Abstract
Leukocytes are unmatched migrators capable of traversing barriers and tissues of remarkably varied structural composition. An effective immune response relies on the ability of its constituent cells to infiltrate target sites. Yet, unwarranted mobilization of immune cells can lead to inflammatory diseases and tissue damage ranging in severity from mild to life-threatening. The efficacy and plasticity of leukocyte migration is driven by the precise spatiotemporal regulation of the actin cytoskeleton. The small GTPases of the Rho family (Rho-GTPases), and their immediate downstream effector kinases, are key regulators of cellular actomyosin dynamics and are therefore considered prime pharmacological targets for stemming leukocyte motility in inflammatory disorders. This review describes advances in the development of small-molecule inhibitors aimed at modulating the Rho-GTPase-centric regulatory pathways governing motility, many of which stem from studies of cancer invasiveness. These inhibitors promise the advent of novel treatment options with high selectivity and potency against immune-mediated pathologies.
Linked Articles
This article is part of a themed section on Cytoskeleton, Extracellular Matrix, Cell Migration, Wound Healing and Related Topics. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-24
Introduction
Tissue injury triggers the rapid and transient release of soluble molecules that result in leukocyte homing to the site of injury. This process of immune cell recruitment in response to damage is termed inflammation. In an appropriate immune response, efficient pathogen elimination and the removal of antigenic material are achieved through transient non-destructive inflammation. However, antigen persistence may lead to chronic inflammation, characterized by tissue remodelling, destruction and defective healing (Ariel and Timor, 2013). Leukocyte motility and recruitment are at the heart of the inflammatory response. For this reason, targeting leukocyte migration constitutes an important treatment strategy for curbing immune responses. The selective modulation of immune trafficking in the treatment of pathologies has been successful in dampening excessive inflammation (autoimmunity and diseases associated with chronic inflammation; Mackay, 2008; Griffith and Luster, 2013; Di Gennaro and Haeggstrom, 2014) or boosting the host immune response (cancer and immune-deficiency disorders; Mellman et al., 2011; Restifo et al., 2012).
The pivotal role of immune cell migration is highlighted by the existence of many different disorders caused by defective immune cell mobilization. Immune-mediated inflammatory disorders are a heterogeneous group of diseases characterized by aberrant cytokine production and unresolved inflammation resulting from unwarranted immune responses (Williams and Meyers, 2002). Among these diseases are autoimmune conditions and inflammatory allergic disorders. Some examples of autoimmune diseases are autoimmune hepatitis, type I diabetes mellitus, rheumatoid arthritis (RA), Sjögren's syndrome, systemic lupus erythematous, multiple sclerosis (MS), psoriasis and inflammatory bowel disease (Crohn's disease and ulcerative colitis) (Beyaert et al., 2013). Examples of inflammatory allergic disorders include bronchial asthma, atopic dermatitis, rhinitis and food allergies. In autoimmune diseases, the immune system reacts against ‘self’, resulting in tissue destruction and eventual loss of function. The causes that underlie autoimmunity are still largely ignored, but there is evidence that autoimmune disorders arise as a combination of genetic makeup and unresolved inflammation (Atassi and Casali, 2008).
Similarly to autoimmune diseases, inflammatory allergic disorders are hyperactivity reactions. In conditions such as asthma and atopic dermatitis, however, the immune response is not directed against self, but against exogenous antigens that would be tolerated in a non-pathological context (Galli et al., 2008). Another significant inflammatory condition is the immune reaction associated with transplant allograft rejection. Unlike autoimmunity, transplant rejection is triggered by an acute challenge to an otherwise homeostatic immune system (Hautz et al., 2010). Animal models of autoimmunity, transplantation and allergic inflammation have been invaluable in the study of these complex conditions. However, the lack of understanding of what drives the chronicity of these diseases has been a considerable obstacle in translating successful treatment strategies from animal models to human disease (Feldmann and Steinman, 2005; Gaston, 2011; Beyaert et al., 2013).
One of the main strategies in the treatment of inflammatory disorders has been to impair immune cell recruitment by: (i) blocking adhesion to the endothelium and extravasation into the target tissue; (ii) modulating leukocyte influx and retention by targeting chemo-attractant receptors (Mackay, 2008); and (iii) curbing immune cell migration within the interstitium and target tissue (Friedl and Weigelin, 2008).
This review will focus on the emerging therapeutic potential of small-molecule inhibitors (SMIs) against Rho-GTPases in targeting the migratory capacity of immune cells in inflammatory disorders.
Leukocyte migration in the immune response
Leukocytes include both innate (granulocytes, monocytes, macrophages, dendritic cells, NK cells and others) and adaptive immune cells (B and T lymphocytes). All leukocytes are generated in the bone marrow, from where they are released into the vasculature. They then leave the bloodstream for tissue surveillance and exertion of effector functions. Some leukocytes then exit organs through lymphatic venules and eventually re-enter the blood stream. The requirement for such shuttling implies that leukocytes need to move effectively in a wide variety of tissue environments and be able to penetrate the physical barriers of endothelial walls and extracellular matrices. Leukocytes are uniquely endowed with the ability to navigate almost any tissue environment in the body by virtue of their aptitude to readily switch between different migration modes to overcome tissue-specific obstructions, and likely their capacity to survive and migrate effectively without adhesion signals (Ivetic and Ridley, 2004; Friedl and Weigelin, 2008).
Leukocyte traffic from the bloodstream through the endothelium into target tissues involves a multistep process that includes: (i) tethering and rolling on the endothelial surface; (ii) crawling; (iii) firm adhesion; (iv) transmigration (diapedesis) and (v) interstitial migration (reviewed in Ley et al., 2007; Muller, 2011). After extravasating and traversing the interstitial space, leukocytes arrive at target sites. The ability of leukocytes to move efficiently within the extravascular compartment is fundamental to tissue surveillance and pathogen elimination. For instance, the anti-tumour activity of effector T-cells has been shown to depend on their ability to migrate and navigate effectively within the tumour (Mrass et al., 2006; 2008). Each step of leukocyte migration relies on a well-coordinated sequence of signalling events that exert a tight spatiotemporal regulation of cytoskeletal dynamics (Renkawitz and Sixt, 2010). The small GTPases of the Rho family are fundamental cytoskeletal regulators in immune cell migration (Ivetic and Ridley, 2004).
The cytoskeleton and Rho-GTPases in immune cell migration
Leukocyte migration is, as any form of animal cell migration, mediated by polarized shape changes and mechanical interactions with the extracellular environment, driven by the cytoskeleton and associated proteins. The cytoskeleton is a dynamic scaffold of protein filaments pervading the cellular cytoplasm. Not only does it confer rigidity and structure to the cell, but it is also instrumental for intracellular transport and subcellular organization. Its components are also notably responsible for driving cell movement and cell division. The cytoskeleton is composed of three different classes of protein filaments, each assembled from non-covalently bound protein monomers: microfilaments (F-actin), microtubules and intermediate filaments. The main cytoskeletal components responsible for migratory force generation are F-actin and myosin II motor proteins (collectively called actomyosin), which form a contractile and cross-linked network that subtends the plasma membrane, namely the cell cortex (Salbreux et al., 2012).
Filamentous actin
Actin filaments have a diameter of 7–9 nm and are right-handed double-helical assemblies of parallel protofilaments, which in turn are composed of linear homopolymers of actin monomers (G-actin). G-actin is a highly conserved globular yet polar protein, consisting of four domains between which a cleft can bind to either an ATP or ADP nucleotide. The so-called nucleotide state of the actin monomer, determined by whether it is bound to an ATP or ADP, confers upon G-actin dynamic properties that affect its associative characteristics (Pollard and Cooper, 2009). The actin monomers are aligned in a head-to-tail fashion, endowing microfilaments with an inherent polarity, as the extremities of the filaments therefore expose opposite ends of the terminal G-actins. Actin filaments are straight and rigid, and have persistence lengths in the order of ∼15 μm (Howard, 2001). Aside from the nucleotide state of the available G-actin pool, the extent and ultrastructure of the polymerized actin network in a cell is dependent on nucleators (e.g. Arp2/3 complex and formins), G-actin-binding proteins (e.g. profilin), severing proteins (e.g. cofilin) and nucleation-promoting factors (NPFs) [e.g. Scar/WAVE and Wiskott–Aldrich syndrome protein (WASp)/N-WASp].
Myosin
Myosins are members of the superfamily of actin-specific molecular motors. Non-muscle myosin II (henceforth, simply myosin) is the main driving force of the cytoskeleton in immune cells, and is characterized by its ability to convert ATP hydrolysis into mechanical work. Myosin is a hexameric protein, composed of three pairs of peptides that associate to form a myosin monomer: two long heavy chains (MHC), two regulatory light chains (MRLC) and two essential light chains (ELC). Myosin ATPase activity is highly dependent on phosphorylation of MRLC, and to some extent the MHC. The main kinase responsible for myosin activation through phosphorylation is the myosin light-chain kinase (MLCK). Due to their association into anti-parallel bundles, myosins can ‘walk’ along actin filaments in a polarized fashion and exert forces on the F-actin network by pulling on filaments with respect to one another (Vicente-Manzanares et al., 2009).
Cytoskeletal regulation by Rho-GTPases
The forces generated by the actomyosin machinery can be either protrusive or contractile and give rise to various types of cellular protrusions that facilitate cell motility (Charras and Paluch, 2008; Vicente-Manzanares et al., 2009; Bergert et al., 2012). Regulation of the dynamics of the actomyosin cortex affords cells the ability to migrate using a specific method of locomotion, traditionally categorized into mesenchymal and amoeboid modes (Yoshida and Soldati, 2006; Keren et al., 2008). Mesenchymal migration relies on the generation of lamellipodia at the leading edge of the cell, where polymerization of filamentous actin generates protrusive forces that extend the plasma membrane into relatively stable thin sheets, and is prevalent in rigid environments that are amenable to strong adhesion. In contrast, amoeboid migration is typified by the adoption of a rounded morphology and a highly dynamic leading edge with frequent extensions and retractions of protrusions. Polarization of actomyosin dynamics is essential for the concerted propulsion of the cell in a given direction. Cellular F-actin and myosin have a myriad interaction partners that play important roles in the regulation of the actomyosin cytoskeleton. For a proper execution of convoluted processes such as immune cell locomotion, the polymerization dynamics of actin, the organization of actin networks and myosin contractility all need to be tightly regulated.
