Abstract
Monoclonal antibodies (mAbs) have been a spectacular clinical and commercial success in the treatment of cancer and autoimmune diseases. Many of these mAbs (for example, OKT3, Campath-1H, rituximab and infliximab) are against surface or secreted products of lymphocytes. However, mAbs can have a variety of adverse effects including fever, chills and nausea. This is probably a result of cytokine release, which is most seriously manifested as a ‘cytokine storm' as highlighted by the TGN1412 (anti-CD28) trial. Prediction of adverse effects of mAbs would be clinically advantageous and numerous in vitro assays attempting to predict adverse effects have been reported. Here, we report an in vivo humanized mouse model to detect adverse effects in response to OKT3, Campath-1H or the polyclonal Ab preparation anti-thymocyte globulin. We found that the administration of each of these Abs to humanized mice led to acute clinical symptoms such as piloerection, hypomotility and hypothermia, particularly when delivered via the intravenous route. A cytokine storm occurred in the humanized mice receiving OKT3. This model system is a potentially useful tool to predict adverse effects and select initial doses for first-in-human trials. We would advocate this in vivo model, in addition to current in vitro preclinical testing, as a more representative and robust means of assessing potential adverse effects of mAb before their human use.
Muromonab-CD3 (OKT3) was the first of the monoclonal antibodies (mAbs) to be approved for clinical use in 1986 and is used to immunosuppress transplant recipients. Since then, mAbs have become a medical and commercial success especially for cancer and autoimmune diseases. mAbs against CD20 (rituximab) for non-Hodgkin's lymphoma, vascular endothelial growth factor (bevacizumab) for colorectal cancer, ErbB2 (trastuzumab) for breast cancer and tumor necrosis factor (TNF; infliximab and adalimumab) for rheumatoid arthritis are ‘blockbuster' drugs. According to the Global and Chinese Monoclonal Antibody Industry Report, 2013–2017, the global market for mAb in 2012 was $78 billion; this is expected to rise to $141 billion by 2017.
Part of the reason for the success of mAbs is their specificity. Nevertheless, clinical toxicity such as fever and chills can occur at the time of infusion and may be associated with a more severe ‘cytokine storm' or cytokine release syndrome (CRS). CRS is characterized by the systemic release of mainly inflammatory cytokines, predominantly TNF-α and interferon-γ (IFN-γ), usually 1–2 h after infusion, followed by interleukin-6 (IL-6) and IL-10 and, in some cases, IL-2 and IL-8.1 CRS has been seen with several mAbs including campath (alemtuzumab), muromonab-CD3, rituximab, tosituzumab, CP-870, 893, LO-CD2a/BTI-322 and TGN1412.2 In the case of the anti-CD28 mAb TGN1412, CRS was life threatening with suspected organ failure in all six previously healthy volunteers. Whether the preclinical testing (on human lymphocytes in vitro and cynomolgus macaques in vivo) failed to herald the danger or the data were not interrogated punctiliously remains debatable.3 Studies with cynomolgus macaques may have been misleading owing to the differences in CD28 expression between this monkey species and humans.4 Although in vitro studies and standard animal models can be useful, the TGN1412 example underscores the need for additional models more representative of humans for acute toxicity testing of therapeutic mAb. In this paper, we describe the use of a humanized mouse as a screen for the adverse effects of three clinically used Abs, two monoclonal and one polyclonal.
Humanized mice have been used for testing the efficacy of Abs against human cells. These include prevention of graft versus host disease,5 induction of regulatory T cells6 and mimicking the side effects of anti-CTLA4 Ab.7
In this model, we aim to assess clinical signs (appearance, behavior and body temperature) and perform laboratory testing to quantify plasma cytokines and lymphocyte activation markers immediately ex vivo. We performed dose titration, which can be prohibitively expensive in primate models, and used two routes of administration namely intraperitoneal (IP) and intravenous (IV) to reflect slow and rapid infusion, respectively.
