Abstract
Objective
Arthritis activity assessments in psoriatic arthritis (PsA) have traditionally relied on tender and swollen joint counts, but in rheumatoid arthritis, multiple studies have demonstrated subclinical inflammation using modern imaging. The aim of this study was to compare clinical examination and ultrasound (US) findings in an early PsA cohort.
Methods
Forty-nine disease-modifying antirheumatic drug–naive patients with recent-onset PsA (median disease duration 10 months) underwent gray-scale (GS) and power Doppler (PD) US of 40 joints plus tender and swollen joint counts of 68/66 joints. GS and PD were scored on a 0–3 semiquantitative scale for each joint. Clinically active joints were defined as tender and/or swollen and US active joints were defined as a GS score ≥2 and/or a PD score ≥1.
Results
The most common sites for subclinical synovitis were the wrist (30.6%), knee (21.4%), metatarsophalangeal (MTP) joints (26.5–33.7%), and metacarpophalangeal joints (10.2–19.4%). Excluding MTP joints and ankles, 37 (75.5%) of 49 patients had subclinical synovitis with a median of 3 (interquartile range [IQR] 1–4) joints involved. In contrast, clinical overestimation of synovitis occurred most commonly at the shoulder (38%) and ankle (28.6%). Twelve of 49 patients were classified clinically as having oligoarthritis; of these, subclinical synovitis identified 8 (75%) as having polyarthritis with an increase in their median joint count from 3 (IQR 1–4) to 6 (IQR 5–7).
Conclusion
This study has demonstrated that subclinical synovitis, as identified by US, is very common in early PsA and led to the majority of oligoarthritis patients being reclassified as having polyarthritis. Further research is required into the relationship of such subclinical synovitis to structural progression.
INTRODUCTION
ClinicalTrials.gov identifier: NCT01106079. ISRCTN: 30147736.
Dr. Freeston’s work was supported by Arthritis Research UK (Clinician Scientist, grant 19335). Dr. Coates’s work was supported by Arthritis Research UK (Clinical Research Fellow, grant 18364). The Tight Control of Psoriatic Arthritis (TICOPA) study was supported by Arthritis Research UK (grant 18825).
Drs. Freeston and Coates and Drs. Helliwell and Conaghan contributed equally to this work.
Dr. Emery has received consulting fees, speaking fees, and/or honoraria (less than $10,000 each) and/or has provided expert testimony for AbbVie, BMS, Lilly, Merck, Novartis, Pfizer, Roche, and UCB. Dr. Helliwell has received consulting fees, speaking fees, and/or honoraria (less than $10,000 each) from Pfizer, Celgene, BMS, UCB, AbbVie, and Eli Lilly. Dr. Conaghan has received consulting fees, speaking fees, and/or honoraria (less than $10,000) from Novartis.
The emphasis placed on treating inflammatory arthritis as early as possible to minimize damage and functional disability has been shown to be effective in rheumatoid arthritis (RA) (1,2), and the concept has been extended to other inflammatory arthritides such as psoriatic arthritis (PsA). Such a treatment strategy requires accurate assessment of disease activity. Traditionally, clinical examination (CE) in the form of tender and swollen joint counts has been used to assess disease activity, particularly in the absence of reliable PsA-specific biomarkers. The 68/66 joint count has been recommended for use in PsA (3) because reduced joint counts miss a significant proportion of active disease.
Ultrasound (US) has been shown to be a sensitive method, particularly when compared to CE, for assessing both disease activity and damage in inflammatory arthritis, including PsA (4,5). In RA, multiple studies have shown subclinical joint disease using US assessment of disease activity (6–8) and its subsequent association with erosion progression (9,10).
Significance & Innovations.
Approximately 75% of patients with early psoriatic arthritis have evidence of subclinical synovitis, with a median of 3 additional joints affected.
Subclinical disease is most common in the wrist, knee, and metacarpophalangeal joints.
The majority of patients with clinical oligoarthritis actually have polyarticular disease if subclinical synovitis is considered.
Wakefield et al have highlighted the occurrence of subclinical disease in 13% of joints scanned in a mixed recent-onset oligoarthritis cohort using US (7). More recently, studies in psoriasis cohorts (without clinical evidence of PsA) have shown a high prevalence of synovitis and enthesopathy (11–13). Naredo et al demonstrated US synovitis in 3.2% of joints in their psoriasis cohort that did not have musculoskeletal disease (n = 162), compared to 1.3% in their control group of patients with other skin diseases and no musculoskeletal disease (n = 60; P < 0.0005) (11). A non–contrast-enhanced magnetic resonance imaging study of the feet of 26 psoriasis patients (with clinically asymptomatic feet) and 10 controls showed joint effusion/synovitis in 46% of patients, but in none of the controls (14). However, US was not used in this study. A small study of psoriasis patients with recent-onset joint symptoms has shown presence of gray-scale (GS) and power Doppler (PD) US activity in clinically inactive joints (15), but the potential presence of subclinical synovitis in PsA has not been addressed.
