Abstract
Haemolytic disease of the fetus and newborn (HDFN) may occur when maternal IgG antibodies against red blood cells (RBCs), often anti-RhD (anti-D) antibodies, cross the placenta and mediate the destruction of RBCs via phagocytic IgG-Fc-receptors (FcγR). Clinical severity is not strictly related to titre and is more accurately predicted by the diagnostically-applied monocyte-based antibody-dependent cellular cytotoxicity (ADCC), a sensitive test with relatively low specificity. This suggests that other factors are involved in the pathogenesis of HDFN. Binding of IgG to FcγR requires the N-linked glycan at position 297 in the IgG-Fc-region, consisting of several different glycoforms. We therefore systematically analysed IgG-derived glycopeptides by mass spectrometry from 70 anti-D IgG1 antibodies purified from the plasma of alloimmunized pregnant women. This revealed a variable decrease in Fc-fucosylation in the majority of anti-D IgG1 (even down to 12%), whereas the total IgG of these patients remained highly fucosylated, like in healthy individuals (>90%). The degree of anti-D fucosylation correlated significantly with CD16 (FcγRIIIa)-mediated ADCC, in agreement with increased affinity of defucosylated IgG to human FcγRIIIa. Additionally, low anti-D fucosylation correlated significantly with low fetal-neonatal haemoglobin levels, thus with increased haemolysis, suggesting IgG-fucosylation to be an important pathological feature in HDFN with diagnostic potential.
Keywords: haemolytic disease of the fetus and newborn, anti-RhD or anti-D alloantibodies, IgG-glycosylation, IgG-fucosylation
Haemolytic disease of the fetus or newborn (HDFN) arises due to maternal alloimmunization against paternally inherited fetal red blood cell antigens, most commonly Rh-D. These anti-D alloantibodies are transported across the placenta, bind to D-positive fetal red blood cells (RBCs) and subsequently engage with phagocytic-IgG Fc-receptors (FcγR), resulting in red blood cell clearance. Clinically, this can result in fetal anaemia, jaundice, hydrops and stillbirth (Urbaniak & Greiss, 2000). The administration of anti-D immunoprophylaxis to women at risk has greatly reduced the immunization rate (Koelewijn et al, 2008). Furthermore, the occurrence of severe fetal/neonatal complications in alloimmunized women is prevented in most developed countries due to the nationwide red cell antibody screening programmes (Engelfriet et al, 2003). Once an alloantibody is recognized, pregnant women are carefully monitored to start timely treatment. We have previously shown that the most sensitive laboratory test for predicting fetal red cell destruction is the monocyte-based antibody-dependent cellular cytotoxicity (ADCC) assay (Oepkes et al, 2001). For that reason, all pregnant women in The Netherlands with red blood cell alloantibodies are monitored using this assay and are only referred for Doppler flow measurement when the ADCC assay rises above a certain threshold. This suggests that the interaction of the antibody with phagocytic cells is an important factor determining the occurrence of red cell haemolysis. The strength of the interaction between IgG and FcγR, and hence the strength of the phagocyte response, depends on several factors, including the IgG subclass involved, the FcγR polymorphic make-up of the patient, and IgG-Fc glycosylation (Hogarth & Pietersz, 2012) (Kapur et al, 2014a).
IgG antibodies are glycoproteins harbouring a branched sugar moiety attached to asparagine (Asn) 297 in the Fc domain. The glycan is required for binding of IgG to FcγR. Furthermore, slight variations in this glycan's composition modulate its affinity (Sondermann et al, 2000; Shields et al, 2002; Ferrara et al, 2011). The Asn297-linked glycan comprises an invariant structure consisting of an N-acetylglucosamines (GlcNAc) and mannoses, but can be found either with or without fucose and bisecting GlcNAc, including various amounts of galactose and sialic acid. The presence of a bisecting GlcNAc is a known modification of the biantennary N-glycans of human IgG. This monosaccharide in the 2,4-position is linked to the innermost mannose of the N-glycan core structure. The relative abundance of these glycans is altered for total IgG in various clinical settings, including pregnancy (Rook et al, 1991; van de Geijn et al, 2009; Selman et al, 2012), cancer (Saldova et al, 2007; Kodar et al, 2012), autoimmunity (Parekh et al, 1985; Rook et al, 1991; Bond et al, 1996, 1997; Alavi et al, 2000; van de Geijn et al, 2009; Selman et al, 2011, 2012; Bondt et al, 2013) and infectious diseases (Parekh et al, 1989). For most of these glycan modifications, the reported changes in FcγR binding are modest, however, the lack of core fucose results in much stronger, up to 50-fold increased, binding to human FcγRIII, due to glycan-glycan interactions between Asn297 in IgG1 and Asn162 in FcγRIIIa and FcγRIIIb (Shibata-Koyama et al, 2009a; Ferrara et al, 2011; Mizushima et al, 2011). We have recently shown that anti-human platelet antigen (HPA)-1a IgG1 antibodies, formed during pregnancy against HPA-1a positive fetal platelets, can display a pronounced decrease in Fc-fucose, resulting in an increased binding affinity for FcγRIIIa/b, enhanced platelet phagocytosis, and a more severe fetal or neonatal alloimmune thrombocytopenia (FNAIT) (Kapur et al, 2014b).
Here we investigated if similar low Fc-fucosylation can be found in pregnancy-induced anti-D allo-IgG1 antibodies, and if skewed anti-D IgG1 fucosylation may explain the discrepancy between anti-D titre and clinical severity, which is observed in some cases.
Methods
Patient samples
Anti-D alloantibodies were diagnosed at Sanquin, Amsterdam, The Netherlands, and samples were generally acquired in the third trimester (n = 70). Diagnosis was made using the indirect antiglobulin test with the addition of polyethylene glycol and the use of a polyclonal anti-IgG (Sanquin reagents, Sanquin, Amsterdam). The titre was determined with a twofold dilution in a tube indirect antiglobulin test (no additions, incubation 30 min at 37°C, three washes, polyclonal anti-IgG and monoclonal anti-C3d) against RBCs with a R2R2 (ccDDEE) phenotype. Clinical data was obtained retrospectively by contacting obstetric care-givers. Samples were obtained with informed consent from the patients in accordance with the Declaration of Helsinki.