Results
Abs bind T cells in hu-SCID
In general, 10 days after adoptive transfer of human peripheral blood mononuclear cells (PBMCs) our hu-severe combined immunodeficiency (SCID) mice have a ratio of human CD45 cells to mouse CD45 cells of 3:1 and 0.4:1 in the spleen and blood, respectively. This corresponds to 1–2 × 107 human CD45 cells in the spleen and 1 × 106 per ml in blood.
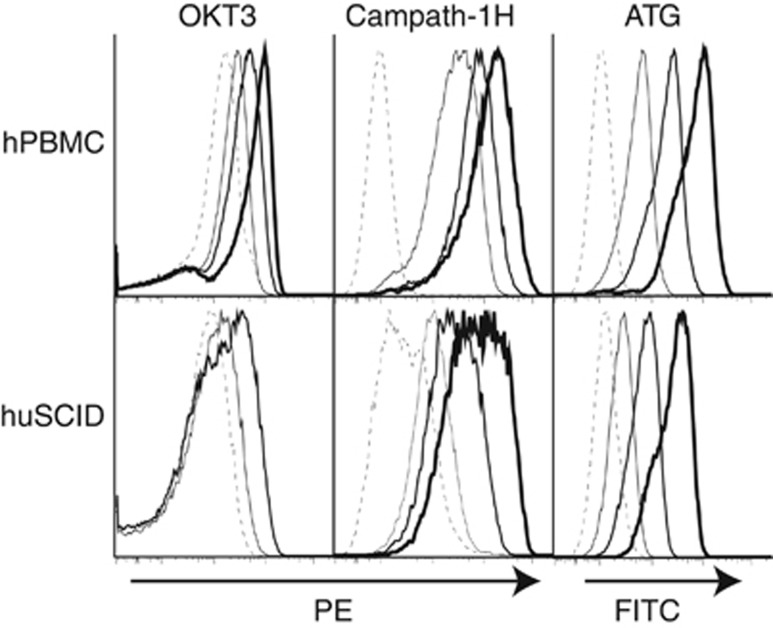
OKT3 and Campath-1H bind CD3 and CD52, respectively, on human lymphocytes. Anti-thymocyte globulins (ATGs) are polyclonal IgG preparations that bind human lymphocytes. Each has been reported to produce adverse effects acutely after infusion.2, 8 Human cells isolated from the spleen of a hu-SCID mouse 10 days after adoptive transfer of human PBMCs were shown to still bind OKT3, Campath-1H and ATG (Figure 1).
Figure 1.
Titration of mAb binding to human PBMCs by flow cytometry. Serially diluted OKT3, Campath-1H or ATG were incubated with fresh hPBMCs or human cells isolated from the spleen of a hu-SCID mouse 10 days after adoptive transfer of PBMCs. Binding of OKT3 was detected with goat anti-mouse IgG. Binding of Campath-1H was detected with goat anti-human IgG. Binding of ATG was detected with goat anti-rabbit IgG. Isotype controls at all concentrations showed no binding. Dash, thin, medium and thick lines indicate increasing doses (OKT3 0, 9.8, 39, 156 ng ml−1; Campath-1H 0, 625, 2500, 10 000 ng ml−1; ATG 0, 625, 2500, 10 000 ng ml−1).
