-fold lower for patients with index ≤ 0.9 (0.4 vs 5.4 per 1,000) and 4-fold lower for patients with index ≤ 1.5 (1.3 vs 5.4 per 1,000) compared with all anti-JCV antibody–positive patients.5 For patients with an anti-JCV antibody index > 1.5 with no prior immunosuppressant use, the relative risk of PML increased from 0.6 to 1.0 per 1,000 patients over 1 to 24 months of natalizumab exposure and from 5.2 to 8.1 per 1,000 patients over 25 to 48 months of exposure compared with the current PML risk stratification algorithm as of September 2012.5 As previously described, selection of 1 optimal cutpoint would not provide flexibility in clinical practice based on individual risk tolerance; thus, various index thresholds were used in Tables1, 2, and 3.
Analysis of longitudinal data from 2 clinical studies demonstrated that approximately 96% of baseline anti-JCV antibody–negative patients maintained a negative serostatus or remained consistently below the anti-JCV antibody index threshold of 0.9 and thereby at potentially lower risk of PML over at least 18 months. Higher rates of serostatus change have been reported especially in cases where patients initially tested negative with the first-generation anti-JCV antibody assay and were then retested with the second-generation assay.29,30 The PML risk in patients who change serological status may differ based on anti-JCV antibody index. In addition, 2 recent studies31,32 have shown that JCV DNA is detectable in blood compartments of natalizumab-treated MS patients who test negative for anti-JCV antibodies in serum. These data may suggest that the presence of anti-JCV antibodies is not a direct surrogate marker for JCV exposure but rather a marker of increased viral burden in an individual due to either an ongoing infection or periodic reactivation of the virus from latency. Our data collected from pre-PML samples shows the presence of anti-JCV antibodies in 232 of 234 natalizumab-treated patients who had a blood sample available prior to PML diagnosis.5 Therefore, the presence of anti-JCV antibodies in serum or plasma has been confirmed to be relevant for PML risk stratification, whereas the presence of detectable JCV DNA in blood in the absence of detectable anti-JCV antibodies has yet to be shown useful for PML risk stratification.
Longitudinal analysis of samples from PML patients with no prior immunosuppressant use also showed that 96% (24 of 25) maintained an anti-JCV antibody index > 0.9 at all available time points and often over several months prior to PML development. These results may indicate that patients who consistently have higher levels of anti-JCV antibodies in blood are at higher risk of PML than patients who are intermittently positive.
Although several publications have commented on the possibility of rising anti-JCV antibody titers being predictive of PML, there are few clinical data to support this hypothesis. In the Warnke et al publication,33 modest increases in the level of anti-JCV antibodies were shown in 5 PML patients, but only at the time of clinical diagnosis of PML. In our analyses to date, natalizumab-treated PML patients with samples available at least 3 to 6 months prior to clinical diagnosis of PML have typically shown consistently higher anti-JCV antibody levels. The study published by Trampe et al10 demonstrated higher anti-JCV antibody levels (normalized OD) in PML compared with non-PML patients, similar to our findings; however, the dynamic range of the signal in the first-generation assay used in that study was not sufficient to delineate lower and higher PML risk based on anti-JCV antibody levels.12,34 All calculations of PML risk estimates herein were therefore done using the anti-JCV antibody index output of the second-generation anti-JCV antibody assay.12
Our analysis is based on a hypothesis that continues to evolve and has some limitations. The total number of PML cases included in the analyses (n = 71) was relatively small, and additional cases may influence the results. Current data in patients with prior immunosuppressant use do not show an association between higher index and PML risk. The underlying biological explanation for this effect is unknown. The authors postulate that a potential effect on the immune system and therefore on antibody production may impact the ability of MS patients treated with immunosuppressants to mount an effective antibody response to JCV. However, this difference was not observed at the level of seropositivity to JCV but rather only in the level of antibodies. Moreover, prior immunosuppressant use in the natalizumab population is decreasing over time.
Efforts are ongoing to collect serum or plasma samples from natalizumab-treated patients through Biogen Idec–sponsored clinical studies as well as through collaborations with treating neurologists to further strengthen the observation regarding the association of higher index with PML risk and the effect of immunosuppressants. Recent analysis of additional samples from natalizumab-treated PML and non-PML patients supports the findings outlined in this paper, and will be a subject of future publication.