Purification and IgG quantification of anti-D antibodies from sera
Anti-D alloantibodies were purified from serum by incubation of the serum with D-positive RBC (500 μl serum for an anti-D titre of 256 or lower, and for a titre of 512, 250 μl of serum was used; all incubated with 500 μl packed D-positive RBC), for 1 h at 37°C. Similarly, D-negative RBC incubated with D-positive serum, as well as D-positive RBC incubated with normal human serum (NHS), were used as negative controls. This was followed by six washes with cold phosphate-buffered saline (PBS). Antibodies were then eluted from the RBC by the addition of eluting buffer (low-pH glycine buffer of Gamma ELU-KIT™ II; Immucor, Inc., Norcross, GA, USA) and the pH of the antibody-containing eluate (supernatant) was neutralized by addition of 250 μl of basic Tris-PBS (214 mmol/l Tris, 22 mmol/l Na2HPO4). The specificity of the eluate was confirmed by gelcards (DiaMed GmbH, Bio-Rad Laboratories, Inc., Hercules, CA, USA; six microtubes containing rabbit anti-human IgG within the gel matrix). The amount of IgG1 and IgG3 in the eluate was determined by enzyme-linked immunosorbent assay (ELISA) in microtitre plates (NUNC-Immuno, Maxisorp; Sigma Aldrich, St Louis, MO, USA) coated with either mouse-anti-human IgG1 Fc (clone MH161-1, Sanquin) or with mouseanti- human IgG3 hinge (clone MH163-1, Sanquin reagents). For calculations of IgG1 and IgG3 concentrations, fully human recombinant antibodies IgG1κ and IgG3 GDob1 (Vidarsson et al, 2006; Stapleton et al, 2011) were used as standards. Each eluate was only considered to contain purified anti-D IgG if all negative controls gave a result below the threshold, and a positive reaction was obtained with D-positive cells only.
IgG purification from eluates
IgG1 was isolated from plasma and eluates from D+ RBC by subclass-specific affinity chromatography using 10 μl CaptureSelect™ non-glycosylated nanobody-VH anti-human IgG1 affinity beads in 96-well plate format with 0·7 ml/well filter plates (Orochem, Naperville, IL, USA). Serum (2 μl) or affinity-purified anti-D antibodies (200–4000 ng as determined by ELISA) were applied to the affinity beads in 200 μl of PBS, followed by a 1-h incubation at room temperature with shaking. Then the IgG1 affinity beads were washed by filtration three times with 200 μl PBS and three times with 200 μl water. Bound IgG1 was eluted with 100 mmol/l formic acid (100 μl; Fluka, Steinheim, Germany) and dried by vacuum centrifugation. The purified IgG1 was digested overnight at 37°C with 200 ng trypsin (Promega, Leiden, The Netherlands) in 40 μl 25 mmol/l ammonium bicarbonate buffer (Fluka). Samples were stored at −20°C until further use.
Reverse-phase solid phase extraction of glycopeptides
IgG1 Fc glycopeptides were enriched and desalted by reverse phase (RP) solid phase extraction (SPE) using Chromabond C18ec (C-18) beads (Marcherey-Nagel, Düren, Germany). The C-18 beads were activated by 80% acetonitrile (ACN) containing 0·1% trifluoroacetic acid (TFA; Fluka) and conditioned with 0·1% TFA. Tryptic IgG1 digests (40 μl) were added to 150 μl 0·1% TFA, loaded onto C-18 beads, followed by three washes with 100 μl 0·1% TFA. Elution of the IgG glycopeptides were performed with 100 μl of 18% ACN containing 0·1% TFA to minimize co-elution of interfering peptides. The purified glycopeptides were dried by vacuum centrifugation and stored at −20°C until mass spectrometric analysis.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of IgG Glycopeptides
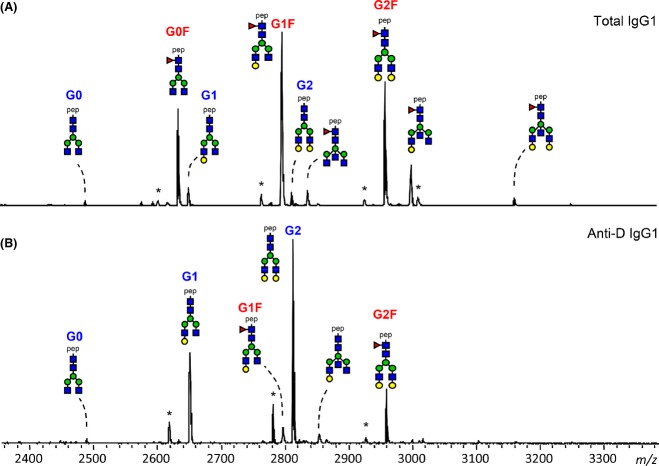
Analysis of reverse-phase solid phase extraction (RP-SPE) purified IgG1 Fc-glycopeptides was performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Samples were dissolved in 40 μl of water, and 2 μl aliquots were spotted onto MTP 384 polished steel target plates (Bruker Daltonics, Bremen, Germany) and allowed to dry at room temperature. Subsequently, 2 μl of 5 mg/ml 4-chloro-α-cyanocinnamic acid (Cl-CCA; 95% purity; Bionet Research, Camelford, UK) in 70% ACN was applied on top of each sample and allowed to dry. Glycopeptides were analysed on an UltrafleXtreme MALDI-TOF/TOF mass spectrometer (Bruker Daltonics), which was operated in the positive-ion reflectron mode. Ions between 1000 and 3700 m/z were recorded. To allow homogeneous spot sampling, a random walk laser movement with 100 laser shots per raster spot was applied, and each IgG glycopeptide sum mass spectrum was generated by accumulation of 2000 laser shots. Mass spectra were internally calibrated using a list of known glycopeptides (G0, G0F, G1, G1F, G2, G2F species, see Fig1).
Fig 1.
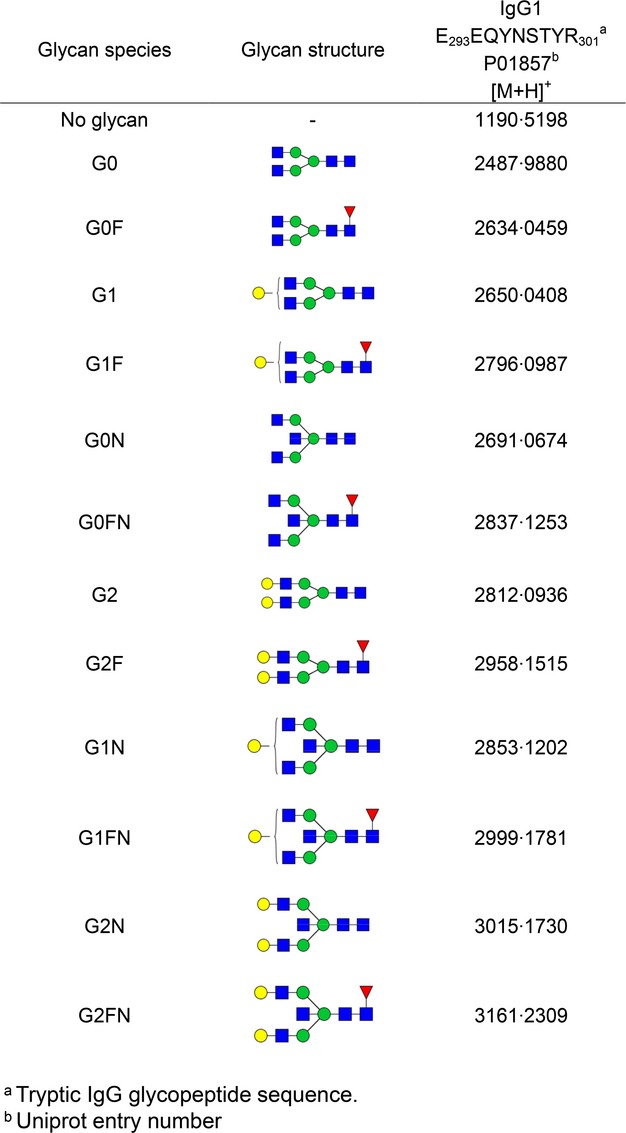
Theoretical masses of tryptic glycopeptides of human IgG1. Glycan structural features are given in terms of number of galactoses (G0, G1, G2), fucose (F) and bisecting N-acetylglucosamine (N). G0, agalactosylated glycan without core fucose; G0F, agalactosylated glycan with core fucose; G1, monogalactosylated glycan without core fucose, etc. Blue square, N-acetylglucosamine; green circle, mannose; yellow circle, galactose; red triangle, fucose.