Gross signs after injection of Abs
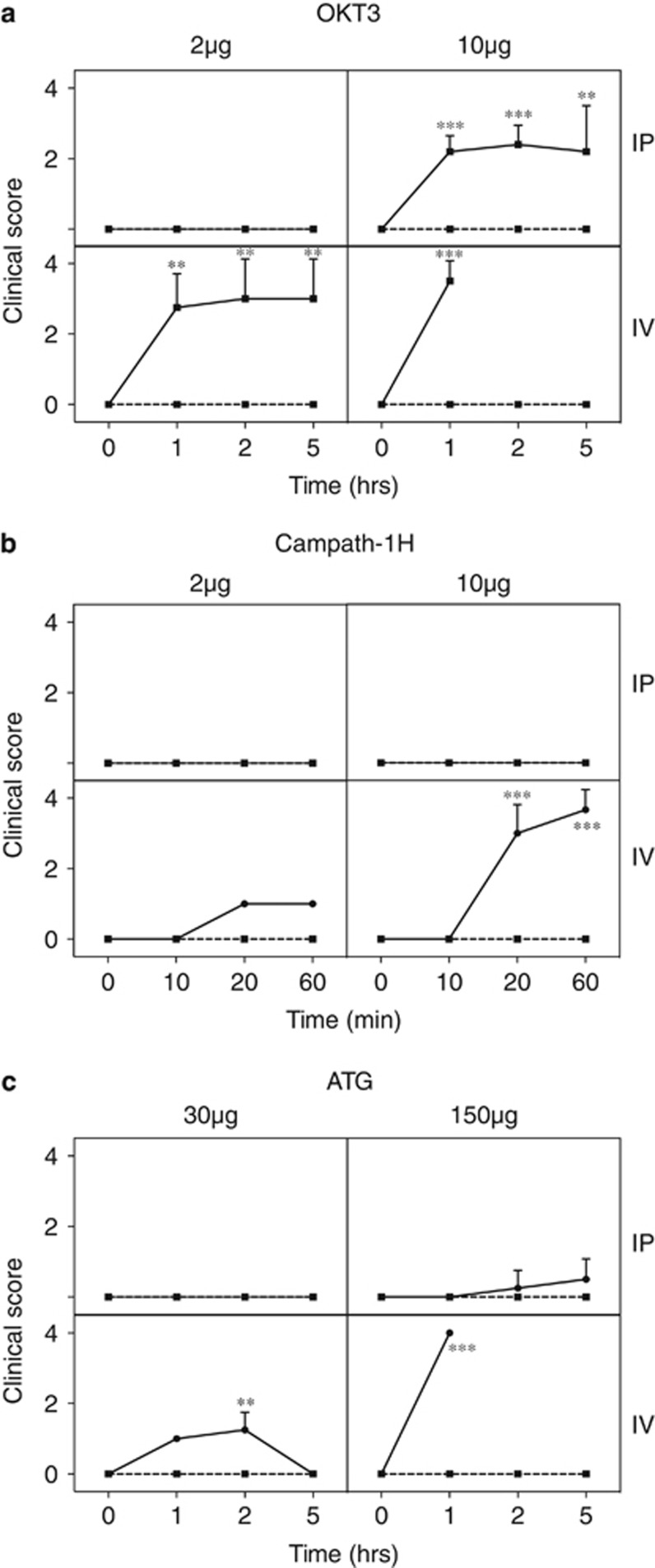
The typical human doses for OKT3 and Campath-1H are 0.083 mg kg−1 and 0.5 mg kg−1, respectively. For a mouse weighing 20 g, this translates to 1.6 μg OKT3 and 10 μg Campath (based on body weight) or 4.8 μg OKT3 and 30 μg Campath (based on body surface area).9 Initially, we covered both the methods by choosing 2 or 10 μg for OKT3; for Campath-1H, the mice became severely ill at the 10 μg dose, so the higher 30 μg dose was waived.
In addition, both IV and IP routes were included as the latter may mimic more closely mAbs given by slow infusion. OKT3 was injected into hu-SCID mice and at 1, 2 and 5 h the clinical scores were assigned (Figure 2a). At the lower dose of 2 μg by the IP route all hu-SCID mice were unaffected. Hu-SCID mice that received 10 μg OKT3 IP had moderate clinical scores (hunched, hypomotile), and one of five mice became moribund and was killed at 2 h. When 2 μg OKT3 was administered IV, hu-SCID mice had a moderate to severe reaction within 1 h. Two of the four mice required euthanasia, whereas the remaining two mice although showing moderate illness slowly recovered over 5 h. The reaction to 10 μg OKT3 IV was so severe that all mice were killed at the first hour time point.
Figure 2.
