Abstract
Introduction:
Secondhand smoke (SHS) is a significant cause of acute respiratory illness (ARI) and 5 times more common in indigenous children. A single-blind randomized trial was undertaken to determine the efficacy of a family centered SHS intervention to reduce ARI in indigenous infants in Australia and New Zealand.
Methods:
Indigenous mothers/infants from homes with ≥1 smoker were randomized to a SHS intervention involving 3 home visits in the first 3 months of the infants’ lives (plus usual care) or usual care. The primary outcome was number of ARI-related visits to a health provider in the first year of life. Secondary outcomes, assessed at 4 and 12 months of age, included ARI hospitalization rates and mothers’ report of infants’ SHS exposure (validated by urinary cotinine/creatinine ratios [CCRs]), smoking restrictions, and smoking cessation.
Results:
Two hundred and ninety-three mother/infant dyads were randomized and followed up. Three quarters of mothers smoked during pregnancy and two thirds were smoking at baseline (as were their partners), with no change for more than 12 months. Reported infant exposure to SHS was low (≥95% had smoke-free homes/cars). Infant CCRs were higher if one or both parents were smokers and if mothers breast fed their infants. There was no effect of the intervention on ARI events [471 intervention vs. 438 usual care (reference); incidence rate ratio = 1.10, 95% confidence intervals (CI) = 0.88–1.37, p = .40].
Conclusions:
Despite reporting smoke-free homes/cars, mothers and their partners continue to smoke in the first year of infants’ lives, exposing them to SHS. Emphasis needs to be placed on supporting parents to stop smoking preconception, during pregnancy, and postnatal.
Introduction
In Australia and New Zealand (NZ), deaths and hospitalization among indigenous children aged 0–4 years, due to acute respiratory illness (ARI), is higher compared with nonindigenous children (1–3). Secondhand smoke (SHS) exposure is the most modifiable risk factor for ARI among these populations. The effect of parental smoking on the frequency and severity of respiratory illness, asthma, otitis media, and chronic middle ear effusion is strongest in younger children (4–11). The World Health Organization has prioritized the need to educate parents about the impact of SHS on children’s health (12), especially among populations with high smoking rates. Indigenous Australians and NZ Māori are twice as likely to smoke as their nonindigenous counterparts (13,14), and thus indigenous children in these countries have greater SHS exposure (3,15,16).
A systematic review of 36 randomized trials that investigated family/carer tobacco control programs for reducing children’s exposure to SHS found insufficient evidence to support any one intervention (17). However, a modest effect was found for intensive parental counseling that focused on changing attitudes and behaviors, premised on behavior change theory (18), as opposed to changing knowledge alone. More recent trials support this finding (19,20). Qualitative research on smoking in remote Northern Territory Aboriginal communities has found that indigenous parents/carers are concerned about the health effects of SHS (21,22). In addition to positive role modeling, the health of their children is their primary motivation to quit (23). NZ Māori similarly stop smoking for the benefit of their children (24,25). These data suggest that a family-based SHS intervention that focuses more on the welfare of children as opposed to adults stopping smoking may be particularly salient among these two indigenous populations. Thus, we undertook a randomized trial with blinded outcome assessment to determine the impact of a culturally appropriate, family-centered SHS program (where we involved as many extended family members as possible and focused on strategies for reducing SHS exposure in children, which included positive role modeling, within family support and smoking cessation support for all) on the respiratory health of indigenous infants in Australia and NZ. We hypothesized that such a program would reduce the number of ARI-related visits to a health provider in the first year of infants’ lives.
Methods
Participants
The rationale and methodology for this trial has been reported previously (26) and is summarized below. Between December 2009 and January 2012, Community Workers (CWs) in Darwin, Australia, and Auckland, NZ, approached potentially eligible mothers through antenatal clinics and hospital birth records. Mother/infant dyads were eligible if (a) the infant was aged between 0–5 weeks; (b) the mother self-identified as Māori or Australian Aboriginal/Torres Strait Islander; (c) the mother was aged ≥16 years; (d) the mother was a current smoker or the infant lived in a household where there was at least one other smoker; (e) the mother resided permanently with the infant in Darwin/Greater Darwin area of Australia or within the Counties Manukau District Health Board region, NZ; (f) the infant was a singleton or the first born in a multiple pregnancy delivery; and (g) the mother spoke English and/or Māori. Ethics approval was obtained from the Menzies Human Research Ethics Committee (Australia) and the Northern Region Human Ethics Committee (NZ).
Randomization and Blinding
Eligible and interested mothers were consented and mother/infant dyads were randomized in a 1:1 ratio to one of two arms by central computer, using blocked randomization stratified by country (Australia, NZ). Participants were not blinded to treatment allocation, but research staff assessing the primary outcome were blinded.
Intervention
The control group received “usual care” comprising standard management by hospital and primary care providers, which ranged from brief quit advice to the provision of cessation treatment. In the intervention group, all mothers (and family members that were present) who smoked received usual care plus behavioral “coaching” about the dangers of SHS exposure to children, commitment to smoking restrictions in the home/car, positive role modeling, and strategies for overcoming obstacles to making smoke-free changes. Those who smoked were also given either brief advice or more intensive counseling to quit (depending on how receptive they were) and offered free nicotine replacement therapy (NRT; 21mg patches and/or 4mg gum) and/or a Quitline referral, unless it became clear as part of the conversation with them that they were not interested in such options. The program was founded on Māori and Aboriginal holistic models of health (24,27,28) and was delivered by CWs (who were mainly indigenous and received identical training in motivational interviewing and program delivery) through three face-to-face home visits conducted over the first 3 months of the infants’ lives. Both groups received brief health promotion messages (focused on immunization, infant nutrition/breast feeding, and safe sleeping for baby) from the CWs at baseline and when the infants were 4 and 12 months of age.
Outcomes
Baseline data were collected through a face-to-face visit at the mothers’ homes, as close as possible to 6 week after the infants’ birth. Baseline measures are described in detail in the published protocol (26). In the intervention group, at baseline, 2 months, and 3 months, a mix of quantitative and qualitative process evaluation indicators was collected related to the program delivery, including the amount of the program that was delivered, commitment to smoke-free changes, and parent satisfaction. No information was collected on usual care practices.