Peripheral arthritis in PsA has typically been divided into polyarthritis (≥5 joints involved) and oligoarthritis (<5 joints involved) (16,17). Oligoarthritis accounts for a significant proportion of patients presenting with PsA. In the original series by Moll and Wright (17), oligoarthritis accounted for more than 70% of PsA patients and in subsequent series has ranged from 14–37% (16,18,19). Oligoarthritis has typically been considered a milder form of the disease, and there are few evidence-based therapies available for this subgroup. Recent European League Against Rheumatism (EULAR) recommendations for the treatment of PsA provide an algorithm for treatment where the presence of polyarthritis (≥5 active joints) is an adverse prognostic factor that should result in more rapid escalation of therapy (20). However, patients with oligoarthritis experience a significant impact on function and quality of life, particularly in PsA (21). Imaging with US may be able to differentiate more accurately between oligo- and polyarticular disease to help guide therapeutic decisions.
The aim of this study was therefore to compare CE and US findings in an early PsA cohort to explore the frequency of subclinical synovitis and identify which joints commonly have subclinical disease. Given the common classification of PsA into oligoarthritis and polyarthritis, we aimed to see if subclinical synovitis affected patients' classification into these subgroups.
SUBJECTS AND METHODS
Forty-nine patients with recent-onset PsA, defined as a disease duration <24 months, enrolled in the Tight Control of Psoriatic Arthritis (TICOPA) trial (22), and 8 control subjects without joint pain or diagnoses of psoriasis or arthritis were recruited. All gave informed, written consent. Ethical approval for this study was given by the North East-York Research Ethics Committee. Patients fulfilled the PsA criteria of the Classification of Psoriatic Arthritis Study Group (23) and had never taken disease-modifying antirheumatic drugs (DMARDs). They had clinically active disease, defined as ≥1 active (tender and/or swollen) joint, ≥1 tender enthesis, or a Bath Ankylosing Spondylitis Disease Activity Index score ≥4 for axial disease.
Non-US assessment
All patients and controls had a clinical assessment, including a 68/66 tender/swollen joint count. A classification of oligoarthritis (≤4 joints) or polyarthritis (≥5 joints) by CE was based on the 68/66 clinical joint count. Other assessments included measurement of rheumatoid factor, anti–cyclic citrullinated antibody, and body mass index. Active clinical joint involvement was defined as ≥1 tender and/or swollen joint.
US assessment
US (GS and PD) of a standard set of 40 joints (a subset of those examined clinically) was performed on the same day as the clinical assessment by 1 of 2 experienced ultrasonographers (JEF [n = 38 scans] and JLN [n = 11 scans]) using a Philips HDI 5000 machine employing 12–5-MHz and 15–7-MHz linear transducers.
The ultrasonographer scanning the patient was unaware of the CE findings of the patients. PD was assessed using a pulse repetition frequency of 750 Hz and medium wall filter, and gain was adjusted until the background signal was removed. Bilateral posterior glenohumeral joints (n = 50 PsA, control data not available), olecranon fossa (n = 98 PsA, n = 16 control), wrists (n = 98 PsA, n = 16 control), metacarpophalangeal (MCP) joints 1–5 (n = 488 PsA, n = 80 control), proximal interphalangeal (PIP) joints 1–5 (n = 488 PsA, n = 80 control), knees (n = 98 PsA, n = 16 control), tibiotalar (n = 98 PsA, n = 16 control), and metatarsophalangeal (MTP) joints 1–5 (n = 490 PsA, n = 80 control) were examined. Each joint was scanned in both longitudinal and transverse planes. For the wrist, the radiocarpal, intercarpal, and ulnar-carpal joints were scanned and the highest GS and PD scores for any compartment were used as the overall score in this analysis. For the knee, the suprapatellar pouch and medial and lateral gutters were scanned and the highest GS and PD scores for any compartment were used in this analysis as the overall score.
The GS/PD joint scoring system used was that of the EULAR/Outcome Measures in Rheumatology system, which was developed on smaller joints but shown to be applicable to larger joints with good reliability (Keen H: unpublished observations). GS and PD were scored separately on a 0–3 semiquantitative scale (as used in RA studies 6) for each joint imaged. Because of the difficulties in distinguishing swollen synovium from effusion, these features were combined into one overall GS score, as per Bruyn et al (24). A GS score ≥2 and/or a PD score ≥1 were used to identify US active joints, since low-level GS changes can be seen in normal subjects (25). The probe positions and landmarks used were in line with Backhaus et al (26).
Statistical analysis
The proportion of both patients and joints with subclinical synovitis was calculated. When defining symmetric disease, symmetry was defined as a symmetry number of ≥0.5, where the symmetry number was calculated as the number of joints as symmetric pairs divided by the total number of joints involved (27). The level of agreement between the 2 sonographers and between CE and US when detecting synovitis was quantified descriptively as the proportion of joints in which both methods exactly agreed over the presence or absence of synovitis (percentage exact agreement [PEA]), proportions of category-specific negative and positive agreement (Sp0 and Sp1 for absence and presence of synovitis, respectively), and the proportions of joints where CE and US disagreed in either direction (US > CE, US < CE). Category-specific agreement was defined as the proportion of the total number of positive or negative ratings (CE + US) that were concordant. The kappa statistic was also calculated and supplemented with the prevalence-adjusted bias-adjusted kappa (PABAK) statistic to give an indication of the extent to which differences in the overall level of synovitis identified by each assessment method together with imbalances in the proportions of joints with and without synovitis affected the calculated value of kappa.
RESULTS
Clinical assessment
The clinical characteristics of the early PsA cohort are shown in Table1. Although clinical arthritis was not a requirement for entry, all patients did have active arthritis of ≥1 joint at the time of inclusion. Thirty (61%) of 49 PsA patients and no control subjects were taking nonsteroidal antiinflammatory drugs (NSAIDs). Since patients had active disease at the time of assessment and were not receiving treatment with DMARDs, they were allowed to continue on NSAIDs for symptomatic relief.