Acute clinical signs after injection of mAb. Hu-SCID mice were scored clinically after injection of OKT3 (a), Campath-1H (b) or ATG (c). Score: 0=normal activity; 1=normal activity, piloerection, tiptoe gait; 2=hunched, reduced activity but still mobile; 3=hypomotile but mobile when prompted; 4=moribund; killed. The number of mice for each group was 4–5 and data are presented as mean+s.d. *P<0.05, **P<0.01 and ***P<0.001 compared with controls at the same time point.
From preliminary experiments, clinical signs from Campath-1H treatment appeared much earlier than with OKT3; hence we performed a time course of up to 1 h rather than 5 h (Figure 2b). Hu-SCID mice that received 2 or 10 μg Campath-1H via the IP route had no clinical signs. Campath-1H (2 μg) IV educed mild clinical signs but all mice survived the 1 h time course. Mice that received 10 μg Campath-1H IV became ill within 20 min (average clinical score of 3) and all were moribund within 1 h and killed. Overall, for both mAbs, the severity of gross signs increased in order according to: 2 μg IP<10 μg IP<2 μg IV<10 μg IV.
The polyclonal Ab ATG is typically given at 1.5–5 mg kg−1 in humans, equating to 30–100 μg (weight) or 90–300 μg (surface area) per mouse. In preliminary experiments, we had already observed no clinical signs at the 2 μg and 10 μg doses that we had used for OKT3 and Campath-1H. We therefore decided to test 30 and 150 μg (Figure 2c). At 30 μg ATG IP clinical signs were absent and at 150 μg ATG IP the signs were very mild. Hu-SCID mice that received 30 μg ATG IV showed mild clinical signs and recovered over the 5-h monitoring period, but mice that received 150 μg ATG IV rapidly became moribund and were killed after 1 h. Although the doses were different, the pattern of gross signs was similar for OKT3 and Campath-1H, increasing from low-dose IP and high-dose IP to low-dose IV and high-dose IV.
Neither hu-SCID mice injected with control IgG nor SCID mice treated with OKT3, Campath-1H or ATG exhibited clinical signs, even when extremely high doses (0.5 mg) were given to the latter. Therefore, the effects described above were not due to nonspecific toxicity of the agents themselves.
Hypothermia after injection of Ab
Both hypothermia and hyperthermia have been observed in the CRS. After administration of 10 μg of OKT3, either IP or IV, rectal temperatures dropped profoundly from 37 °C to below 32 °C within 1 h (Figure 3a). With the lower dose of 2 μg, the IV route resulted in a transient hypothermia (~32 °C) at 1 h that partially recovered after 5 h (Figure 3a); the modest changes after IP route were similar to those found with injection of control human IgG (Figure 3a).
Figure 3.
Hypothermia induction after injection of mAb. Rectal temperature was measured in hu-SCID mice injected with control IgG or OKT3 (a), Campath-1H (b) or ATG (c). The number of mice for each group was 4–5 and data are presented as mean+s.d. *P<0.05, **P<0.01 and ***P<0.001 compared with controls at the same time point.
Campath-1H had a profound effect on temperature, with the 10 μg IV dose causing a drop of >5 °C by 1 h (Figure 3b). High-dose IP and low-dose IV showed similar decreases in temperature over the 1-h time course. Low-dose IP showed a similar trend to the control injected mice with a very slight decrease in body temperature over the time course (presumably owing to multiple bleeds over the time course).
After administration of ATG, the disease was so acute with the 150 μg IV dose that mice were killed within 1 h and the rectal temperatures were not measured. Mice that received the 30 μg dose of ATG IV showed transient hypothermia (~33 °C) at 1 h that recovered after 5 h (Figure 3c). Either doses of ATG administered IP showed a similar trend to the control injected mice.
