Abstract
Introduction
Radiotherapy management of patients with brain metastases most commonly involve a whole‐brain radiation therapy (WBRT) regime, as well as newer techniques such as stereotactic radiosurgery (SRS) and intensity modulated radiotherapy (IMRT). The long treatment times incurred by these techniques indicates the need for a novel technique that has shorter treatment times, whilst still producing highly conformal treatment with the potential to deliver escalated doses to the target area. Volumetric modulated arc therapy (VMAT) is a dynamic, highly conformal technique that may deliver high doses of radiation through a single gantry arc and reduce overall treatment times. The aim of this systematic review is to determine the feasibility and benefits of VMAT treatment in regard to overall survival rates and local control in patients with brain metastases, in comparison with patients treated with WBRT, SRS and IMRT.
Methods
A search of the literature identified 23 articles for the purpose of this review. Articles were included on the basis they were human‐based studies, with sample sizes of more than five patients who were receiving treatment for 1–10 metastatic brain lesions.
Results
VMAT was found to be highly conformal, have a reduced treatment delivery time and incurred no significant toxicities in comparison with WBRT, SRS and IMRT.
Conclusion
Compared to other treatment techniques, VMAT proved to have fewer toxicities than conventional WBRT, shorter treatment times than SRS and similar dose distributions to IMRT plans. Future prospective studies are needed to accurately assess the prognostic benefits of VMAT as well as the occurrence of late toxicities.
Keywords: Brain metastases, IMRT, radiation therapy, stereotactic radiosurgery, volumetric modulated arc therapy
Introduction
Whole brain radiotherapy (WBRT) has traditionally been the primary treatment modality for patients diagnosed with multiple brain metastases. Brain metastases are diagnosed in 20–40% of patients with systemic cancers, with the most common primary sites arising from lung, breast and gastro‐intestinal cancers.1, 2, 3 Some patients who develop brain metastases remain asymptomatic; in others, brain metastases can produce debilitating neurological symptoms including increased intracranial pressure, altered mental status, headaches, nausea, seizures, aphasia, ataxia and visual defects.1, 4 Whilst the incidence of brain metastases appears to be rising, the overall prognosis for these patients remains poor; hence there is a need to investigate advancements in modern treatment techniques.3
WBRT using opposing lateral beams is the standard palliative radiotherapy treatment for patients with brain metastases and is used as an adjuvant treatment to surgical resection and steroid therapy.5 It may also be used as a stand‐alone treatment in patients who have unresectable metastatic tumours.5, 6 WBRT is effective in treating solitary metastatic lesions but is less effective in patients who suffer from multiple metastatic lesions and is associated with poor prognosis and survival rates (3–6 months).3, 6, 7
Whilst conventional WBRT techniques remain routinely used in every day practice, newer techniques, such as stereotactic radiosurgery (SRS) and intensity modulated radiation therapy (IMRT) are becoming the standard form of treatment for patients with more advanced tumour progression.1, 3, 5, 8
SRS is a minimally invasive, highly conformal technique, and as such, is able to deliver large doses to well‐defined target areas within the brain, making it an effective technique for treating patients with multiple brain lesions.8 Additionally, SRS has the capability to deliver the entire treatment dose in a single fraction.8, 9 The downside of this is that longer treatment times are required to deliver the dose, varying from 30 min for a single lesion to several hours for multiple lesions, with treatment time increasing with the number of lesions requiring treatment.8 Treatment time is further influenced by whether a cone‐based or MLC‐based SRS technique has been used.8, 10 Whilst cone‐based SRS treatment is commonly used to treat small lesions, MLC‐based SRS has been shown to produce better dose conformity and reduced treatment times when used to treat larger, or multiple, lesions.8, 10 As such, only studies utilising MLC‐based SRS treatments have been included in this review.
IMRT provides highly conformal treatment to be delivered without including the surrounding healthy tissue, and can allow for dose escalation to the target volume.11 IMRT requires multiple fields to produce an optimal dose distribution, and as such, incurs longer overall treatment delivery times.11 However, both SRS and IMRT are time‐consuming for the patient and staff involved with both the planning and treatment processes.12
Volumetric modulated arc therapy (VMAT) is a highly conformal intensity modulated technique utilising either a single or multiple arcs that can improve coverage of the target volume while sparing normal tissue in comparison to conventional radiotherapy treatment.2, 3, 12, 13 The dynamic nature of VMAT results in treatment times being significantly reduced, due to the absence of multiple field arrangements.2, 7 For patients with multiple brain metastases; radiotherapy treatment using a highly conformal technique such as VMAT could improve local control and overall survival rates, as well as improve the overall treatment delivery in terms of decreased treatment times and potential for dose escalation.1, 13 Several studies have identified the potential to use VMAT therapy to deliver simultaneous integrated boost (SIB) doses to the traditional WBRT treatment regimes, whereby VMAT treatment is delivered primarily to the site of the metastases to provide conformal dose escalation to that area.1, 12, 14
The aim of this review was to determine the feasibility and benefits of VMAT treatment in regard to overall survival rates and local control in patients with multiple brain metastases. This review will evaluate the use of VMAT compared with other important treatment modalities, such as conventional WBRT, SRS and IMRT. Specifically, this review will evaluate the evidence base to address the following questions:
What are the treatment benefits, toxicity characteristics and local control rates associated with VMAT treatment in patients with brain metastases?
