Abstract
Exercise intolerance is the primary chronic symptom in heart failure with preserved ejection fraction (HFpEF), the most common form of HF in older patients, however its pathophysiology is not well understood. Recent data suggest that peripheral factors, such as skeletal muscle dysfunction, may be important contributors. Therefore, 38 participants, 23 HFpEF patients (69±7 years) and 15 age-matched healthy controls (HC), underwent magnetic resonance imaging and cardiopulmonary exercise testing to assess for: skeletal muscle (SM), intermuscular fat (IMF), subcutaneous fat (SCF), total thigh, and thigh compartment areas (TC) and peak exercise oxygen consumption (peak VO2). There were no significant inter-group differences in total thigh area, TC, SCF, or SM. However, in HFpEF versus HC, IMF area (35.6±11.5 vs. 22.3±7.6 cm2, p=0.01), percent IMF/TC (26±5 vs. 20±5%, p=0.005), and the ratio of IMF/SM (0.38±0.10 vs. 0.28±0.09, p=0.007) were significantly increased, while percent SM/TC was significantly reduced (70±5 vs. 75 ±5, p=0.009). In multivariate analyses, IMF area (partial r= -0.51, p=0.002) and IMF/SM ratio (partial r= -0.45, p=0.006) were independent predictors of peak VO2 while SM area was not (partial r=-0.14, p=0.43). Thus, older HFpEF patients have greater thigh IMF and IMF/SM ratio compared to HC, and these are significantly related to their severely reduced peak VO2. These data suggest that abnormalities in skeletal muscle composition may contribute to the severely reduced exercise capacity in older HFpEF patients. This implicates potential targets for novel therapeutic strategies in this common, debilitating disorder of older persons.
Keywords: Heart failure, exercise, aging
INTRODUCTION
The primary chronic symptom in HFpEF patients is severe exercise intolerance, measured objectively as decreased peak oxygen uptake (peak VO2)1-4. The mechanisms for decreased peak VO2 are not well understood, however, ‘peripheral’ non-cardiac factors may contribute to reduced exercise tolerance, and may be the major contributor to its improvement following exercise training2,5. Specifically, using dual energy x-ray absorptiometry (DXA), we recently reported that older HFpEF patients had reduced percent total and leg lean body mass compared to age-matched healthy controls6. Moreover, the slope of the relationship of peak VO2 with percent leg lean mass was markedly reduced in HFpEF versus healthy controls (HC). These data suggested that the ‘quality’ of skeletal muscle may be abnormal and contribute to the reduced peak VO2 found in older HFpEF patients. Currently, there is no information regarding skeletal muscle composition and its relationship to exercise capacity in HFpEF patients. The purpose of this study was to test the hypothesis that older HFpEF patients have adverse skeletal muscle composition and that this contributes to their severe exercise intolerance.
METHODS
As previously described in studies reported from our laboratory1,2,5-8. HFpEF was defined as symptoms and signs of HF according to the National Health and Nutrition Examination Survey HF clinical score of ≥3 and the criteria of Rich et al9,10. Age-matched, sedentary HC were recruited and screened and excluded if they had any chronic medical illness, were on any chronic medication, had current complaints or an abnormal physical examination (including blood pressure ≥ 140/90 mmHg), had abnormal results on the screening tests (electrocardiogram, cardiopulmonary exercise, and spirometry), or were regularly exercising
Doppler echocardiograms were performed and LV filling patterns were categorized as previously described1,2,7,8,11.
Exercise testing was performed as previously described in the upright position on an electronically braked bicycle using a staged protocol starting at 12.5 W for 2 minutes, increasing to 25 W for 3 minutes, and with 25 W per 3-minute increments thereafter to exhaustion1,2,5,7,8. Breath-by-breath gas exchange data were measured continuously (CPX-2000; MedGraphics; Minneapolis, MN) during exercise and averaged every 15 seconds, and peak values were averaged from the last two 15-second intervals during peak exercise.
The left thigh of each subject was scanned with a 1.5T Horizon (General Electric Medical Systems, Milwaukee, Wisconsin) whole body magnetic resonance imaging system. A scout film of the inferior head of femur was obtained by single phase, multi-slice coronal acquisition. Slices were 10 mm thick with a 5-mm gap, had a 256 × 128 matrix, a 40-cm field of view, a 30° flip angle, an auto repetition time, and a minimum echo time. Straight axial scans superior to the inferior head of the femur were obtained by single phase, multi-slice acquisition. Slices were 8 mm thick with a 50-mm gap, had a 256 × 128 matrix, a 14-20 cm field of view, a 20 ° flip angle, an auto repetition time, and minimum echo time.