Circulating cytokine concentrations after Ab administration
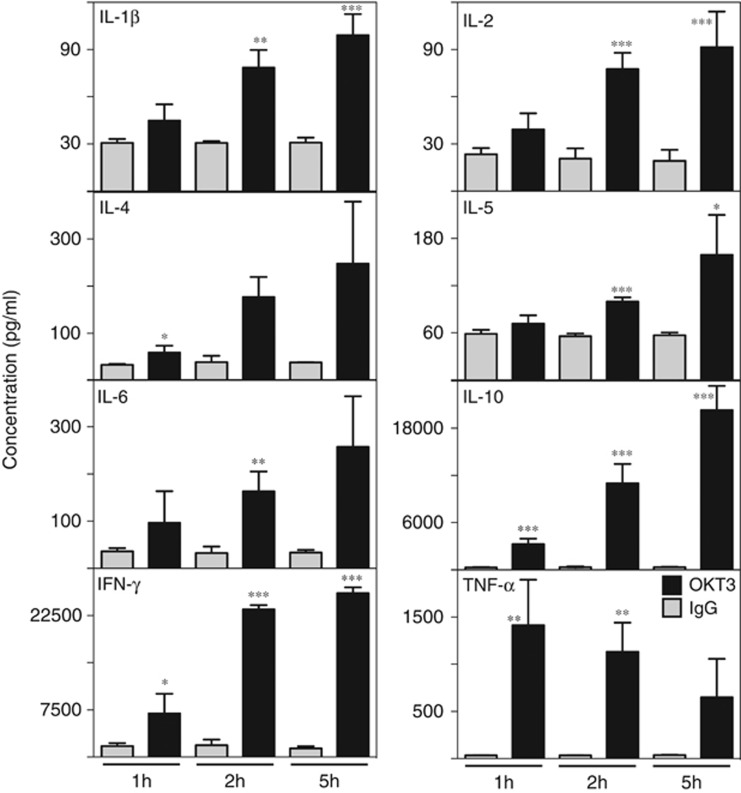
To ascertain whether a cytokine storm was induced, cytokines (human IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13, IFN-γ and TNF-α) were assayed in the plasma of hu-SCID mice following injection of mAbs. High-dose (10 μg) OKT3 IP induced the production of multiple cytokines, namely; IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10 and IFN-γ, the concentrations of which increased over the 5-h time course (Figure 4); TNF-α production was also induced but peaked earlier (at 1 h). As found previously,10 cytokine concentrations returned to normal by 24 h. IL-12(p70) and IL-13 concentrations were very low (similar to the IgG-treated controls) and are not shown here. Hu-SCID mice that received the high-dose OKT3 IV were killed after 1 h (see above), and therefore cytokines could not be assayed beyond this time point. From these samples, most cytokines were induced at similar concentrations to the 1-h time point of the high-dose IP group, with a couple of exceptions. Lower concentrations of IL-4 and TNF-α were detected in comparison with the high-dose IP group (data not shown).
Figure 4.
Induction of cytokines after injection of OKT3. Hu-SCID mice were injected with 10 μg OKT3 (black bars) or IgG control (gray bars) IP. Mice were bled at 1, 2 and 5 h and circulating cytokine concentrations measured. Sensitivity (limit of detection) of analytes was 1 ng ml−1. The number of mice for each group was 4–5 and data are presented as mean+s.d. *P<0.05, **P<0.01, ***P<0.001 compared with controls at the same time point.
Injection of 2 μg OKT3, whether IP or IV, induced very little detectable cytokine at any time point. Given that mice had substantial clinical scores and hypothermia at 1 h after 2 μg OKT3 IV, it would appear that the clinical readout is more sensitive than the biochemical readout of circulating cytokine concentrations, that is, at lower doses of mAbs, mice became sick before plasma cytokines were elevated.
Mice given Campath-1H, whether 2 or 10 μg IV or IP, did not exhibit plasma cytokine concentrations above those in the IgG-treated control mice at the end of 1 h time (data not shown). In addition, cytokines were not detected in mice given ATG at any dose or by any route (data not shown), again demonstrating that the clinical readout is more sensitive than the biochemical.