How does VMAT compare with other important treatment modalities, including conventional WBRT, SRS and IMRT, in terms of the treatment benefits (i.e., treatment times), toxicity characteristics and local control rates achieved in patients with brain metastases?
Methods
The literature review carried out for this article was undertaken exclusively via electronic resources in October 2013. The literature search strategy, databases searched, and search terms used are schematically demonstrated in the Figure 1 flow diagram.
Figure 1.
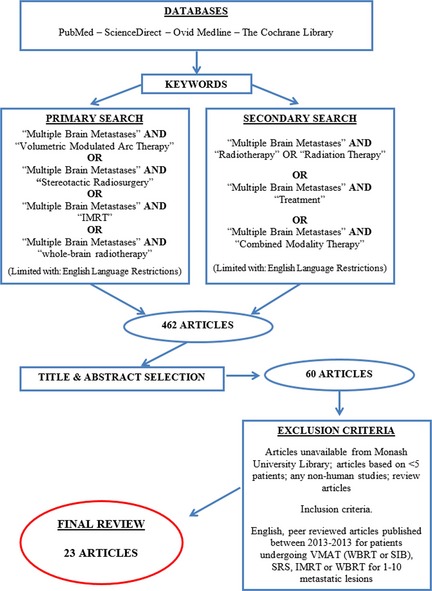
Literature search methods. Flow diagram of the electronic search conducted for the purpose of this review.
Results
Study designs returned from the search included: randomised control trials, clinical trials, cohort studies and retrospective studies. After applying the inclusion and exclusion criteria to determine full eligibility, 23 articles (7 VMAT, 6 SRS, 5 WBRT and 5 IMRT) were deemed relevant to be included within this review.
What are the treatment benefits, toxicity characteristics and local control rates associated with VMAT treatment in patients with multiple brain metastases?
Seven articles based on the use of VMAT in patients with brain metastases were identified in our search, detailed in Tables 1 and 2. Upon review of the returned articles, many of them included patients with solitary metastases. As it was not possible to remove the patients with solitary metastases from the larger cohorts within these papers, the scope of the review was revised to allow inclusion of VMAT for patients with single cranial lesions, when they were reported within an article also including patients with multiple metastases.
Table 1.
Use of VMAT in patients with brain metastases
| Source | Sample size | Dose regimes | Treatment planning | |||
|---|---|---|---|---|---|---|
| PTV margins | MU | Arcs & energy | Treatment time | |||
| Awad et al.14 | 30 patients (1–8 lesions) |
50 Gy/15fx 30 Gy/15fx WBRT |
GTV + 2 mm | N/A | 2 arcs 6 MV | 3.43 min |
| Hsu et al.2 | 10 patients (1–3 lesions) | 32.25 Gy/15fx | GTV + 2 mm | 400/min | 1 arc 6 MV | 3–4 min |
| Huang10 | 17 patients (2–5 lesions) | N/A | GTV + 2 mm | 600/min | 3–5 partial arcs 6 MV | N/A |
| Lagerwaard et al.12 | 8 patients (1–5 lesions) |
40 Gy/5fx 20 Gy/5fx WBR 20 Gy/5fx SIB |
GTV + 2 mm | 530/min | 2 arcs 6 MV | 3 min |
| Lee et al.1 | 9 patients (4–10 lesions) |
30 Gy/12fx + 15 Gy/6fx (boost) OR 48 Gy/12fx with SIB |
GTV + 1–2 mm | N/A | 2 arcs (8 patients) 3 arcs (1 patient) 6 MV | 3 min |
| Wang et al.13 | 12 patients (2–12 lesions) | 18–20 Gy/ 1fx (SRS) | CTV + 2 mm | 600/min | 2 arcs 6 MV | 7.1 mina |
| Weber et al.3 | 29 patients (1–4 lesions) | 40 Gy/10fx | GTV + 3 mm | 600/min | 2 arcs 6 MV | N/A |
Gy, Gray; fx, fractions; MV, megavoltage; GTV, gross tumour volume; PTV, planning treatment volume; MU, monitor units; WBR, whole brain radiotherapy; SIB, simultaneous integrated boost; N/A, not available.