Complex and as yet incompletely mapped signalling pathways are responsible for the precise spatiotemporal regulation of cellular actomyosin. The central components of these pathways are small GTPases of the Rho family (Rho-GTPases), notably Rho, Rac and Cdc42 (Hall, 2012). Rho-GTPases govern both contractile and expansive forces required for cell polarization and translocation, and are intricately linked to the overarching regulation of the actomyosin cytoskeleton. Rho-GTPases are found in two inter-convertible states, namely an active GTP-bound state and an inactive GDP-bound state, and can thus function as molecular switches. Exchanges in the GTP- and GDP-state cycle are regulated by guanine nucleotide dissociation inhibitors (GDIs), GTPase-activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs) (Figure 1; Spiering and Hodgson, 2011). GDIs associate with GDP-bound GTPases and prevent these from membrane-association, in effect sequestering them in an inactive sate. GEFs catalyse GDP-to-GTP nucleotide exchange and thereby activate Rho-GTPases. GAPs promote GTP hydrolysis, leading to Rho-GTPase inactivation. In their active state, Rho-GTPases interact with over 60 different target effectors (Etienne-Manneville et al., 2002). Despite the number of potential binding partners, active Rho-GTPases interact with specific effectors at any one time. Interactions between Rho-GTPases and their effectors trigger distinct changes in the actomyosin cytoskeleton. The Rho-GTPases can mediate signalling cascades that result in actin polymerization and network formation, as well as regulate myosin contractility. A model of the intricate pathways downstream of Rho-GTPase activation and the degree of crosstalk between the different Rho-GTPases is illustrated in Figure 2.
Figure 1.
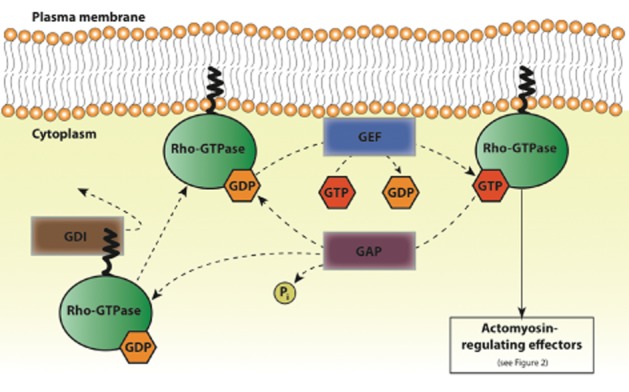
Rho-GTPase activation and inactivation by GEFs, GAPs and GDIs. Schematic of the activation cycle of Rho-GTPases (such as Rho, Rac and Cdc42), by GEFs, GAPs and GDIs. GDIs associate with GDP-bound Rho-GTPases and sequester them in an inactive state. Dissociation of the GDI from the Rho-GTPase allows for its anchoring to the plasma membrane via a prenyl group. GEFs catalyse GDP to GTP exchange and thus activate Rho-GTPases for interaction with downstream actomyosin-regulating effectors (detailed in Figure 2). GAPs stimulate the hydrolysis of GTP into GDP and phosphate (Pi) and thereby contribute to Rho-GTPase inactivation. GDP-bound Rho-GTPases are then again sequestered by GDIs or reactivated by GEFs.
Figure 2.
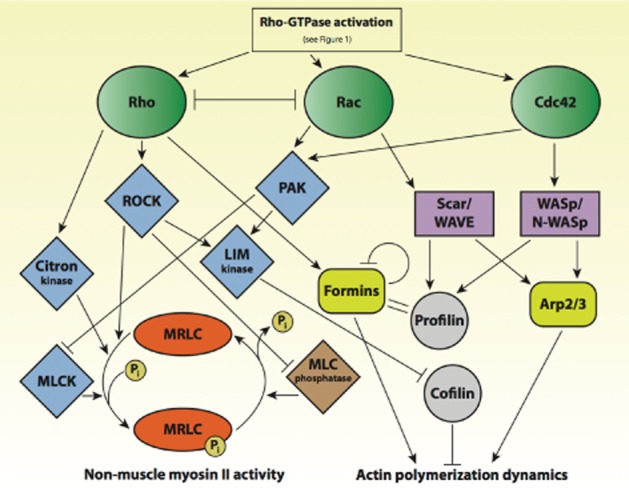
Regulation of cellular actomyosin dynamics by Rho-GTPases. Simplified regulatory pathway highlighting the central role of the Rho-GTPases Rho, Rac and Cdc42 in controlling actomyosin dynamics in cells. Rho and Rac inhibit one another and influence myosin contractility through effector kinases such as ROCK, PAK, Citron- and Lim kinase. MLCK phosphorylates MRLC, thereby promoting myosin activity. ROCK further inhibits myosin light-chain phosphatase, thus doubly contributing to myosin activation. Rho also activates formins, which together with G-actin-binding profilin, promotes actin polymerization. The nucleation-promoting factors Scar/WAVE and WASp/N-WASp are activated by both Rac and Cdc42, and induce actin polymerization either via Arp2/3 complex (branched actin networks) or via profilin-activation (unbranched actin networks). Rho affects actin polymerization dynamics via LIM kinase-mediated inhibition of cofilin, which as an actin-severing protein influences the balance between G- and F-actins.
Rho (RhoA and RhoC) is mainly involved in the activation of myosin, mediated through the phosphorylation of myosin regulatory light chain (MRLC) via Rho-associated coiled-coil-forming kinase (ROCK), MLCK and Citron kinase. ROCK additionally inhibits myosin light-chain phosphatase, thus doubly contributing to myosin activation. Rho also activates the formin group of actin nucleators, responsible for rapidly polymerizing unbranched F-actin. Finally, Rho contributes to actin polymerization and the regulation of the network ultrastructure by inhibiting cofilin via LIM kinase (LIM domain kinases 1 and 2). Rac and Rho inhibit one another. Both Rac and Cdc42 activate p21-activated kinases (PAK) and thereby inhibit MLCK. Through PAK, they also activate LIM kinase, which inhibits cofilin. Rac and Cdc42 are mainly involved in the activation of both branched and unbranched actin network formation. Growth of branched actin involves Arp2/3-complex activation, mediated in the case of Rac via the NPFs Scar/WAVE, and in the case of Cdc42 via the NPFs WASp/N-WASp. The polymerization of unbranched actin is promoted via the interaction of the aforementioned NPFs with profilin and formins (Raftopoulou et al., 2004; Lee and Dominguez, 2010; Pertz, 2010).
Rho-GTPases and cell motility
The selective activation of Rho-GTPases and their effectors results in the precise regulation of actin networks required to form the varying structures involved in cell motility, notably protrusions such as lamellipodia, filopodia and blebs (Pollard and Borisy, 2003; Charras et al., 2006). Simultaneous but spatially segregated activation of Rho-GTPases can result in the polarization of cells, such as required for sustained motility. For instance, during mesenchymal migration, cells activate Rho at the rear of the cell, thereby favouring contractile network formation. Conversely, Rac and Cdc42 are activated at the leading edge, favouring the rapid actin polymerization dynamics of lamellipodia (Burridge and Wennerberg, 2004). The polarized formation of Rac-dependent lamellipodia at the leading edge of motile cells is the hallmark of mesenchymal migration (Bergert et al., 2012). In contrast, RhoA- and myosin-dependent contraction of the cell rear and expansive protrusive activity at the leading edge gives rise to amoeboid motility (Lammermann and Sixt, 2009). Amoeboid motility is often observed in fast-moving cells such as leukocytes, which are thought to be able to undergo a blebbing migration mode (Charras and Paluch, 2008; Fackler and Grosse, 2008; Lammermann et al., 2008).
Crucially, aberrant regulation of Rho-GTPases and consequent migratory dysfunction in leukocytes has been implicated in numerous pathologies, notably inflammatory diseases (Hall, 2012) and cancers (Vega and Ridley, 2008). Because of their central role in regulating cytoskeletal dynamics and cell migration, Rho-GTPases have emerged as highly attractive pharmacological targets, particularly in inflammatory disorders where blocking tissue infiltration by leukocytes can be both prophylactic and therapeutic (Zhao and Pothoulakis, 2003).
Inhibiting Rho-GTPases in immune cell migration
A precise spatiotemporal regulation of Rho-GTPases and their effectors is vital for cell migration, division, adhesion, endo- and exocytosis, polarization, as well as general cellular homeostasis. Because of their key role in such a wide variety of cellular processes, misregulation of Rho-GTPases is associated with a range of diseases characterized by aberrant cytoskeletal dynamics, notably developmental defects, immunodeficiencies and tumour metastasis (Ligeti et al., 2012). As such, Rho-GTPases and their immediate downstream effector kinases are prime targets for pharmacological modulation of the cytoskeletal dynamics of cells. By disrupting the cytoskeleton through Rho-GTPase modulation, both cell migration and tissue integrity can be influenced.
The vast majority of pharmaceutical developments targeting the cytoskeleton, and the Rho-GTPases specifically, have thus far focused on inhibiting proliferation in various malignancies. As the main regulators of the actin cytoskeleton, Rho-GTPases do in fact play pivotal roles in cell division. However, Rho-GTPases are even more prominent determinants of cell migration. Of late, efforts have, therefore, turned to curbing the migratory capacity of cancer cells, and of relevance to this review, immune cells in the context of inflammatory diseases, via inhibition of Rho-GTPases. Table 1 lists all the SMIs covered in the following sections.
Table 1.