Data processing and evaluation were performed with FlexAnalysis Software (Bruker Daltonics) and Microsoft Excel, respectively. The data were baseline subtracted and the intensities of a defined set of 12 glycopeptides (six glycoforms for IgG1 with core-fucose and six glycoforms for IgG1 without core-fucose; see Fig1) were defined automatically for each spectrum.
Relative intensities of IgG Fc glycopeptides were obtained by integrating the first isotopic peaks followed by normalization to the total IgG1 specific glycopeptide intensities. The level of galactosylation was calculated from the relative intensities of various Fc N-glycopeptides according to the formula: (G1 + G1F + G1FN + G1N) × 0·5 + G2 + G2F + G2N + G2FN + G2NS. The prevalence of bisecting GlcNAc was determined by summing the relative intensities of all bisected glycoforms (G0N, G0FN, G1N, G1FN, G2N and G2FN). The incidence of IgG1 fucosylation was evaluated by summing all fucosylated IgG1 Fc N-glycoforms (G0F + G1F + G0FN + G2F + G1FN +G2FN).
Natural killer (NK)-cell and monocyte based ADCC
D-positive RBCs were labelled with radioactive chromium 51 (51Cr) and incubated with maternal serum containing anti-D antibodies at an equal titre of 128. Peripheral blood mononuclear cells were isolated from blood taken from healthy volunteers, via density gradient centrifugation over Ficoll (GE Healthcare, Uppsala, Sweden, 1·077 g/ml) and platelets were removed by washing. Monocytes were depleted by adherence to plastic tissue culture flasks for 1 h at 37°C, after which the non-adherent fraction was taken and allowed to adhere to plastic tissue culture flasks for 1 h at 37°C. The non-adherent cells were washed and re-suspended in RPMI medium with 20% human AB serum and incubated for 1·5 h at 37°C in triplicate in U-well microplates with opsonized 51Cr-labelled D-positive RBCs (3 × 106/ml, 150 000 per well), in a total volume of 100 μl and an effector to target cell ratio of 15:1. Maximum lysis was obtained with the addition of 150 μl 5% saponine to 50 μl of RBCs and was used to calculate the degree of lysis (%). Similarly, the monocyte-ADCC was performed as described previously (Oepkes et al, 2001) with 1·5 × 106/ml monocytes (75 000 per well and derived from a pool of 70–100 donors, stored in liquid nitrogen). Cytotoxic lysis was assessed by counting the 51Cr-activity in the supernatant and expressed as a percentage of lysis produced by a pooled polyclonal anti-D standard according to a calibration curve.
Statistical analysis
Analysis was performed using GraphPad Prism 5·01 (La Jolla, CA, USA). Tests were applied as indicated and the level of significance was set at P < 0·05.
Results
Fc-glycosylation of anti-D IgG1 in pregnancy
IgG1 was purified from anti-D containing sera from 70 pregnant women and from the specific anti-D alloantibodies, which were obtained from these sera via acidic elution from D-positive RBCs. Tryptic IgG1-derived glycopeptides encompassing the Asn297-Fc glycan were subsequently analysed by MALDI-TOF-MS. Quantities of the 12 most abundant IgG1-Fc glycopeptides were examined (Fig2). For total IgG1, the Fc glycosylation profile was abundant in fucosylated glycan structures (G0F, G1F, G2F, Fig3A), as described for normal human IgG1 (Wuhrer et al, 2009; Selman et al, 2012; Bakovic et al, 2013; Kapur et al, 2014b). In contrast, the anti-D specific IgG1-derived glycopeptides mostly consisted of glycoforms lacking core fucose (G1 and G2, Fig3B, demonstrating the mass spectrometry profile of one pregnant woman). Systematic analysis of the 70 pregnancy-induced anti-D IgG1 sera for Fc-galactosylation, -bisection (bisecting GlcNAc) and -fucosylation, showed that the levels of Fc-galactosylation were significantly increased (Fig4A) and the levels of Fc-bisection significantly decreased (Fig4B), when compared to total IgG1. Strikingly, the levels of Fc-fucosylation were variably decreased, in some cases with levels of core fucosylation down to 12%, while fucosylation was fairly constant for total IgG1 (on average 93·3 ± 4·6%, Fig4C), which is comparable to the total IgG1-fucosylation in a non-pregnancy setting (92·9%) (Selman et al, 2012). Furthermore, we observed a weak but significant negative correlation between anti-D IgG1-fucosylation and galactosylation (R2 = 0·06 P = 0·046), but no significant correlation between bisection and galactosylation or bisection and fucosylation (Fig S1).
Fig 2.
IgG1 glycosylation profiles of a pregnant woman with anti-D. Tryptic IgG1 Fc glycopeptides of total IgG1 (A) and anti-D specific IgG1 (B), affinity-purified from the serum of a pregnant woman, were measured by positive-ion reflectron mode matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. The profile shown represents specific antibodies with a low fucosylation of 16%, while the total IgG1 was highly fucosylated (94%). For the assignment and the definitions of the glycopeptides signals see Fig1. Pep, peptide moiety; *, contaminant.
Fig 3.