Longer time due to stereotactic dose being delivered.
Table 2.
Dosimetry, toxicity, local control and survival rates of brain metastases patients treated with VMAT
| Source | Conformity index (CI) | Toxicity | Local control/overall survival |
|---|---|---|---|
| Awad et al.14 | Mean CI = 8.6a |
‐Minimal acute toxicity: ‐Alopecia, headache, vomiting ‐Grade I toxicity‐ 5 patients ‐Grade I/II toxicity‐ 2 patients ‐Grade II toxicity‐ 3 patients ‐Grade IV late toxicity‐ 1 patient |
‐Mean survival = 9.4 months ‐81% of lesions controlled 3.5 months post‐radiotherapy |
| Hsu et al.2 | Mean CI = 0.73 ± 0.10 | N/A | N/A |
| Huang10 | Mean CI = 1.43 ± 0.3 | N/A | N/A |
| Lagerwaard et al.12 | Mean CI = 1.3 ± 0.3 | N/A | N/A |
| Lee et al.1 | N/A |
‐Grade I pruritus in 1 patient ‐No toxicity of grade 3 or above was found during or after treatment. |
Survival at: ‐6 months = 66.7% ‐12 months = 41.7% ‐Mean OS= 9 months ‐Mean local progression‐free survival = not achieved |
| Wang et al.13 | Mean CI = 1.6 ± 0.4 | ‐Grade I/II alopecia in 31% of patients | N/A |
| Weber et al.3 | N/A | ‐Alopecia observed in less than a third of the patients |
‐6 months OS = 72% in patients who underwent prior surgical resection ‐6 months OS = 66.9% in patients with good performance status ‐79% local and distant control achieved |
N/A, not available in these studies; OS, overall survival.
There was a high percentage of major deviations within this study due to the GTV volumes measuring <1 cm3.
Table 1 identified a total of 115 patients within these studies, with the number of metastatic lesions reported per patient ranging from 1 to 10 brain metastases. The studies reported the range of arcs used to deliver the VMAT treatment to be between one to three arcs, with the exception of one study which required the use of three to five partial arcs. The majority of the studies discussed the use of VMAT to deliver SIB doses to the site of the metastases alone, with the exception of two studies which delivered VMAT to the whole brain. Treatment delivery times for the majority of the articles was found to be 3 min based on VMAT treatments, involving one to three arcs of the gantry, with the exception of the study by Wang13 which used VMAT to deliver a stereotactic dose of 18–20 Gy in the single fraction, thus requiring a longer time to deliver the treatment.
Table 2 depicted the conformity indexes (CI), toxicity characteristics, local control and overall survival rates of the patients treated with VMAT.
The CI values shown on Table 2 ranged from 0.7 ± 0.1 to 8.6. CI values close to 1.0 represent high levels of dose conformity to the planning target volume (PTV). Awed et al.'s14 study reported a CI of 8.6 acknowledging a high percentage of major deviations within their study due to the GTV volumes measuring <1 cm³. Despite this, the results indicate the potential for achieving highly conformal dose distributions using VMAT; but also highlight the limitations of achieving conformity when treating very small volumes.12
Toxicities as demonstrated in Table 2 were minimal in VMAT patients and typically only incurred mild alopecia, headache and symptoms of nausea. Studies that used VMAT to deliver SIB doses used typical dose regimes ranging from 40 to 50 Gy delivered in 12–15 fractions and in these studies, no significant toxicities were reported aside from mild alopecia.1, 13, 14 Similarly, in the two studies that reported the use of VMAT whole‐brain treatment, mild alopecia was reported in a third of the patients and no other significant toxicities were reported.3, 10
Local control, where reported, is shown in Table 2. Two of the studies indicated that VMAT treatment resulted in 79–81% local control being reported.1, 3, 14 Median overall survival times ranging from 9 to 10 months were also reported in two studies.
How does VMAT compare with other important treatment modalities, including conventional WBRT, SRS and IMRT, in terms of the treatment benefits (i.e. treatment times), toxicity characteristics and local control rates achieved in patients with multiple brain metastases?