Table 1.
Clinical characteristics of the early PsA cohort*
| Early PsA (n = 49) | |
|---|---|
| Age, mean ± SD years | 42.8 ± 12.5 |
| Women | 23 (46.9) |
| RF positive | 1 (2) |
| Anti-CCP positive | 2 (4) |
| Symptom duration, median (IQR) months | 10 (4–13) |
| Arthritis at presentation | 49 (100) |
| Enthesitis at presentation | 37 (75.5) |
| Inflammatory back disease at presentation | 13 (26.5) |
| Current skin psoriasis | 44 (89.8) |
| PASI score, median (IQR) | 2.6 (1.2–4.7) |
| Nail psoriasis | 30 (61.2) |
| mNAPSI score, median (IQR) | 1 (0–9) |
| Tender joint count (total 68), median (IQR) | 7 (4–15) |
| Swollen joint count (total 66), median (IQR) | 4 (3–9) |
Values are the number (percentage) unless indicated otherwise. PsA = psoriatic arthritis; RF = rheumatoid factor; anti-CCP = anti–cyclic citrullinated peptide antibody; IQR = interquartile range; PASI = Psoriasis Area and Severity Index; mNAPSI = modified Nail Psoriasis Severity Index.
A total of 47 (96%) of 49 patients had ≥1 joint showing subclinical synovitis; in these patients, the median number of joints with subclinical synovitis was 4 (interquartile range [IQR] 2–7, range 1–19). Subclinical PD changes were identified in 17 (34.7%) of 49 patients; in these patients, the median number of joints with subclinical PD was 1 (IQR 1–2, range 1–4). Excluding MTP joints and ankles, 37 (75.5%) of 49 patients had subclinical synovitis (median 3 [IQR 1–4], range 1–11); 15 (30.6%) of 49 had subclinical PD changes (median 1 [IQR 1–2], range 1–4). The distribution of subclinical synovitis defined by the combination of GS and/or PD US signal in different joints assessed is shown in Figure 1A. Subclinical synovitis (defined as a GS score ≥2 and/or a PD score ≥1 in a joint, in the absence of tenderness or swelling) was seen in 12.4% (237 of 1,908) of the joints scanned (16.6% [237 of 1,431] of the clinically normal joints) and PD signal was present in 1.6% (30 of 1,908) of all joints scanned (2.1% [30 of 1,431] of the clinically inactive joints). Excluding MTP joints and ankles, subclinical synovitis was seen in 9.8% (130 of 1,320) of the joints scanned (13.5% [130 of 963] of clinically inactive joints), whereas subclinical PD was seen in 1.8% (24 of 1,320) of the joints scanned (2.5% [24 of 963] of clinically inactive joints). Subclinical synovitis was seen most frequently in the wrist (30.6% [30 of 98 joints]), knee (21.4% [21 of 98 joints]), MTP1 (26.5% [26 of 98 joints]), and MTP2 (33.7% [33 of 98 joints]). The MCP2 (16.3% [16 of 98 joints]), MCP5 (19.4% [19 of 98 joints]), and MTP3 (10.2% [10 of 98 joints]) also showed subclinical activity to a slightly lesser degree.
Figure 1.
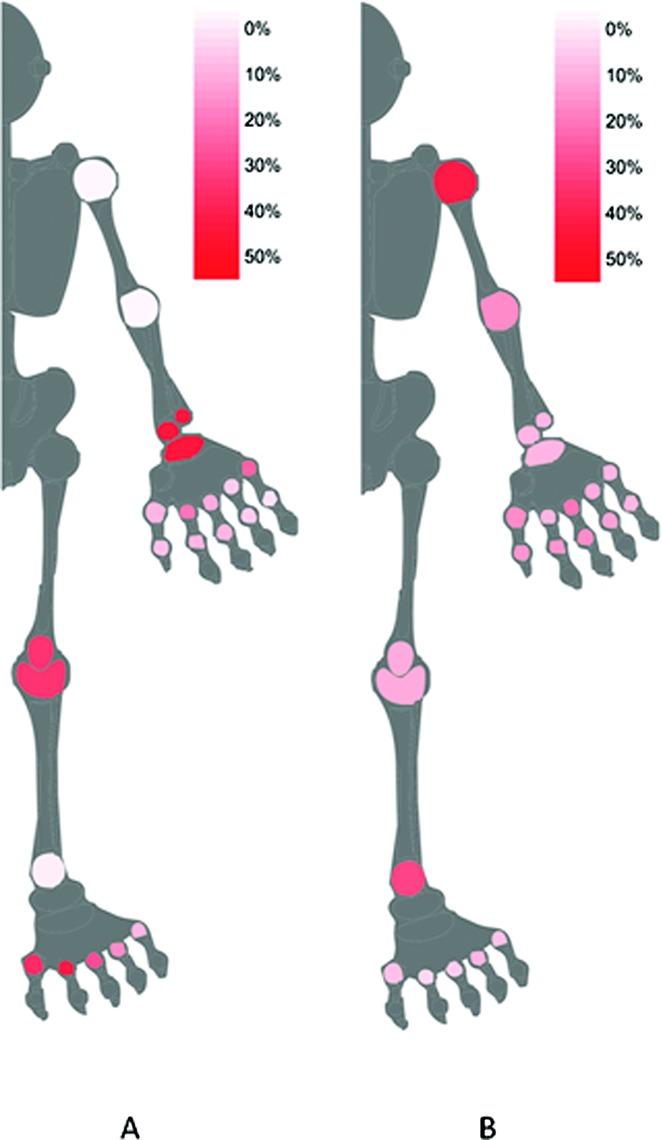
Graphical summary (homunculus) of A, subclinical synovitis (shown in red), graded according to the proportion of joints in which ultrasound (US) was positive (gray-scale [GS] score ≥2 or power Doppler [PD] score ≥1 present) and clinical examination (CE) was negative (no tenderness or swelling) and B, apparent clinical overestimation of synovitis (shown in red), graded according to the proportion of joints in which US was negative (GS score ≥2 or PD score ≥1 absent) and CE was positive (tenderness and/or swelling present). White = 0% of joints; red = 50% of joints.