List and status of SMIs of Rho-GTPases
| Rho-GTPase pathway | Target molecule | Name of compound | Therapeutic status | Model system/pathology | References |
|---|---|---|---|---|---|
| Rho | ROCK | Fasudil (HA-1077) | Approved | Hypertension/inflammation | Takayasu et al., 1986; Satoh et al., 1999; Masumoto et al., 2002; Fujita et al., 2010; Ding et al., 2011; Kojonazarov et al., 2012; Li et al., 2012a; Liu et al., 2013 |
| Dimethylfasudil (H-1152) | In vivo | Hypertension models/inflammation | Sasaki et al., 2002; Warfel and D'Agnillo, 2011; Breyer et al., 2012; O et al., 2012 | ||
| Y-27632 | In vivo | Hypertension/inflammation | Uehata et al., 1997; Kawaguchi et al., 2004; Tasaka et al., 2005; Prakash et al., 2008; Schaafsma et al., 2008; Slotta et al., 2008; Chen et al., 2011 | ||
| WF-536 | In vivo | Metastatic cancer models | Nakajima et al., 2003a,b, | ||
| Y-39983 (RKI-983) | In vivo | Glaucoma | Tokushige et al., 2007 | ||
| SNJ-1656 | Phase II clinical trial | Glaucoma | Tanihara et al., 2008 | ||
| SB-772077-B | In vivo | Hypertension models | Doe et al., 2007; Dhaliwal et al., 2009 | ||
| GSK269962A | In vivo | Hypertension models | Doe et al., 2007 | ||
| K-115 | Phase II clinical trial | Glaucoma | Fang et al., 2010; Tanihara et al., 2013 | ||
| SR-3677 | Ex vivo | Glaucoma | Feng et al., 2008 | ||
| AR-12286 | Phase II clinical trial | Glaucoma | Williams et al., 2011 | ||
| SAR407899 | Phase II clinical trial | Erectile dysfunction | Lohn et al., 2009 | ||
| PT-262 | In vitro | Lung carcinoma cells | Tsai et al., 2011 | ||
| Azaindole 1 | In vitro | Hypertension models | Kast et al., 2007 | ||
| Rho | Rhosin | In vitro | Breast cancer cells | Shang et al., 2012 | |
| Y16 | In vitro | Breast cancer cells | Shang et al., 2013 | ||
| CCG-1423 | In vitro | Prostate cancer cells | Evelyn et al., 2007; 2010 | ||
| Rho/ROCK | Rhodblocks | In vitro | Cell division Drosophila cells | Castoreno et al., 2010 | |
| Vav | CHS-111 | In vitro | Neutrophils | Chang et al., 2009; 2011 | |
| Rac | Rac | NSC23766 | In vitro/in vivo | Prostate cancer invasion/inflammation | Gao et al., 2004; Akbar et al., 2006; Vockel and Vestweber, 2013 |
| Rac/Cdc42 | EHop-016 | In vitro | Breast cancer cells | Montalvo-Ortiz et al., 2012 | |
| Rac | Compounds 7, 11 | In vitro | Breast cancer cells | Hernandez et al., 2010 | |
| EHT-1864 | In vitro/in vivo | Fibroblasts/Alzheimer's disease model | Desire et al., 2005; Shutes et al., 2007; Onesto et al., 2008 | ||
| Cdc42 | Cdc42 | Secramine | In vitro | Primary smooth muscle cells/renal epithelial cells | Pelish et al., 2006; Xu et al., 2006; Lengfeld et al., 2012 |
| CID29950007 | In vitro | Ovarian cancer/lymphoma cell lines | Hong et al., 2013 | ||
| CID44216842 | In vitro | Ovarian cancer/lymphoma cell lines | Hong et al., 2013 | ||
| ZCL278 | In vitro | Metastatic prostate cancer cells | Friesland et al., 2013 | ||
| AZA1 | In vivo | Prostate cancer | Zins et al., 2013 | ||
| N-WASp | 187-1 | In vitro | Primary synovial isolates | Peterson et al., 2001; Connolly et al., 2011 | |
| Wiskostatin | In vitro | Melanoma and T-cells | Peterson et al., 2004; Haller et al., 2006; Kovacs et al., 2006 | ||
| PAK | K252 | In vivo | Neurological disease models | Kaneko et al., 1997 | |
| KT-D606 | In vitro | Immortalized fibroblasts | Nheu et al., 2002 | ||
| CEP-1347 | In vitro | Immortalized fibroblasts | Nheu et al., 2002 | ||
| OSU-03012 | In vitro | Thyroid cancer cells | Porchia et al., 2007 | ||
| IPA-3 | In vitro | HEK-293 cells | Deacon et al., 2008 | ||
| Λ-FL172/Λ-FL411 | In vitro | Schwannoma cancer cells | Maksimoska et al., 2008 | ||
| PF-3758309 | In vivo | Tumour xenograft models | Murray et al., 2010 | ||
| LCH-7749944 | In vitro | Gastric cancer cells | Zhang et al., 2012 | ||
| FRAX597 | In vivo | Schwannoma tumour model/neurofibromatosis | Licciulli et al., 2013 | ||
| All | Rho/Rac/Cdc42 | Berberine | In vitro | Nasopharyngeal cancer cells | Tsang et al., 2009 |
The table summarizes the SMIs targeting Rho-GTPases discussed in this review, specifying the Rho-GTPase pathway and target molecule. The therapeutic status of the compound is indicated as either being used in vitro, in vivo, in clinical trials or as having been approved for clinical use. The model system or pathology in which the drug is being applied is given, along with pertinent references.
Rho and its kinases
Notwithstanding the central role of small GTPases of the Rho family in key cellular processes and in a wide variety of pathologies, attempts to develop pharmacological agents modulating the relevant biochemical pathways have thus far been largely unproductive. This task has been found to be particularly challenging because: (i) small GTPases are generally globular in shape with limited druggable pockets exposed; (ii) the high affinity with which GTP binds to its exposed allosteric site hinders out-competition by SMIs; and (iii) the diversity and complexity of the downstream pathways governed by single Rho-GTPases make it difficult to target a specific cellular process. Thus, as the ATP-binding affinity of kinases is much lower than that of the GTP-binding affinity of RhoA, the development of SMIs against kinases such as ROCK has been much more successful (Vigil and Der, 2013). ROCK misregulation has been shown to significantly contribute to the pathophysiology of cancers, hypertension, glaucoma and most relevant to this review, inflammatory diseases such as asthma, diabetes, RA and MS (LoGrasso and Feng, 2009). Therefore, successful pharmacological treatments of inflammatory disorders have tended to target Rho kinases rather than RhoA itself.
Rho kinase (ROCK)
A major class of SMIs targeting the pathways of Rho-GTPases are isoquinoline-based compounds. Fasudil, historically referred to as HA-1077, is the most extensively studied isoquinolinesulfonamide targeting ROCK. It was originally developed in the 1980s as an intracellular calcium ion antagonist and noted for its vasoregulatory properties in a canine model of cerebral vasospasm (Hidaka et al., 1984; Takayasu et al., 1986). It was later discovered to be an effective Ser/Thre kinase inhibitor with a wide spectrum of kinase targets, including ROCK (Uehata et al., 1997; Nagumo et al., 2000). Fasudil binds competitively to the ribose region in the ATP-binding motif of ROCK, thereby inhibiting ROCK-mediated actomyosin dynamics (Nagumo et al., 2000). Fasudil was trialled and later approved for the treatment of cerebral vasospasms (Masumoto et al., 2002; Olson, 2008; Suzuki et al., 2008; Velat et al., 2011), as well as pulmonary hypertension (Fukumoto et al., 2005; Ishikura et al., 2006; Fujita et al., 2010; Kojonazarov et al., 2012) in human patients. Various animal models of cancer have revealed that fasudil can block tumour invasiveness and metastasis (Ying et al., 2006). Importantly, fasudil has been successfully used to treat inflammation by interfering with leukocyte recruitment to sites of inflammation. Fasudil was shown to inhibit neutrophil infiltration in a cerebral model of infarction (Satoh et al., 1999). Furthermore, in both mouse models and human cells isolated from RA patients, fasudil treatment reduced synovial inflammation and production of pro-inflammatory cytokines (He et al., 2008). In diabetes models, fasudil decreased monocyte adhesion to endothelial cells and lowered levels of leukocyte-associated chemoattractants (Li et al., 2012a). Fasudil has demonstrated anti-inflammatory effects via ROCK-mediated modification of the endothelium. It inhibited neutrophil tissue infiltration in mouse models of acute lung injury and systemic inflammation (Li et al., 2010; Ding et al., 2011). In experimental autoimmune encephalomyelitis, fasudil treatment resulted in decreased immune cell infiltration and tissue destruction, especially during disease onset (Sun et al., 2006; Liu et al., 2013). The anti-inflammatory effects of fasudil can result from either changes in endothelial permeability and modifications of cytokine production that facilitate leukocyte transmigration (Tasaka et al., 2005; Li et al., 2010), and/or from the alteration of cytoskeletal dynamics in the leukocytes themselves (Heasman et al., 2010). Fasudil-based treatments have a long history of safe clinical application with relatively mild side effects, and are thus emerging as a promising therapeutic recourse for inflammatory diseases. However, fasudil is not specific for Rho kinases and has been shown to target other kinases involved in cytoskeleton remodelling (Anastassiadis et al., 2011). This may have both beneficial effects in overcoming inherent redundancies in cytoskeletal regulation as well as adverse consequences in terms of specificity.
A derivative of fasudil, discovered in 2002, dimethylfasudil or H-1152, is a more selective inhibitor of ROCK (Sasaki et al., 2002). It has been shown to possess endothelium-modulating effects similar to those of fasudil (Breyer et al., 2012; O et al., 2012; Wang et al., 2012) and could, therefore, potentially be further developed for use in manipulating leukocyte extravasation. It was recently also suggested as a promising agent in maintaining the integrity of the endothelial barrier upon lethal anthrax toxin challenge (Warfel and D'Agnillo, 2011).
Y-27632, an isoquinoline-based synthetic pyridine derivative, is another very extensively used ROCK inhibitor, both in vitro as an invaluable tool in the study of cytoskeletal regulation and in in vivo models of inflammatory disease. Y-27632, first developed as a potent smooth muscle relaxant to relieve hypertension, selectively targets ROCK by competitive inhibition of its ATP-binding pocket through interactions in two distinct regions (Uehata et al., 1997; Yamaguchi et al., 2006). This drug has been successfully employed in various animal models to curb leukocyte migration and reduce inflammation. In ischaemia/reperfusion and liver injury models, treatment with Y-27632 reduced inflammation, macrophage infiltration and tissue damage (Prakash et al., 2008), as well as reducing liver damage by suppressing leukocyte infiltration and inflammatory cytokines (Kawaguchi et al., 2004; Thorlacius et al., 2006; Slotta et al., 2008). Y-27632 blocked renal interstitial inflammation by dampening monocyte infiltration in chronic allograph nephropathy in rats (Liu et al., 2009). In animal models of asthma, Y-27632 inhalation resolved symptoms and reduced the number of eosinophils, macrophages and neutrophils recovered by bronchoalveolar lavage (Henry et al., 2005; Schaafsma et al., 2008). In a study of acute lung injury, Y-27632 treatment attenuated lung oedema and neutrophil infiltration of the lung parenchyma, probably as a result of ROCK-inhibition-mediated cytoskeletal rearrangement of the endothelium (Tasaka et al., 2005). Y-27632 has also successfully inhibited the invasive migration of carcinoma cells in vitro (Chen et al., 2011). Recently, however, Y-27632 has been reported to inhibit cytoskeleton-associated kinases other than ROCK, and this has prompted new efforts for the development of more selective ROCK inhibitors (Anastassiadis et al., 2011). Similar to fasudil, the anti-inflammatory effects of Y-27632 probably result from a combination of both endothelial modulation and migratory inhibition of immune cells.