Images were transferred to an image analysis workstation and the slice corresponding to a constant location of the mid-thigh was selected in a manner as previously described12. The cross sectional area of skeletal muscle (SM), subcutaneous fat (SCF), intermuscular fat (IMF), and bone were measured using commercially available software (Tomovision, Montreal, Quebec). Total thigh area was calculated as the sum of all 4 components (SM, SCF, IMF, bone) and thigh compartment area (TC) was calculated as the sum of SM, IMF, and bone. To determine the intra-observer reproducibility of SM, SCF and IMF areas, duplicate measurements from 10 randomly chosen subjects were analyzed in a blinded fashion. The Pearson correlation coefficients for SM, SCF and IMF were 0.99, 0.99, and 0.90 respectively. These values are similar to previous reported values that range from 0.94-0.99, representing excellent reproducibility13.
Descriptive statistics are reported as means and standard deviations for continuous variables and as n (number in the category) and percent for categorical variables. Intergroup comparisons were made by independent samples t-tests for continuous variables, by Fisher's exact tests for binomial variables, and by Chi-square tests for categorical variables. Comparisons of peak exercise variables between groups were made by analysis of covariance, adjusting for age and gender. Likewise, comparisons of thigh composition variables between groups were made by analysis of covariance, adjusting for body surface area. The relationships between SM, IMF and IMF/SM and peak VO2 were assessed by Pearson correlations. Finally, a multivariate regression model was used to assess predictors of peak VO2. Competing variables were selected a priori based on their known impact on peak VO2. A two-tailed p-value of <0.05 was required for significance.
RESULTS
The patients were clinically stable (NYHA class II and III) with typical characteristics of HFpEF, including advanced age, female preponderance, abnormal LV diastolic filling, left ventricular (LV) hypertrophy, increased left atrial size, and severely reduced peak exercise VO2 (Table 1). HFpEF and HC were well matched for age and gender. Body weight, body mass index (BMI) and body surface area were higher for HFpEF than HC (Table 1), however the mean HFpEF BMI was similar to that observed in multiple, large population-based studies of older patients with HFpEF, including CHS, Framingham, and Olmsted County studies14,15.
Table 1.
Participant Characteristics
| Variable | HFPEF (N=23) | HC (N=15) | p-value |
|---|---|---|---|
| Age (years) | 69 ± 7 | 70 ± 8 | 0.78 |
| Women | 15 (65%) | 11 (73%) | 0.73 |
| White | 16 (70%) | 13 (87%) | 0.27 |
| Weight (kg) | 84 ± 19 | 67 ± 12 | 0.003 |
| Body mass index (kg/m2) | 30.4 ± 5.7 | 24.6 ± 2.9 | <0.001 |
| Body surface area (m2) | 1.9 ± 0.2 | 1.7 ± 0.2 | 0.01 |
| Systolic blood pressure (mmHg) | 146 ± 22 | 129 ± 15 | 0.02 |
| Diastolic blood pressure (mmHg) | 84 ± 13 | 77 ± 7 | 0.06 |
| Left ventricular mass (g) | 212 ± 72 | 167 ± 29 | 0.08 |
| Left ventricular mass/end diastolic volume ratio | 3.21 ± 1.36 | 2.60 ± 0.64 | 0.27 |
| Left atrial diameter (cm) | 3.6 ± 0.7 | 3.0 ± 0.4 | 0.02 |
| Ejection Fraction (%) | 63.5 ± 10.0 | 63.5 ± 10.5 | 0.99 |
| Diastolic Filling | |||
| Normal | 1 (4%) | 12 (80%) | <0.001 |
| Impaired relaxation | 14 (61%) | 3 (20%) | |
| Pseudonormal | 6 (26%) | 0 (0%) | |
| Restrictive | 1 (4%) | 0 (0%) | |
| Indeterminate (atrial fibrillation) | 1 (4%) | 0 (0%) | |
| B-type natriuretic peptide (pg/ml) | 50 ± 44 | 23 ± 12 | 0.07 |
| History of hypertension | 21 (91%) | -- | -- |
| Diabetes mellitus | 6 (26%) | -- | -- |
| New York Heart Association class | |||
| II | 11 (48%) | -- | -- |
| III | 12 (52%) | -- | -- |
| Medications | |||
| Diuretics | 15 (65%) | -- | -- |
| Angiotensin receptor blockers | 7 (30%) | -- | -- |
| Angiotensin converting enzyme Inhibitors | 7 (30%) | -- | -- |
| Beta blockers | 7 (30%) | -- | -- |
| Calcium channel blockers | 8 (35%) | -- | -- |
| Nitrates | 2 (9%) | -- | -- |
Values are mean ± SD, or n (%).