Table2 shows further detail of US findings, with the number of joints with US synovitis defined by GS alone, PD alone, and the combination of GS or PD activity. Positive GS scores, as opposed to positive PD scores, were the predominant contributor to the US changes in the MTP joints; the percentage of joints with a PD score ≥1 was 3.1% in MTP1, 1.0% in MTP2, and 0.0% in MTP3 compared to 13.3% in the wrist and 12.2% in the knee. Symmetry of joint involvement was assessed in 25 patients who had 8 joint pairs scanned. Considering clinical assessment alone, 52% (13 of 25) had symmetric arthritis. When subclinical disease as seen on US was included, a further 6 patients had symmetric arthritis, while 2 patients changed from symmetric to asymmetric arthritis. Excluding the MTP joints and ankles, considering clinical assessment alone, 40% (10 of 25) had symmetric arthritis; when subclinical disease as seen on US was included, a further 4 patients had symmetric arthritis, while 1 patient changed from symmetric to asymmetric arthritis. Subclinical synovitis was not significantly associated with psoriasis severity (as measured by the Psoriasis Area and Severity Index score) (data not shown).
Table 2.
Joints with US synovitis*
| US definition of synovitis | |||
|---|---|---|---|
| GS ≥2 | PD ≥1 | GS ≥2 or PD ≥1 | |
| Shoulder† | 2 (1/50) | 0 (0/50) | 2 (1/50) |
| Elbow‡ | 5 (5/98) | 2 (2/98) | 5 (5/98) |
| Wrist | 46 (45/98) | 21 (21/98) | 50 (49/98) |
| MCP1 | 14 (13/96) | 6 (6/96) | 15 (14/96) |
| MCP2 | 36 (35/98) | 15 (15/98) | 36 (35/98) |
| MCP3 | 19 (19/98) | 11 (11/98) | 21 (21/98) |
| MCP4 | 10 (10/98) | 4 (4/98) | 11 (11/98) |
| MCP5 | 24 (24/98) | 3 (3/98) | 24 (24/98) |
| PIP1 | 14 (13/96) | 8 (8/96) | 14 (13/96) |
| PIP2 | 22 (22/98) | 10 (10/98) | 22 (22/98) |
| PIP3 | 21 (21/98) | 9 (9/98) | 21 (21/98) |
| PIP4 | 12 (12/98) | 2 (2/98) | 12 (12/98) |
| PIP5 | 9 (9/98) | 3 (3/98) | 9 (9/98) |
| Knee | 42 (41/98) | 18 (18/98) | 43 (42/98) |
| Ankle§ | 6 (6/98) | 1 (1/98) | 6 (6/98) |
| MTP1 | 34 (33/98) | 3 (3/98) | 35 (34/98) |
| MTP2 | 47 (46/98) | 3 (3/98) | 47 (46/98) |
| MTP3 | 36 (35/98) | 3 (3/98) | 36 (35/98) |
| MTP4 | 20 (20/98) | 0 (0/98) | 20 (20/98) |
| MTP5 | 13 (13/98) | 3 (3/98) | 13 (13/98) |
| Total | 22 (423/1,908) | 7 (125/1,908) | 23 (433/1,908) |
Values are the percentage (number/total). US = ultrasound; GS = gray-scale; PD = power Doppler; MCP = metacarpophalangeal; PIP = proximal interphalangeal; MTP = metatarsophalangeal.
Posterior glenohumeral joint.
Olecranon fossa.
Tibiotalar joint.
There were 8 control patients scanned (all women) with a mean ± SD age of 49.8 ± 9.6 years. Although 75% of the controls (6 of 8) had subclinical synovitis in ≥1 joint (median 2, range 0–8) and 6% of the joints scanned (18 of 304) were affected, in contrast to the PsA patients, almost all of the affected joints were located in the feet and none had active PD; 16 were MTP joints, all with a GS score of 2 but with a PD score of 0. The remaining 2 joints (0.9% of 224 joints scanned, excluding MTP joints and ankles) were the 2 knees of 1 control patient also with a GS score of 2 but with a PD score of 0.
