Abstract
BACKGROUND & PURPOSE
Prompt thrombolytic therapy with intravenous alteplase reduces disability after acute ischemic stroke. In an exploratory analysis we examined whether long-term survival varied by baseline characteristics after alteplase.
METHODS
In this open-treatment, international, randomized, controlled trial, ischemic stroke patients were randomly allocated <6h of onset to intravenous alteplase (0·9 mg/kg) plus standard care (n=1515) or standard care alone (n=1520). We followed patients to death, censoring when last known to be alive. We grouped patients by delay to randomization, and ‘good’ or ‘poor’ predicted prognosis (calculated from baseline NIHS score and age). We present absolute mortality differences between treated and control groups at 7 days, 6 months and 18 months post stroke.
RESULTS
Alteplase was not associated with a significant increase in mortality within 18 months [0.6% (95%CI:−2.9% to +4.2) P=0.72] in all patients with complete vital status (99.9%, 3034/3035). In patients randomized <3 hrs of stroke, 18 month mortality was lower in the alteplase treated group than the control group [40.6%(95%CI:42.6–52.7) vs 47.8%(95%CI:35.5–45.3)P=0.0434].The difference in 18 month mortality between alteplase-treated and control patients was greater in patients who were randomized early (<3h) compared to late (3-6h) [+9%(95%CI:1–17)P=0.0317]. Alteplase led to a greater improvement in 18 month survival in patients with a poor prognosis than in patients with a good prognosis [+8% (95%CI: 2–14)P=0.0091].
CONCLUSIONS
These exploratory analyses of the IST-3 trial support improving acute stroke patients’ access to earlier alteplase treatment; treatment of patients with poor prognosis; and further randomized controlled trials in minor stroke to replicate these findings.
CLINICAL TRIAL REGISTRATION
www.controlled-trials.com. Unique identifier: ISRCTN25765518
INTRODUCTION
When given soon after onset of acute ischemic stroke, thrombolytic therapy with intravenous alteplase leads to an improvement in both short term1 and long term2 functional outcome. While time to treatment is the key determinant of the effect of alteplase on functional outcome, the factors that modify the effect of alteplase on long term mortality after stroke are less clear. Alteplase had no statistically significant effect on mortality by 90 days when studied in randomized, controlled trials. 1, 3 However, in the short term (7 days) thrombolysis leads to an increase in the risk of death (22 per 1000 treated), principally due intracranial hemorrhage.1 These data suggest that stroke patients treated with alteplase who survive the acute phase are likely to gain a survival benefit from alteplase over the medium term that offsets any early increase in mortality due to bleeding. This survival benefit may be because stroke survivors who are less disabled due to alteplase treatment live longer after stroke4, and it is possible that survival benefits accrue with time.
The balance between the early hazard of fatal intracranial hemorrhage and potential long term survival benefits may vary in different patients. In patients treated earlier – in whom the medium term benefits of alteplase on functional outcome are larger - alteplase treatment may lead to a long term survival benefit that is difficult to demonstrate in the medium term. In ischemic stroke patients with a good prognosis, who are at a low absolute risk of death in the long term without alteplase treatment, any early increase in mortality with alteplase (from intracranial hemorrhage) may not be outweighed by a reduction in death in the longer term. In order to investigate whether the effect of alteplase on long term survival in acute ischemic stroke patients was dependent on the delay to treatment or baseline prognosis, we performed an exploratory analysis of the third International Stroke Trial (IST-3), a large randomized trial of alteplase in ischemic stroke that collected data on survival up to 18 months of stroke.2, 5
METHODS
Ethics statement
IST-3 was approved by the Multi-centre Research Ethics Committees, Scotland (reference MREC/99/0/78), and by local ethical committees. Patients or a valid proxy gave written consent to participate. This trial was registered (ISRCTN25765518).