Derivatives of Y-27632 similarly blocking ROCK-ATP binding have been developed for in vivo use with varying success. WF-536 was tested as an inhibitor of invasive tumour cell migration, and was found to reduce pulmonary metastasis in metastatic mouse models without any observable associated toxicity (Nakajima et al., 2003a,b,). Y-39983, also known as RKI-983, is structurally similar to Y-27632, but 30 times more potent in inhibiting ROCK activity (Tokushige et al., 2007). Topical administration of this drug in mice and monkeys resulted in lower intraocular pressure with few side effects. SNJ-1656, an ophthalmic solution of Y-39983, proved safe and effective in the treatment of glaucoma in human patients (Tanihara et al., 2008), and is currently in phase II clinical trials in Japan for the treatment of glaucoma, by Senju and Novartis Pharmaceuticals. These latest ROCK inhibitors show great promise for their use in treating a wider range of inflammatory disorders in the near future.
Given the promising results of the application of fasudil and Y-27632 and its derivatives in the clinic, great efforts have been invested in the discovery of other more powerful and more specific ROCK inhibitors. There has been a recent burst in the identification of different structures that are proving more potent and selective than their predecessors. Examples of these new classes of ATP-competitive ROCK inhibitors are the aminofurazan compounds, tetrahydroisoquinoline-based amides, isoquinolinesulfonamide K-115 and pyridine-thyazol compounds such as SR-3677. Aminofurazan compounds such as SB-772077-B and GSK269962A, have shown promising effects on various animal models of hypertension (Doe et al., 2007; Stavenger et al., 2007; Dhaliwal et al., 2009). Tetrahydroisoquinoline-based amides, K-115 (Fang et al., 2010; Tanihara et al., 2013) and SR-3677 (Feng et al., 2008), have shown encouraging results in animal models of glaucoma. In fact, K-115, from Kowa Pharmaceutical, is currently in phase II clinical trials for the treatment of glaucoma in human patients. Other ROCK inhibitors currently in clinical trials include Aerie Pharmaceuticals' AR-12286 (Williams et al., 2011) in phase II also for the treatment of glaucoma, and Sanofi's SAR407899 (Lohn et al., 2009) in phase II for the treatment of erectile dysfunction.
Additional ROCK inhibitors in early stages of development are quinolinedione-based compounds such as the PT-262 (Tsai et al., 2011), and azaindole 1, a potent orally active ROCK inhibitor that shows promise in the treatment of hypertension (Kast et al., 2007).
RhoA
RhoA directly influences contractile force generation via myosin-driven motor activity as well as actin polymerization kinetics.
A Rho-specific inhibitor named rhosin, was recently identified by rational design and virtual screening using published structures of protein : protein interactions of RhoA and its GEF leukaemia-associated RhoGEF (LARG) (Shang et al., 2012). Shang et al. found that rhosin specifically inhibits RhoA and RhoC interaction with GEFs. Rhosin therefore prevents RhoA activation and downstream MLC phosphorylation, filamentous actin formation and focal adhesion assembly without perturbing endogenous Cdc42 or Rac signalling. Rhosin was evaluated in vitro in a variety of cancer cell models, including physiological three-dimensional mammospheres, and was found to readily inhibit the motility and invasiveness of breast cancer cells in a dose-dependent manner (Shang et al., 2012). A major advantage of rhosin as a cytoskeletal inhibitor is its specificity for RhoA and RhoC, both prominent regulators of actin cytoskeleton dynamics during immune cell migration, and negligible interaction with other Rho-GTPases. However, rhosin binds RhoA with low affinity, and μM concentrations are therefore required to achieve adequate inhibition.
More recently, the same authors reported a novel compound, Y16, which inhibits RhoA by specifically recognizing the opposite interactive surfaces between RhoA and its GEF LARG to that of rhosin (Shang et al., 2013). Thus, Y16 and rhosin were considered amenable to being used in combination. Although Y16 treatment on its own did not show a significant improvement over rhosin in in vitro experiments, their combined use had a clear synergistic effect on migration, invasiveness and proliferation in a three-dimensional breast cancer model (Shang et al., 2013). Such a combined application of Y16 and rhosin provides added potency and specificity for the inhibition of RhoA activation in cells. Furthermore, it proves the efficacy of a strategy based on the rational design of combination inhibitors directly targeting Rho-GTPases, generally considered of poor ‘druggability’. It will be interesting to see whether the in vivo combination treatment of Y16 and rhosin, or other further optimized drug pairs, is successfully applicable in inflammatory disease models.
In another approach, a phenotypic screen using cultured Drosophila cells pre-sensitized by partial knockdown of RhoA, identified SMIs that enhanced the knockdown phenotype (Castoreno et al., 2010). The phenotypic criterion evaluated in the screen was the ability of the cells to complete cytokinesis following treatment, which would therefore be relevant to the development of mitogenic cancer drugs. This type of phenotypic screening approach sought to uncover inhibitors of the RhoA pathway in general, including components upstream and downstream of RhoA. Eight compounds exhibited a phenotype and were collectively called Rhodblocks, numbered from 1 to 8. Rhodblocks 1, 3 and 6 were found to be potent inhibitors of the Rho pathway, with Rhodblock 6 determined to specifically attenuate Rho kinase activity (Castoreno et al., 2010). Although the exact mechanisms of action of these newly discovered inhibitors remain to be elucidated, they hold promise for use as cell migration modulators because of their specific inhibition of cytoskeletal regulation via the Rho pathway.
In addition to its prominent role in actin cytoskeleton regulation, RhoA is also involved with the regulation of gene transcription mediated via the serum response factor and the oncogene megakaryoblastic leukaemia 1. Evelyn and colleagues took advantage of this additional RhoA function in designing a luciferase-based high-throughput screen for specific RhoA pathway inhibitors (Evelyn et al., 2007). They identified CCG-1423 as an inhibitor of RhoA-mediated transcription and, following compound optimization and in vitro Matrigel invasion experiments using prostate cancer cells, further showed its effectiveness in inhibiting invasive migration (Evelyn et al., 2010). CCG-1423 has successfully been used in mice models; however, its potential for inhibition of immune cell migration remains to be tested.
Another prospective modulator of immune cell migration via RhoA activity-inhibition is CHS-111, a novel compound described in Chang et al. (2009). CHS-111 was subsequently found to reduce levels of active GTP-bound RhoA by blocking the activation of the RhoA-specific GEF Vav. Consequently, CHS-111 impedes the recruitment of RhoA and ROCK to the plasma membrane and limits the phosphorylation of myosin light chain 2, as shown in stimulated rat neutrophils (Chang et al., 2011). CHS-111 inhibits neutrophil migration in a dose-dependent manner without affecting degranulation. It should be noted that CHS-111 was also found to inhibit Rac2 activation in stimulated neutrophils (Chang et al., 2009), which might help overcome the inherent redundancies in cytoskeletal regulation and may, therefore, prove especially efficacious in curbing leukocyte migration. However, such lack of specificity could also lead to undesired effects. In vivo testing of this new compound is still outstanding.
Rac
Rac GTPases are pleiotropic modulators of a variety of important cellular processes, including actin polymerization dynamics and the formation of migratory protrusions such as lamellipodia. Rac regulates actin polymerization through PAK- and LIM kinase-mediated inhibition of cofilin, as well as through Arp2/3 complex branched actin nucleation. Misregulation of Rac activity has been implicated in various pathologies, including invasive cancers and immunodeficiency.
NSC23766, a first generation of Rac-specific SMI, was identified in a computer-based virtual screen and was found to inhibit Rac activity by blocking Rac-GTP loading without affecting RhoA or Cdc42 (Gao et al., 2004). It was later established that NSC23766 prevented GEF-mediated activation (Akbar et al., 2006). In both in vitro and in vivo systems, it was shown that NSC23766 inhibited tumour cell transformation and invasion, lamellipodia formation and haematopoietic progenitor cell mobilization (Gao et al., 2004; Akbar et al., 2006). NSC23766 also inhibited platelet aggregation (Akbar et al., 2006). In an in vitro system, Vockel and Vestweber showed that adhesion of leukocytes to the endothelium triggers a signalling cascade that involves Rac1 activation for the dissociation of intercellular junctions within the endothelium (Vockel and Vestweber, 2013). Such endothelial ‘loosening’ facilitates efficient leukocyte transmigration, which is blocked by NSC23766. It will be interesting to see the outcome of the application of this novel drug in animal models of inflammatory disease.
A derivative of NSC23766, EHop-016, is a more potent and more effective SMI than its parent compound. It inhibits Rac1 and Rac3, but also Cdc42 by preventing GEF-mediated activation by competitive binding. EHop-016 specifically interferes with the interaction between Rac and its GEF Vav. In vitro EHop-016 treatment inhibited lamellipodia formation and directed migration in invasive metastatic breast cancer cells (Montalvo-Ortiz et al., 2012). Also related to NSC23766, Compounds 7 and 11 were generated by molecular docking,and were found to be two to three times more potent than NSC23766 (Hernandez et al., 2010). Similar to the related EHop-016, these compounds inhibited cell spreading and lamellipodia formation in metastatic breast cancer cells with no observable effect on normal epithelial cells. Importantly, the cytoskeletal alterations caused by these compounds were more dramatic than those observed in the presence of NSC23766 (Hernandez et al., 2010). Molecular docking and virtual screening also lead to the discovery of five new SMIs against Rac1 (as yet unnamed; Ferri et al., 2009). These compounds proved significantly more effective than the reference compound NSC23766 in inhibiting Rac1 and resulted in a very similar cytoskeletal phenotype.
Another interesting SMI, EHT-1864 (Desire et al., 2005) binds tightly to Rac1 (also to Rac2 and to a lesser extent Rac3) by blocking it in an inactive state, and thus inhibiting inherent Rac-mediated cytoskeletal changes. EHT-1864 was effective in reducing plaque formation and associated neuronal toxicity in a guinea pig model of Alzheimer's disease (Desire et al., 2005). Treatment of NIH-3T3 cells with EHT-1864, resulted in inhibition of Rac1-dependent lamellipodia formation and cellular transformation in vitro (Shutes et al., 2007; Onesto et al., 2008).
The evidently successful Rac-dependent inhibition of cytoskeletal rearrangements in a wide variety of cell types, including immune cells, highlights the potential therapeutic value of SMIs against Rac GTPases in the treatment of immune diseases.