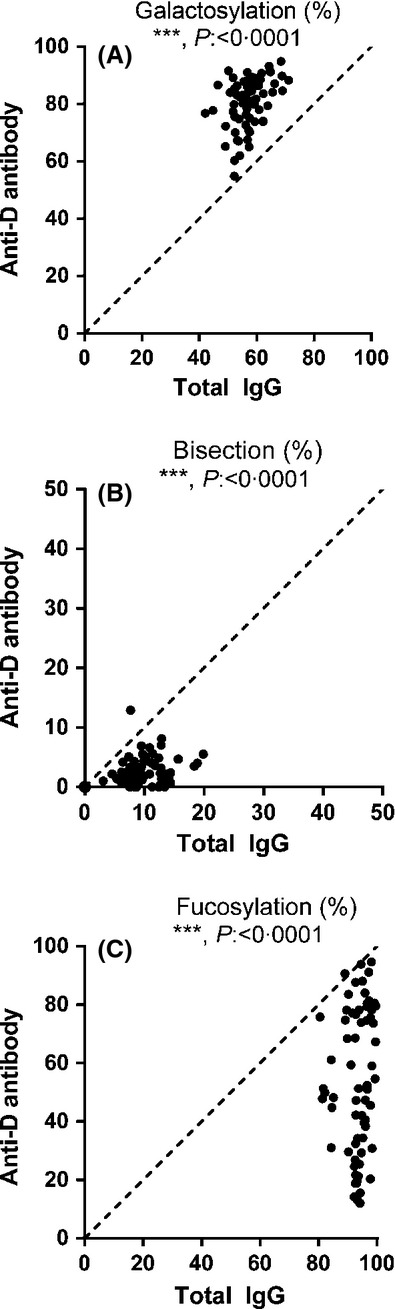
Anti-D IgG1 in pregnancy displays pronounced lowered Fc-fucosylation. Relative expression levels of major IgG-Fc Asn-297 glycoforms for both total IgG1 (x-axis) and antigen-specific IgG1 (y-axis) for 70 pregnancy-induced anti-D serum samples (A–C). Serum populations were analysed for Fc-galactosylation (A), Fc-bisection (bisecting N-acetylglucosamine) (B) and Fc-fucosylation (C). The statistical outcome between two-tailed paired t-test analysis of total IgG1 versus. specific antibodies is listed in each panel. The diagonal, dotted lines represent the theoretical equal glycosylation of total IgG1 and anti-D IgG1. Normal total IgG1 values (non-pregnancy setting): galactose (61·92%), bisection (13·80%) and fucose (92·86%) (Selman et al, 2012).
Fig 4.
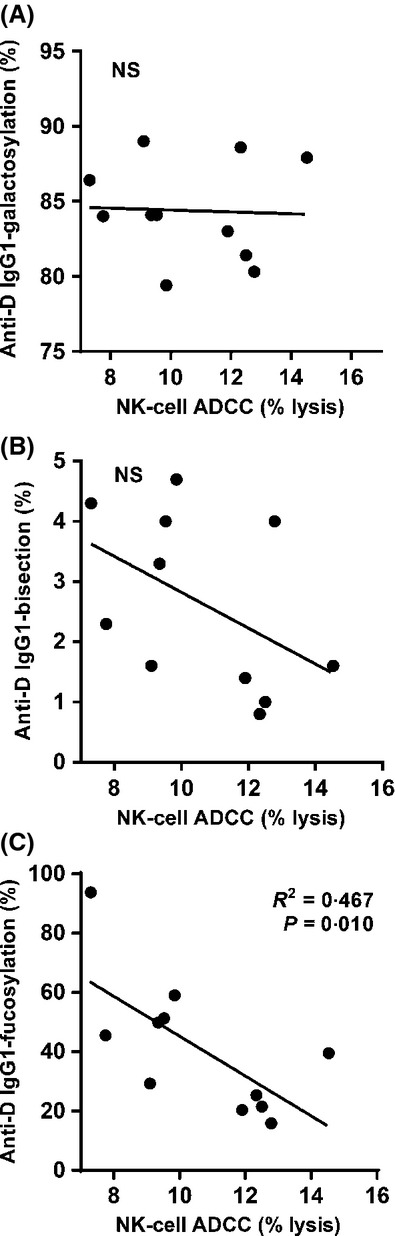
Low anti-D IgG1-fucosylation induces stronger NK-cell mediated ADCC. Natural killer (NK)-cell ADCC versus the levels of galactosylation (A), bisection (B) and fucosylation (C) found in IgG1 anti-D. For the NK-cell ADCC only samples containing enough material and with an anti-D titre of 1/128 were plotted. Statistical analyses were performed using two-tailed (A, B) or one-tailed (C) Pearson correlation with significance set at P = 0·05.
Fc-fucosylation levels of anti-D IgG1 predict NK-cell mediated ADCC activity
As monocyte ADCC is used to monitor anti-D activity for diagnostic purposes, because it is more predictive for disease severity of HDFN than the antibody titre (Oepkes et al, 2001), we first investigated if glycosylation of anti-D IgG1 affected anti-D activity in this assay. A very weak, albeit significant, correlation between monocyte ADCC and the level of galactosylation was found (Fig S2). No significant correlation was found between monocyte ADCC and bisection or fucosylation. However, as IgG-Fc fucosylation only affects binding to monocyte FcγRIIIa, which is not well expressed on circulating monocytes (∼5% of cells show low expression), we also tested the functional capacity of pregnancy-induced anti-D IgG1 antibodies to mediate RBC lysis through CD16+ (FcγRIIIa) NK cell-mediated ADCC. Sufficient material from 11 samples with equal titres, and glycoprofiled in Fig4, was available to perform this assay. This set of samples did not show any significant correlation between anti-D IgG1 galactosylation or bisection and NK-cell ADCC (Fig5A, B, respectively), and also not with the monocyte ADCC (Fig S3). However, in line with the strong FcγRIIIa expression on NK cells, the degree of anti-D IgG1 fucosylation correlated significantly with NK-cell mediated ADCC, with a lower degree of anti-D Fc-fucosylation corresponding to increased NK-cell mediated ADCC (Fig5C). No correlations were found between the anti-D titre and the levels of IgG1-galactosylation, -bisection, or -fucosylation (Fig S4).
Fig 5.
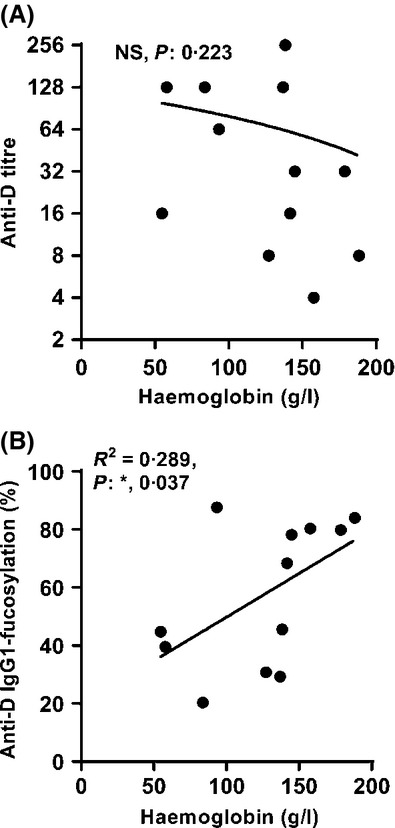
Anti-D IgG1 with lower core fucosylation induces more severe haemolysis. No significant correlation was found between anti-D titre and Haemoglobin levels (A). However, the degree of IgG1-anti-D fucosylation in pregnancy correlated significantly with fetal or neonatal haemoglobin levels (B). Statistical analyses were performed using one-tailed Pearson correlation with significance set at P = 0·05. NS: not significant.