IST-3 study design and participants
The details of the IST-3 study protocol,6 statistical analysis plan,7 and primary outcomes5 have been published previously. In brief, ischemic stroke patients (with no upper age limit) who could start alteplase treatment within 6 hours of symptom onset, with no clear indication for, or clear contraindication to alteplase, and in whom the randomising clinician was substantially uncertain about the risks and benefits of alteplase, were randomized 1:1 to standard care with an infusion of 0.9mg/kg alteplase or standard care without alteplase between 2000 and 2011. Both groups were managed within a stroke unit with intravenous access and monitoring of blood pressure and other physiological variables. Treatment with alteplase was not blinded. Baseline data were collected by a web-based or telephone randomization system prior to treatment allocation. At 7 days, randomising clinicians reported whether patients were ‘independent of activities of daily living’ or ‘not independent of daily living’. The trial event adjudication committee defined ‘symptomatic post-rtPA ICH’ (SICH) as a clinically significant deterioration or death within the first 7 days of treatment and evidence of either significant brain parenchymal hemorrhage (local or distant from the infarct) or significant hemorrhagic transformation of an infarct on brain imaging.7
The dates of death up to 18 months post randomization were ascertained from central death registries in the UK, Norway and Sweden. In other countries, dates of death were ascertained by contacting the patient’s primary care physician or hospital co-ordinators. In a few cases, deaths were ascertained during the process of follow up for disability at 6 and 18 months. Functional outcome was measured at 6 months with the Oxford Handicap Scale (OHS), which is very similar to the modified Rankin Score. 8
Statistical analysis
In our survival analysis, we compared the survival of acute ischemic stroke patients treated with alteplase + standard care with patients managed with standard care alone (control) over 18 months, analysed in their randomly allocated groups ( i.e. intention to treat analysis).
We examined the effect of delay to randomization on long term survival in the subgroups 0-3 hours versus 3-6 hours and 0-4.5 hrs versus 4.5-6 hrs. These thresholds were chosen as the marketing authorisation for the use of alteplase is within 3 hours of stroke onset in the United States, and within 4.5 hours of stroke onset from Europe and other regions. 9
We examined subgroups of patients defined by their probability of death or dependence at 3 months, calculated from their status at randomization with a previously developed and validated clinical prediction model based on the NIHSS score and age,10 at a threshold of predicted risk of 50%. The choice of this threshold was arbitrary, but pre-specified. We defined a predicted risk of death or dependence of <50% as ‘good prognosis’ and a predicted risk of ≥50% as ’poor prognosis’. To support this analysis we separately explored the components of this predictive model, age (dichotomised at 80) and the NIH Stroke Scale (NIHSS) (dichotomised at 5), and performed sensitivity analyses at different thresholds of predicted risk (20% and 40%); in patients randomized within 4.5 hrs; and in patients who received their allocated treatment (‘on treatment analysis’).
We used time from stroke onset to death within the first 548 days (18 months) of follow up as the primary outcome, and censored either at the end of follow up, or withdrawal of consent (for more details, please see supplementary materials at http://stroke.ahajournals.org).
Anticipating non-proportional hazards that would preclude Cox-regression analysis, we calculated the cumulative mortality differences between alteplase and control at 7 days, 6 months and 18 months, using Kaplan Meier estimates. We tested interactions between treatment and delay by measuring the statistical significance of the difference in mortality differences between groups [e.g. (mortalitycontrol - mortalityalteplas) for patients <3 hours post stroke minus (mortalitycontrol - mortality-alteplas) for patients 3–6 hours post stroke]. The interactions between predicted prognosis and treatment were tested similarly. We adjusted further for the confounding effects of delay and predicted prognosis as continuous variables using Cox regression in different time periods (0-7, 7-183 and 183-548 days) and using logistic regression with death by 18 months as the dependent variable in those patients for whom follow up to 18 months was planned. We tested multiplicative interactions between treatment and delay or prognosis with a likelihood ratio test. We report p-values with reference to a nominal two-sided significance level of 0.05, though the type 1 error rate is unknown as they are exploratory.
We wrote and published the statistical analysis plan for this work (available at www.dcn.ed.ac.uk/dcn/staff/displaystaff.asp?RecordId=174) before undertaking the analyses but after the publication of primary IST-3 report.5
RESULTS
We analysed all patients randomized in IST-3, bar one for whom we were unable to calculate a survival time (1520 control vs. 1514 alteplase). Important prognostic factors were balanced between groups (please see http://stroke.ahajournals.org, supplementary table I). We were unable to calculate mortality hazard ratios between alteplase treated and control patients as there was good evidence that the hazards of death over 18 months were non-proportional (test for nonproportionality p=0.0011), principally due to an excess of deaths within 7 days in the alteplase group.
By 7 days, in all patients, patients allocated to alteplase had an excess mortality over control of 3.5% (95%CI 1.5 to 5.4), though no such excess at 6 months [0.1% (95%CI: −3.1 to +3.2)] or 18 months [−0.6% (95%CI: −4.1 to +2.9)]. (Table 1)
Table 1.