Cdc42
The Rho-GTPase Cdc42 is one of the main cytoskeletal modulators. Cdc42 participates in a variety of cytoskeletal processes such as polarity establishment, migration, adhesion, membrane ruffling and intracellular trafficking (Cerione, 2004). Cdc42 is required for the formation of lamellipodia and filopodia. These cell protrusions are central to certain migration modes and are formed, via WASp, by Arp2/3 and formin-nucleated actin networks. Cdc42 is associated with carcinogenesis and metastasis, hence significant effort has been invested in the development of SMI targeting the Cdc42 signalling pathway. Defects in Cdc42 signalling are associated with various human diseases including neurodegenerative conditions (Auer et al., 2011), cancer and metastasis, and genetic disorders such as Fanconi anaemia (Stengel and Zheng, 2011). Notably, Fanconi anaemia is characterized by a variety of haematological abnormalities including defects in adhesion and homing of haematopoietic progenitors, a feature attributed, at least in part, to Cdc42 dysfunction (Zhang et al., 2008).
The first selective inhibitor of Cdc42 discovered was secramine. An analogue of the natural product galanthamine, it was found by high-throughput phenotypic screening for molecules that interfered with membrane trafficking (Pelish et al., 2006). Secramine prevents the activation and membrane availability of Cdc42 by stabilizing its interaction with GDIs. In vitro experiments revealed secramine to inhibit cell spreading (Xu et al., 2006) and to interfere with Golgi polarization during migration in a wound-healing assay (Pelish et al., 2006). Treatment of mouse primary smooth muscle cells with secramine inhibited secretion of collagen, suggesting that Cdc42 inhibition could be useful in the treatment of atherosclerotic plaque formation in cardiovascular diseases (Lengfeld et al., 2012). In platelets, secramine reduced integrin-mediated adhesion to collagen (Pula and Poole, 2008). CID29950007 (also ML141) and its related analogue CID44216842 are novel SMIs of Cdc42 that act by blocking nucleotide binding with no effect on RhoA or Rac (Surviladze et al., 2012; Hong et al., 2013). These compounds inhibited migration and filopodia formation in cultured fibroblasts, and chemotaxis in ovarian cancer cells. Importantly, in an in vitro model of leukocyte adhesion, CID29950007 blocked integrin binding to its endothelial receptor (vascular cell adhesion molecule 1) (Hong et al., 2013), suggesting the potential usefulness of this compound in immune cell trafficking. ZCL278, another novel selective SMI of Cdc42, was discovered by virtual screening and found to inhibit Cdc42 activity by directly blocking GEF binding (Friesland et al., 2013). This compound was tested in a variety of in vitro models and was shown to inhibit migration in prostate cancer cells and filopodia formation in fibroblasts and primary neurons (Friesland et al., 2013). A derivative of NSC23766, AZA1 was identified by virtual screening and found to inhibit Cdc42 as well as Rac1 by blocking GEF binding (Zins et al., 2013). In vitro use of AZA1 resulted in altered cytoskeletal dynamics, reduced migration, lamellipodia and filopodia formation in prostate cancer cells, while in vivo administration decreased tumour growth and increased survival in a mouse xenograft model of prostate cancer (Zins et al., 2013). Given that most of the Cdc42 inhibitors mentioned earlier act upon different molecular targets and via differing mechanisms, it will be interesting to see whether they are amenable to safe use in combination and whether therapeutic potency can thereby be increased. Berberine, a natural isoquinoline alkaloid, is an inhibitor of all three Rho-GTPases. Its active ingredient, Coptidis rhizome, was traditionally used in Chinese medicine. In in vitro studies using various cancer cell types, low doses of berberine treatment inhibited migration and invasion, while higher doses resulted in mitotic arrest (Tsang et al., 2009).
Wiskott–Aldrich syndrome proteins
In an attempt to develop novel pathway inhibitors, efforts have also been focused on targeting direct downstream effectors of Cdc42 such as WASp, including the ubiquitously expressed N-WASp. WAS family proteins are key NPFs of the Arp2/3 complex. WASp can bind both the Arp2/3 complex and actin monomers and have two crucial stimulating roles in Arp2/3-driven actin polymerization within cells (Pollard and Cooper, 2009). On the one hand, WASp induce activation of conformational changes in the Arp2/3 complex itself, on the other hand, WASp help recruit, via intermediary binding, actin monomers to the Arp2/3 complex (Chesarone and Goode, 2009). Importantly, haematopoietic WASp, is a key multifunctional cytoskeletal regulator in immune cells with roles in immune-target cell interactions, apoptosis, signalling and leukocyte migration. The significance of this NPF in immunity is illustrated by its name WASp, from its involvement in Wiskott–Aldrich syndrome, a group of immunological disorders caused by inactivating mutations in the WASP gene and characterized by increased susceptibility to infections, eczema and thrombocytopoenia (Thrasher and Burns, 2010).
The N-WASp inhibitor 187-1 is a synthetic 14-residue cyclodimeric peptide identified in a high-throughput screen of xenopus cell-free extracts aimed to identify novel inhibitors of actin polymerization (Peterson et al., 2001). 187-1 inhibits N-WASp by locking it in its auto-inhibited conformation. In vitro experiments using synovial tissue from patients with RA showed that treatment with 187-1 resulted in altered cytoskeletal rearrangements accompanied by reduced cellular migration and invasion (Connolly et al., 2011). A few years later, using an identical procedure, Peterson and collaborators discovered wiskostatin, another N-WASp inhibitor that similarly prevents the activation of the Arp2/3 complex by stabilizing N-WASp activity in its inactive state (Peterson et al., 2004). Treatment with this drug decreased membrane ruffling in melanoma cells (Kovacs et al., 2006) and blocked T-cell activation in cultured lymphocyte lines (Haller et al., 2006). However, in another study, wiskostatin was shown to interfere with energy stores, protein synthesis and processing, cellular processes unlikely to be mediated by N-WASp (Guerriero and Weisz, 2007). Such an unwarranted effect cautions against the use of wiskostatin as a specific N-WASp inhibitor in in vivo models.
P21-activated kinases
PAK are downstream effectors of both Cdc42 and Rac GTPases. PAKs are involved in cell cycle progression and proliferation; however, they are best known for their role in cell polarity and plasticity, through modulation of the actin cytoskeleton. Some of the cytoskeletal effects of PAK activation include cell elongation, and loss of focal adhesions and stress fibres (Manser et al., 1997). PAKs are also involved in the modulation of endosomal membrane transport, membrane ruffling, filopodia and lamellipodia formation (Szczepanowska, 2009). PAK signalling deregulation is associated with tumourigenesis and cancer progression, neurodegenerative diseases and infection (Chan and Manser, 2012). There are two types or PAKs. Group I PAKs PAK1, PAK2 and PAK3 are directly activated via interaction with Rac and Cdc42, while group II PAKs (4 to 6) do not directly interact with Rho-GTPases (Chan and Manser, 2012). The role of PAK as an important effector of Rho-GTPases and their role in cytoskeletal modulation and cell cycle progression have made them attractive targets for drug discovery.
Staurosporine and its relative K252, are natural, potent broad spectrum kinase inhibitors that act by competitively binding to the allosteric ATP site of their target (Kaneko et al., 1997; Yi et al., 2010). KT-D606 and CEP-1347, derived from K252, are more selective than their predecessors, and effectively suppress H-Ras-mediated transformation in immortalized fibroblasts. KT-D606 and CEP-1347, however, inhibit both PAK and MLK3 kinases, and are not potent enough to be effective in vivo (Nheu et al., 2002). OSU-03012 is another ATP-competitive inhibitor of PAK. It was originally derived from the COX inhibitor celocoxib as a PDK1 antagonist, but was later rediscovered as an even more potent suppressor of PAK activity (Porchia et al., 2007). In vitro treatment of thyroid cancer cells with OSU-03012 resulted in reduced proliferation and migration (Porchia et al., 2007). IPA-3 is a more selective inhibitor of the PAKs (Deacon et al., 2008). This compound binds the regulatory domain of PAK, stabilizing the kinase in its auto-inhibited conformation (Deacon et al., 2008). However, IPA-3 is unstable under physiological conditions and is unlikely to be effective in vivo (Viaud and Peterson, 2009). Two other preferential inhibitors of PAK1, Λ-FL172 and Λ-FL411, were generated by modifying other compounds with bulky ruthenium-scaffolds to selectively target the wide ATP-binding groove of PAK1 (Maksimoska et al., 2008). Λ-FL172 was highly selective for group I PAKs and effective at sub-micromolar concentrations in vitro. These compounds are currently under development for in vivo use (Maksimoska et al., 2008). PF-3758309 was identified using high-throughput screening of structure-based SMI libraries (Murray et al., 2010). PF-3758309, a pyrrolo–pyrazole compound, is a potent ATP-competitive inhibitor of PAK. It effectively blocks the activity of PAK1, PAK4, PAK5 and PAK6, and is less potent against the remaining PAK2 and PAK3. PF-3758309 was tested in a variety of xenograft cancer models and was shown to effectively block tumour growth (Murray et al., 2010). More recently, another ATP-competitive inhibitor of PAK4 was discovered, LCH-7749944 (with lower potencies against PAK1, PAK5 and PAK6). In vitro treatment of invasive gastric cancer cells with LCH-7749944 resulted in reduced proliferation, filopodia formation, migration and invasion (Zhang et al., 2012). The latest addition to the list is the pyridopyrimidinone FRAX597. FRAX597 was identified by high-throughput screening as a potent ATP-competitive inhibitor of group I PAKs (Licciulli et al., 2013). FRAX597 was shown to decrease proliferation in cultured Schwannoma cells and reduce tumour development in an orthotropic model of neurofibromatosis (Licciulli et al., 2013). The majority of these compounds have been developed as potential cancer treatments and are still in their infancies. However, three of the compounds (PF-3758309, LCH-7749944 and FRAX597) have already shown beneficial effects on animal cancer models, suggesting therapeutic promise. It will be interesting to see the development of SMIs targeting PAK in the clinic and their potential application to other diseases, particularly inflammatory diseases.
Prenyltransferases
Another relevant strategy to block small Rho-GTPase activity is through inhibition of protein prenylation, a post-translational modification that consists in the addition of hydrophobic moieties. The transfer of farnesyl or geranylgeranyl modifications onto Rho-GTPases by prenyltransferases is required for membrane recruitment and substrate activation. A vast number of compounds against prenyltransferases have been developed and more than 70 clinical cancer trials have been performed so far with disappointing results.
Prenylation is responsible for membrane-targeting of Rho-GTPases, whereas this review focuses on the inhibition of the interaction of Rho-GTPases and their downstream effectors; we shall, therefore, not cover this set of drugs in further detail. These groups of compounds and their clinical applications have been extensively reviewed elsewhere (Basso et al., 2006; Holstein and Hohl, 2012; Li et al., 2012b).