Outcome data were collected at 4 and 12 months of age via a face-to-face visit at mothers’ homes. The primary outcome was rate of health provider presentations for new primary episodes of ARI in the first year of life, obtained from the mothers and confirmed by two study clinicians in each country (blinded to treatment allocation), who reviewed the infants’ health provider and hospital clinic records. In Australia, 20 participants had their primary outcome data reviewed by clinicians in NZ, in order to assess intercountry reliability. Secondary outcomes included rate of hospitalizations for ARI, mothers’ breast feeding status (29) and self-report of smoking restrictions in the home (“Is smoking ever allowed inside the house?”—reported as Yes/No, but multiple options were allowed, e.g., Yes—but restricted to certain rooms only, Yes—in any room but only when the infant is not there) and car (“Is smoking allowed inside any car when your infant is in it?”—Yes/No option). Data were also collected on the mothers’ report of their infants’ exposure to SHS in the last 7 days, specifically whether the infant had been near (within arm’s length) people smoking cannabis or an open fire used for cooking/heating or a camp fire, whether they had been around tobacco smoke (e.g., in the same room in a house as someone that was smoking, in a car with someone that was smoking, or sitting outside within arm’s length of someone who was smoking), and whether they had been cared for in other houses or childcare where people smoked. Mothers’ self-report of a quit attempt was also asked, defined as not smoking a cigarette for ≥24hr, as was the presence of day- and nighttime coughing by the infant over the last 2 days (using verbal category descriptive scores from 0–5, as per Chang et al. (30).
To confirm SHS exposure in the last 2–3 days, a single urine sample was collected from each infant (31) at baseline, 4 months, and 12 months of age (26). Samples were tested for urinary cotinine and creatinine using gas chromatography/mass spectrometry. Results are expressed as the cotinine/creatinine ratio (CCR, ng/mg), with values of ≥30ng/mg indicating that the infant was exposed to SHS (sensitivity 80%, specificity 100%) (32).
Sample Size/Analyses
A study of disease burden and clinic attendances for young indigenous children in two remote Northern Territory communities found the median number of presentations for upper respiratory illness in the first year of life was 7.5 (interquartile range 4–11) and for lower respiratory illness, 2.5 (interquartile range 1–5) (33). Our clinical experience with infants and limited data from children suggested that there would be much fewer episodes of ARI in urban compared with remote settings (34). Thus, it was estimated that an average of three visits per year would occur in the control group. Each population had a sample size estimate of 210 mother/infants dyads, which provided 90% power (p = .05) to detect a 25% reduction in new episodes of ARI in the intervention group compared with the usual care group (based on three health provider visits per year in the usual care group and 2.25 visits in the intervention group), assuming a Poisson distribution and a 10% loss to follow-up. Combining data from the two countries (n = 420) provided 90% power (p = .05) to detect an 18% reduction in the primary outcome.
All analyses were undertaken using SAS Version 9.3. Complete case analysis was undertaken, and sensitivity analyses using a modified intention-to-treat (ITT) approach (excluding those randomized but who did not enter the protocol) with a multiple imputation method (using 50 imputations) applied to the missing data (35) and analyses adjusted for potential confounding factors. The incidence rate for ARI between the two groups was analyzed using negative binomial regression as there was evidence of overdispersion. The incidence rate ratio (IRR) and 95% confidence intervals (95% CI) were reported (with usual care as the reference). Dichotomous outcomes were compared using chi-square tests and continuous outcomes were compared using T-tests or Mann–Whitney tests. Due to the skewed nature of the CCR data, they were log transformed and presented as geometric means. The difference between groups in log-transformed CCR are presented as a ratio of geometric means (95% CI) and adjusted (using linear regression) for infants’ birth weight and baseline measures of CCR, mothers’ age, education, smoking status, breast feeding status, and crowding index. For log-transformed values that were not significantly different, the 95% CI of the ratio included 1. Intra-rater and intercountry agreement for grading of the primary outcome assessment were assessed using the Kappa statistic (unweighted).
Results
Overall, 228 mother/infant dyads were recruited in NZ (115 intervention, 113 usual care) and 93 in Australia (46 intervention, 47 usual care). Recruitment took 14 months in NZ and stopped at 28 months in Australia due to not reaching the recruitment target within the budgeted timeframe. More potential participants in Australia declined to participate (56%) than those in NZ (31%, p < .001), and more were noncontactable in NZ (39% NZ vs. 7% Australia, p < .001). Rates of withdrawal and ineligibility after randomization and before baseline assessment did not differ between country or by treatment group (Figure 1).
Figure 1.
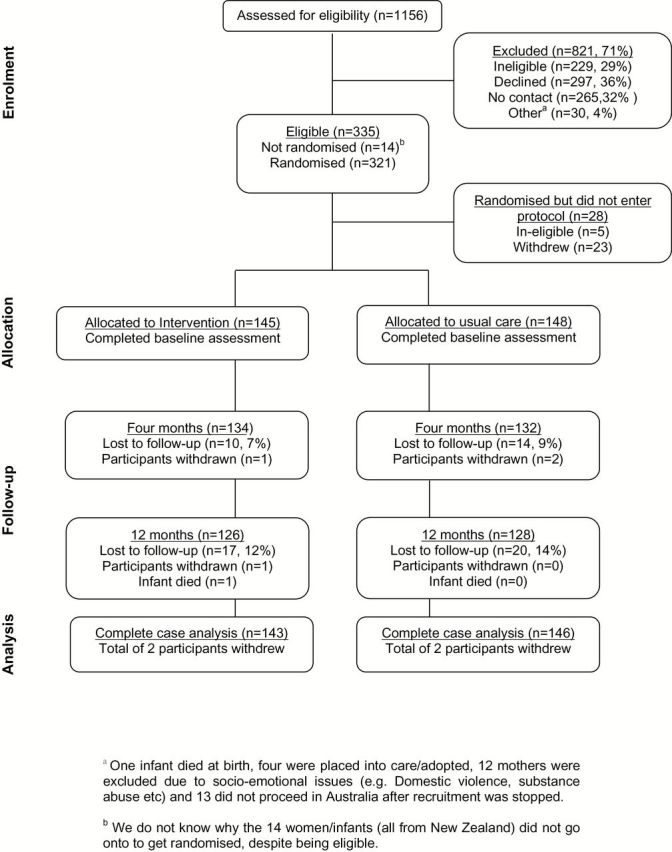
Flowchart of recruitment and retention of participants throughout the trial (New Zealand and Australia combined).