Kaplan Meier estimates of mortality with 95% CIs at 7 days, 6 months and 18 months post stroke, with absolute difference between treatment arms at each time point (control minus alteplase).
| Control (%) |
Alteplase (%) |
Difference (control-alteplase) (%)* |
||||
|---|---|---|---|---|---|---|
| Days since enrolment | Deaths (n) | K-M estimate (95% CI) | Deaths (n) | K-M estimate (95% CI) | Estimate (95% CI) | P-value |
| All patients | ||||||
| 7 | 102 | 6.71 (5.44 to 7.96) | 154 | 10.17 (8.64 to 11.68) | −3.46 (−5.44 to −1.49) | 0.0006 |
| 183 | 407 | 26.80 (24.54 to 29.00) | 407 | 26.90 (24.63 to 29.10) | −0.10 (−3.25 to 3.06) | 0.9510 |
| 548 | 539 | 36.56 (34.03 to 38.99) | 529 | 35.93 (33.41 to 38.35) | 0.63 (−2.86 to 4.13) | 0.7235 |
| Delay to randomization | ||||||
| Time to randomization, < 3 hrs | ||||||
| 7 | 35 | 8.86 (6.01 to 11.62) | 41 | 10.10 (7.12 to 12.98) | −1.24 (−5.29 to −2.82) | 0.5497 |
| 183 | 142 | 35.95 (31.04 to 40.51) | 130 | 32.02 (27.33 to 36.41) | 3.93 (−2.63 to 10.49) | 0.2401 |
| 548 | 184 | 47.84 (42.55 to 52.65) | 161 | 40.62 (35.54 to 45.30) | 7.22 (0.21 to 14.23) | 0.0434 |
| Time to randomization, ≥ 3 hrs | ||||||
| 7 | 67 | 5.96 (4.56 to 7.33) | 113 | 10.20 (8.40 to 11.96) | −4.24 (−6.50 to −1.99) | 0.0002 |
| 183 | 265 | 23.59 (21.06 to 26.03) | 277 | 25.03 (22.43 to 27.54) | −1.44 (−5.00 to 2.12) | 0.4287 |
| 548 | 355 | 32.58 (29.72 to 35.33) | 368 | 34.20 (31.29 to 37.00) | −1.62 (−5.62 to 2.38) | 0.4270 |
| Time to randomization, < 4.5 hrs | ||||||
| 7 | 74 | 7.44 (5.80 to 9.06) | 110 | 11.19 (9.20 to 13.14) | −3.75 (−6.30 to −1.19) | 0.0041 |
| 183 | 306 | 30.81 (27.88 to 33.62) | 297 | 30.22 (27.29 to 33.03) | 0.59 (−3.47 to 4.65) | 0.7753 |
| 548 | 398 | 41.12 (37.91 to 44.16) | 379 | 39.42 (36.23 to 42.45) | 1.70 (−2.70 to 6.11) | 0.4489 |
| Time to randomization, ≥ 4.5 hrs | ||||||
| 7 | 28 | 5.32 (3.39 to 7.22) | 44 | 8.29 (5.91 to 10.60) | −2.96 (−5.99 to 0.07) | 0.0552 |
| 183 | 101 | 19.23 (15.79 to 22.53) | 110 | 20.76 (17.23 to 24.13) | −1.53 (−6.35 to 3.30) | 0.5353 |
| 548 | 141 | 27.89 (23.84 to 31.72) | 150 | 29.41 (25.31 to 33.28) | −1.52 (−7.12 to 4.08) | 0.5941 |
| Subgroups of differing stroke severity | ||||||
| Predicted risk of death or dependence by three months < 0.5 (‘good prognosis’) | ||||||
| 7 | 8 | 1.49 (0.46 to 2.50) | 14 | 2.69 (1.29 to 4.07) | −1.21 (−2.93 to 0.52) | 0.1713 |
| 183 | 36 | 6.71 (4.57 to 8.81) | 45 | 8.67 (6.22 to 11.06) | −1.96 (−5.18 to 1.26) | 0.2323 |
| 548 | 55 | 10.71 (7.99 to 13.36) | 74 | 15.03 (11.80 to 18.15) | −4.32 (−8.48 to −0.17) | 0.0415 |
| Predicted risk of death or dependence by three months ≥ 0.5 (‘poor prognosis’) | ||||||
| 7 | 94 | 9.57 (7.71 to 11.39) | 140 | 14.08 (11.89 to 16.22) | −4.51 (−7.35 to −1.67) | 0.0018 |
| 183 | 371 | 37.78 (34.67 to 40.74) | 362 | 36.42 (33.36 to 39.35) | 1.36 (−2.90 to 5.62) | 0.5330 |
| 548 | 484 | 50.60 (47.30 to 53.70) | 455 | 46.78 (43.52 to 49.85) | 3.82 (−0.67 to 8.32) | 0.0956 |
Note one patient wasexcluded from all analyses as follow up time was missing.