Concluding remarks
Because of their central role in actin cytoskeleton regulation, targeting the Rho family of small GTPases, and their immediate downstream effectors, is a promising strategy for curbing the migration of leukocytes. Pharmacological inhibition of immune cell motility can be highly beneficial in a vast number of inflammatory disorders where excessive tissue infiltration can cause significant damage. The lack of effective and selective therapies for such diseases has failed to resolve their chronicity and consequently required the continued use of immune-suppressive treatments, such as corticosteroids, with potential side effects. Advances in drug design technologies and high-throughput experimental assays have brought about a novel class of highly specific SMIs that have proven their worth in the laboratory. Precise characterizations, dose optimizations and the production of improved derivatives are now leading to successful applications of drugs that target Rho-GTPase in animal models, and clinical trials. The application of SMIs for inhibiting Rho-GTPases is particularly attractive in the context of immune cell migration in inflammatory disease, because of the potential for developing leukocyte- or migration mode-specific drugs. The directed inhibition of a particular Rho-GTPase regulatory pathway can lead to the complete inhibition of specific cytoskeletal dynamics, thus potentially allowing for both potency and specificity. Rho-GTPase-targeted cytoskeletal modulation can lead to changes in endothelial cohesion or the cell-intrinsic capacity for leukocyte motility and can, therefore, be adapted to suit the pathophysiology of particular inflammatory diseases. The use of combination treatments of SMIs targeting Rho-GTPases is an interesting prospect currently being tested.
A key consideration for the future is the development of inhibitors that target a specific subset of leukocytes without diminishing the capacity of other subsets to perform their immunodefensive roles. The general inhibition of leukocyte trafficking results in the host being more susceptible to infections and having a diminished capacity for the homeostatic immuno-surveillance of tissues, which could potentially lead to oncogenic events being left unchecked. However, higher numbers of macrophages, mast cells and neutrophils detected within the tumour environment correlate with a poorer prognosis, whereas a greater infiltration of the tumour by lymphocytes correlates with a favourable prognosis (de Visser et al., 2006). Furthermore, chronic inflammation increases the risk of carcinogenesis. It, therefore, remains to be established to what extent the benefits of inhibiting leukocyte migration in an inflammatory context outweigh the long-term effects on the risk of developing cancer.
The recent developments discussed in this review are yet to be fully translated into clinical applications, and the next few years will surely see the emergence of eagerly awaited effective treatments for a set of conditions experiencing a steep rise in global incidence.
Acknowledgments
M. B. is supported by the Sydney Medical School at The University of Sydney and the Cure Cancer Australia Foundation. M. B. and W. W. are supported by fellowships from the Cancer Institute New South Wales. W. W. is supported by the Australian National Health and Medical Research Council and the Australian Research Council.
Glossary
- GAP
GTPase-activating protein
- GDI
guanine nucleotide dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- NPF
nucleation-promoting factor
- SMI
small-molecule inhibitor
Conflict of interest
The authors have no conflict of interest to declare.
References
- Akbar H, Cancelas J, Williams DA, Zheng J, Zheng Y. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol. 2006;406:554–565. doi: 10.1016/S0076-6879(06)06043-5. [DOI] [PubMed] [Google Scholar]
- Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1039–1045. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Timor O. Hanging in the balance: endogenous anti-inflammatory mechanisms in tissue repair and fibrosis. J Pathol. 2013;229:250–263. doi: 10.1002/path.4108. [DOI] [PubMed] [Google Scholar]
- Atassi MZ, Casali P. Molecular mechanisms of autoimmunity. Autoimmunity. 2008;41:123–132. doi: 10.1080/08916930801929021. [DOI] [PubMed] [Google Scholar]
- Auer M, Hausott B, Klimaschewski L. Rho GTPases as regulators of morphological neuroplasticity. Ann Anat. 2011;193:259–266. doi: 10.1016/j.aanat.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AD, Kirschmeier P, Bishop WR. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res. 2006;47:15–31. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]
- Bergert M, Chandradoss SD, Desai RA, Paluch E. Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc Natl Acad Sci U S A. 2012;109:14434–14439. doi: 10.1073/pnas.1207968109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaert R, Beaugerie L, Van Assche G, Brochez L, Renauld JC, Viguier M, et al. Cancer risk in immune-mediated inflammatory diseases: IMID and cancer risk. Mol Cancer. 2013;12:98. doi: 10.1186/1476-4598-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer J, Samarin J, Rehm M, Lautscham L, Fabry B, Goppelt-Struebe M. Inhibition of Rho kinases increases directional motility of microvascular endothelial cells. Biochem Pharmacol. 2012;83:616–626. doi: 10.1016/j.bcp.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Castoreno AB, Smurnyy Y, Torres AD, Vokes MS, Jones TR, Carpenter AE, et al. Small molecules discovered in a pathway screen target the Rho pathway in cytokinesis. Nat Chem Biol. 2010;6:457–463. doi: 10.1038/nchembio.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerione RA. Cdc42: new roads to travel. Trends Cell Biol. 2004;14:127–132. doi: 10.1016/j.tcb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Chan PM, Manser E. PAKs in human disease. Prog Mol Biol Transl Sci. 2012;106:171–187. doi: 10.1016/B978-0-12-396456-4.00011-0. [DOI] [PubMed] [Google Scholar]
- Chang LC, Lin RH, Huang LJ, Chang CS, Kuo SC, Wang JP. Inhibition of superoxide anion generation by CHS-111 via blockade of the p21-activated kinase, protein kinase B/Akt and protein kinase C signaling pathways in rat neutrophils. Eur J Pharmacol. 2009;615:207–217. doi: 10.1016/j.ejphar.2009.04.050. [DOI] [PubMed] [Google Scholar]
- Chang LC, Huang TH, Chang CS, Tsai YR, Lin RH, Lee PW, et al. Signaling mechanisms of inhibition of phospholipase D activation by CHS-111 in formyl peptide-stimulated neutrophils. Biochem Pharmacol. 2011;81:269–278. doi: 10.1016/j.bcp.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- Charras GT, Hu CK, Coughlin M, Mitchison TJ. Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175:477–490. doi: 10.1083/jcb.200602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang D, Guo Z, Zhao J, Wu B, Deng H, et al. Rho kinase phosphorylation promotes ezrin-mediated metastasis in hepatocellular carcinoma. Cancer Res. 2011;71:1721–1729. doi: 10.1158/0008-5472.CAN-09-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone MA, Goode BL. Actin nucleation and elongation factors: mechanisms and interplay. Curr Opin Cell Biol. 2009;21:28–37. doi: 10.1016/j.ceb.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly M, Veale DJ, Fearon U. Acute serum amyloid A regulates cytoskeletal rearrangement, cell matrix interactions and promotes cell migration in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1296–1303. doi: 10.1136/ard.2010.142240. [DOI] [PubMed] [Google Scholar]
- Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desire L, Bourdin J, Loiseau N, Peillon H, Picard V, De Oliveira C, et al. RAC1 inhibition targets amyloid precursor protein processing by gamma-secretase and decreases Abeta production in vitro and in vivo. J Biol Chem. 2005;280:37516–37525. doi: 10.1074/jbc.M507913200. [DOI] [PubMed] [Google Scholar]
- Dhaliwal JS, Badejo AM, Jr, Casey DB, Murthy SN, Kadowitz PJ. Analysis of pulmonary vasodilator responses to SB-772077-B [4-(7-( (3-amino-1-pyrrolidinyl)carbonyl)-1-ethyl-1H-imidazo(4,5-c)pyridin-2-yl)-1,2,5-oxadiazol-3-amine], a novel aminofurazan-based Rho kinase inhibitor. J Pharmacol Exp Ther. 2009;330:334–341. doi: 10.1124/jpet.109.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gennaro A, Haeggstrom JZ. Targeting leukotriene B4 in inflammation. Expert Opin Ther Targets. 2014;18:79–93. doi: 10.1517/14728222.2013.843671. [DOI] [PubMed] [Google Scholar]
- Ding RY, Zhao DM, Zhang ZD, Guo RX, Ma XC. Pretreatment of Rho kinase inhibitor inhibits systemic inflammation and prevents endotoxin-induced acute lung injury in mice. J Surg Res. 2011;171:e209–e214. doi: 10.1016/j.jss.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Doe C, Bentley R, Behm DJ, Lafferty R, Stavenger R, Jung D, et al. Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther. 2007;320:89–98. doi: 10.1124/jpet.106.110635. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Evelyn CR, Wade SM, Wang Q, Wu M, Iniguez-Lluhi JA, Merajver SD, et al. CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther. 2007;6:2249–2260. doi: 10.1158/1535-7163.MCT-06-0782. [DOI] [PubMed] [Google Scholar]
- Evelyn CR, Bell JL, Ryu JG, Wade SM, Kocab A, Harzdorf NL, et al. Design, synthesis and prostate cancer cell-based studies of analogs of the Rho/MKL1 transcriptional pathway inhibitor, CCG-1423. Bioorg Med Chem Lett. 2010;20:665–672. doi: 10.1016/j.bmcl.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Yin Y, Chen YT, Yao L, Wang B, Cameron MD, et al. Tetrahydroisoquinoline derivatives as highly selective and potent Rho kinase inhibitors. J Med Chem. 2010;53:5727–5737. doi: 10.1021/jm100579r. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435(7042):612–619. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- Feng Y, Yin Y, Weiser A, Griffin E, Cameron MD, Lin L, et al. Discovery of substituted 4-(pyrazol-4-yl)-phenylbenzodioxane-2-carboxamides as potent and highly selective Rho kinase (ROCK-II) inhibitors. J Med Chem. 2008;51:6642–6645. doi: 10.1021/jm800986w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri N, Corsini A, Bottino P, Clerici F, Contini A. Virtual screening approach for the identification of new Rac1 inhibitors. J Med Chem. 2009;52:4087–4090. doi: 10.1021/jm8015987. [DOI] [PubMed] [Google Scholar]
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- Friesland A, Zhao Y, Chen YH, Wang L, Zhou H, Lu Q. Small molecule targeting Cdc42-intersectin interaction disrupts Golgi organization and suppresses cell motility. Proc Natl Acad Sci U S A. 2013;110:1261–1266. doi: 10.1073/pnas.1116051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Fukumoto Y, Saji K, Sugimura K, Demachi J, Nawata J, et al. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels. 2010;25:144–149. doi: 10.1007/s00380-009-1176-8. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91:391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston B. The biochemistry of asthma. Biochim Biophys Acta. 2011;1810:1017–1024. doi: 10.1016/j.bbagen.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Griffith JW, Luster AD. Targeting cells in motion: migrating toward improved therapies. Eur J Immunol. 2013;43:1430–1435. doi: 10.1002/eji.201243183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero CJ, Weisz OA. N-WASp inhibitor wiskostatin nonselectively perturbs membrane transport by decreasing cellular ATP levels. Am J Physiol Cell Physiol. 2007;292:C1562–C1566. doi: 10.1152/ajpcell.00426.2006. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- Haller C, Rauch S, Michel N, Hannemann S, Lehmann MJ, Keppler OT, et al. The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J Biol Chem. 2006;281:19618–19630. doi: 10.1074/jbc.M513802200. [DOI] [PubMed] [Google Scholar]
- Hautz T, Brandacher G, Zelger B, Gorantla VS, Lee AW, Pratschke J, et al. Immunologic aspects and rejection in solid organ versus reconstructive transplantation. Transplant Proc. 2010;42:3347–3353. doi: 10.1016/j.transproceed.2010.09.020. [DOI] [PubMed] [Google Scholar]
- He Y, Xu H, Liang L, Zhan Z, Yang X, Yu X, et al. Antiinflammatory effect of Rho kinase blockade via inhibition of NF-kappaB activation in rheumatoid arthritis. Arthritis Rheum. 2008;58:3366–3376. doi: 10.1002/art.23986. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Carlin LM, Cox S, Ng T, Ridley AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J Cell Biol. 2010;190:553–563. doi: 10.1083/jcb.201002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PJ, Mann TS, Goldie RG. A rho kinase inhibitor, Y-27632 inhibits pulmonary eosinophilia, bronchoconstriction and airways hyperresponsiveness in allergic mice. Pulm Pharmacol Ther. 2005;18:67–74. doi: 10.1016/j.pupt.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Hernandez E, De La Mota-Peynado A, Dharmawardhane S, Vlaar CP. Novel inhibitors of Rac1 in metastatic breast cancer. P R Health Sci J. 2010;29:348–356. [PubMed] [Google Scholar]
- Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Holstein SA, Hohl RJ. Is there a future for prenyltransferase inhibitors in cancer therapy? Curr Opin Pharmacol. 2012;12:704–709. doi: 10.1016/j.coph.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Hong L, Kenney SR, Phillips GK, Simpson D, Schroeder CE, Noth J, et al. Characterization of a Cdc42 protein inhibitor and its use as a molecular probe. J Biol Chem. 2013;288:8531–8543. doi: 10.1074/jbc.M112.435941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. 2001. Mechanics of motor proteins and the cytoskeleton.
- Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, et al. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112:165–176. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Saito Y, Saito H, Matsumoto T, Matsuda Y, Vaught JL, et al. Neurotrophic 3,9-bis[ (alkylthio)methyl]-and-bis(alkoxymethyl)-K-252a derivatives. J Med Chem. 1997;40:1863–1869. doi: 10.1021/jm970031d. [DOI] [PubMed] [Google Scholar]
- Kast R, Schirok H, Figueroa-Perez S, Mittendorf J, Gnoth MJ, Apeler H, et al. Cardiovascular effects of a novel potent and highly selective azaindole-based inhibitor of Rho-kinase. Br J Pharmacol. 2007;152:1070–1080. doi: 10.1038/sj.bjp.0707484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi A, Ohmori M, Fujimura A. Partial protective effect of Y-27632, a Rho kinase inhibitor, against hepatic ischemia-reperfusion injury in rats. Eur J Pharmacol. 2004;493:167–171. doi: 10.1016/j.ejphar.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, Mogilner A, et al. Mechanism of shape determination in motile cells. Nature. 2008;453(7194):475–480. doi: 10.1038/nature06952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojonazarov B, Myrzaakhmatova A, Sooronbaev T, Ishizaki T, Aldashev A. Effects of fasudil in patients with high-altitude pulmonary hypertension. Eur Respir J. 2012;39:496–498. doi: 10.1183/09031936.00095211. [DOI] [PubMed] [Google Scholar]
- Kovacs EM, Makar RS, Gertler FB. Tuba stimulates intracellular N-WASp-dependent actin assembly. J Cell Sci. 2006;119(Pt 13):2715–2726. doi: 10.1242/jcs.03005. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21:636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453(7191):51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- Lee SH, Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol Cells. 2010;29:311–325. doi: 10.1007/s10059-010-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengfeld J, Wang Q, Zohlman A, Salvarezza S, Morgan S, Ren J, et al. Protein kinase C delta regulates the release of collagen type I from vascular smooth muscle cells via regulation of Cdc42. Mol Biol Cell. 2012;23:1955–1963. doi: 10.1091/mbc.E11-06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li H, Peng W, Jian W, Li Y, Li Q, Li W, et al. ROCK inhibitor fasudil attenuated high glucose-induced MCP-1 and VCAM-1 expression and monocyte-endothelial cell adhesion. Cardiovasc Diabetol. 2012a;11:65. doi: 10.1186/1475-2840-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang W, Cheng S, Cao D, Prent M. Isoprenoids and related pharmacological interventions: potential application in Alzheimer's disease. Mol Neurobiol. 2012b;46:64–77. doi: 10.1007/s12035-012-8253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu Y, Wang Z, Zhang XH, Wu WK. Fasudil attenuates lipopolysaccharide-induced acute lung injury in mice through the Rho/Rho kinase pathway. Med Sci Monit. 2010;16:BR112–BR118. [PubMed] [Google Scholar]
- Licciulli S, Maksimoska J, Zhou C, Troutman S, Kota S, Liu Q, et al. FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of NF2-associated schwannomas. J Biol Chem. 2013;288:29105–29114. doi: 10.1074/jbc.M113.510933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligeti E, Welti S, Scheffzek K. Inhibition and termination of physiological responses by GTPase activating proteins. Physiol Rev. 2012;92:237–272. doi: 10.1152/physrev.00045.2010. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Yu J, Feng L, Hou S, Liu Y, et al. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS ONE. 2013;8:e54841. doi: 10.1371/journal.pone.0054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Gu M, Wu Y, Zhu P, Zhang W, Yin C, et al. Therapeutic effect of Y-27632 on chronic allograft nephropathy in rats. J Surg Res. 2009;157:e117–e127. doi: 10.1016/j.jss.2008.10.018. [DOI] [PubMed] [Google Scholar]
- LoGrasso PV, Feng Y. Rho kinase (ROCK) inhibitors and their application to inflammatory disorders. Curr Top Med Chem. 2009;9:704–723. doi: 10.2174/156802609789044452. [DOI] [PubMed] [Google Scholar]
- Lohn M, Plettenburg O, Ivashchenko Y, Kannt A, Hofmeister A, Kadereit D, et al. Pharmacological characterization of SAR407899, a novel rho-kinase inhibitor. Hypertension. 2009;54:676–683. doi: 10.1161/HYPERTENSIONAHA.109.134353. [DOI] [PubMed] [Google Scholar]
- Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat Immunol. 2008;9:988–998. doi: 10.1038/ni.f.210. [DOI] [PubMed] [Google Scholar]
- Maksimoska J, Feng L, Harms K, Yi C, Kissil J, Marmorstein R, et al. Targeting large kinase active site with rigid, bulky octahedral ruthenium complexes. J Am Chem Soc. 2008;130:15764–15765. doi: 10.1021/ja805555a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, et al. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo-Ortiz BL, Castillo-Pichardo L, Hernandez E, Humphries-Bickley T, De la Mota-Peynado A, Cubano LA, et al. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J Biol Chem. 2012;287:13228–13238. doi: 10.1074/jbc.M111.334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, et al. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203:2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrass P, Kinjyo I, Ng LG, Reiner SL, Pure E, Weninger W. CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity. 2008;29:971–985. doi: 10.1016/j.immuni.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A. 2010;107:9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagumo H, Sasaki Y, Ono Y, Okamoto H, Seto M, Takuwa Y. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am J Physiol Cell Physiol. 2000;278:C57–C65. doi: 10.1152/ajpcell.2000.278.1.C57. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Hayashi K, Egi Y, Katayama K, Amano Y, Uehata M, et al. Effect of Wf-536, a novel ROCK inhibitor, against metastasis of B16 melanoma. Cancer Chemother Pharmacol. 2003a;52:319–324. doi: 10.1007/s00280-003-0641-9. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Katayama K, Tamechika I, Hayashi K, Amano Y, Uehata M, et al. WF-536 inhibits metastatic invasion by enhancing the host cell barrier and inhibiting tumour cell motility. Clin Exp Pharmacol Physiol. 2003b;30:457–463. doi: 10.1046/j.1440-1681.2003.03855.x. [DOI] [PubMed] [Google Scholar]
- Nheu TV, He H, Hirokawa Y, Tamaki K, Florin L, Schmitz ML, et al. The K252a derivatives, inhibitors for the PAK/MLK kinase family selectively block the growth of RAS transformants. Cancer J. 2002;8:328–336. doi: 10.1097/00130404-200207000-00009. [DOI] [PubMed] [Google Scholar]
- O E, Lee SW, Lee HS, Lim HS, Ahn HY, Shin JC, et al. Enhancement of endothelial progenitor cell numbers and migration by H1152, a Rho kinase specific inhibitor. Biosci Biotechnol Biochem. 2012;76:172–175. doi: 10.1271/bbb.110468. [DOI] [PubMed] [Google Scholar]
- Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onesto C, Shutes A, Picard V, Schweighoffer F, Der CJ. Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Methods Enzymol. 2008;439:111–129. doi: 10.1016/S0076-6879(07)00409-0. [DOI] [PubMed] [Google Scholar]
- Pelish HE, Peterson JR, Salvarezza SB, Rodriguez-Boulan E, Chen JL, Stamnes M, et al. Secramine inhibits Cdc42-dependent functions in cells and Cdc42 activation in vitro. Nat Chem Biol. 2006;2:39–46. doi: 10.1038/nchembio751. [DOI] [PubMed] [Google Scholar]
- Pertz O. Spatio-temporal Rho GTPase signaling – where are we now? J Cell Sci. 2010;123(Pt 11):1841–1850. doi: 10.1242/jcs.064345. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Lokey RS, Mitchison TJ, Kirschner MW. A chemical inhibitor of N-WASp reveals a new mechanism for targeting protein interactions. Proc Natl Acad Sci U S A. 2001;98:10624–10629. doi: 10.1073/pnas.201393198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, Bickford LC, Morgan D, Kim AS, Ouerfelli O, Kirschner MW, et al. Chemical inhibition of N-WASp by stabilization of a native autoinhibited conformation. Nat Struct Mol Biol. 2004;11:747–755. doi: 10.1038/nsmb796. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326(5957):1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porchia LM, Guerra M, Wang YC, Zhang Y, Espinosa AV, Shinohara M, et al. 2-amino-N-{4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phenyl} acetamide (OSU-03012), a celecoxib derivative, directly targets p21-activated kinase. Mol Pharmacol. 2007;72:1124–1131. doi: 10.1124/mol.107.037556. [DOI] [PubMed] [Google Scholar]
- Prakash J, de Borst MH, Lacombe M, Opdam F, Klok PA, van Goor H, et al. Inhibition of renal rho kinase attenuates ischemia/reperfusion-induced injury. J Am Soc Nephrol. 2008;19:2086–2097. doi: 10.1681/ASN.2007070794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pula G, Poole AW. Critical roles for the actin cytoskeleton and cdc42 in regulating platelet integrin alpha2beta1. Platelets. 2008;19:199–210. doi: 10.1080/09537100701777303. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Renkawitz J, Sixt M. Mechanisms of force generation and force transmission during interstitial leukocyte migration. EMBO Rep. 2010;11:744–750. doi: 10.1038/embor.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbreux G, Charras G, Paluch E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012;22:536–545. doi: 10.1016/j.tcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[ (4-methyl-5-isoquinoline)sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol Ther. 2002;93:225–232. doi: 10.1016/s0163-7258(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Satoh S, Kobayashi T, Hitomi A, Ikegaki I, Suzuki Y, Shibuya M, et al. Inhibition of neutrophil migration by a protein kinase inhibitor for the treatment of ischemic brain infarction. Jpn J Pharmacol. 1999;80:41–48. doi: 10.1254/jjp.80.41. [DOI] [PubMed] [Google Scholar]