A total of 293 participants were available for follow-up, with a total 12-month loss-to-follow-up (including withdrawal and death) of 13% (39/293) and with no significant difference by treatment group, country, or their interaction. No significant differences in baseline data between the two countries were found, and thus the two data sets were combined (Table 1), with results presented hereafter.
Table 1.
Baseline Characteristics of Infant and Mother (New Zealand and Australia Combined)
| Variables | Intervention group, N = 145 (%) | Usual care group, N = 148 (%) |
|---|---|---|
| Infants: female | 58 (40) | 68 (46) |
| Infants: mean age at baseline, weeks (SD) | 6.3 (2.7) | 6.0 (2.7) |
| Infants: country | ||
| New Zealand | 108 (74) | 108 (73) |
| Australia | 37 (26) | 40 (27) |
| Infants: mean gestational age at birth, weeks (SD) | 39.3 (1.3) | 39.3 (1.5) |
| Infants: mean birth weight, kilograms (SD) | 3.3 (0.5) | 3.3 (0.6) |
| Infants: unwell since birtha | 44 (30) | 36 (24) |
| Infants: coughing | ||
| No daytime cough | 111 (77) | 119 (80) |
| No nighttime cough | 123 (85) | 128 (87) |
| Mothers: mean age at baseline in years (SD) | 26.8 (6.5) | 25.3 (5.8) |
| Mothers: highest level of education | ||
| ≤Secondary school | 104 (72) | 114 (77) |
| TAFE/polytechnic/university | 41 (28) | 34 (23) |
| Mothers: marital statusb | ||
| Married/defacto/living with partner | 72 (50) | 91 (62) |
| Divorced/separated/widowed | 8 (6) | 20(14) |
| Never married | 44 (30) | 25 (17) |
| Refused to answerc | 21 (15) | 12 (8) |
| Mothers: breast feeding state | ||
| Yes—exclusive | 43 (30) | 49 (33) |
| Yes—full | 29 (20) | 31 (21) |
| Yes—partial | 42 (29) | 40 (27) |
| No | 31 (21) | 28 (19) |
| Mothers: smoked during pregnancy | 114 (79) | 105 (71) |
| Mothers: reduced amount smoked during pregnancye | 74 (65) | 71 (68) |
| Mothers: current smoking status | ||
| Current smoker | 105 (72) | 88 (60) |
| Ex-smoker | 22 (15) | 37 (25) |
| Never smoked | 18 (12) | 23 (16) |
| Mothers: frequency of smokingf | ||
| At least weekly | 95 (90) | 83 (94) |
| Less than weekly | 10 (10) | 5 (6) |
| Mothers: number of cigarettes smoked per dayf | ||
| ≤10 | 54 (51) | 63 (72) |
| 11–20 | 38 (35) | 20 (23) |
| 21–30 | 6 (6) | 2 (2) |
| ≥31 | 1 (1) | 1 (1) |
| Missing data | 6 (6) | 2 (2) |
| Mothers: time to first cigarettef | ||
| ≤30min of waking | 48 (49) | 31 (37) |
| >30min of waking | 50 (51) | 53 (63) |
| Mothers: quit attempt in last 12 monthsf | 49 (47) | 44 (50) |
| Mothers: mean self-efficacy score (SD)g | 3.3 (0.8) | 3.3 (0.8) |
| Household: mean crowding index (SD)d | 2.0 (0.7) | 2.0 (0.7) |
| Household: mean number of children in house aged under 5 years (SD) | 1.9 (1.0) | 1.9 (1.0) |
SD = standard deviation; TAFE = Technical and Further Education Institution.
aThe specific question was “Has your infant been unwell and needed to go to the health clinic, general practitioner or hospital since he/she was born?”
b X 2; p = .002.
cAll but three participants were from New Zealand. In New Zealand, different levels of social support are offered depending on marital status. It is likely that some women in the study did not wish to disclose their marital status, in case their access to certain social support was jeopardized.
dDefined as the number of people currently sleeping in the house divided by the number of rooms in the house where people were sleeping
eIn those that smoked during pregnancy.
fIn current smokers.
gBelief in their ability to quit this time, measured on a scale of 1–5, where one was very low and five was very high.
In the intervention group at baseline, 2 months, and 3 months, all but 2, 8, and 17 households, respectively, received the intervention. Not all parts of the program were implemented in every household, due to the mother and/or family members wanting to move the conversation on or stop the interview. Across the baseline, 2-month and 3-month visits, only 2%–7% of the mothers/family members failed to agree to smoking restrictions inside the home and car.
Primary Outcome
The rate of health provider presentations for new primary episodes of ARI in the first year of life did not differ significantly between the groups (intervention: 471 events; usual care: 438 events; IRR = 1.10, 95% CI = 0.88–1.37, p = .40). Results were similar for ITT analysis (IRR = 1.10, 95% CI = 0.88–1.36, p = .41) and after adjusting for infants’ birth weight, mother’s age at baseline, education, smoking status, breast feeding status, and crowding index (IRR = 1.07, 95% CI = 0.86–1.34, p = .53). No differences in the primary outcome were found according to new episodes of upper respiratory tract infection (intervention: 315 events; usual care: 278 events; IRR = 1.16, 95% CI = 0.92–1.46, p = .22), lower respiratory tract infection (intervention: 147 events; usual care: 167 events; IRR = 0.90, 95% CI = 0.65–1.25, p = .53), otitis media (intervention: 105 events; usual care: 95 events; IRR = 1.13, 95% CI = 0.74–1.73, p = .58), or rate of hospitalizations for ARI (intervention: 53 events; usual care: 44 events; IRR = 1.23, 95% CI = 0.70–2.15, p = .47). Between country, interrater agreements for lower respiratory tract infection, upper respiratory tract infection, and otitis media were high (κ = 0.84, 0.78, and 0.79, respectively).