positive numbers indicate less mortality with alteplase. We estimated the standard error for the difference in survivorship functions using the individual standard errors of the K-M estimates for control and alteplase and calculated the 95% point-wise confidence bands and associated P-values.
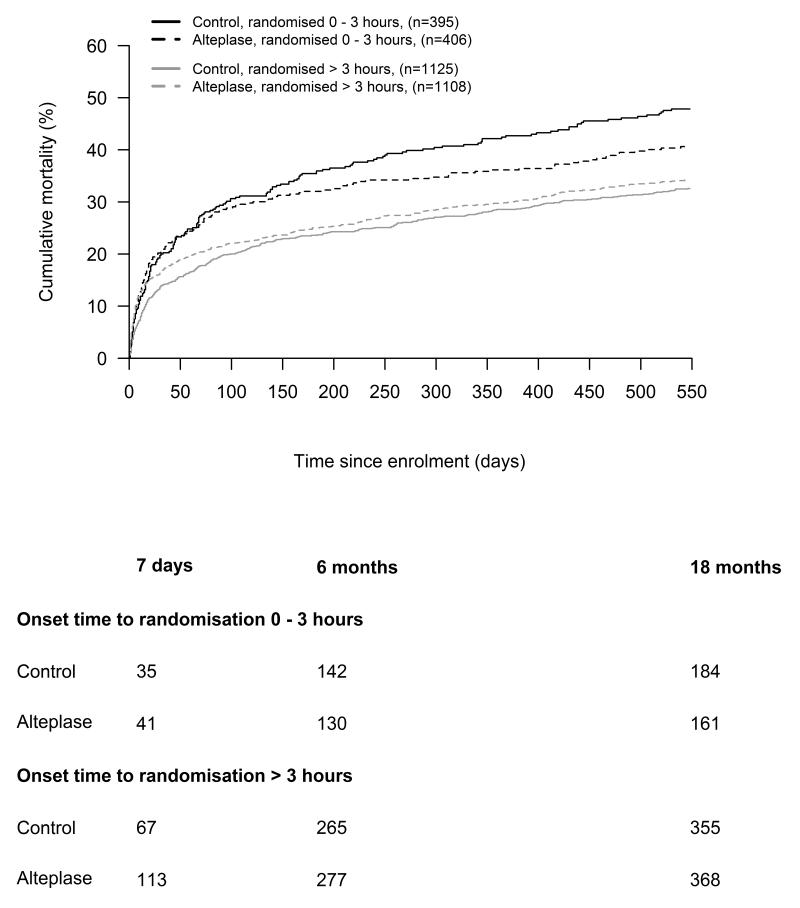
There was evidence to suggest that the absolute effect of alteplase on mortality varied by time to randomization. There was modification of the difference in 18 month mortality between alteplase and control patients in the direction of improved survival in those randomized <3 hrs. A test for interaction, the difference in 18 month mortality differences with alteplase between early and late groups, [+8.8% (95% CI: 0.8 to 16.9, P = 0.03)] suggested improved long term mortality with earlier alteplase treatment. However, when comparing patients treated 0-4.5hrs with patients treated >4.5 hrs the difference of effect was in the same direction but not statistically significant (+3.2 % 95% CI: −3.9 to 10.4, P = 0.38). (Figure 1)
Figure 1.