Apparent clinical overestimation of synovitis occurred most commonly at the shoulder (38% [19 of 50 joints]) and ankle (28.6% [28 of 98 joints]), as shown in Table3 and Figure 1B. None of these shoulder or ankle joints had any evidence of US synovitis (all had a GS score of 0 and a PD score of 0). The shoulder joints were all deemed clinically active due to tenderness; none were swollen. Half of the ankle joints were swollen (50% [14 of 28]) and the majority (86% [24 of 28]) were tender. This may represent patients reporting pain from other causes, limitations or misinterpretation of CE, or a limitation of the sensitivity of US to identify synovitis, particularly in larger joints where a limited sonographic window may be a concern. However, rates of clinical overestimation were lower in the knee, and the majority of those found to be clinically active but inactive on US had a GS score of 1 (69% [9 of 13]).
Table 3.
Agreement between CE (tender and/or swollen) and US (GS ≥2 or PD ≥1) synovitis, by joint type*
| PEA, % | Sp0, % | Sp1, % | US > CE, % | CE > US, % | κ (95% CI) | PABAK | |
|---|---|---|---|---|---|---|---|
| Shoulder† | 62 | 76 | 10 | 0 | 38 | 0.06 | 0.24 |
| Elbow‡ | 83 | 90 | 37 | 0 | 17 | 0.31 | 0.65 |
| Wrist | 57 | 64 | 48 | 31 | 12 | 0.14 | 0.14 |
| MCP1 | 78 | 86 | 49 | 4 | 18 | 0.37 | 0.56 |
| MCP2 | 71 | 78 | 58 | 16 | 12 | 0.36 | 0.42 |
| MCP3 | 69 | 79 | 42 | 10 | 20 | 0.23 | 0.38 |
| MCP4 | 80 | 88 | 29 | 7 | 13 | 0.17 | 0.60 |
| MCP5 | 68 | 80 | 24 | 19 | 12 | 0.05 | 0.36 |
| PIP1 | 78 | 87 | 40 | 6 | 16 | 0.28 | 0.56 |
| PIP2 | 78 | 85 | 59 | 6 | 16 | 0.44 | 0.56 |
| PIP3 | 78 | 85 | 59 | 5 | 17 | 0.45 | 0.56 |
| PIP4 | 81 | 88 | 42 | 5 | 14 | 0.32 | 0.62 |
| PIP5 | 88 | 93 | 57 | 1 | 11 | 0.51 | 0.76 |
| Knee | 65 | 72 | 55 | 21 | 13 | 0.27 | 0.30 |
| Ankle§ | 70 | 82 | 26 | 1 | 29 | 0.17 | 0.40 |
| MTP1 | 63 | 75 | 31 | 27 | 10 | 0.09 | 0.26 |
| MTP2 | 60 | 70 | 40 | 34 | 6 | 0.17 | 0.20 |
| MTP3 | 69 | 79 | 46 | 22 | 8 | 0.27 | 0.38 |
| MTP4 | 74 | 84 | 32 | 14 | 11 | 0.17 | 0.48 |
| MTP5 | 79 | 88 | 16 | 11 | 10 | 0.04 | 0.58 |
| Total | 73 | 82 | 43 | 12 | 15 | 0.25 | 0.46 |
| Total¶ | 74 | 83 | 47 | 10 | 16 | 0.30 | 0.48 |
CE = clinical examination; US = ultrasound; GS = gray-scale; PD = power Doppler; PEA = percentage exact agreement; Sp0 = agreement specific to a score of 0; Sp1 = agreement specific to a score of 1; 95% CI = 95% confidence interval; PABAK = prevalence-adjusted bias-adjusted kappa; MCP = metacarpophalangeal; PIP = proximal interphalangeal; MTP = metatarsophalangeal.
Posterior glenohumeral joint.
Olecranon fossa.
Tibiotalar joint.
Excluding MTP joints/ankles.
Of the 49 patients, 38 were classified as having polyarthritis and 11 were classified as having oligoarthritis on clinical assessment. Of the 11 clinical oligoarthritis patients, US evidence of subclinical synovitis identified 9 (81.8%) as having polyarthritis. The clinical oligoarthritis patients (n = 11) had a median joint count of 3 (IQR 3–4, range 1–4). Of these, the 9 patients who were reclassified as having polyarthritis had an increase in their median joint count from 3 (IQR 3–4, range 1–4) to 7 (IQR 6–11, range 6–14). In the polyarthritis group, US assessment was associated with an increase in the median joint count from 12 (IQR 8–20, range 5–55) to 19 (IQR 14–27, range 7–55). Excluding MTP joints and ankles, of the 49 patients, 37 were classified as having polyarthritis and 12 were classified as having oligoarthritis on clinical assessment. Of the 12 clinical oligoarthritis patients, US evidence of subclinical synovitis identified 8 (75.0%) as having polyarthritis. The clinical oligoarthritis patients (n = 12) had a median joint count of 3 (IQR 1–4, range 1–4). Of these, the 8 patients who were reclassified as having polyarthritis had an increase in their median joint count from 3 (IQR 1–4, range 1–4) to 6 (IQR 5–7, range 5–9). In the polyarthritis group, US assessment was associated with an increase in the median joint count from 9 (IQR 7–19, range 5–46) to 14 (IQR 9–21, range 5–46). If we allowed US evidence of the presence or absence of synovitis to revise the clinical joint count up or down, then 6 of 12 of those with clinical oligoarthritis were found to have polyarthritis (increased from median 4 [IQR 1.75–4] to 6 [IQR 5–7.25]), while 3 of 37 patients with clinical polyarthritis were found to have oligoarthritis. The joint counts of these 3 individuals changed as follows: 5 joints revised to 1 (wrists, MCP1, and MCP3 discrepant), 6 joints revised to 4 (elbow, shoulder, and wrist CE > US, MCP3 CE < US), and 7 joints revised to 3 (shoulder, MCP2, and both PIP2 and PIP3 CE > US, PIP1 CE < US).