Conventional WBRT
Table 3 reported five studies that identified patients who were treated with WBRT. 553 patients were identified in these studies, each presenting with one to five brain metastases. Typical dose regimes for these patients ranged from 20 Gy in five fractions to 30–40 Gy in 10 fractions. Toxicities were identified in the majority of the studies and ranged from acute toxicities such as alopecia, headache and nausea, to late‐induced radiation effects. Local control ranged from 29.2 to 29.5% at 6 months and 16.5–60.8% at 12 months. Overall survival was poor, ranging from 8 weeks post‐treatment to 14.5 ± 1.3 months post‐treatment.
Table 3.
Use of conventional WBRT to treat patients with brain metastases, including treatment characteristics, associated toxicities, local control and overall survival rates
| Source | Sample size | Dose regimes | Planning | Treatment time | Toxicity | Local control/overall survival |
|---|---|---|---|---|---|---|
| Barnes et al.4 | 137 patients (1–4 lesions) | 20 Gy/5fx | N/A | N/A | ‐Neurological symptoms | ‐MS = 2.5 months |
| Casanova et al.6 | 83 patients (1–3 lesions) | 30–40 Gy/10fx | 2 opposing lateral beams 6 MV | N/A |
‐Minimal late radiation‐induced toxicity ‐Alopecia in 33.9% of patients. ‐No gross neurocognitive dysfunction. ‐Asthenia in 9.8% of patients |
‐MS = 14.5 ± 1.3 months ○ 6 month LCR = 29.5% ○ 12 month LCR = 60.8% |
| Gerrard et al.7 | 38 patients (>2 lesions) | 30 Gy/10fx | 2 opposing lateral beams 6 MV | N/A | N/A |
‐OS was poor ○ MS = 2 months post‐treatment |
| Hauswald et al.15 |
87 patients (1–5 lesions) |
30 Gy/10fx 40 Gy/20fx SIB |
2 opposing lateral beams 6 MV |
N/A |
‐Acute side effects in 97% patients:: ○ Headache ○ Fatigue ○ Nausea ○ Dizziness ‐Late side effect ○ Ongoing fatigue |
‐MS = 3.5 months ○ 6 month SR = 29.2% ○ 12 month SR = 16.5% ○ 24 month SR = 8.6% |
| Li et al.16 | 208 patients (>2 lesions) | 30 Gy/10fx | N/A | N/A | ‐Some deterioration in neurocognitive functioning | ‐MS = 5 months |
Gy, Gray; fx, fractions; MV, megavoltage; SIB, simultaneous integrated boost; MS, median survival; LCR, local control rate; SR, survival rate; OS, overall survival; N/A, not available in these studies.
Stereotactic radiosurgery
Table 4 reported six studies based on patients treated with SRS. 901 patients were identified with the number of brain metastases ranging from 1 to 15. The majority of the stereotactic dose regimes ranged from 12 to 25 Gy in a single fraction and treatment times were reported to range from 9 min per lesion to being treated over several days. The studies identified stereotactic treatment to be associated with higher incidences of late toxicities, with four of the studies reporting the occurrence of radiation necrosis in patients. Neurological deficits including seizures, motor and cognitive deficits were also reported, as was the incidence of acute toxicities such as headache and haemorrhaging of the lesions. Local control of lesions was reported to range from 89% to 90% at 6 months, 76–92% at 12 months and 78–84% at 24 months. Overall survival rates at 12 months ranged from 44% to 58% and 24–84% at 24 months.
Table 4.