Cumulative mortality plots of alteplase-treated versus control patients split by onset time to randomization (0 to 3 hours vs. > 3 hours). The difference of the differences in 18 month mortality in the 0=3 hrs and the >3 hrs groups was 8.8% (95% CI: 0.8 to 16.9, P-value = 0.0317)
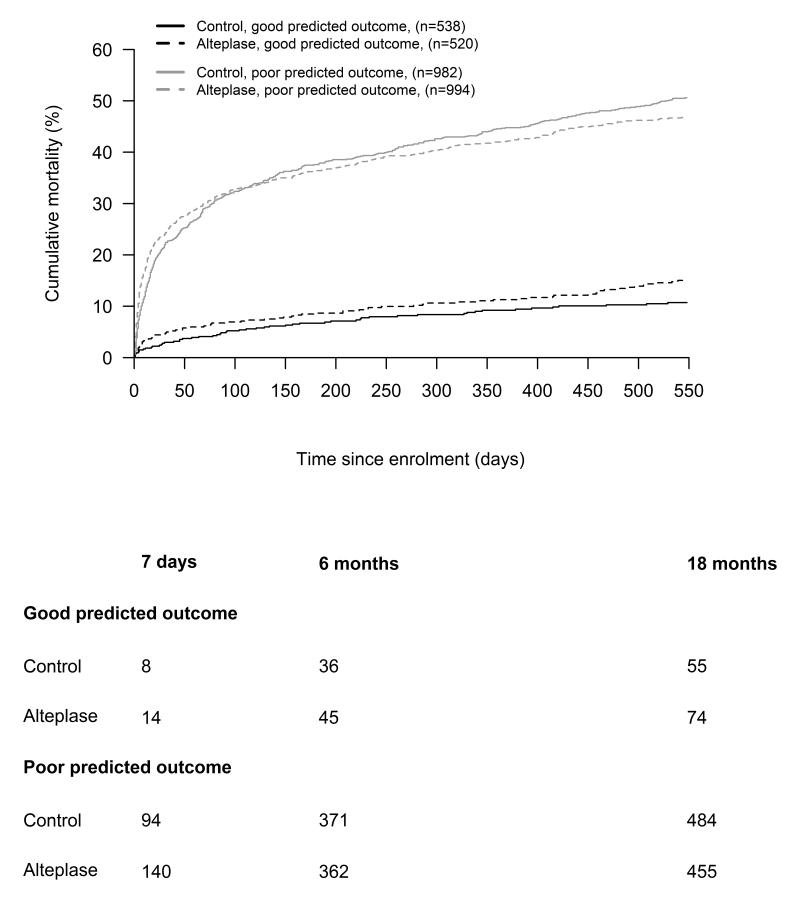
There was evidence to suggest that the absolute effect of alteplase on 18 month mortality varied by baseline prognosis. There was modification of the difference in 18 month mortality in the direction of greater survival with alteplase in poor prognosis patients. The test for interaction, the difference of 18 month mortality differences with alteplase between good and poor prognosis groups was statistically significant [−8.1% (95% CI: 2.0 to 14.2) P = 0.0091] (Figure 2). The direction of this difference was similar – though not consistently statistically significant - with different thresholds of predicted risk of poor outcome to define good prognosis (<20% −2.2 (95% CI: −8.9 to 4.5) P=0.5178, and <40% −8.15 (95% CI −14.3 to −2.0 P=0.0090); in patients randomized less than 4.5 hours after stroke (−6.2%, 95%CI:-31.8 to 19.5 P=0.1307 ) and in an ‘on treatment’ analysis (−9.4%, 95%CI:−15.8 to −2.9 P=0.00470). When patients were grouped by components of the prognostic score, the difference of differences in 18 month mortality between alteplase and control groups were smaller and not statistically significant (NIHSS <5 vs ≥5=−4.0 (95% CI: −10.6 to 2.7 P = 0.2412), age ≤80 vs >80=−1.8 (95% CI: −8.5 to 4.8 P = 0.5879). (please see supplementary materials http://stroke.ahajournals.org, table II)
Figure 2.
Cumulative mortality plots of alteplase-treated versus control patients split by predicted functional outcome (good <50% vs. poor ≥50%). The difference of the differences between alteplase treated and control patients in the good prognosis and poor prognosis groups was −8.1% (95% CI: 2.0 to 14.2 P = 0.0091) at 18 months.
We further adjusted for the confounding effects of prognosis and delay in Cox regression models in different periods. After adjustment, the hazard ratio of early death (0-7 days) was increased by alteplase (HR 1.54, 95%CI: 1.19–2.00), with no evidence of modification by time to randomization (Pinteraction =0.2371) or predicted prognosis (Pinteraction =0.9386). For late death (183-548 days), there was evidence that worse predicted prognosis reduced the hazard ratio of later death with alteplase treatment (Pinteraction=0.0066), but the reduction in late death with earlier treatment was not significant (Pinteraction =0.1994) (please see supplementary materials http://stroke.ahajournals.org, supplementary table III). In an adjusted logistic regression analysis of all patients followed up until 18 months (N=2348) alteplase led to lower odds of all deaths by 18 months in patients with a poorer compared to better predicted prognosis (Pinteraction =0.005), and non-significantly lower odds of death by 18 month in patients treated earlier rather than later after stroke (Pinteraction =0.499).