Future analytical directions may include exploring the value of a longitudinal, continuous model to further improve PML risk estimation over time for an individual patient. This may allow for increased precision in PML risk estimates and for modeling the possible association between different risk factors over time. Efforts from several other investigators have also been focused on identifying markers to better predict PML risk on an individual-patient basis.35,36
In conclusion, anti-JCV antibody index appears to differentiate PML risk in natalizumab-treated anti-JCV antibody–positive MS patients with no prior immunosuppressant use, with higher anti-JCV antibody index patients having an increased risk of PML compared with lower anti-JCV antibody index patients. Most patients who changed serological status from anti-JCV antibody negative to positive over 18 months maintained a lower index. The stability of PML risk estimates over time based on anti-JCV antibody index continues to be evaluated to confirm these results.
Acknowledgments
The study was funded by Biogen Idec, which also provided funding for editorial support in the development of the manuscript.
Freelance writer Michelle McDermott, PharmD, wrote the first draft of the manuscript based on input from authors, and Joshua Safran of Infusion Communications copyedited and styled the manuscript per journal requirements. Biogen Idec reviewed and provided feedback on the manuscript to the authors. The authors had full editorial control of the manuscript and provided their final approval of all content.
Authorship
T.P. and M.S. contributed equally to this work.
Potential Conflicts of Interest
All authors have equity interests in and are currently or were formerly employed by Biogen Idec.
References
- 1.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 2.O'Connor P, Goodman A, Kappos L, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS Study. Neurology. 2014;83:78–86. doi: 10.1212/WNL.0000000000000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butzkueven H, Kappos L, Pellegrini F, et al. Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp-2013-306936. 2014 Feb 14 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 5.Biogen Idec. Medical information website. Available at: https://medinfo.biogenidec.com/medinfo. Accessed September 2012 to August 30, 2014.
- 6.Bozic C, Richman S, Plavina T, et al. Anti-JCV antibody prevalence in patients with relapsing multiple sclerosis receiving or considering treatment with natalizumab: baseline results of STRATIFY-2. Neurology. 2012;78:S41. [Meeting Abstracts 1]: .002. [Google Scholar]
- 7.Bozic C, Richman S, Plavina T, et al. Anti-John Cunningham virus antibody prevalence in multiple sclerosis patients: baseline results of STRATIFY-1. Ann Neurol. 2011;70:742–750. doi: 10.1002/ana.22606. [DOI] [PubMed] [Google Scholar]
- 8.Olsson T, Achiron A, Alfredsson L, et al. Anti-JC virus antibody prevalence in a multinational multiple sclerosis cohort. Mult Scler. 2013;19:1533–1538. doi: 10.1177/1352458513477925. [DOI] [PubMed] [Google Scholar]
- 9.Outteryck O, Ongagna JC, Duhamel A, et al. Anti-JCV antibody prevalence in a French cohort of MS patients under natalizumab therapy. J Neurol. 2012;259:2293–2298. doi: 10.1007/s00415-012-6487-5. [DOI] [PubMed] [Google Scholar]
- 10.Trampe AK, Hemmelmann C, Stroet A, et al. Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort. Neurology. 2012;78:1736–1742. doi: 10.1212/WNL.0b013e3182583022. [DOI] [PubMed] [Google Scholar]
- 11.Plavina T, Lee S, Berman M, et al. Longitudinal stability of anti-JC virus antibody status in multiple sclerosis patients: results of STRATIFY-1. Neurology. 2013;80:S30. 001. [Google Scholar]
- 12.Lee P, Plavina T, Castro A, et al. A second generation ELISA (STRATIFY JCV™ DxSelect™) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J Clin Virol. 2013;57:141–146. doi: 10.1016/j.jcv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Bohl DL, Brennana DC, Ryschkewitschc C, et al. BK virus antibody titers and intensity of infections after renal transplantation. J Clin Virol. 2008;43:184–189. doi: 10.1016/j.jcv.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastrana DV, Wieland U, Silling S, et al. Positive correlation between Merkel cell polyomavirus viral load and capsid-specific antibody titer. Med Microbiol Immunol. 2012;201:17–23. doi: 10.1007/s00430-011-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Touzé A, Le Bidre E, Laude H, et al. High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29:1612–1619. doi: 10.1200/JCO.2010.31.1704. [DOI] [PubMed] [Google Scholar]
- 16.Plavina T, Subramanyam M, Bloomgren G, et al. JCV antibody index stratifies PML risk in natalizumab-treated MS patients. Available at: https://cmscactrims.confex.com/cmscactrims/2013/webprogram/Paper1642.html. Accessed November 25, 2013.