Use of SRS to treat patients with multiple brain metastases, including treatment characteristics, associated toxicities, local control and overall survival rates
| Source | Sample size | Dose regimes | Treatment/planning | Toxicity | Local control/overall survival | |
|---|---|---|---|---|---|---|
| PTV margins | Treatment time | |||||
| Breneman et al.9 | 53 patients (1–15 lesions) | 12–22 Gy/1fx | N/A | Patients with several lesions were treated over several sessions |
‐Adverse events‐5 patients ‐Radiation necrosis‐ 2 patients ‐Haemorrhaging‐ 3 patients ‐Post‐treatment oedema‐ 17 lesions (responded to corticosteroid therapy) |
‐LC‐ ○ 90% at 6 months ○ 80% at 12 months ○ 78% at 18 months ○ 78% at 24 months ‐OS ○ 70% at 6 months ○ 44% at 12 months |
| Ernst‐Stecken et al.17 | 51 patients (1–4 lesions) | 30–35 Gy/5fx | GTV + 3 mm | N/A | ‐Increased incidences of oedema and new or larger volumes of necrosis occurred if more than 23 cm3 of normal brain tissue received more than 4 Gy per fraction. These patients required long‐term management with steroid medications. |
‐CR‐ 66.7% of patients ‐PR‐ 18.1% of patients ‐NR‐ 12.5% of patients ‐Progression of new metastatic lesions‐ 2.8% of patients ‐LC‐ ○ 6 months = 89% ○ 12 months = 76% ‐MS‐ ○ 11 months |
| Hunter et al.18 | 64 patients (5–10 lesions) |
24 Gy/1fx for lesions <2 cm 18 Gy/1fx for lesions > 2 cm but <3 cm 15 Gy/1fx for lesions>3 cm |
N/A | N/A | No significant toxicities | N/A |
| Minnitti et al.19 | 206 patients (1–3 lesions) |
20 Gy/1fx for lesions <4.3 cm3
18 Gy/1fx for lesions 4.3–14.1 cm3 16–16 Gy/1fx for lesions >14.1 cm3 |
GTV + 1–2 mm | 10 min/lesion |
‐Brain radionecrosis in 24% of patients ‐Neurological deficits in 13.1% of patients: ‐Seizure ‐Motor deficits ‐Cognitive deficits ‐Speech deficits ‐Other complications: ‐Headache (5%) ‐Hydrocephalus (2%) ‐Haemorrhage (2%) |
‐LC‐ ○ 12 months = 92% ○ 24 months = 84% ‐SR‐ ○ 12 months = 58% ○ 24 months = 24% ‐Intracranial tumour progression at either distant or local sites ‐74 patients. |
| Nath et al. 20108 | 26 patients (2–13 lesions) | 14–25 Gy/1fx | CTV + 1 mm | 9–38.9 min |
‐Acute toxicity ‐3 patients ‐Acute Grade 2 events (seizure and a transient worsening of pre‐existing visual symptoms that resolved with corticosteroids)‐ 2 patients ‐Acute Grade 3 event 8 days post‐ RT (hemiparesis after haemorrhaging of a treated lesion)‐ 1 patient ‐Late toxicity radionecrosis (requiring corticosteroids and surgical intervention)‐ 1 patient |
‐SR‐ ○ 6 months = 50% ○ 12 months = 38% |
| Rodrigues et al.5 | 501 patients (1–4 lesions) |
18 Gy/1fx OR 24 Gy/3fx |
GTV + 2 mm | N/A | N/A | N/A |
Gy, Gray; fx, fractions; GTV, gross tumour volume; PTV, planning treatment volume; MS, median survival; OS, overall survival; LC, local control; SR, survival rate; CR, complete response; PR, partial response; NR, no response; N/A, not available in these studies.
Intensity modulated radiotherapy
Table 5 identified five studies that treated patients using IMRT. Ninety patients were identified in the studies, presenting with 1–13 brain metastases. Two of the studies reported treatment times, ranging from 2.79 to 30 min. Toxicities associated with IMRT were minimal, with mild alopecia and dermatitis being reported in one of the studies, whilst the other studies did not report any acute toxicity. Two of the studies reported CIs ranging from 0.7 to 1.4 ± 0.1. Local control of the metastatic lesions ranged from 59% to 72% and the median overall survival ranged from 2 to 13 months.
Table 5.
Use of IMRT to treat patients with multiple brain metastases, including treatment characteristics, associated toxicities, local control and overall survival rates
| Source | Sample size | Dose regimes | Treatment/planning | Toxicity | Local control/ overall survival | Conformity index (ci) | |
|---|---|---|---|---|---|---|---|
| Ptv margins | Treatment time | ||||||
| Beal et al.20 | 41 patients (1–2 lesions) | 30 Gy/5fx | GTV + 0.5 cm | N/A | ‐No acute toxicities |
‐MS = 13 months ‐OS‐ ○ 12 months = 59% ‐LC‐ ○ 12 months = 72% ‐Freedom from progression elsewhere in the brain‐ 59% at 12 months |
N/A |
| Clark et al.21 | 8 patients (1–13 lesions) | 45 Gy/15fx | GTV + 3 mm | 30 mins (total treatment time) |
‐Grade 2 fatigue‐ 2 patients ‐Grade 2 alopecia‐ 1 patient ‐Grade 1 dermatitis and alopecia‐ 7 patients ‐No toxicities >Grade 2. |
‐LC in 16/17 lesions | N/A |
| Edwards et al.22 | 11 patients (1–4 lesions) |
30 Gy/10fx 40 Gy/10fx SIB |
GTV + 3 mm | N/A |
‐No acute toxicities ‐No brain complications |
‐7/11 patients survived and show no evidence of disease progression or local recurrence at 2–9 months post‐RT | N/A |
| Hermento et al.23 | 20 patients (1–2 lesions) | 50 Gy/30fx | CTV + 0.5 cm | N/A | N/A | N/A | Mean CI = 1.38 ± 0.10 |
| Liang et al.11 |
10 patients (1–3 lesions) |
30 Gy/10 40 Gy/10fx SIB |
GTV + 2 mm | 2.79 mins | ‐No acute toxicities | N/A | Mean CI = 0.682 |
Gy, Gray; fx, fractions; GTV, gross tumour volume; PTV, planning tumour volume; CI, conformity index; MS, median survival; OS, overall survival; LC, local control; N/A, not available in these studies.