Secondary Outcomes
Mothers’ Report on Infants’ Exposure to SHS
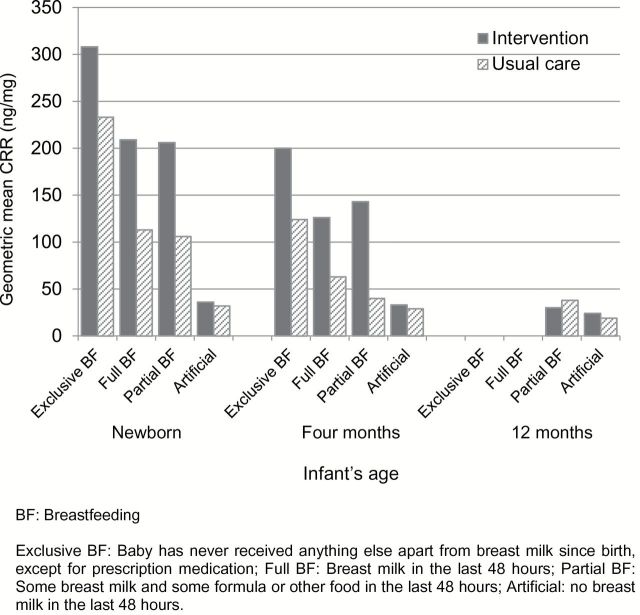
Three quarters of mothers smoked during their pregnancy, and two thirds were current smokers at baseline (Table 1). No significant change in smoking prevalence and intensity was seen by group over the first year of the infants’ lives (Table 2). Of the 238 mothers (80%) who had a partner at baseline, 164 (69%) reported that their partner smoked, with no difference in this proportion by group or over time (Table 2). The geometric mean CCRs were significantly higher if parents smoked (mother and partner smoked: 365ng/mg; mother only smoked: 245ng/mg; partner only smoked: 27ng/mg) compared with neither parent smoking (25ng/mg; p < .0001). Breast feeding rates were high at baseline, with this proportion declining over time and no difference noted by group (Table 2). Mean CCRs were significantly higher at baseline and 4 months in those mothers breast feeding their infants compared with artificial feeding (both p < .001), with no difference at 12 months (p = .09; Figure 2). Overall, mean CCRs declined over time to <30ng/mg by 12 months of age, indicative of little SHS exposure (Table 2). After adjusting for baseline variables (including baseline CCR), there was no significant difference in mean CCRs between the groups at 4 months (ratio of geometric mean difference = 1.39, 95% CI = 0.99–1.94, p = .06) or 12 months of age (ratio of geometric mean difference = 0.97, 95% CI = 0.69–1.37, p = .87).
Table 2.
Secondhand Smoke Exposure Over Time (New Zealand and Australia Combined)
| Baseline | 4 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| Intervention, n/N (%) | Usual care, n/N (%) | Intervention, n/N (%) | Usual care, n/N (%) | Intervention, n/N (%) | Usual care, n/N (%) | |
| Full smoking ban in home | 136/145 (94) | 141/148 (95) | 125/134 (93) | 123/132 (93) | 117/126 (94) | 120/128 (95) |
| Smoking ban in car | 140/145 (97) | 143/148 (97) | 130/134 (97) | 129/132 (98) | 119/126 (95) | 123/128 (97) |
| Mother is current smoker | 105/145 (72) | 88/148 (60) | 94/134 (70) | 78/132 (59) | 83/126 (66) | 70/128 (55) |
| Mother smokes ≤20 per day | 92/105 (88) | 83/88 (94) | 87/94 (93) | 74/78 (95) | 73/83 (88) | 64/70 (91) |
| Partner is current smokera | 83/116 (72) | 81/122 (66) | 75/109 (69) | 72/108 (67) | 59/99 (60) | 72/109 (66) |
| Infant is breast fedb | 114/145 (79) | 120/148 (81) | 83/134 (62) | 87/132 (66) | 34/126 (27) | 34/128 (27) |
| Members of the household smoke inside | 13/145 (9) | 11/148 (7) | 10/134 (8) | 13/132 (10) | 10/126 (8) | 7/128 (5) |
| In last seven days, infant has been cared for in another place where people smokec | 16/145 (11) | 26/148 (18) | 10/134 (8) | 11/132 (8) | 13/126 (10) | 10/128 (8) |
| In last seven days, infant has been around tobacco smokec, d | 17/145 (12) | 20/148 (14) | 14/134 (10) | 10/132 (8) | 23/126 (18) | 15/128 (12) |
| In last seven days, infant has been near (within arm’s length) of an open fire for cooking or heating or camp firec | 5/145 (3) | 6/148 (4) | 11/134 (8) | 5/132 (4) | 2/126 (2) | 7/128 (5) |
| In last seven days, infant has been near (within arm’s length) of people smoking cannabisc | 0/145 (0) | 3/148 (2) | 3/134 (2) | 1/132 (1) | 0/126 (0) | 1/128 (1) |
| Geometric mean cotinine/creatinine ratio (ng/mg)e | 157.6 | 112.2 | 84.8 | 47.5 | 25.0 | 21.8 |
aIn those mothers with partners.
bDefined as breast fed exclusively, fully, or partially.
cDefined as number of infants with ≥1 day of exposure.
dDefined as in the same room with someone that is smoking, in a car with someone smoking, or sitting outside within arm’s length of someone who is smoking—includes when holding the infant.
eReadings of ≥30ng/mg indicate secondhand smoke exposure.
Figure 2.
Cotinine/creatinine ratio in infants’ urine, according to feeding status over time.
Smoking bans were reportedly well established with ≥95% smoke-free homes/cars at baseline, and mothers’ report of infants’ exposure to SHS in the last 7 days was relatively low. Both variables did not differ by group and did not significantly change over time (Table 2). Overall, in the last 7 days ≤18% of infants were potentially exposed to SHS through being cared for in other places where people smoke, being in the same room in a house with someone that was smoking, being in a car with someone that was smoking, or sitting outside within arm’s length of someone who was smoking (Table 2). In the last 7 days, ≤8% of infants had been near an open fire for cooking/heating, or a camp fire, and ≤2% of infants had been near people smoking cannabis (Table 2).
Quitting Behavior
At baseline, almost half of the 193 mothers that were current smokers had tried to quit smoking in the last 12 months (Table 1). When asked about their belief in their ability to quit smoking this time, measured on a scale of 1–5, where one was very low and five was very high, mothers had a mean self-efficacy score of 3.3 (SD = 0.8; Table 1). Across the baseline, 2-month, and 3-month visits, 24%–30% of the mothers in the intervention group that smoked agreed to quit smoking (p = .55), 65%–87% were offered free NRT (70% acceptance at baseline, which was significantly more than at 2 and 3 months: 32% and 41%, respectively, p = .0004), and 20%–43% were offered Quitline referrals (2-month data were significantly greater than those at baseline and 1 month: p = .0006, all refused the offer at baseline and 2 months; 7% accepted the offer at 3 months). Follow-up of infants at 4 and 12 months of age showed no difference between the groups in mothers’ quitting behavior over time. At 4 months, 24% (41/172) of the current smokers had made a quit attempt, and 33% (51/153) at 12 months. No difference in 7-day point prevalence abstinence for mothers was seen between the two groups when their infants were 4 months (n = 25, 19% intervention vs. n = 34, 26% usual care, p = .16) or 12 months of age (n = 22, 18% intervention vs. n = 33, 26% usual care, p = .10).