Peak exercise VO2, workload, exercise time, and 6-minute walk distance were severely reduced in HFpEF versus HC (Table 2). Peak systolic and diastolic blood pressures were not significantly different between HFpEF and HC (Table 2). Although respiratory exchange ratio was slightly lower in HFpEF versus HC, the mean was ≥1.15 in both groups, indicating exhaustive exercise effort. As described in prior reports from our group and others, peak heart rate was reduced in HFpEF versus controls2.
Table 2.
Peak exercise performance
| Variable | HFPEF | HC | p-value | HFPEF | HC | p-value |
|---|---|---|---|---|---|---|
| Raw Data | Adjusted Data* | |||||
| Oxygen uptake (ml/min) | 1177 ± 256 | 1348 ± 419 | 0.12 | 1236 ± 57 | 1451 ± 72 | 0.02 |
| Oxygen uptake (ml/kg/min) | 14.3 ± 3.1 | 20.1 ± 4.5 | <0.001 | 14.5 ± 0.8 | 20.6 ± 1.0 | <0.001 |
| Carbon dioxide production (ml/min) | 1330 ± 292 | 1630 ± 471 | 0.02 | 1395 ± 65 | 1743 ± 82 | 0.002 |
| Exercise time (min.) | 8.5 ± 2.3 | 11.7 ± 3.4 | 0.001 | 8.9 ± 0.5 | 12.4 ± 0.7 | <0.001 |
| Workload (watts) | 61 ± 20 | 86 ± 31 | 0.004 | 65 ± 5 | 93 ± 6 | <0.001 |
| Respiratory exchange ratio | 1.15 ± 0.09 | 1.22 ± 0.08 | 0.01 | 1.14 ± 0.02 | 1.21 ± 0.02 | 0.02 |
| Heart rate (beats/min) | 130 ± 25 | 153 ± 13 | 0.002 | 127 ± 4 | 149 ± 6 | 0.003 |
| Systolic blood pressure (mmHg) | 193 ± 22 | 193 ± 19 | 0.92 | 192 ± 5 | 193 ± 6 | 0.92 |
| Diastolic blood pressure (mmHg) | 92 ± 12 | 89 ± 11 | 0.50 | 92 ± 3 | 90 ± 3 | 0.56 |
| Ventilation/carbon dioxide slope | 31.3 ± 3.8 | 32.9 ± 3.0 | 0.18 | 31.7 ± 0.7 | 33.4 ± 0.9 | 0.12 |
| 6-minute walk distance (m) | 428 ± 73 | 554 ± 54 | <0.001 | 430 ± 15 | 559 ± 18 | <0.001 |
Raw data are presented as mean ± standard deviation.
adjusted for age and gender and presented as least square means ± standard error.
No significant difference in SCF, SM, bone, total thigh, or TC areas was found between groups (Table 3). However, despite no difference in SCF, IMF area, IMF area as a proportion of TC area, and the ratio of IMF/SM was significantly increased in HFpEF versus HC (Table 3). SM as a proportion of TC was significantly reduced in HFpEF (Table 3). Adjusting for height, height2.7, femur length, or body mass index instead of body surface area did not alter these results, other than a modest reduction in the significance level (to p=0.07) when IMF area was adjusted for body mass index. Figure 1 shows examples of MRI axial images of the mid-thigh from subjects from each group. Notably, IMF area was substantially increased in the HFpEF patient compared to the HC despite similar SCF area.
Table 3.