Discussion
What are the treatment benefits, toxicity characteristics and local control rates associated with VMAT treatment in patients with multiple brain metastases?
The findings of our review indicate that VMAT is a safe, highly conformal treatment technique that may be used to deliver SIBs and whole brain radiotherapy to patients with multiple brain metastases.2, 12
VMAT offers the ability to significantly reduce treatment times, meaning that VMAT treatments are a suitable technique for minimising patient discomfort and reducing patient movement that can occur with other treatment modalities that require significantly longer treatment delivery times.2, 12
The results from this systematic review indicate that dose escalation can be safely delivered using VMAT without incurring significant toxicities.24 Good local control was observed in patients treated with VMAT, and the median overall survival times reported ranged from 9 to 10 months in two studies (Table 2) indicate a potential improvement in survival times compared to conventional WBRT which reports a survival rate of 2–5 months (Table 3).2
Good dose conformity was observed in the CI values reported for most studies utilising VMAT, except when treating small lesions. This is an important finding as higher conformity indicates that there is reduced irradiation of the surrounding healthy tissue, which may significantly reduce the likelihood of neurological deficits and radiation‐induced brain necrosis from occurring and thus improve patient's quality of life.25
Clinically, the potential for dose escalation in brain metastases due to the ability of VMAT to deliver highly conformal doses could lead to improved local control and subsequently, improved prognosis in these patients.24
How does VMAT compare with other important treatment modalities, including conventional WBRT, SRS and IMRT, in terms of the treatment benefits (i.e., treatment times), toxicity characteristics and local control rates achieved in patients with multiple brain metastases?
VMAT versus Conventional WBRT
The studies we identified that used conventional WBRT failed to report the treatment delivery times associated with delivering the WBRT regimes so we were unable to compare the two techniques in this regard. Our findings did, however, identify differences in the toxicities produced between the two treatment techniques. Whilst our VMAT findings reported minimal toxicities associated with treatment, all but one of our WBRT articles reported significant toxicities associated with treatment.4, 6, 15, 16 The articles reported that WBRT was associated with acute toxicities such as headache, nausea and alopecia, produced some decline in neurocognitive functioning and showed minimal radiation‐induced late toxicities, with fatigue being the main symptom.4, 6, 15, 16 Variances were also reported in local control and survival rates between the two treatments. The studies we identified reported WBRT to achieve local control ranging from 29.2% to 29.5% at 6 months and 16.5–60.8% at 12 months. In comparison, VMAT achieved 79–81% local control, further indicating the benefits of being able to deliver dose escalation to the localised metastases site.24 Overall survival rates identified in the WBRT patients was poor, ranging from 8 weeks to 14.5 ± 1.3 months post‐treatment.4, 6, 15, 16 VMAT appeared to be associated with the better overall survival rate, however, given the limitations of our study and the smaller patient populations that comprised our VMAT study population; the accuracy of these findings are not conclusive.
VMAT versus SRS
Of the SRS articles identified in this review, three discussed the treatment times associated with delivering single fraction stereotactic treatment. SRS treatment times increased with the number of metastases requiring treatment and were reported to range from 9 min to requiring several days to deliver the one fraction.8, 9, 19 The longer treatment times involved with SRS justify why the accuracy of the immobilisation equipment used in SRS treatments is of such critical importance in ensuring patient movement is kept as minimal as possible.5, 8, 9, 19 In comparison, VMAT times were significantly shorter, indicating patient discomfort may be reduced with the use of VMAT.3 Four of the SRS articles identified brain necrosis as a late toxicity induced by the SRS treatment – this is a late effect commonly experienced due to the high doses delivered.8, 9, 17, 19 Whilst our study did not identify VMAT treatment to be associated with any late toxicity, the lack of retrospective studies on the use of VMAT does limit our findings and we cannot conclusively state that VMAT is associated with less toxicity than SRS. In regard to local control and overall survival, the studies on SRS reported slightly better local control of lesions than the VMAT control rates (SRS = 89–90% at 6 months, vs. VMAT = 79–81% at 6 months) and reported similar survival rates, however, given that our VMAT findings are based on data from only two studies, these results are not conclusive.8, 9, 17, 19
VMAT versus IMRT
IMRT and VMAT were found to be the most similar of all the techniques. Given that VMAT is essentially IMRT with the added capability of delivering treatment through the use of arc therapy, this was not a surprising finding as the two techniques would be expected to achieve similar results.23 IMRT treatments are preferentially used over conventional whole‐brain treatment due to their higher conformity and ability to spare critical structures.23 The downside of IMRT is that it requires the use of several fields to achieve a homogenous‐dose distribution and this incurs longer overall treatment times.11, 23 The studies used in this review demonstrated that VMAT treatment times were superior to those of the reported IMRT times which ranged from 2.79 to 30 min.11, 21 Comparable results were identified in regard to the toxicities incurred by the two techniques, with both techniques only reporting minimal toxicities of alopecia and dermatitis1, 13, 14, 21 Local control and survival rates reported in the IMRT studies were similar to the VMAT rates, with IMRT local control ranging from 59% to 72% and the median overall survival ranging from 2 to 13 months.20, 21, 22 Future studies should compare the ability of VMAT treatment to spare critical structures in comparison to IMRT treatment so that accurate toxicity data can be obtained.