Low fucosylation of IgG1 anti-D is associated with low fetal or neonatal haemoglobin levels
IgG-opsonized RBCs are cleared quickly through FcγR-receptor mediated pathways in the liver and spleen (Armour et al, 2006). We therefore tested if the haemoglobin levels in the fetus or neonate were associated with IgG1-anti-D fucosylation in these patients in a pilot-study. All patients with dominant IgG1 anti-D for whom the haemoglobin levels were recorded before any intra-uterine transfusion (IUT), were included (n = 12) (Table SI). In agreement with lowered anti-D IgG1 core-fucosylation and its increased affinity to FcγRIIIa (Kapur et al, 2014b), we found the degree of IgG1-anti-D Fc-fucosylation, and not the titre, to correlate significantly with the haemoglobin levels (Fig5, R2 = 0·289 and P = 0·037).
Discussion
In the current study we investigated the IgG1 Fc-glycosylation patterns of 70 patients with pregnancy-induced anti-D antibodies. The glycosylation pattern of the anti-D specific IgG1 was compared to that of total IgG1 from the same patient. The antigen-specific IgG-antibodies were first affinity purified using D-positive RBCs, and then using anti-IgG1 beads, after which tryptic glycopeptides were analysed by mass spectrometry. Despite the presence of IgG3 anti-D in some patients, we focused solely on IgG1-Fc glycosylation in pregnancy. IgG3-derived glycopeptide detection by mass spectrometry is complex due to the presence of multiple IgG3 allotypes in the population, which have identical mass to glycopeptides of either IgG2 or IgG4 (Wuhrer et al, 2007; Stapleton et al, 2011). In general, the anti-D IgG1 antibodies displayed elevated levels of galactosylation and lowered bisection, but, most prominently, a decrease in core fucosylation. Importantly, the affinity purification procedure of IgG1 with acidic elution did not result in changes of IgG Fc glycosylation profiles. This was assessed by comparing the glycosylation profile of a highly core-fucosylated monoclonal antibody before and after affinity purification (not shown) (Wuhrer et al, 2009; Kapur et al, 2014b). In addition, the changes observed were reproducible upon re-measuring (Selman et al, 2012) and highly variable between patients, with some patients showing no changes in anti-D glycosylation patterns compared to total IgG1. Therefore, the immunopurification procedure is unlikely to alter the degree of fucosylation.
The most striking finding in the current study is the highly variable but potent decrease in core-fucose levels observed for anti-D IgG1 antibodies in pregnancy, with 34 out of 70 samples showing a Fc-fucosylation of <50%, whereas fucose levels in total serum IgG are generally not reported to be lower than 90% (Wuhrer et al, 2009; Selman et al, 2012; Bakovic et al, 2013; Kapur et al, 2014b). Recently, we described a similar decrease of IgG1-Fc fucosylation in anti-HPA-1a IgG1 antibodies in FNAIT, which we did not observe for anti-human leucocyte antigen (HLA) antibodies in platelet transfusion refractoriness refractory thrombocytopenia (RT) (Kapur et al, 2014b). Although both FNAIT and HDFN are antibody-mediated disorders in pregnancy, we have also observed low anti-D Fc-fucosylation in male hyperimmunized anti-D (HID)-donors (R. Kapur, L. Della Valle, O. Verhagen, A. Hipgrave Ederveen, P. Ligthart, M. de Haas, B. Kumpel, M. Wuhrer, C.E. van der Schoot, G. Vidarsson, unpublished observations). Although this is not a natural event, it indicates that the regulation of the Fc-fucosylation is not a pregnancy-related phenomenon. In refractory thrombocytopenia patients, the HLA-class I antibodies did not display low Fc-fucosylation (Kapur et al, 2014b). Therefore, low Fc-fucosylation seems to depend on the nature of the recognized antigen, perhaps on how and where the antigen is presented to the immune system (expressed on solid tissue or blood-borne cells with easier access to the spleen). This kind of skewing of core fucosylation in antigen-specific IgG has also been described in a recent study on anti-human immunodeficiency virus (HIV) responses (Ackerman et al, 2013). In this study, prominent inter-individual variation was also observed. Remarkably, the individuals who were able to spontaneously control HIV showed a lower level of fucosylation of HIV-specific IgG. The lower level of IgG fucosylation in these elite controllers was accompanied by a decreased level of FUT8, the main fucosyltransferase responsible for core fucosylation, and an increased level of the FUCA2 fucosidase, in peripheral IgG+ B cells. It will be informative to investigate the level of glycosyltransferases and fucosidases in the D-specific B cells (Dohmen et al, 2005).
Half-life and transplacental IgG-transport are mainly mediated by the neonatal Fc-receptor (FcRn). However, these processes are not affected by glycosylation, as deglycosylated IgGs have normal half-life (Kaneko et al, 2006), and because fetal- and maternal IgG have identical glycosylation (Einarsdottir et al, 2013). In addition, crystallographic data indicates that Fc-glycans are not involved in binding to FcRn (West & Bjorkman, 2000).
Although we have not monitored different time-points within one pregnancy, we do not expect the fucosylation to change much during pregnancy and to be rather stable over time. This hypothesis is based on our previously published data for anti-HPA-1a antibodies in pregnancy, which demonstrated the lowered Fc-fucosylation to be present up to 7 years after delivery (Kapur et al, 2014b). Our preliminary data of hyperimmunized anti-D donors also suggests that anti-D fucosylation levels remain fairly constant over time (R. Kapur, L. Della Valle, O. Verhagen, A. Hipgrave Ederveen, P. Ligthart, M. de Haas, B. Kumpel, M. Wuhrer, C.E. van der Schoot, G. Vidarsson, unpublished observations).