As part of the intervention program, other family members that smoked were also supported to quit smoking. When the infants were ~1 month old, in 37 families other members of the household (N = 58, mean = 1.6 people/household, SD = 0.8) were given a brief cessation intervention or more intensive counseling to stop smoking, of which 32 people in 17 (50%) families agreed to quit. From 53 households, a total of 73 family members were offered free NRT (of which 61 people or 84% accepted). Thirteen people from 12 households were offered a referral to Quitline (of which none accepted). Similar findings were found when the infants were seen at 2 and 3 months of age (data not reported).
Other Outcomes
Across the baseline (Table 1), 4-month, and 12-month visits, 77%–88% of infants had no parent-reported daytime cough and 75%–93% had no nighttime cough, with no statistical differences over time or by group. The majority (n = 126, 93%) of the 135 mothers in the intervention group who were interviewed when their infant was 3 months old felt that the program was “helpful” or “very helpful” for reducing SHS exposure in their child. Almost all (n = 131, 97%) were “satisfied” or “very satisfied” with the program.
Discussion
This trial tested whether a family-based SHS intervention focusing predominately on the health of infants, as opposed to smoking cessation in adults, had any effect on the number of health provider presentations by infants for ARI over a 12-month period. Although previous research suggested that such an intervention would lead to reduced exposure to SHS for infants (17,36), we found no effect on SHS exposure, parental smoking, or ARI. Mothers reported exposure of the infant to SHS was low. However, mean urinary CCRs in the infants at baseline and at 4 months of age were consistent with SHS exposure. A recent U.S. trial (n = 138) looking at the effectiveness of a community-based motivational intervention to reduce SHS exposure in children under six in low-income communities reported similar findings (37).
Possible explanations for why the trial infants had high CCRs in the first 4 months of life are addressed below and include exposure to maternal nicotine/cotinine via breast milk, and/or unreported or underestimated SHS exposure by mothers. It is unlikely that the CCRs observed can be solely explained by high smoking rates in pregnancy, as prenatal exposure to nicotine is not measureable 3–6 weeks after birth (38), which was when the first urine samples were collected. Elevated CCRs in breast-fed infants has previously been noted (39–42) and is likely due to the transmission of nicotine metabolites in the milk of mothers who smoked and/or were exposed to high levels of SHS.
Breast milk cotinine levels have been shown to peak 30–60min after smoking 1–2 cigarettes and dissipate after 3hrs (43). Cotinine does not appear to have any pharmacological or toxicological properties of concern and thus is unlikely by itself to cause any adverse health effects for infants (42). However, it is unknown whether carcinogenic substances present in SHS are transferred to breast milk and thus to infants. Breast feeding has been shown to modify the effect of maternal smoking, such that the risk of ARI is decreased (44). As the infants aged and became increasingly mobile, their mean CCRs declined. Use of NRT by mothers trying to quit smoking may also have resulted in increased nicotine exposure for infants via breast milk (45), leading to increased CCRs. Approximately 50% of mothers in the intervention arm were offered NRT, but we did not record whether they used it, and it is unknown how many mothers in the usual care arm were offered and used NRT as part of usual care.
Unreported SHS exposure may also be an explanation for the elevated CCRs. Previous research has shown that the association between infant cotinine levels and parental smoking is in part due to cosleeping and minimum room temperature (46). We did not consider these variables and thus are unable to comment on their influence. Underestimating SHS exposure is another possibility. Some infants may have been exposed to SHS in spite of families having smoke-free rules, or without their mother recalling this exposure.
Some of the trials’ strengths are that it was conducted in line with CONSORT guidelines, assessment of the primary outcome was blinded, and family members were involved. The complex reasons for the slow recruitment (especially in Australia) and high participant retention rate are discussed in a recent paper by Glover et al. (47). A number of limitations should be acknowledged: (a) mothers’ reported infant SHS exposure and breast feeding status may be affected by social desirability bias. Advertising campaigns ran in both countries before and during the study period promoting the importance of breast feeding, smoking cessation, and not exposing children to SHS. Consequently, breast feeding may have been over-reported and SHS exposure under-reported, biasing the results toward the null (although not differentially). No measurements of home/car air quality were taken as verification of reported infant SHS exposure; (b) the number of ARI events was considerably lower than previously reported in a remote indigenous community setting (48), meaning our findings may not be generalizable to populations with higher rates of ARI. In Darwin, we hand-searched all hospital records and the primary care records of the largest Aboriginal Medical Service. However, we may have missed cases that attended mainstream primary care services and were not reported by mothers. In NZ, we electronically searched primary care and hospital records within the region for ARI cases but with limited data linkage cases of ARI that occurred outside of the region may have been missed (again, this bias was unlikely to be differential); (c) the mothers that we were unable to contact or those that declined to participate may have been the ones who did not have strong smoke-free policies in place and thus were the ones this intervention could have helped the most; (d) the total number of cigarettes smoked in the home is reported as a key predictor of cotinine levels in children (49). However, we did not assess this variable as indigenous Australians report significant sharing of cigarettes (23), making accurate measurement of “cigarettes smoked per day” difficult. In hindsight, we should have asked this question and acknowledged the potential misclassification; (e) as part of the intervention, information was provided to mothers about the negative health effects (including ARI) of SHS exposure on children. It can be hypothesized that mothers increased awareness of ARI may have increased their engagement with health care specialists about their infants’ health, thereby reducing any differences between the groups; (f) the proportion of smoke-free homes and cars reported in this study were much higher than reported by adults in population surveys (3,15,16,50,51), which raises some concerns about the generalizability of our findings to other populations; (g) we did not record use of NRT by mothers trying to quit smoking, so are unable to discuss the effect of NRT use on CCRs; and (h) we did not record “partner” versus “other family member” involvement in the study, so are unable to report findings according to these two groups.