Limitations
The authors recognise that there are several limitations that should be considered for this article. VMAT is still a relatively new technology compared to the other technologies considered in this review. As such, there is a lack of studies prospectively comparing VMAT with traditional methods and a lack of data on long‐term toxicities associated with VMAT. As new research is published, any documenting outcomes and toxicities associated with VMAT compared with traditional treatment regimes should be carefully considered, especially for late toxicities. Additionally, our study did not control for any specific patient characteristics, such as age, type of disease, prior treatment etc.; hence the accuracy of our findings may not be representative of the entire brain metastases population. This indicates there is potential for future studies to be conducted to assess the long‐term effects of VMAT treatment, as well as the need for specific studies that do control for patient characteristics to be conducted.
This review was also limited to articles that could be accessed through the Monash University article retrieval system. Whilst this comprehensive database enabled us to access all the major peer reviewed journals, it is possible that some relevant literature was not returned in our search.
Conclusion
This systematic review identifies VMAT as a safe and feasible technique for the treatment of patients with brain metastases. VMAT treatment was found to incur shorter treatment times than other important treatment modalities, incurred minimal toxicities and produced favourable local control and survival outcomes. Given the limited amount of literature currently available on the use of VMAT in brain metastases patients, this review was unable to conclusively determine the benefits that VMAT treatment has over the other common treatment techniques. This indicates the need for further research to be conducted, in particular, the need for prospective studies, preferably in the form of a randomised control trial, to be conducted to assess any late‐toxicities that may arise with the use of VMAT, as well as to accurately assess any improvement in prognosis that VMAT incurs.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The author thanks Charlotte Sale and Kellie Knight for their editorial support in the writing of this review.
Journal of Medical Radiation Sciences 61 (2014) 267–276
References
- 1. Lee SH, Lee KC, Choi J, et al. Clinical application of RapidArc volumetric modulated arc therapy as a component in whole brain radiation therapy for poor prognostic, four or more multiple brain metastases. Radiother Oncol 2012; 30: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu F, Carolan H, Nichol A, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1‐3 brain metastases: a feasibility study using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2010; 76: 1480–5. [DOI] [PubMed] [Google Scholar]
- 3. Weber DC, Caparrotti F, Laouiti M, Malek K. Simultaneous in‐field boost for patients with 1 to 4 brain metastasis/es treated with volumetric modulated arc therapy: a prospective study on quality‐of‐life. Radiother Oncol 2011; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnes EA, Chow E, Tsao MN, et al. Physician expectations of treatment outcomes for patients with brain metastases referred for whole brain radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 187–92. [DOI] [PubMed] [Google Scholar]
- 5. Rodrigues G, Gonzalez‐Maldonado S, Bauman G, Senan S, Lagerwaard F. A statistical comparison of prognostic index systems for brain metastases after stereotactic radiosurgery or fractionated stereotactic radiation therapy. Clin Oncol 2013; 25: 227–35. [DOI] [PubMed] [Google Scholar]
- 6. Casanova N, Mazouni Z, Bieri S, Combescure C, Pica A, Weber DC. Whole brain radiotherapy with a conformational external beam radiation boost for lung cancer patients with 1‐3 brain metastasis: a multi institutional study. Radiother Oncol 2010; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gerrard GE, Prestwich RJ, Edwards A, et al. Investigating the palliative efficacy of whole‐brain radiotherapy for patients with multiple‐brain metastases and poor prognostic features. Clin Oncol 2003; 15: 422–8. [DOI] [PubMed] [Google Scholar]