The lowered core-fucosylation of the antigen-specific IgG1 antibodies is particularly interesting because we and others have found that lowered Fc-fucosylation facilitates stronger binding to FcγRIIIa (Shields et al, 2002; Niwa et al, 2005; Masuda et al, 2007; Peipp et al, 2008; Shibata-Koyama et al, 2009a,b; Junttila et al, 2010; Kapur et al, 2014b) and FcγRIIIb (Peipp et al, 2008; Shibata-Koyama et al, 2009a; Kapur et al, 2014b), but not other FcγR (Peipp et al, 2008; Kapur et al, 2014b. This agrees with the increased binding to FcγRIII that takes place through glycan-glycan interactions of the N-linked Fc-glycan at position 297 with the glycan at position 162 that is unique to the human family (Ferrara et al, 2006, 2011). We found this decreased Fc-fucosylation to resulted in enhanced platelet-phagocytosis mediated by FcγRIIIa-positive monocytes or FcγRIIIb-positive PMN. In addition, the V158-isoform of FcγRIIIa is associated with a faster clearance of D-positive RBCs (Kumpel et al, 2003; Miescher et al, 2004), suggesting that FcγRIIIa is an important receptor in IgG-opsonized RBC clearance. We therefore tested the functional effect of pregnancy-induced anti-D antibodies in FcγRIIIa-mediated ADCC using sera with equal titres. A significant correlation between IgG1-anti-D fucosylation and FcγRIIIa (NK-cell)-mediated ADCC was found, with a lower degree of Fc-fucosylation corresponding to higher NK-cell mediated ADCC. No correlation was found between fucosylation levels and monocyte-mediated ADCC, which mainly occurs through FcγRI (Ruegg & Jungi, 1988; Kumpel et al, 2002). Nevertheless, monocyte-mediated ADCC was previously found to be a more specific parameter (Oepkes et al, 2001) for predicting fetal red cell destruction than anti-D titre, even though FcγRIIIa-negative peripheral monocytes do not strongly participate in RBC removal in vivo (Armour et al, 2006). This is probably due to the fact that the monocyte-mediated ADCC also takes the interaction of the antibody with the phagocytic FcγRI into account. Although monocyte-based ADCC is extremely sensitive, as shown by the fact that severe fetal anaemia does not occur when ADCC results remain <50% (Oepkes et al, 2001), in terms of predicting severe anaemia the specificity is relatively low with only 43% of fetuses with high ADCC levels (>80%) being severely anaemic. The present study provides a possible explanation for this, as we now show a large individual variation in core-fucosylation levels among anti-D antibodies, low levels of which predict higher affinity to FcγRIIIa, which is not detected by monocyte ADCC.
We also found a positive correlation between fetal or neonatal haemoglobin levels and IgG1-anti-D Fc fucosylation in a small group of patients. Unfortunately, we were unable to include more samples from our original cohort (70 samples) due to availability and because the analysis was restricted to samples with identical titre in certain cases, which may have resulted in a random selection bias. However, the data seem to suggest that monitoring the degree of IgG Fc-fucosylation, either directly or by NK-cell ADCC, might improve the specificity of laboratory screening.
We also observed increased Fc-galactosylation of total IgG1, which was more pronounced for D-specific IgG1. This was not unexpected, as total IgG1-galactosylation increases in pregnancy (Rook et al, 1991; van de Geijn et al, 2009; Selman et al, 2012), and because we previously found that decreased core fucosylation goes hand-in-hand with increased galactosylation in anti-HPA-1a IgG1 found in FNAIT (Kapur et al, 2014b). However, there does not seem to be a direct causal relationship between the level of fucosylation and galactosylation in general, as decreased fucosylation and galactosylation has been observed in anti-HIV IgG1 (Ackerman et al, 2013).
Due to the fact that we measured the samples in the mass spectrometer in positive-ion mode for increased sensitivity, we were not able to obtain reliable data for sialylation. However, based on our previous analysis of patient anti-HPA1a antibodies and in vitro generated antibodies [where we found that increased galactosylation generally resulted in increased sialylation, given that the substrate for sialyltransferases (adding α2-6 sialic acid) are β1,4 galactose residues (Anthony et al, 2008)], a similar increase in sialylation of the anti-D specific IgG1 was expected. This fits with a recent report showing 16% sialylation of anti-D specific IgG compared to 8% for total IgG (Winkler et al, 2013). Most importantly, addition of galactose to the Asn297 glycan in IgG has been shown to result in decreased binding to FcγR (Li et al, 2006), which is further reduced by the addition of sialic acid in mice (Kaneko et al, 2006). This is supported by a recent report showing that lack of galactosylation enhances the pathogenic activity of IgG1 anti-RBC autoantibodies (Ito et al, 2014). Together this makes it very unlikely that the increased galactosylation we observed, with or without possible increased sialylation, will positively affect ADCC. Although the degree of galactosylation did correlate significantly with monocyte ADCC, the effect was marginal at best with no such correlation being noted for the NK-cell mediated ADCC. However, we cannot exclude that a possible effect is masked by the concomitant decrease in fucosylation, which has the opposite effect due the increased binding to FcγRIIIa.
In conclusion, the IgG-glycosylation we find in pregnancy responses against RhD bear remarkable similarities to what we have previously reported for anti-platelet responses. This holds true, in particular, with the potent lowering of core-fucosylation of the antigen-specific IgG. In addition, the lowered Fc core-fucose in anti-D IgG1 was associated with increased FcγRIIIa-mediated ADCC and decreased fetal or neonatal haemoglobin levels, suggesting this type of glycosylation to be an important biomarker and therapeutic target in HDFN. For the improved assessment of fetuses that are at high risk of HDFN, the measurement of the degree of anti-D IgG fucosylation directly by mass-spectrometry may currently be too time-consuming for routine use. The NK-cell mediated ADCC may therefore be a better variable to measure, because this would be easier to incorporate into current laboratory screening procedures, as the testing of monocyte-mediated ADCC is already established.
Acknowledgments
This work was supported by grants from Sanquin (PPOC-09-025) (R.K. and R.V.), the Landsteiner Foundation for Blood Transfusion (Grant 1229) (M.S.), and the European Union Seventh Framework Programme (FP7-Health-F5-2011) under Grant Agreement No. 278535 (HighGlycan) (M.W. and A.H.E.)
Authorship
R.K. performed all purifications of anti-D alloantibodies from serum, confirmed the specificity of the eluate by gelcards, co-ordinated the ADCC experiments and analysed clinical data; L.D.V. gathered serum samples together with P.L., determined the amount of IgG1 and IgG3 in the eluate via ELISA; M.S. gathered clinical data; A.H.E. prepared samples, conducted the mass spectrometry and processed the raw mass spectrometry data with M.W., R.V. isolated cells for the ADCC, P.L. provided cells for antibody purifications from serum; R.K., G.V., M.W. made the figures and tables, R.K. performed statistical analysis, G.V. conceived the study and G.V., C.E.v.d.S. and M.W. supervized the study; M.d.H. co-supervized the study; R.K., G.V, C.E.v.d.S., M.W. wrote the paper, which was critically revised and approved by all authors, and all authors contributed to analysis and interpretation of data.
Conflict of interest
The authors declare no competing financial interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Overview of samples used for analysis of anti-D titer/fucosylations versus fetal (F) or neonatal (N) hemoglobin levels.
Fig S1. The relationships between the degree of glycosylation of anti-D IgG1 for (A) galactosylation and fucosylation, (B) galactoylation and bisection, and (C) fucosylation and bisection.
Fig S2. The relationship between monocyte ADCC and anti-D IgG1-galactosylation, bisection and fucosylation.
Fig S3. Monocyte-mediated ADCC towards RBC versus glycosylation of anti-D IgG1 using the same set of samples as in Fig 3, shown for (A) galacosylation, (B) bisection, (C) fucosylation.