For a subset of joints (1,198 of 1,320, excluding MTP joints and ankles), patient-reported pain in the joint was available. Of the clinically active joints, 44% (141 of 324) were reported to be painful. Of the clinically inactive joints (without tenderness or swelling), 8% (69 of 874) were reported to be painful; 18% (20 of 114) of joints with subclinical synovitis were reported to be painful compared to 6% (49 of 760) of joints without subclinical synovitis. At the patient level, there were 8 patients who had ≥1 painful, clinically inactive joint (median 2 [IQR 1–4], range 1–11). There was no substantive correlation between the number of painful, clinically inactive joints and the number of joints with subclinical synovitis (n = 47; ρ = 0.134, P = 0.370).
When comparing overall agreement between clinical assessment and US assessment (excluding MTP joints and ankles), the PEA was 74% (982 of 1,320) when clinical involvement was defined as tender and/or swollen and US involvement was defined as a GS score ≥2 and/or a PD score ≥1. Agreement on negative results (i.e., no clinical or US synovitis) was 83% (1,666 ratings concordant of 2,004 ratings) and agreement on the presence of synovitis was 47% (298 of 636). The PABAK statistic was 0.48. In summary, the overall PEA was relatively high, but this was largely due to the predominance of zero scores.
A total of 62.5% (30 of 48; 1 patient's NSAID status was unknown) of patients were taking NSAIDs at the time of their assessment, which is known to potentially affect US results (28). Therefore, agreement was specifically investigated to see if NSAID use may have affected the results. The PEA between CE (tender and/or swollen) and US (GS score ≥2 and/or PD score ≥1) assessments was similar in NSAID users compared to nonusers (PEA 76% versus 72%); agreement over the presence of synovitis was slightly better, but the proportions of patients with over- and underestimation of synovitis when comparing clinical and US assessments were very similar. The kappa statistic was higher in NSAID users than nonusers (0.38 versus 0.15), which could have been due to a higher prevalence of US synovitis in this group (23.6% of joints scanned versus 18.1%); the PABAK indicated that agreement was similar in the 2 groups (0.52 versus 0.44) (Table4).
Table 4.
Impact of NSAIDs on agreement between CE and US synovitis, excluding metatarsophalangeal joints and ankles*
| Clinical assessment | US synovitis assessment, GS ≥2 or PD ≥1 | |
|---|---|---|
| No NSAIDs | NSAIDs | |
| Tender and/or swollen joint | ||
| PEA | 72 (348/486) | 76 (612/808) |
| Sp0 | 82 (628/766) | 84 (994/1,190) |
| Sp1 | 33 (68/206) | 54 (230/426) |
| US > CE | 11 (54/486) | 9 (76/808) |
| US < CE | 17 (84/486) | 15 (120/808) |
| κ (95% CI) | 0.15 (0.05–0.25) | 0.38 (0.31–0.45) |
| PABAK | 0.44 | 0.52 |
Values are the percentage (number/total) unless indicated otherwise. NSAIDs = nonsteroidal antiinflammatory drugs; CE = clinical examination; US = ultrasound; GS = gray-scale; PD = power Doppler; PEA = percentage exact agreement; Sp0 = agreement specific to a score of 0; Sp1 = agreement specific to a score of 1; 95% CI = 95% confidence interval; PABAK = prevalence-adjusted bias-adjusted kappa.
US inter- and intrareader reliability
GS scores (≤1 = absent, ≥2 = present) were moderately repeatable (JEF: PEA 79.6%, Sp0 75.6%, Sp1 82.5%, κ = 0.58 [95% confidence interval (95% CI) 0.37–0.80], PABAK 0.59; JLN: PEA 96.3%, Sp0 96.2%, Sp1 96.4%, κ = 0.93 [95% CI 0.82–1.00], PABAK 0.93) and interreader agreement was moderate (PEA 75.9%, Sp0 74.5%, Sp1 77.2%, κ = 0.52 [95% CI 0.29–0.75], PABAK 0.52). PD scores (0 = absent, ≥1 = present) were highly repeatable (JEF: PEA 93.9%, Sp0 85.7%, Sp1 96.2%, κ = 0.82 [95% CI 0.58–1.00], PABAK 0.88; JLN: PEA 100.0%, Sp0 100.0%, Sp1 100.0%, κ = 1.00, PABAK 1.00) and interreader agreement was substantial (PEA 90.9%, Sp0 80.0%, Sp1 94.1%, κ = 0.74 [95% CI 0.47–1.00], PABAK 0.82).
DISCUSSION
This study has shown that subclinical synovitis, as identified by US, is very common in early PsA, with 96% of patients having evidence of subclinical synovitis in at least 1 joint. Subclinical synovitis (defined as a GS score ≥2 and/or a PD score ≥1 in a joint, in the absence of tenderness or swelling) was seen most frequently in the wrist (30.6% of joints), knee (21.4% of joints), MTP1 (26.5% of joints), and MTP2 (33.7% of joints). Of note, the GS component of the US changes was driving the scores in the MTP joints, which may suggest an element of mechanical/noninflammatory etiology in the MTP joints rather than active PsA alone. However, high proportions of other joints, particularly the wrists and knees, showed both GS synovial hypertrophy and PD signal, indicating increased blood flow. Clinically, this finding of such significant subclinical disease is particularly important, since US evidence of subclinical synovitis led to 81.8% of oligoarthritis patients being reclassified as having polyarthritis. Some tender and/or swollen joints were not found to have US evidence of disease activity. This may result from tenderness related to surrounding structures such as the entheses/bursae, misinterpretation of swelling by the clinical assessor, or limited sensitivity of US to identify synovitis. The last issue is particularly of concern in larger joints such as the shoulder, ankle, and elbow, where a higher discordance was found.