Research has shown that having smoke-free homes (17,36) and smoking outside or away from infants can reduce SHS exposure but does not offer complete protection as the dust, air, and surfaces within homes remains contaminated (52). Future research therefore needs to focus on not only supporting mothers, their partners, and other family members to stop smoking but also on how to reduce children’s exposure to third-hand smoke. Personalized feedback on indoor air quality and CCR levels to families also has the potential to increase the effectiveness of future interventions (19,53). Future research should also investigate why these populations had no interest in Quitline support, although qualitative research from NZ (n = 168, 53% Māori) suggests awareness of the service, time required, and personal relevance may play some role (54).
In summary, our family-centered intervention to reduce exposure to SHS had no effect on rates of ARI in indigenous infants or on smoking and quitting behavior. These findings suggest that simply having smoke-free homes and cars is not sufficient to protect children from exposure to SHS—all household members who smoke should stop smoking from the time of conception and should continue to be smoke-free after the child is born (55). Furthermore, breast feeding should continue to be encouraged, but smoking while breast feeding should be discouraged.
Funding
This work was supported by the National Health and Medical Research Council of Australia (545203); the Health Research Council of NZ (09/626); Cure Kids NZ (3525) and the James Russell Lewis Trust, New Zealand (13787/15734).
Contributors
DT, AC, and VJ conceived the original idea for the trial; NW, DT, VJ, MG, CB, AT, AC, PM, NB, RB, VP, CS, DF, Toni Mason, and Kane Ellis sought funding and wrote the protocol. VJ, TvB, DW, and EH managed the day-to-day running of the trial, including management of staff involved in participant follow-up. CB, AT, VJ, and DT acted as medical reviewers of the clinical records data in the study. VP provided statistical advice for the trial and carried out all data analyses. The paper was written by NW with input from all coauthors. NW will act as guarantor for the paper.
Declaration of interests
All authors declare that (1) no authors have received support from any companies for the submitted work; (2) CB has previously undertaken research on behalf of NicoNovum, but prior to the purchase of the company by RJ Reynolds. NW has provided consultancy to the manufacturers of smoking cessation medications, received honoraria for speaking at a research meeting, and received benefits in kind and travel support from a manufacturer of smoking cessation medications. MG has provided consultancy to the manufacturers of smoking cessation medications; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) all authors have no non-financial interests that may be relevant to the submitted work. NW, CB, MG, and VP have also undertaken two trials of very low nicotine content cigarettes, which were purchased from two different tobacco companies. The companies concerned had no role in development of the study design, data collection, data analysis, data interpretation, or writing of the trial publications.
Acknowledgments
This trial (called Healthy Starts in Australia and Te Piripohotanga in New Zealand) is a collaboration between Menzies School of Health Research, Danila Dilba Health Service, Cancer Council Victoria and Quit Victoria, The University of Auckland (specifically NIHI and the Centre for Tobacco Control Research) and Counties Manukau District Health Board. All data management, study monitoring, and data analysis for the trial was undertaken by NIHI, Auckland, New Zealand. Thanks go to all families/whānau involved in the study. In New Zealand, specific thanks are given to the Eseta Nichols, Kristine Day, Trudy Carle, Julie Nyman, and NIHI staff involved in the study (Colin Howe, John Fa’atui, Stephen Boswell, Terry Holloway, Lyn Cummings, Vanessa Singh, and Sheila Fisher). In addition, thanks are given to Nicola MacDonald, Whakawhetu (Māori SIDS), Betty-Lou Iwikau at Te Kaahui Ora - Māori Health Unit, Community Midwives, Module 10 staff, Middlemore Lab/Super Clinic Lab, LabPlus, Turuki Health Care, Papakura Marae (Health Services), South Auckland region Plunket services, South Auckland GP practices, Quitline, Te Unga Waka Marae, and Radio Waatea. In Australia, specific thanks are given to Kane Ellis, Toni Mason, Elizabeth Heenan, Karen Kairupan, Ramya Ramamoorthi, Patricia Rankine, Cyan Earnshaw, Sian Graham, Helen Kassman-Reid, Leslie Johnson, Danila Dilba, Royal Darwin Hospital, and Darwin general practices. The trial was designed, conducted and analysed by the researchers independent of all funders.
References
- 1. Carville KS, Lehmann D, Hall G, et al. Infection is the major component of the disease burden in Aboriginal and non-Aboriginal Australian children: a population-based study. Pediatr Infect Dis J. 2007;26(3):210–216. [DOI] [PubMed] [Google Scholar]
- 2. O’Grady KAF, Torzillo PJ, Chang AB. Hospitalisation of Indigenous children in the Northern Territory for lower respiratory illness in the first year of life. Med J Aus. 2010;192(10):586–590. https://www.mja.com.au/journal/2010/192/10/hospitalisation-indigenous-children-northern-territory-lower-respiratory-illness Accessed August 5, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Robson B, Harris R. Hauora: Māori Standards of Health IV. A Study of the Years 2000–2005. Wellington, New Zealand: Te Rōpū Rangahau Hauora a Eru Pōmare; 2007. http://www.otago.ac.nz/wellington/research/erupomare/projects/otago019494.html. Accessed August 5, 2014 [Google Scholar]
- 4. Alati R, Mamun AA, O’Callaghan M, Najman JM, Williams GM. In utero and postnatal maternal smoking and asthma in adolescence. Epidemiology. 2006;17:138–144. [DOI] [PubMed] [Google Scholar]
- 5. Anderson HR, Cook DG. Health effects of passive smoking 2: passive smoking and sudden infant death syndrome: review of the epidemiological evidence. Thorax. 1997;52(11):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113(suppl 4):1007–1015. Online ISSN: 1098–4275. [PubMed] [Google Scholar]