There were no important differences in 18 month mortality when comparing patients allocated alteplase and those allocated control between men and women (P for difference of difference 0.901) or between different baseline stroke subtypes classified with the OCSP scale.
The early excess mortality with alteplase compared to control was largely due to symptomatic intracranial hemorrhage. By 18 months, in patients who avoided intracranial hemorrhage, the survival benefit of alteplase compared with control was +4.13% (95%CI: 0.43–7.83, P-value = 0.0287). However, this comparison is non-randomized, and should be interpreted with caution.
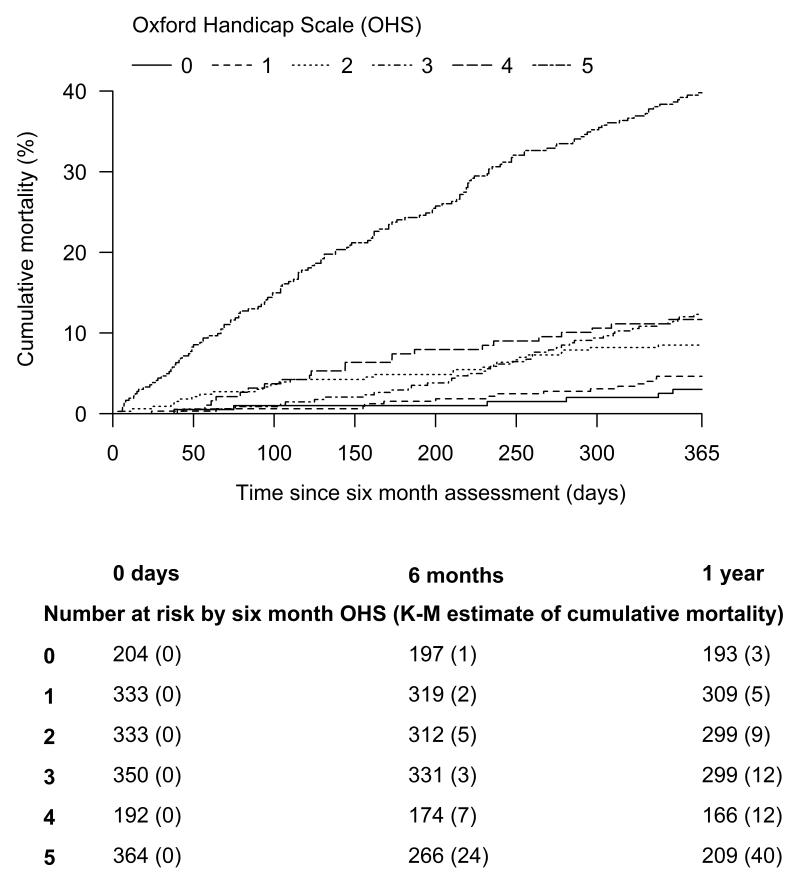
Patients who were dependent at 7 days follow up had greater mortality by 18 months days after stroke than patients who were independent at 7 days (proportion of 7 day survivors dead at 548 days 44% vs 11%). Patients with less disability in the medium term (6 months) had better survival than patients with more disability (Figure 3). For patients with a 6 month OHS of 0, 1, 2, 3, 4 and 5 the proportion who died over the next 12 months were 3%, 5%, 9%, 12%, 12%, and 40% respectively.
Figure 3.
The cumulative mortality of 6 month survivors, grouped by Oxford Handicap Scale measured at 18 months.
DISCUSSION
We have shown that although alteplase given within 6 hours of ischemic stroke does not lead to any significant change in overall mortality at 18 months, there was some evidence in our exploratory analysis that the effect of alteplase on mortality differed by time to randomization. Consistent with previous findings that earlier treatment leads to better functional outcome at 3-6 months (though no clear difference in mortality is evident at that time point)11, treatment less than three hours after stroke was associated with better 18 month survival than later treatment, though this association was attenuated after adjustment for predicted prognosis and could have been due to confounding or chance.