Limitations of our study include the scanning protocol and the use of NSAIDs by some patients. It was not possible to blind the ultrasonographer to the presence of psoriatic plaques adjacent to scanned joints, which may have led to increased attribution of pathology in the respective joints. Although 40 joints were scanned in each patient, there were some joints not assessed by US, and the prevalence of subclinical disease in these joints is unknown. This study did not address the significance of subclinical disease in terms of the potential for structural progression. It is not currently known whether there is a particular level of subclinical disease that results in joint damage in PsA, although this area has been addressed in RA (10). Further work is required to answer this important research question. This study also only aimed to address peripheral joint disease activity and did not address other domains of the disease such as skin and entheseal disease or the overall impact of disease on patients. These other factors should be taken into account when addressing disease activity and severity in patients with PsA.
With respect to NSAID use, overall there was slightly better agreement between clinical and US assessment in NSAID users compared with non-NSAID users. A greater proportion of NSAID users had synovitis when assessed by US. This higher frequency of synovitis may be expected, since patients with worse symptoms are more likely to be taking NSAIDs and there may still be an underestimation if some synovitis is suppressed by these therapies. This is a cross-sectional rather than longitudinal comparison; therefore, the results do not allow us to infer about the suppressive effects of NSAIDs on synovitis. However, the use of NSAIDs did not greatly affect the levels of subclinical synovitis or the overestimation of clinical synovitis, ensuring that there was no major bias introduced by those patients taking NSAIDs in the cohort.
These study data are in line with studies showing evidence of subclinical joint inflammation in cohorts of psoriasis (11) and psoriasis “with joint symptoms” (15), which may represent a disease continuum with early PsA. Naredo et al demonstrated US synovitis in 3.2% of joints in their psoriasis cohort compared to 1.3% in controls (P < 0.0005) (11). Nigg et al, using US in their cohort of psoriasis patients with joint symptoms, showed that 52% of joints with a GS score ≥2 and 30% of joints showing PD activity were clinically inactive (15).
Although oligoarthritis accounts for approximately 30% of cases of PsA, it has been less studied than polyarthritis, and this lack of research impacts the availability of evidence-based therapies. In an early US study of 80 patients with oligoarthritis (<12 months of symptoms), 13% (107 of 826) of asymptomatic (nonpainful) joints had US-detected synovitis (7). Interestingly, 36% of their patients with oligoarthritis were reclassified as polyarticular following the US assessment. The number of patients with evidence of psoriasis was not reported in that study, nor was a Doppler US technique employed. Ten-year outcomes in a cohort of patients with oligoarthritis have shown a significant impact on function and quality of life, with poorer results seen for those with PsA (21). The prevalence of subclinical synovitis in this cohort may partly explain the relatively poor outcomes for those with oligoarticular PsA. Given that current treatment recommendations published by the EULAR recommend stratifying the treatment algorithm by oligo- and polyarticular disease (20), the existence of such significant subclinical synovitis may alter treatment decisions for patients.
In summary, subclinical synovitis was a common finding in this study of early PsA, leading to reclassification of a high number of patients from oligoarthritis to polyarthritis. These preliminary data require confirmation in a large study with longitudinal followup to determine the effect on structural progression.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Conaghan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Freeston, Coates, Emery, Helliwell, Conaghan.
Acquisition of data. Freeston, Coates, Nam, Moverley, Emery, Helliwell.
Analysis and interpretation of data. Freeston, Coates, Hensor, Wakefield, Emery, Conaghan.