- 7. Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108(1):18–24. [DOI] [PubMed] [Google Scholar]
- 8. Fergusson DM, Horwood LJ, Shannon FT, Taylor B. Parental smoking and lower respiratory illness in the first three years of life. J Epidemiol Commun Health. 1981;35:180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haustein K. Smoking and poverty. Eur J Cardiovasc Prev Rehab. 2006;13(3):312–318. [DOI] [PubMed] [Google Scholar]
- 10. Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. 2011;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwok M-K, Schooling CM, Ho L-M, et al. Early life second hand smoke exposure and serious infectious morbidity during the first eight years: evidence from Hong Kong’s “Children of 1997” birth cohort. Tob Cont. 2008;17:263–270. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization. Policy Recommendations on Protection From Exposure to Second-hand Tobacco Smoke: Policy Recommendations. Geneva, Switzerland: World Health Organization; 2007. http://whqlibdoc.who.int/publications/2007/9789241563413_eng.pdf?ua=1. Accessed August 5, 2014. [Google Scholar]
- 13. Borrelli B, McQuaid EL, Novak SP, Hammond SK, Becker B. Motivating Latino caregivers of children with asthma to quit smoking: a randomized trial. J Consul Clin Psychol. 2010;78(1):34–43. [DOI] [PubMed] [Google Scholar]
- 14. Bailey E, Morris P, Kruske S, Cates C, Chang A. Culture-specific programs for children and adults from minority groups who have asthma. Cochr Data Syst Rev. 2009;4. [DOI] [PubMed] [Google Scholar]
- 15. Angstman S, Patten CA, Renner CC, et al. Tobacco and other substance use among Alaska native youth in western Alaska. Am J Health Behav. 2007;31(3):249–260. [DOI] [PubMed] [Google Scholar]
- 16. Australian Bureau of Statistics; Australian Institute of Health and Welfare. The health and welfare of Australia’s Aboriginal and Torres Strait Islander peoples, October 2010 (pp. Cat. No. 4704.4700). Canberra, Australia: Australian Bureau of Statistics; 2010. http://www.abs.gov.au/ausstats/abs@.nsf/mf/4704.0/ Accessed August 5, 2014. [Google Scholar]
- 17. Priest N, Roseby R, Waters E, et al. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochr Data Syst Rev. 2008;(4):1-58. Art. No.: CD001746. [DOI] [PubMed] [Google Scholar]
- 18. Borland R. Background paper: theories of behavior change in relation to environmental tobacco control to protect children. In: International Consultation on Environmental Tobacco Control Smoke and Child Health. Geneva, Switzerland: World Health Organization; 1999. Available at: Last accessed 5 August 2014: pgs 1-16. http://www.who.int/tobacco/media/en/borland.pdfhttp://www.who.int/toh/TFI/consult.htm. [Google Scholar]
- 19. Wilson I, Semple S, Mills LM, et al. REFRESH - reducing families exposure to secondhand smoke in the home: a feasability study. Tob Cont. 2013;22(e8):1–10. [DOI] [PubMed] [Google Scholar]
- 20. Wilson SR, Farber HJ, Knowles SB, Lavori PW. A randomized trial of parental behavioural counseling and cotinine feedback for lowering environmental tobacco smoke exposure in children with asthma. Chest. 2011;139(3):581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gould GS, Munn J, Avuri S, Hoff S, Cadet-James Y, McEwan A, Clough AR. “Nobody smokes in the house if there’s a new baby in it”: Aboriginal perspectives on tobacco smoking in pregnancy and in the household in regional NSW Australia [published online ahead of print September 05, 2013]. Women Birth. 2013;26(4):246–253. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22. Gould GS, Munn J, Watters T, McEwan A, Clough AR. Knowledge and views about materal tobacco smoking and barriers for cessation in Aboriginal and Torres Strait Islanders: a systematic review and meta-ethnography. Nicotine Tob Res. 2013;15(5):863–874. [DOI] [PubMed] [Google Scholar]
- 23. Johnston V, Thomas DP. Smoking behaviours in a remote Australian indigenous community: the influence of family and other factors. Soc Sci Med. 2008;67(11):1708–1716. [DOI] [PubMed] [Google Scholar]
- 24. Glover M. Analysing smoking using Te Whare Tapa Wha. New Zeal J Psychol. 2005;34(1):13–19. http://www.psychology.org.nz/wp-content/uploads/NZJP-Vol341-2005-2-Glover2.pdf. Accessed August 5, 2014. [Google Scholar]
- 25. Glover M, Nosa V, Gentles D, Watson D, Paynter J. Do New Zealand Maori and Pacific ‘walk the talk’ when it comes to stopping smoking? A qualitative study of motivation to quit. J Smok Cess. 2013:1–8. [Google Scholar]
- 26. Johnston V, Walker N, Thomas D, et al. The study protocol for a randomized controlled trial of a family-centred tobacco control program about environmental tobacco smoke (ETS) to reduce respiratory illness in Indigenous infants. BMC Pub Health. 2010;10(114):1–10. http://www.biomedcentral.com/1471–2458/10/114 Accessed August 5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Aboriginal Health Strategy Working Party (Australia). A National Aboriginal Health Strategy: Report of the National Aboriginal Health Strategy Working Party. Canberra, Australia: Australian Government Publishing Service; 1989. http://www.health.gov.au/internet/publications/publishing.nsf/Content/mental-pubs-w-wayforw-toc~mental-pubs-w-wayforw-bac~mental-pubs-w-wayforw-bac-str Accessed August 5, 2014. [Google Scholar]
- 28. New South Wales Health. Principles for Better Practice Aboriginal Health Promotion - the Sydney Consensus Statement. Sydney, Australia: New South Wales Health, Australia; 2002. http://www0.health.nsw.gov.au/pubs/2004/pdf/principlesaborginal.pdf. Accessed August 5, 2014. [Google Scholar]
- 29. Coubrough L. Breastfeeding Definitions for Monitoring the National Health Outcomes Targets for New Zealand: Review of the Evidence and Recommendations. A Final Report for Ministry of Health. Wellington, New Zealand: Ministry of Health; 1999. http://www.moh.govt.nz/notebook/nbbooks.nsf/0/65454BE64D7D1C3ACC257A5C00089216/$file/Breastfeeding%20definitions.pdf. Accessed August 5, 2014. [Google Scholar]
- 30. Chang AB, Newman RG, Carlin JB, Phelan PD, Robertson CF. Subjective scoring of cough in children: parent-completed vs child completed diary cards vs an objective method. Eur Respir J. 1998;11:462–466. [DOI] [PubMed] [Google Scholar]