Fig S4. The relationship between anti-D titre and anti-D IgG1-galactosylation, bisection and fucosylation.
References
- Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C. Alter G. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. The Journal of Clinical Investigation. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi A, Arden N, Spector TD. Axford JS. Immunoglobulin G glycosylation and clinical outcome in rheumatoid arthritis during pregnancy. Journal of Rheumatology. 2000;27:1379–1385. [PubMed] [Google Scholar]
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC. Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour KL, Parry-Jones DR, Beharry N, Ballinger JR, Mushens R, Williams RK, Beatty C, Stanworth S, Lloyd-Evans P, Scott M, Clark MR, Peters AM. Williamson LM. Intravascular survival of red cells coated with a mutated human anti-D antibody engineered to lack destructive activity. Blood. 2006;107:2619–2626. doi: 10.1182/blood-2005-03-0989. [DOI] [PubMed] [Google Scholar]
- Bakovic MP, Selman MH, Hoffmann M, Rudan I, Campbell H, Deelder AM, Lauc G. Wuhrer M. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. Journal of Proteome Research. 2013;12:821–831. doi: 10.1021/pr300887z. [DOI] [PubMed] [Google Scholar]
- Bond A, Alavi A, Axford JS, Youinou P. Hay FC. The relationship between exposed galactose and N-acetylglucosamine residues on IgG in rheumatoid arthritis (RA), juvenile chronic arthritis (JCA) and Sjogren's syndrome (SS) Clinical and Experimental Immunology. 1996;105:99–103. doi: 10.1046/j.1365-2249.1996.d01-741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A, Alavi A, Axford JS, Bourke BE, Bruckner FE, Kerr MA, Maxwell JD, Tweed KJ, Weldon MJ, Youinou P. Hay FC. A detailed lectin analysis of IgG glycosylation, demonstrating disease specific changes in terminal galactose and N-acetylglucosamine. Journal of Autoimmunity. 1997;10:77–85. doi: 10.1006/jaut.1996.0104. [DOI] [PubMed] [Google Scholar]
- Bondt A, Selman MH, Deelder AM, Hazes JM, Willemsen SP, Wuhrer M. Dolhain RJ. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. Journal of Proteome Research. 2013;12:4522–4531. doi: 10.1021/pr400589m. [DOI] [PubMed] [Google Scholar]
- Dohmen SE, Mulder A, Verhagen OJ, Eijsink C, Franke-van Dijk ME. van der Schoot CE. Production of recombinant Ig molecules from antigen-selected single B cells and restricted usage of Ig-gene segments by anti-D antibodies. Journal of Immunological Methods. 2005;298:9–20. doi: 10.1016/j.jim.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Einarsdottir HK, Selman MH, Kapur R, Scherjon S, Koeleman CA, Deelder AM, van der Schoot CE, Vidarsson G. Wuhrer M. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconjugate Journal. 2013;30:147–157. doi: 10.1007/s10719-012-9381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelfriet CP, Reesink HW, Judd WJ, Ulander VM, Kuosmanen M, Koskinen S, Rouger P, Morelati F, Tantalo V, Fujii T, de Haas M, van der Schoot CE, Overbeeke M, Koelewijn J, Bonsel G, Vrijkotte T, Zupanska B, Martin-Vega C, Parra LR, de Silva M, Contreras M, Panzer S, Ulm B. Mayr WR. Current status of immunoprophylaxis with anti-D immunoglobin. Vox Sanguinis. 2003;85:328–337. doi: 10.1111/j.0042-9007.2003.364_1.x. [DOI] [PubMed] [Google Scholar]
- Ferrara C, Stuart F, Sondermann P, Brunker P. Umana P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. Journal of Biological Chemistry. 2006;281:5032–5036. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umana P. Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, Hazes JM. Dolhain RJ. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Research and Therapy. 2009;11:R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth PM. Pietersz GA. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nature Reviews Drug Discovery. 2012;11:311–331. doi: 10.1038/nrd2909. [DOI] [PubMed] [Google Scholar]
- Ito K, Furukawa JI, Yamada K, Tran NL, Shinohara Y. Izui S. Lack of galactosylation enhances the pathogenic activity of IgG1 but not IgG2a anti-erythrocyte autoantibodies. The Journal of Immunology. 2014;192:581–588. doi: 10.4049/jimmunol.1302488. [DOI] [PubMed] [Google Scholar]
- Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, Crocker L, Pabonan O, Baginski T, Meng G, Totpal K, Kelley RF. Sliwkowski MX. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Research. 2010;70:4481–4489. doi: 10.1158/0008-5472.CAN-09-3704. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F. Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Kapur R, Einarsdottir HK. Vidarsson G. IgG-effector functions: “The Good, The Bad and The Ugly”. Immunology Letters. 2014a doi: 10.1016/j.imlet.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Kapur R, Kustiawan I, Vestrheim A, Koelman CA, Visser R, Einarsdottir HK, Porcelijn L, Jackson D, Kumpel B, Deelder AM, Blank D, Skogen B, Killie MK, Michaelsen TE, de Haas M, Rispens T, van der Schoot CE, Wuhrer M. Vidarsson G. A prominent lack of IgG1 Fc-fucosylation of platelet-alloantibodies in pregnancy. Blood. 2014b;123:471–480. doi: 10.1182/blood-2013-09-527978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodar K, Stadlmann J, Klaamas K, Sergeyev B. Kurtenkov O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconjugate Journal. 2012;29:57–66. doi: 10.1007/s10719-011-9364-z. [DOI] [PubMed] [Google Scholar]
- Koelewijn JM, de Haas M, Vrijkotte TG, Bonsel GJ. van der Schoot CE. One single dose of 200 microg of antenatal RhIG halves the risk of anti-D immunization and hemolytic disease of the fetus and newborn in the next pregnancy. Transfusion. 2008;48:1721–1729. doi: 10.1111/j.1537-2995.2008.01742.x. [DOI] [PubMed] [Google Scholar]
- Kumpel BM, Beliard R, Brossard Y, Edelman L, de Haas M, Jackson DJ, Kooyman P, Ligthart PC, Monchatre E, Overbeeke MA, Puillandre P, de Romeuf C. Wilkes AM. Section 1C: assessment of the functional activity and IgG Fc receptor utilisation of 64 IgG Rh monoclonal antibodies. Coordinator's report. Transfusion Clinique et Biologique. 2002;9:45–53. doi: 10.1016/s1246-7820(01)00215-4. [DOI] [PubMed] [Google Scholar]
- Kumpel BM, de Haas M, Koene HR, van de Winkel JG. Goodrick MJ. Clearance of red cells by monoclonal IgG3 anti-D in vivo is affected by the VF polymorphism of Fcgamma RIIIa (CD16) Clinical and Experimental Immunology. 2003;132:81–86. doi: 10.1046/j.1365-2249.2003.02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Sethuraman N, Stadheim TA, Zha D, Prinz B, Ballew N, Bobrowicz P, Choi BK, Cook WJ, Cukan M, Houston-Cummings NR, Davidson R, Gong B, Hamilton SR, Hoopes JP, Jiang Y, Kim N, Mansfield R, Nett JH, Rios S, Strawbridge R, Wildt S. Gerngross TU. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nature Biotechnology. 2006;24:210–215. doi: 10.1038/nbt1178. [DOI] [PubMed] [Google Scholar]