There was support for our hypothesis that patients with a good prognosis after acute stroke might not gain a survival advantage from alteplase. In our analysis, patients with a poor prognosis had greater reduction in long term mortality than patients with a good prognosis, and there was some evidence for a small increase in 18 month mortality with alteplase treatment in patients with a good prognosis. A plausible explanation is that early hazard due to SICH is outweighed by a survival advantage due to a small reduction in severe disability in patients with a poor prognosis, though not in patients with a good prognosis.
The strengths of this study are the large number of patients randomized, the inclusion of older patients, and those with more severe stroke, and the length and completeness of follow up. We have studied all-cause mortality where the ascertainment of the outcome and its timing is objective, so bias in outcome ascertainment is unlikely and the lack of blinding is not relevant.
The study has a number of limitations. Although we studied 1068 deaths in 3034 stroke patients, it is likely that the study was underpowered to determine all the important factors that modify the effect of alteplase on mortality. The Stroke Thrombolysis Trialists’ Collaboration individual patient data meta-analysis of all patients randomized in trials of intravenous alteplase has important information on short- and medium-term mortality, to which we add with longer term mortality estimates.1 IST-3 was an open label study; this aspect has been discussed in great detail before,12 and while this may have biased the observed estimate of effects on functional outcome, it is hard to conceive how an open design might bias estimates of effect on late mortality. The effects of alteplase on disability in IST-3 were entirely consistent with the effects of alteplase in previous trials,11 and our observations in this study run counter to the expected direction of information bias. Although using survivor functions may have reduced statistical power to examine the effect of factors modifying mortality our conclusions are supported by more powerful Cox and logistic regression modelling. These analysis adjusted for the well-recognised confounding of time to randomization by stroke severity (i.e. patients with more severe strokes tend to be randomised into trials earlier). 1 We only collected cause of death up to 7 days after stroke, so cause specific differences in long term mortality were not available. Whilst a protocol was used for this statistical analysis, this was written after knowledge of the primary results of IST-3, and therefore these results are exploratory (i.e. there is a risk of a type 1 error) and should be put in the context of a number of secondary analyses of the data; a p-value of <0.05 should not be taken as strong evidence that the results were not due to chance. These finding therefore should be replicated. This may be achieved through studying long term data in other studies: the only other existing study with long term follow up, the NINDS trial, did not report an early increased risk of death with alteplase, so may be less generalizable to clinical practice where fatal symptomatic intracranial hemorrhage is not infrequent 13, 14.
The main cause of the increase in early mortality with alteplase treatment is symptomatic intracranial hemorrhage. There is no reliable way to detect a group of patients at a high risk of intracranial hemorrhage who do not benefit from alteplase using clinical or simple imaging biomarkers. 14 A future blood or imaging marker that reliably identified a group of patients at high risk of intracranial hemorrhage and who did not benefit from alteplase, or a new thrombolysis agent or treatment regimen with similar or increased benefits to alteplase with a reduced risk of intracranial hemorrhage would therefore be extremely useful.
In a meta-analysis of all available data, there was no evidence that the relative effects of alteplase on medium term mortality or functional outcome (mRS 0-1) or on long term quality of life were materially different across the range of the components of the prognostic score (NIHSS and age). 1,3 In conclusion, in patients with a good prognosis, the balance between a modest absolute improvement in disability with alteplase versus a potential small increase in mortality in the long term needs to be further investigated. These results therefore support the rationale for further randomized trials of alteplase versus control in patients with good prognosis, such as the Potential of rtPA for Ischemic Strokes with Mild Symptoms [PRISMS] study which is recruiting patients <3 hrs post stroke (http://www.clinicaltrials.gov, NCT02072226). Our data support the use of alteplase in patients with a poor prognosis, despite their increase risk of intracranial hemorrhage, as it does not lead to increased mortality in the long term and leads to an improvement in functional outcome.