Thigh Composition Measured by Magnetic Resonance Imaging
| Variable | HFPEF | HC | p-value | HFPEF | HC | p-value |
|---|---|---|---|---|---|---|
| Raw Data | Adjusted Data* | |||||
| Total thigh area (cm2) | 233 ± 54 | 221 ± 30 | 0.46 | 226 ± 9 | 233 ± 11 | 0.63 |
| Subcutaneous fat (cm2) | 95 ± 49 | 110 ± 37 | 0.33 | 97 ± 10 | 107 ± 12 | 0.55 |
| Thigh compartment area (cm2) | 138 ± 34 | 112 ± 25 | 0.02 | 128 ± 4 | 126 ± 4 | 0.68 |
| Femur (cm2) | 6.1 ± 0.9 | 5.9 ± 1.3 | 0.57 | 5.8 ± 0.2 | 6.2 ± 0.2 | 0.17 |
| Skeletal muscle (cm2) | 96 ± 27 | 83 ± 22 | 0.14 | 89 ± 3 | 94 ± 4 | 0.40 |
| Intermuscular fat (cm2) | 35.6 ± 11.5 | 22.3 ± 7.6 | <0.001 | 33.4 ± 1.8 | 25.7 ± 2.2 | 0.01 |
| Intermuscular fat/ thigh compartment (%) | 26 ± 5 | 20 ± 5 | 0.002 | 26 ± 1 | 20 ± 1 | 0.005 |
| Skeletal muscle/ thigh compartment (%) | 70 ± 5 | 75 ± 5 | 0.008 | 69 ± 1 | 75 ± 1 | 0.009 |
| Intermuscular fat/skeletal muscle | 0.38 ± 0.10 | 0.28 ± 0.09 | 0.003 | 0.38 ± 0.02 | 0.28 ± 0.03 | 0.007 |
Thigh compartment = skeletal muscle + intermuscular fat + femur. Raw data are presented as mean ± standard deviation.
adjusted for body surface area and presented as least square means ± standard error.
Figure 1.
MRI axial image of the mid-thigh in a HFpEF and a HC subject. Red= skeletal muscle; green = Intermuscular fat; blue = subcutaneous fat; purple = femoral cortex; yellow = femoral medulla. Intermuscular fat (green) is substantially increased in the HFpEF patient compared to HC despite similar subcutaneous fat.
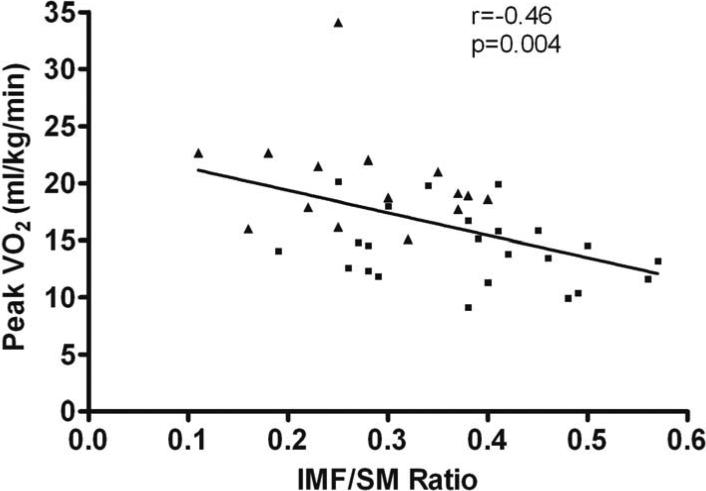
In univariate analyses with all subjects combined, there was a significant inverse relationship between IMF area and peak VO2 (r=-0.49, p=0.002), while SM area was not significantly associated with peak VO2 (r=-0.10, p=0.57). Further, IMF/SM ratio was significantly inversely related to peak VO2 (r=-0.46, p=0.004, Figure 2). When analyses were restricted to HFpEF patients, the inverse relationship between IMF and peak VO2 remained significant (r=-0.53, p=0.01) and the inverse relationship between IMF/SM ratio and peak VO2 remained as a trend (r=-0.28, p=0.19).
Figure 2.
Relationship between intermuscular fat/skeletal muscle ratio and peak oxygen uptake in HFpEF and HC. Solid squares=HFpEF; solid triangles=HC.
In multivariate analyses that included the a priori selected variables age, gender, IMF area, and SM area as competing predictors of peak VO2, IMF area was an independent predictor of peak VO2 (partial r=-0.51, p=0.002) while SM area was not (partial r=-0.14, p=0.43). Additionally, with IMF/SM ratio in the multivariate model (instead of IMF and SM area), IMF/SM ratio was an independent predictor of peak VO2 (partial r=-0.45, p=0.006). Adding body mass index to the multivariate models moderately weakened the partial correlations of IMF area and IMF/SM ratio with peak VO2 to trends (p-values of 0.15 and 0.16, respectively). However, when thigh SCF area, a relevant region-specific measure of adiposity, was included in the multivariate models, IMF area (partial r=-0.50, p=0.003) and IMF/SM ratio (partial r=-0.44, p=0.008) remained as independent predictors of peak VO2.