- 8. Nath SK, Lawson JD, Simpson DR, et al. Single‐isocenter frameless intensity‐modulated stereotactic radiosurgery for simultaneous treatment of multiple brain metastases: clinical experience. Int J Radiat Oncol Biol Phys 2010; 78: 91–7. [DOI] [PubMed] [Google Scholar]
- 9. Breneman JC, Steinmetz R, Smith A, Lamba M, Warnick RE. Frameless image‐guided intracranial stereotactic radiosurgery: clinical outcomes for brain metastases. Int J Radiat Oncol Biol Phys 2009; 74: 702–6. [DOI] [PubMed] [Google Scholar]
- 10. Huang C. in 41 Treatment of multiple brain metastases using stereotactic radiosurgery with single‐isocentre volumetric modulated arc therapy: comparison with conventional dynamic conformal arc and static beam stereotactic radiosurgery [Masters Degree]. Duke University, Durham, NC, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Liang X, Ni L, Hu W, et al. A planning study of simultaneous integrated boost with forward IMRT for multiple brain metastases. Med Dosim 2013; 38: 115–6. [DOI] [PubMed] [Google Scholar]
- 12. Lagerwaard FJ, van der Hoorn EA, Verbakel WF, Haasbeek CJ, Slotman BJ, Senan S. Whole‐brain radiotherapy with simultaneous integrated boost to multiple brain metastases using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2009; 75: 253–9. [DOI] [PubMed] [Google Scholar]
- 13. Wang JZ, Pawlicki T, Rice R, et al. Intensity‐modulated radiosurgery with rapidarc for multiple brain metastases and comparison with static approach. Med Dosim 2012; 37: 31–6. [DOI] [PubMed] [Google Scholar]
- 14. Awad R, Fogarty G, Hong A, Kelly P, Ng D, Santos D, Haydu L. Hippocampal avoidance with volumetric modulated arc therapy in melanoma brain metastases‐ the first Australian experience. Radiother Oncol 2013; 8: 62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hauswald H, Dittmar JO, Habermehl D, Reiken S, Sterzing F, Debus J, Combs SE. Efficacy and toxicity of whole brain radiotherapy in patients with multiple cerebral metastases from malignant melanoma. Radiother Oncol 2012; 7: 130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Bentzen SM, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole‐brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys 2008; 71: 64–70. [DOI] [PubMed] [Google Scholar]
- 17. Ernst‐Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol 2006; 81: 18–24. [DOI] [PubMed] [Google Scholar]
- 18. Hunter GK, Suh JH, Reuther AM, et al. Treatment of five or more brain metastases with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2012; 83: 1394–8. [DOI] [PubMed] [Google Scholar]
- 19. Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiother Oncol 2011; 6: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beal KP, Yamada Y, Chan TA, et al. Hypofractionated IMRT for large, difficult brain metastases. Int J Radiat Oncol Biol Phys 2008; 72(Suppl. 1): S228–9. [Google Scholar]
- 21. Clark G, Plants B, Mallah J, et al. Whole brain radiation therapy (WBRT) with simultaneous integrated boost (SIB) to brain metastasis via image guided, intensity modulated radiation therapy (IG‐IMRT): dosimetric and early clinical experience. Int J Radiat Oncol Biol Phys 2008; 72(Suppl. 1): S241. [Google Scholar]
- 22. Edwards AA, Keggin E, Plowman PN. The developing role for intensity‐modulated radiation therapy (IMRT) in the non‐surgical treatment of brain metastases. Br J Radiol 2010; 83: 133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hermento U, Frija EK, Lii MFJ. Chang EL, Mahajan A, Woo SY. Intensity‐modulated radiotherapy (IMRT) and conventional three‐dimensional conformal radiotherapy for high‐grade gliomas: does IMRT increase the integral dose to normal brain? Int J Radiat Oncol Biol Phys 2007;67:1135–44. [DOI] [PubMed] [Google Scholar]
- 24. Shaffer R, Nichol AM, Vollans E, et al. A comparison of volumetric modulated arc therapy and conventional intensity‐modulated radiotherapy for frontal and temporal high‐grade gliomas. Int J Radiat Oncol Biol Phys 2010; 76: 1177–84. [DOI] [PubMed] [Google Scholar]
- 25. Shibamoto Y, Baba F, Oda K, et al. Incidence of brain atrophy and decline in mini‐mental state examination score after whole‐brain radiotherapy in patients with brain metastases: a prospective study. Int J Radiat Oncol Biol Phys 2008; 72: 1168–73. [DOI] [PubMed] [Google Scholar]