Supplementary Material
Acknowledgments
Sources of Funding IST-3 was funded by United Kingdom (UK) Stroke Association, Health Foundation UK , UK Medical Research Council (MRC) (G0400069, EME 09-800-15); Research Council of Norway; AFA Insurances , Sweden; Swedish Heart Lung Fund ; Foundation of Marianne and Marcus Wallenberg; Stockholm County Council and Karolinska Institute Joint ALF-project grants; the Government of Poland (2PO5B10928); the Australian Heart Foundation (G04S1638); Australian National Health and Medical Research Council (457343); the Swiss National Research Foundation; the Swiss Heart Foundation ; Foundation for health and cardio-/neurovascular research, Basel, Switzerland; the Assessorato alla Sanita, Regione dell’Umbria; Danube University, Krems, Austria. Drug and placebo for the 300 patients in the double-blind component of the start-up phase were supplied by Boehringer Ingelheim. We thank the National Institute for Health Research (NIHR) Stroke Research Network, National Health Service (NHS) Research Scotland, through the Scottish Stroke Research Network, and the National Institute for Social Care and Health Research Clinical Research Centre for their support. Scottish Imaging Network a Platform for Scientific Excellence (SINAPSE) is funded by the Scottish Funding Council and the Chief Scientist Office of the Scottish Executive. Additional support was received from Chest Heart and Stroke Scotland, Designed Access (DesAcc), University of Edinburgh, Danderyd Hospital Research and Development Department, Karolinska Institutet, Oslo University Hospital, and the Dalhousie University Internal Medicine Research Fund. This report presents independent research supported by the NIHR through the UK Stroke Research Network. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. Dr. Whiteley is supported by an MRC Clinician Scientist Fellowship (G0902303) and Mr. Thompson is supported by an MRC Hubs for Trials Methodology Research grant (G0800803).
Footnotes
Conflicts of interest PS has received lecture fees (paid to the Division of Clinical Neurosciences, University of Edinburgh) and travel expenses from Boehringer Ingelheim, was a member of the independent data and safety monitoring board of the RE-LY trial funded by Boehringer Ingelheim for which attendance fees and travel expenses were paid (to the Division of Clinical Neurosciences, University of Edinburgh). RIL has been paid for his role as a member of a conference scientific committee and for lectures by Boehringer Ingelheim and has attended national stroke meetings organised and funded by Boehringer Ingelheim. The other authors have no conflicts of interest to declare.
References
- 1.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014;379:2352–2363. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IST-3 collaborative group Effect of thrombolysis with alteplase within 6 h of acute ischaemic stroke on long-term outcomes (the third international stroke trial [ist-3]): 18-month follow-up of a randomised controlled trial. Lancet Neurol. 2013;12:768–776. doi: 10.1016/S1474-4422(13)70130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. The Lancet. 2013;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruins Slot K, E. B, Dorman P, Lewis S, Dennis M, Sandercock P. Impact of functional status at six months on long term survival in patients with ischaemic stroke: Prospective cohort studies. BMJ. 2008;336 doi: 10.1136/bmj.39456.688333.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IST-3 collaborative group The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [ist-3]): A randomised controlled trial. The Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandercock P, Lindley R, Wardlaw J, Dennis M, Innes K, Cohen G, et al. Update on the third international stroke trial (ist-3) of thrombolysis for acute ischaemic stroke and baseline features of the 3035 patients recruited. Trials. 2011;12:252. doi: 10.1186/1745-6215-12-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IST-3 collaborative group Statistical analysis plan for the third international stroke trial (ist-3); part of a thread of reports of the trial. International Journal of Stroke. 2012;7:186–187. doi: 10.1111/j.1747-4949.2012.00782.x. [DOI] [PubMed] [Google Scholar]
- 8.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: The oxfordshire community stroke project--1981-86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53:16–22. doi: 10.1136/jnnp.53.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [Accessed 17th March 2014];Summary of product characteristics (alteplase) http://www.medicines.org.uk/emc/medicine/308/SPC/actilyse/
- 10.Konig IR, Ziegler A, Bluhmki E, Hacke W, Bath PMW, Sacco RL, et al. Predicting long-term outcome after acute ischemic stroke: A simple index works in patients from controlled clinical trials. Stroke. 2008;39:1821–1826. doi: 10.1161/STROKEAHA.107.505867. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. The Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyden PD. In anticipation of international stroke trial-3 (ist-3) Stroke. 2012;43:1691–1694. doi: 10.1161/STROKEAHA.112.656876. [DOI] [PubMed] [Google Scholar]
- 13.Kwiatkowski TG, Libman RB, Frankel M, Tilley BC, Morgenstern LB, Lu M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. New England Journal of Medicine. 1999;340:1781–1787. doi: 10.1056/NEJM199906103402302. [DOI] [PubMed] [Google Scholar]
- 14.Whiteley WN, Bruins Slot K, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: A systematic review and meta-analysis of 55 studies. Stroke. 2012;43:2904–2909. doi: 10.1161/STROKEAHA.112.665331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.