DISCUSSION
Multiple lines of evidence suggest that in addition to cardiac dysfunction, abnormalities in non-cardiac ‘peripheral’ factors may be important contributors to the severe exercise intolerance observed in older HFpEF patients2,4,5,7,16. The major novel finding is that compared to age-matched healthy adults, older HFpEF patients have increased thigh intermuscular fat (IMF), whether expressed as absolute area or as a proportion of the thigh compartment, and this occurs despite similar amount of subcutaneous fat (SCF). Furthermore, the ratio of IMF/SM is increased and both IMF area and IMF/SM ratio were significant, independent predictors of peak exercise VO2 (Figure 2). These results suggest that skeletal muscle composition is abnormal in older HFpEF patients and may be an important contributor to their severe exercise intolerance.
These results are credible given what is known regarding skeletal muscle composition and its relationship to physical function in other populations17-21. For instance, multiple studies have reported increased fat infiltration in skeletal muscle in older persons with impaired physical function17,18 and that this is associated with slower walking speed and chair rise time17,19, decreased strength21, a faster decline in walking speed20, and an increased risk of mobility limitation21. Also, leg skeletal muscle composition is abnormal in patients with HF and reduced EF, and contributes to their severe exercise intolerance22.
Using DXA, we recently reported that percent body fat and percent leg fat were significantly increased while percent body lean and leg lean mass were significantly reduced in older HFpEF patients versus HC6. Our present findings using MRI to directly characterize thigh muscle composition significantly extend these prior results by identifying abnormal fat infiltration into the skeletal muscle and by potentially explaining the markedly abnormal relationship between percent leg lean mass and peak VO2 in HFpEF patients6. Together, these findings support the concept that altered skeletal muscle composition (remodeling) contributes to exercise intolerance in older HFpEF patients.
Increased IMF area may be present and may contribute to reduced peak VO2 in HFpEF patients via a number of mechanisms. The presence of increased IMF may be due to the dysfunction of muscle satellite cells, whereby these cells acquire features of adipocytes, including the ability to accumulate lipids23. In particular, excess mitochondrial reactive oxygen species has been implicated as one pathway for the adipogenic conversion of muscle satellite cells23. Mechanistically, IMF may compete with active muscle tissue for critical nutritive blood flow during exercise. Specifically, Heinonen et al.24 using positron emission tomography, found that adipose tissue blood flow adjacent to the active muscles increased 7-fold during continuous isometric knee-extension exercise in non-obese younger healthy sedentary women. Thus, increased thigh IMF in older HFpEF patients may ‘steal’ blood that would normally be delivered to the active muscles during exercise and thereby reduce perfusive O2 delivery to the thigh muscle. Furthermore, increased IMF area is associated with reduced mitochondrial mass, biogenesis and oxidative metabolism25. Indeed, Bhella et al, using 31Phosphate magnetic resonance spectroscopy during and after performing static leg lifts, revealed impaired skeletal muscle oxidative metabolism in HFpEF patients4. Thus, a number of potential IMF-mediated structural and biochemical alterations may decrease O2 transport to and/or utilization by the active muscles.
We recently showed that endurance training, the single intervention shown to date to improve exercise capacity in HFpEF, appears to do so primarily by improving peripheral, non-cardiac factors5,7, other than large artery function7, suggesting a role for skeletal muscle. Studies in other populations indicate that both exercise training and caloric restriction can reduce regional as well as total body adiposity and improve exercise capacity and physical function26,27. This is pertinent since ~ 85% of HFpEF patients are overweight / obese14. Other strategies that could improve muscle composition include high protein diet28 which has been shown to reduce IMF and total body fat and increase skeletal muscle29.
We adjusted the thigh composition variables by body surface area because it was significantly different between groups and accounts for both body height and weight. However, when we adjusted the thigh composition variables for height, height2.7, femur length, or body mass index, these did not significantly alter the overall conclusions.
Although the HFpEF patients’ body mass and body surface area were significantly greater than HC, thigh SCF, a relevant region-specific measure of adipose, and skeletal muscle, total thigh, and TC areas were similar between groups. Also, peak VO2 indexed to body mass was reduced by nearly 30% in HFpEF compared to HC. Further, the IMF area in our HFpEF patients was three-fold higher than older (75 years), overweight (body mass index 27.1) women without HF reported by Beavers et al. in the Health, Aging, and Body Composition study (35.6 vs. 10.2 cm2, respectively)20. Thus, our findings are not merely due to differences in body mass or adiposity.