- Masuda K, Kubota T, Kaneko E, Iida S, Wakitani M, Kobayashi-Natsume Y, Kubota A, Shitara K. Nakamura K. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Molecular Immunology. 2007;44:3122–3131. doi: 10.1016/j.molimm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Miescher S, Spycher MO, Amstutz H, de Haas M, Kleijer M, Kalus UJ, Radtke H, Hubsch A, Andresen I, Martin RM. Bichler J. A single recombinant anti-RhD IgG prevents RhD immunization: association of RhD-positive red blood cell clearance rate with polymorphisms in the FcgammaRIIA and FcgammaIIIA genes. Blood. 2004;103:4028–4035. doi: 10.1182/blood-2003-11-3929. [DOI] [PubMed] [Google Scholar]
- Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M. Kato K. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes to Cells. 2011;16:1071–1080. doi: 10.1111/j.1365-2443.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M. Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. Journal of Immunological Methods. 2005;306:151–160. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Oepkes D, van Kamp IL, Simon MJ, Mesman J, Overbeeke MA. Kanhai HH. Clinical value of an antibody-dependent cell-mediated cytotoxicity assay in the management of Rh D alloimmunization. American Journal of Obstetrics and Gynecology. 2001;184:1015–1020. doi: 10.1067/mob.2001.112970. [DOI] [PubMed] [Google Scholar]
- Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, Takeuchi F, Nagano Y, Miyamoto T. Kobata A. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R, Isenberg D, Rook G, Roitt I, Dwek R. Rademacher T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. Journal of Autoimmunity. 1989;2:101–114. doi: 10.1016/0896-8411(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WW, Dechant M, Beyer T, Repp R, van Berkel PH, Vink T, van de Winkel JG, Parren PW. Valerius T. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112:2390–2399. doi: 10.1182/blood-2008-03-144600. [DOI] [PubMed] [Google Scholar]
- Rook GA, Steele J, Brealey R, Whyte A, Isenberg D, Sumar N, Nelson JL, Bodman KB, Young A, Roitt IM, Williams P, Scragg I, Edge CJ, Arkwright PD, Ashford D, Wormald M, Rudd P, Redman CWG, Dwek RA. Rademacher TW. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. Journal of Autoimmunity. 1991;4:779–794. doi: 10.1016/0896-8411(91)90173-a. [DOI] [PubMed] [Google Scholar]
- Ruegg SJ. Jungi TW. Antibody-mediated erythrolysis and erythrophagocytosis by human monocytes, macrophages and activated macrophages. Evidence for distinction between involvement of high-affinity and low-affinity receptors for IgG by using different erythroid target cells. Immunology. 1988;63:513–520. [PMC free article] [PubMed] [Google Scholar]
- Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN, Banks RE, Hutson R, Harvey DJ, Antrobus R, Petrescu SM, Dwek RA. Rudd PM. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17:1344–1356. doi: 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- Selman MH, Niks EH, Titulaer MJ, Verschuuren JJ, Wuhrer M. Deelder AM. IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. Journal of Proteome Research. 2011;10:143–152. doi: 10.1021/pr1004373. [DOI] [PubMed] [Google Scholar]
- Selman MH, Derks RJ, Bondt A, Palmblad M, Schoenmaker B, Koeleman CA, van de Geijn FE, Dolhain RJ, Deelder AM. Wuhrer M. Fc specific IgG glycosylation profiling by robust nano-reverse phase HPLC-MS using a sheath-flow ESI sprayer interface. Journal of Proteomics. 2012;75:1318–1329. doi: 10.1016/j.jprot.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Shibata-Koyama M, Iida S, Misaka H, Mori K, Yano K, Shitara K. Satoh M. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for FcgammaRIIIb and MHC class II expression on the phagocytotic neutrophils. Experimental Hematology. 2009a;37:309–321. doi: 10.1016/j.exphem.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Shibata-Koyama M, Iida S, Okazaki A, Mori K, Kitajima-Miyama K, Saitou S, Kakita S, Kanda Y, Shitara K, Kato K. Satoh M. The N-linked oligosaccharide at Fc gamma RIIIa Asn-45: an inhibitory element for high Fc gamma RIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology. 2009b;19:126–134. doi: 10.1093/glycob/cwn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH. Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. Journal of Biological Chemistry. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Sondermann P, Huber R, Oosthuizen V. Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, Zhao Y, Kleijer M, Sandlie I, de Haas M, Jonsdottir I, van der Schoot CE. Vidarsson G. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nature Communications. 2011;2:599. doi: 10.1038/ncomms1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaniak SJ. Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Reviews. 2000;14:44–61. doi: 10.1054/blre.1999.0123. [DOI] [PubMed] [Google Scholar]
- Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, de Haas M. van de Winkel JG. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood. 2006;108:3573–3579. doi: 10.1182/blood-2006-05-024539. [DOI] [PubMed] [Google Scholar]
- West AP., Jr Bjorkman PJ. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor(,) Biochemistry. 2000;39:9698–9708. doi: 10.1021/bi000749m. [DOI] [PubMed] [Google Scholar]
- Winkler A, Berger M. Ehlers M. Anti-rhesus D prophylaxis in pregnant women is based on sialylated IgG antibodies. F1000Research. 2013;2:169. doi: 10.12688/f1000research.2-169.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Stam JC, van de Geijn FE, Koeleman CA, Verrips CT, Dolhain RJ, Hokke CH. Deelder AM. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics. 2007;7:4070–4081. doi: 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Porcelijn L, Kapur R, Koeleman CA, Deelder A, de Haas M. Vidarsson G. Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. Journal of Proteome Research. 2009;8:450–456. doi: 10.1021/pr800651j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of samples used for analysis of anti-D titer/fucosylations versus fetal (F) or neonatal (N) hemoglobin levels.
Fig S1. The relationships between the degree of glycosylation of anti-D IgG1 for (A) galactosylation and fucosylation, (B) galactoylation and bisection, and (C) fucosylation and bisection.
Fig S2. The relationship between monocyte ADCC and anti-D IgG1-galactosylation, bisection and fucosylation.
Fig S3. Monocyte-mediated ADCC towards RBC versus glycosylation of anti-D IgG1 using the same set of samples as in Fig 3, shown for (A) galacosylation, (B) bisection, (C) fucosylation.
Fig S4. The relationship between anti-D titre and anti-D IgG1-galactosylation, bisection and fucosylation.