- 17.Plavina T, Subramanyam M, Bloomgren G, et al. Use of anti-JC virus antibody index to further define risk of PML in anti-JCV antibody-positive natalizumab-treated patients with MS (0228). Presented at: 23rd Annual Meeting of the European Neurological Society; June 8–11, 2013; Barcelona, Spain. [Google Scholar]
- 18.Focus Diagnostics. STRATIFY JCV DxSelect™ prescribing information. 2013. Available at: http://www.focusdx.com/pdfs/pi/US/EL1950.pdf. Accessed November 25, 2013.
- 19.Randhawa PS, Gupta G, Vats A, et al. Immunoglobulin G, A, and M responses to BK virus in renal transplantation. Clin Vaccine Immunol. 2006;13:1057–1063. doi: 10.1128/CVI.00114-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besson C, Amiel C, Le-Pendeven C, et al. Positive correlation between Epstein-Barr virus viral load and anti-viral capsid immunoglobulin G titers determined for Hodgkin's lymphoma patients and their relatives. J Clin Microbiol. 2006;44:47–50. doi: 10.1128/JCM.44.1.47-50.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drouet E, Brousset P, Fares F, et al. High Epstein-Barr virus serum load and elevated titers of anti-ZEBRA antibodies in patients with EBV-harboring tumor cells of Hodgkin's disease. J Med Virol. 1999;57:383–389. doi: 10.1002/(sici)1096-9071(199904)57:4<383::aid-jmv10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Stevens SJ, Blank BS, Smits PH, et al. High Epstein-Barr virus (EBV) DNA loads in HIV-infected patients: correlation with antiretroviral therapy and quantitative EBV serology. AIDS. 2002;16:993–1001. doi: 10.1097/00002030-200205030-00005. [DOI] [PubMed] [Google Scholar]
- 23.Gosert R, Rinaldo CH, Funk GA, et al. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J Exp Med. 2008;205:841–852. doi: 10.1084/jem.20072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randhawa P, Zygmunt D, Shapiro R, et al. Viral regulatory region sequence variations in kidney tissue obtained from patients with BK virus nephropathy. Kidney Int. 2003;64:743–747. doi: 10.1046/j.1523-1755.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 25.Olsen GH, Andresen PA, Hilmarsen HT, et al. Genetic variability in BK virus regulatory regions in urine and kidney biopsies from renal transplant patients. J Med Virol. 2006;78:384–393. doi: 10.1002/jmv.20551. [DOI] [PubMed] [Google Scholar]
- 26.Nakamichi K, Kishida S, Tanaka K, et al. Sequential changes in the non-coding control region sequences of JC polyomaviruses from the cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. Arch Virol. 2013;158:639–650. doi: 10.1007/s00705-012-1532-3. [DOI] [PubMed] [Google Scholar]
- 27.Carrington M, O'Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–551. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 28.Masutani K, Ninomiya T, Randhawa P. HLA-A2, HLA-B44 and HLA-DR15 are associated with lower risk of BK viremia. Nephrol Dial Transplant. 2013;28:3119–3126. doi: 10.1093/ndt/gft298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Outteryck O, Zéphir H, Salleron J, et al. JC-virus seroconversion in multiple sclerosis patients receiving natalizumab. Mult Scler. 2014;20:822–829. doi: 10.1177/1352458513505353. [DOI] [PubMed] [Google Scholar]
- 30.Van Pesch V, Algoed L, Boucquey D, et al. Use of the JC virus stratify assay in a cohort of natalizumab-treated patients from Belgium. Comparative results between 2011 and 2012. Presented at: 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); October 2–5, 2013; Copenhagen, Denmark. [Google Scholar]
- 31.Berger JR, Houff SA, Gurwell J, et al. JC virus antibody status underestimates infection rates. Ann Neurol. 2013;74:84–90. doi: 10.1002/ana.23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Major EO, Frohman E, Douek D. JC viremia in natalizumab-treated patients with multiple sclerosis. N Engl J Med. 2013;368:2240–2241. doi: 10.1056/NEJMc1214233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warnke C, Ramanujam R, Plavina T, et al. Changes to anti-JCV antibody levels in a Swedish national MS cohort. J Neurol Neurosurg Psychiatry. 2013;84:1199–1205. doi: 10.1136/jnnp-2012-304332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. 2010;68:295–303. doi: 10.1002/ana.22128. [DOI] [PubMed] [Google Scholar]
- 35.Schwab N, Schneider-Hohendorf T, Posevitz V, et al. L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81:1–7. doi: 10.1212/WNL.0b013e3182a351fb. [DOI] [PubMed] [Google Scholar]
- 36.Muñoz-Culla M, Irizar H, Castillo-Triviño T, et al. Blood miRNA expression pattern is a possible risk marker for natalizumab-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult Scler. doi: 10.1177/1352458514534513. 2014 May 22 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