- Schaafsma D, Bos IS, Zuidhof AB, Zaagsma J, Meurs H. The inhaled Rho kinase inhibitor Y-27632 protects against allergen-induced acute bronchoconstriction, airway hyperresponsiveness, and inflammation. Am J Physiol Lung Cell Mol Physiol. 2008;295:L214–L219. doi: 10.1152/ajplung.00498.2007. [DOI] [PubMed] [Google Scholar]
- Shang X, Marchioni F, Sipes N, Evelyn CR, Jerabek-Willemsen M, Duhr S, et al. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem Biol. 2012;19:699–710. doi: 10.1016/j.chembiol.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X, Marchioni F, Evelyn CR, Sipes N, Zhou X, Seibel W, et al. Small-molecule inhibitors targeting G-protein-coupled Rho guanine nucleotide exchange factors. Proc Natl Acad Sci U S A. 2013;110:3155–3160. doi: 10.1073/pnas.1212324110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol Chem. 2007;282:35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
- Slotta JE, Laschke MW, Menger MD, Thorlacius H. Rho-kinase signalling mediates endotoxin hypersensitivity after partial hepatectomy. Br J Surg. 2008;95:976–984. doi: 10.1002/bjs.6082. [DOI] [PubMed] [Google Scholar]
- Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr. 2011;5:170–180. doi: 10.4161/cam.5.2.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenger RA, Cui H, Dowdell SE, Franz RG, Gaitanopoulos DE, Goodman KB, et al. Discovery of aminofurazan-azabenzimidazoles as inhibitors of Rho-kinase with high kinase selectivity and antihypertensive activity. J Med Chem. 2007;50:2–5. doi: 10.1021/jm060873p. [DOI] [PubMed] [Google Scholar]
- Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal. 2011;23:1415–1423. doi: 10.1016/j.cellsig.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Minohara M, Kikuchi H, Ishizu T, Tanaka M, Piao H, et al. The selective Rho-kinase inhibitor Fasudil is protective and therapeutic in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;180:126–134. doi: 10.1016/j.jneuroim.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Surviladze Z, Young SM, Sklar LA. High-throughput flow cytometry bead-based multiplex assay for identification of Rho GTPase inhibitors. Methods Mol Biol. 2012;827:253–270. doi: 10.1007/978-1-61779-442-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Shibuya M, Satoh S, Sugiyama H, Seto M, Takakura K. Safety and efficacy of fasudil monotherapy and fasudil-ozagrel combination therapy in patients with subarachnoid hemorrhage: sub-analysis of the post-marketing surveillance study. Neurol Med Chir (Tokyo) 2008;48:241–247. doi: 10.2176/nmc.48.241. discussion 247–248. [DOI] [PubMed] [Google Scholar]
- Szczepanowska J. Involvement of Rac/Cdc42/PAK pathway in cytoskeletal rearrangements. Acta Biochim Pol. 2009;56:225–234. [PubMed] [Google Scholar]
- Takayasu M, Suzuki Y, Shibuya M, Asano T, Kanamori M, Okada T, et al. The effects of HA compound calcium antagonists on delayed cerebral vasospasm in dogs. J Neurosurg. 1986;65:80–85. doi: 10.3171/jns.1986.65.1.0080. [DOI] [PubMed] [Google Scholar]
- Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch Ophthalmol. 2008;126:309–315. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M. Phase 2 randomized clinical study of a rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156:731–736. doi: 10.1016/j.ajo.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, et al. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol. 2005;32:504–510. doi: 10.1165/rcmb.2004-0009OC. [DOI] [PubMed] [Google Scholar]
- Thorlacius K, Slotta JE, Laschke MW, Wang Y, Menger MD, Jeppsson B, et al. Protective effect of fasudil, a Rho-kinase inhibitor, on chemokine expression, leukocyte recruitment, and hepatocellular apoptosis in septic liver injury. J Leukoc Biol. 2006;79:923–931. doi: 10.1189/jlb.0705406. [DOI] [PubMed] [Google Scholar]
- Thrasher AJ, Burns SO. WASp: a key immunological multitasker. Nat Rev Immunol. 2010;10:182–192. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- Tokushige H, Inatani M, Nemoto S, Sakaki H, Katayama K, Uehata M, et al. Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest Ophthalmol Vis Sci. 2007;48:3216–3222. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Liu HF, Hsu KC, Yang JM, Chen C, Liu KK, et al. 7-Chloro-6-piperidin-1-yl-quinoline-5,8-dione (PT-262), a novel ROCK inhibitor blocks cytoskeleton function and cell migration. Biochem Pharmacol. 2011;81:856–865. doi: 10.1016/j.bcp.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Tsang CM, Lau EP, Di K, Cheung PY, Hau PM, Ching YP, et al. Berberine inhibits Rho GTPases and cell migration at low doses but induces G2 arrest and apoptosis at high doses in human cancer cells. Int J Mol Med. 2009;24:131–138. doi: 10.3892/ijmm_00000216. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389(6654):990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Velat GJ, Kimball MM, Mocco JD, Hoh BL. Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurg. 2011;76:446–454. doi: 10.1016/j.wneu.2011.02.030. [DOI] [PubMed] [Google Scholar]
- Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther. 2009;8:2559–2565. doi: 10.1158/1535-7163.MCT-09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil D, Der CJ. Chapter nine – inhibitors of the ROCK serine/threonine kinases: key effectors of the RhoA small GTPase. In: Fuyuhiko T, editor. The Enzymes. Vol. 33. Waltham: Academic Press; 2013. pp. 193–212. Inhibitors of the Ras Superfamily G-proteins, Part A. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Vockel M, Vestweber D. How T cells trigger the dissociation of the endothelial receptor phosphatase VE-PTP from VE-cadherin. Blood. 2013;122:2512–2522. doi: 10.1182/blood-2013-04-499228. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu H, Chen B, Li Q, Huang X, Wang L, et al. RhoA/ROCK-dependent moesin phosphorylation regulates AGE-induced endothelial cellular response. Cardiovasc Diabetol. 2012;11:7. doi: 10.1186/1475-2840-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel JM, D'Agnillo F. Anthrax lethal toxin-mediated disruption of endothelial VE-cadherin is attenuated by inhibition of the Rho-associated kinase pathway. Toxins (Basel) 2011;3:1278–1293. doi: 10.3390/toxins3101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Meyers JA. Immune-mediated inflammatory disorders (I.M.I.D.s): the economic and clinical costs. Am J Manag Care. 2002;8(21 Suppl):S664–S681. quiz S682–665. [PubMed] [Google Scholar]
- Williams RD, Novack GD, van Haarlem T, Kopczynski C. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am J Ophthalmol. 2011;152:834–841 e831. doi: 10.1016/j.ajo.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Xu B, Pelish H, Kirchhausen T, Hammond GB. Large scale synthesis of the Cdc42 inhibitor secramine A and its inhibition of cell spreading. Org Biomol Chem. 2006;4:4149–4157. doi: 10.1039/b609143a. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Miwa Y, Kasa M, Kitano K, Amano M, Kaibuchi K, et al. Structural basis for induced-fit binding of Rho-kinase to the inhibitor Y-27632. J Biochem. 2006;140:305–311. doi: 10.1093/jb/mvj172. [DOI] [PubMed] [Google Scholar]
- Yi C, Maksimoska J, Marmorstein R, Kissil JL. Development of small-molecule inhibitors of the group I p21-activated kinases, emerging therapeutic targets in cancer. Biochem Pharmacol. 2010;80:683–689. doi: 10.1016/j.bcp.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Biroc SL, Li WW, Alicke B, Xuan JA, Pagila R, et al. The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Mol Cancer Ther. 2006;5:2158–2164. doi: 10.1158/1535-7163.MCT-05-0440. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Soldati T. Dissection of amoeboid movement into two mechanically distinct modes. J Cell Sci. 2006;119(Pt 18):3833–3844. doi: 10.1242/jcs.03152. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang J, Guo Q, Wang Y, Zhou Y, Peng H, et al. LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells. Cancer Lett. 2012;317:24–32. doi: 10.1016/j.canlet.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shang X, Guo F, Murphy K, Kirby M, Kelly P, et al. Defective homing is associated with altered Cdc42 activity in cells from patients with Fanconi anemia group A. Blood. 2008;112:1683–1686. doi: 10.1182/blood-2008-03-147090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Pothoulakis C. Rho GTPases as therapeutic targets for the treatment of inflammatory diseases. Expert Opin Ther Targets. 2003;7:583–592. doi: 10.1517/14728222.7.5.583. [DOI] [PubMed] [Google Scholar]
- Zins K, Lucas T, Reichl P, Abraham D, Aharinejad S. A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice. PLoS ONE. 2013;8:e74924. doi: 10.1371/journal.pone.0074924. [DOI] [PMC free article] [PubMed] [Google Scholar]