- 31. Matt GE, Hovell MF, Quintana PJE, et al. The variability of urinary cotinine levels in young children: implications for measuring ETS exposure. Nicotine Tob Res. 2007;9(1):83–92. [DOI] [PubMed] [Google Scholar]
- 32. Henderson FW, Reid HF, Morris R, et al. Home air nicotine levels and urinary cotinine excretion in preschool children. Am Rev Respir Dis. 1989;140:197–201. [DOI] [PubMed] [Google Scholar]
- 33. Clucas DB, Carville KS, Connors C, Currie BJ, Carapetis JR, Andrews RM. Disease burden and health-care clinic attendances for young children in remote Aboriginal communities of northern Australia. B World Health Organ. 2008;86:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oddy WH, Kickett-Tucker C, De Maio J, et al. The association of infant feeding with parent-reported infections and hospitalisations in the West Australian Aboriginal Child Health Survey. Aus New Zeal J Pub Health. 2008;32(3):207–215. [DOI] [PubMed] [Google Scholar]
- 35. Schafer JL. Analysis of Incomplete Multivariate Data. London, UK: Chapman and Hall; 1997. [Google Scholar]
- 36. Baheiraei A, Kharaghani R, Mohsenifar A, et al. Reduction of secondhand smoke exposure among healthy infants in Iran: randomized controlled trial. Nicotine Tob Res. 2011;13(9):840–847. [DOI] [PubMed] [Google Scholar]
- 37. Rees VW, Keske RR, Aronstein D, et al. Effectiveness of a community-based motivational intervention to reduce child secondhand smoke exposure in low income communities. Presented at: the Society for Research into Nicotine and Tobacco conference, Seattle, USA; 2014. [Google Scholar]
- 38. Llaquet H, Pichini S, Joya X, et al. Biological matrices for the evaluation of exposure to environmental tobacco smoke during prenatal life and childhood. [Review]. Anal Bioanal Chem. 2010;396(1):379–399. [DOI] [PubMed] [Google Scholar]
- 39. Labrecque M, Marcoux S, Weber J-P, Fabia J, Ferron L. Feeding and urine cotinine values in babies whose mothers smoke. Pediatrics. 1989;83:93–97. http://pediatrics.aappublications.org/content/83/1/93 Accessed August 5, 2014. [PubMed] [Google Scholar]
- 40. Luck W, Nau W. Nicotine and cotinine concentrations in serum and urine of infants exposed via passive smoking or milk from smoking mothers. J Pediatr. 1985;107:816–820. [DOI] [PubMed] [Google Scholar]
- 41. Mascola MA, Van Vunakis H, Tager IB, Speizer FE, Hanrahan JP. Exposure of young infants to environmental tobacco smoke: breast-feeding among smoking mothers. [Comparative Study Research Support, U.S. Gov’t, P.H.S.]. Am J Pub Health. 1998;88(6):893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schulte-Hobein B, Schwartz-Bickenbach D, Abt S, Plum C, Nau H. Cigarette smoke exposure and development of infants throughout the first year of life: influence of passive smoking and nursing on cotinine levels in breast milk and infant’s urine. Acta Paediatr. 1992;81(6–7):550–557. [DOI] [PubMed] [Google Scholar]
- 43. Mennella JA, Beauchamp GK. Smoking and the flavor of breast milk. New Engl J Med. 1998;339(21):1559–1560. [DOI] [PubMed] [Google Scholar]
- 44. Woodward A, Douglas RM, Graham NM, Miles H. Acute respiratory illness in Adelaide children: breast feeding modifies the effect of passive smoking. J Epidemiol Commun Health. 1990;44(3):224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ilett KF, Heal TW, Page-Sharp M, Kristensen JH, Kohan R, Hackett LP. Use of nicotine patches in breast-feeding mothers: transfer of nicotine and cotinine into human milk. Clin Pharmacol Ther. 2003;74(6):516–524. [DOI] [PubMed] [Google Scholar]
- 46. Joseph DV, Jackson JA, Westaway J, Taub NA, Petersen SA, Wailoo MP. Effect of parental smoking on cotinine levels in newborns. Arch Dis Child: Fetal Neonat Ed. 2007;92:F484–F488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Glover M, Kira A, Johnston V, et al. A systematic review of barriers and facilitators to participation in randomized controlled trials by Indigenous people from New Zealand, Australia, Canada and the United States [published online ahead of print May 19, 2014]. Global Health Prom. 2014. [DOI] [PubMed] [Google Scholar]
- 48. Kearns T, Clucas D, Connors C, Currie BJ, Carapetis JR, Andrews RM. Clinic attendances during the first 12 months of life for Aboriginal children in five remote communities of Northern Australia. PLoS ONE. 2013;3:e58231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mannino DM, Caraballo R, Benowitz N, Repace J. Predictors of cotinine levels in US children: data from the Third National Health and Nutrition Examination Survey. Chest. 2001;120(3):718–724. [DOI] [PubMed] [Google Scholar]
- 50. ASH New Zealand. National Year 10 ASH Snapshot Survey. Auckland, New Zealand: Ministry of Health, Health Promotion Agency (formerly Health Sponsorship Council) and Action on Smoking and Health; 2012. http://www.ash.org.nz/research-and-information/ash-research/ash-year-10-snapshot-survey/#more-70. Accessed August 5, 2014. [Google Scholar]
- 51. Healey B, Edwards R, Wilson N, Thomson G, Hoek J. The important persisting problem of smoking in cars with children: new data from a multi-year national survey of young people [letter]. New Zeal Med J. 2013;126(1369):86–88. ISSN: 1175 8716. [PubMed] [Google Scholar]
- 52. Matt GE, Quintana PJE, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Cont. 2004;13(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schreier M, Luo X, Lowry S, et al. Utilizing biomarker feedback documenting child exposure to tobacco toxins in second-hand smoke to promote smoke-free homes. Presented at: the Society for Research into Nicotine and Tobacco conference, Seattle, USA; 2014. [Google Scholar]
- 54. Glover M, Nosa V, Watson D, Paynter J. WhyKwit: A Qualitative Study of What Motivates Māori, Pacific Island and Low Socio-economic Peoples in Aotearoa/New Zealand to Stop Smoking. Auckland, New Zealand: University of Auckland, School of Population Health, Centre for Tobacco Control Research; 2010. https://cdn.auckland.ac.nz/assets/fmhs/soph/sch/atc/docs/WhyKwit%20Report.pdf. Accessed August 5, 2014. [Google Scholar]
- 55. Blackburn CM, Bonas S, Spencer NJ, Coe CJ, Dolan A, Moy R. Parental smoking and passive smoking in infants: fathers matter too. Health Edu Res. 2005;20(2):185–194. [DOI] [PubMed] [Google Scholar]