REFERENCES
- 1.Lard LR, Visser H, Speyer I, van der Horst-Bruinsma IE, Zwinderman AH, Breedveld FC, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111:446–51. doi: 10.1016/s0002-9343(01)00872-5. [DOI] [PubMed] [Google Scholar]
- 2.Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford) 2004;43:906–14. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Mease PJ, Krueger G, van der Heidje DM, Antoni C, Helliwell PS, et al. Outcome measures in psoriatic arthritis. J Rheumatol. 2005;32:2262–9. [PubMed] [Google Scholar]
- 4.Weiner SM, Jurenz S, Uhl M, Lange-Nolde A, Warnatz K, Peter HH, et al. Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis: a comparison with radiography, MRI and scintigraphy. Clin Rheumatol. 2008;27:983–9. doi: 10.1007/s10067-008-0835-y. [DOI] [PubMed] [Google Scholar]
- 5.Wiell C, Szkudlarek M, Hasselquist M, Moller JM, Vestergaard A, Norregaard J, et al. Ultrasonography, magnetic resonance imaging, radiography, and clinical assessment of inflammatory and destructive changes in fingers and toes of patients with psoriatic arthritis. Arthritis Res Ther. 2007;9:R119. doi: 10.1186/ar2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug–induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761–73. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- 7.Wakefield RJ, Green MJ, Marzo-Ortega H, Conaghan PG, Gibbon WW, McGonagle D, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63:382–5. doi: 10.1136/ard.2003.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandjbakhch F, Conaghan PG, Ejbjerg B, Haavardsholm EA, Foltz V, Brown AK, et al. Synovitis and osteitis are very frequent in rheumatoid arthritis clinical remission: results from an MRI study of 294 patients in clinical remission or low disease activity state. J Rheumatol. 2011;38:2039–44. doi: 10.3899/jrheum.110421. [DOI] [PubMed] [Google Scholar]
- 9.Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 10.Gandjbakhch F, Foltz V, Mallet A, Bourgeois P, Fautrel B. Bone marrow oedema predicts structural progression in a 1-year follow-up of 85 patients with RA in remission or with low disease activity with low-field MRI. Ann Rheum Dis. 2011;70:2159–62. doi: 10.1136/ard.2010.149377. [DOI] [PubMed] [Google Scholar]
- 11.Naredo E, Moller I, de Miguel E, Batlle-Gualda E, Acebes C, Brito E, et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford) 2011;50:1838–48. doi: 10.1093/rheumatology/ker078. [DOI] [PubMed] [Google Scholar]
- 12.Gisondi P, Tinazzi I, El-Dalati G, Gallo M, Biasi D, Barbara LM, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67:26–30. doi: 10.1136/ard.2007.075101. [DOI] [PubMed] [Google Scholar]
- 13.De Filippis LG, Caliri A, Lo Gullo R, Bartolone S, Miceli G, Cannavo SP, et al. Ultrasonography in the early diagnosis of psoriasis-associated enthesopathy. Int J Tissue React. 2005;27:159–62. [PubMed] [Google Scholar]
- 14.Erdem CZ, Tekin NS, Sarikaya S, Erdem LO, Gulec S. MR imaging features of foot involvement in patients with psoriasis. Eur J Radiol. 2008;67:521–5. doi: 10.1016/j.ejrad.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Nigg AP, Malchus AM, Grunke M, Witt M, Prinz JC, Schulze-Koops H. Detection of inflammation in early psoriatic arthritis by ultrasound: a longitudinal study [abstract] Arthritis Rheum. 2011;63(Suppl):S68. [Google Scholar]
- 16.Helliwell P, Marchesoni A, Peters M, Barker M, Wright V. A re-evaluation of the osteoarticular manifestations of psoriasis. Br J Rheumatol. 1991;30:339–45. doi: 10.1093/rheumatology/30.5.339. [DOI] [PubMed] [Google Scholar]
- 17.Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78. doi: 10.1016/0049-0172(73)90035-8. [DOI] [PubMed] [Google Scholar]
- 18.Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA): an analysis of 220 patients. Q J Med. 1987;62:127–41. [PubMed] [Google Scholar]
- 19.Torre Alonso JC, Rodriguez Perez A, Arribas Castrillo JM, Ballina Garcia J, Riestra Noriega JL, Lopez Larrea C. Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Br J Rheumatol. 1991;30:245–50. doi: 10.1093/rheumatology/30.4.245. [DOI] [PubMed] [Google Scholar]
- 20.Gossec L, Smolen JS, Gaujoux-Viala C, Ash Z, Marzo-Ortega H, van der Heijde D, et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis. 2012;71:4–12. doi: 10.1136/annrheumdis-2011-200350. [DOI] [PubMed] [Google Scholar]
- 21.Bennett AN, Marzo-Ortega H, Tan AL, Hensor EM, Green M, Emery P, et al. Ten-year follow-up of SpA-related oligoarthritis involving the knee: the presence of psoriasis but not HLA-B27 or baseline MRI bone oedema predicts outcome. Rheumatology (Oxford) 2012;51:1099–106. doi: 10.1093/rheumatology/ker420. [DOI] [PubMed] [Google Scholar]
- 22.Coates LC, Navarro-Coy N, Brown SR, Brown S, McParland L, Collier H, et al. The TICOPA protocol (TIght COntrol of Psoriatic Arthritis): a randomised controlled trial to compare intensive management versus standard care in early psoriatic arthritis. BMC Musculoskelet Disord. 2013;14:101. doi: 10.1186/1471-2474-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 24.Bruyn GA, Naredo E, Moller I, Moragues C, Garrido J, de Bock GH, et al. Reliability of ultrasonography in detecting shoulder disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:357–61. doi: 10.1136/ard.2008.089243. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt WA. Doppler sonography in rheumatology. Best Pract Res Clin Rheumatol. 2004;18:827–46. doi: 10.1016/j.berh.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, et al. Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–9. doi: 10.1136/ard.60.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helliwell PS, Hetthen J, Sokoll K, Green M, Marchesoni A, Lubrano E, et al. Joint symmetry in early and late rheumatoid and psoriatic arthritis: comparison with a mathematical model. Arthritis Rheum. 2000;43:865–71. doi: 10.1002/1529-0131(200004)43:4<865::AID-ANR18>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Zayat AS, Conaghan PG, Sharif M, Freeston JE, Wenham C, Hensor EM, et al. Do non-steroidal anti-inflammatory drugs have a significant effect on detection and grading of ultrasound-detected synovitis in patients with rheumatoid arthritis? Results from a randomised study. Ann Rheum Dis. 2011;70:1746–51. doi: 10.1136/annrheumdis-2011-200017. [DOI] [PubMed] [Google Scholar]