Due to the cross-sectional design, the study is unable to determine whether greater IMF area and IMF/SM ratio are causes of, or consequences of, the severely reduced peak VO2 in the HFpEF patients.
By study design, HFpEF patients were ambulatory, stable, had no recent acute hospitalization, and able to do exhaustive exercise testing. As a result, the mean BNP level was less than in patients with acute, decompensated HF. BNP levels are known to be lower in HFpEF than in HFrEF. The BNP levels are similar to other studies of stable HFpEF patients able to undergo maximal exercise testing16,30 and are greater than two-fold increased compared to healthy, age-matched, normal subjects1.
Because tissue Doppler was not performed, the study could not assess its potential contribution to the patients’ exercise intolerance.
Acknowledgments
This study was supported by: NIH Grant R37AG18915 and R01 HL093713 and The Claude D. Pepper Older Americans Independence Center of Wake Forest University NIH Grant P30AG021332
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 2.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of Exercise Intolerance in Elderly Heart Failure Patients With Preserved Ejection Fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 4.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky SB, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of Endurance Exercise Training on Endothelial function and Arterial Stiffness in Older Patients with Heart Failure and Preserved Ejection Fraction: A Randomized, Controlled, Single-Blind Trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney R. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 10.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 11.Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow mediated arterial; dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci. 2013;68:161–167. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones DA, Round JM, Edwards RH, Grindwood SR, Tofts PS. Size and composition of the calf and quadriceps muscles in Duchenne muscular dystrophy. A tomographic and histochemical study. J Neurol Sci. 1983;60:307–322. doi: 10.1016/0022-510x(83)90071-0. [DOI] [PubMed] [Google Scholar]
- 13.Holmback AM, Askaner K, Holtas S, Downham D, Lexell J. Assessment of contractile and noncontractile components in human skeletal muscle by magnetic resonance imaging. Muscle Nerve. 2002;25:251–258. doi: 10.1002/mus.10031. [DOI] [PubMed] [Google Scholar]
- 14.Kitzman DW, Gardin JM, Gottdiener JS, Arnold AM, Boineau R, Aurigemma GP, Marino E, Lyles M, Cushman M, Enright P. For the Cardiovascular Health Study Group. Importance of heart failure with preserved systolic function in patients > or = 65 Years of Age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 15.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 16.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, Redfield MM. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. J Obes Relat Metab Disord. 1999;23:126–132. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 18.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 20.Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, Newman AB, Simonsick EM, Studenski SA, Nicklas BJ, Kritchevsky SB. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi: 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. for the Health ABC Study. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 22.Coats AJ, Clark AL, Piepoli MF, Volterranni M, Poole-Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72:S36–S39. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, Federspil G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297:E987–E998. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 24.Heinonen I, Bucci M, Kemppainen J, Knuuti J, Nuutila P, Boushel R, Kalliokoski KK. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. J Appl Physiol. 2012;112:1059–1063. doi: 10.1152/japplphysiol.00732.2011. [DOI] [PubMed] [Google Scholar]
- 25.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89:1043–1052. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Krichevsky SB. Fat Mass Loss Predicts Gain in Physical Function With Intentional Weight Loss in Older Adults. J Gerontol A Biol Sci Med Sci. 2013;68:80–86. doi: 10.1093/gerona/gls092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motie M, Evangelista LS, Horwich T, Hamilton M, Lombardo D, Cooper DM, Galassetti PR, Fonarow GC. Pro-HEART- A randomized clinical trial to test the effectiveness of a high protein diet targeting obese individuals with heart failure: Rationale, design and baseline characteristics. Contemp Clin Trials. 2013;36:371–381. doi: 10.1016/j.cct.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon MM, Bopp MJ, Easter L, Miller GD, Lyles MF, Houston DK, Nicklas BJ, Kritchevsky SB. Effects of dietary protein on the composition of weight loss in post-menopausal women. J Nutr Health Aging. 2008;12:505–509. doi: 10.1007/BF02983202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, Clinical Phenotype, and Outcomes Associated With Normal B-Type Natriuretic Peptide Levels in Heart Failure With Preserved Ejection Fraction. Am J Cardiol. 2012;110:870–876. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]