OKT3 but not Campath-1H induces activation markers
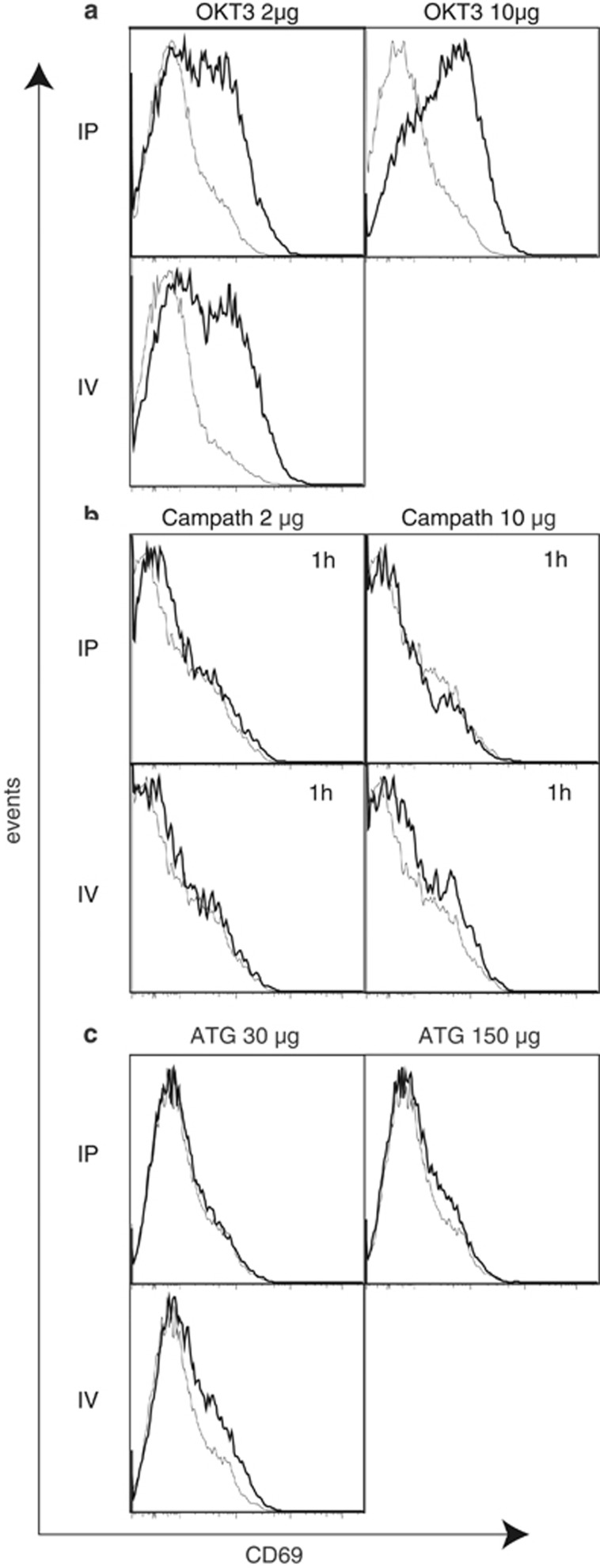
Activation markers (CD25 and CD69) on T cells were analyzed by flow cytometry directly ex vivo. Five hours after injection of OKT3, at low-dose IV and IP and high-dose IP, CD69, but not CD25, was upregulated on human T cells within the spleens of hu-SCID mice (Figure 5a). After high-dose OKT3 IV, mice rapidly became moribund and were killed at 1 h.
Figure 5.
Flow cytometric measurement of activation markers after injecton of mAb. CD69 expression on human CD3+ T cells in the spleen of hu-SCID mice after injection of OKT3 (a), Campath-1H (b), ATG (c) (bold line) or control IgG (gray line). Histograms show a representative plot from each group. No plots are shown for 10 μg OKT3 IV or 150 μg ATG IV as all mice were moribund and killed before the end of the time course.
Activation markers on T cells of mice given Campath-1H were not detected above IgG-treated control mice at any dose or route at the end of the 1-h time course (Figure 5b).
Slight upregulation of CD69 on T cells of hu-SCID mice given 30 μg ATG IV was detected above IgG control mice at the end of the 5-h time course (Figure 5c). Activation markers were not detected in hu-SCID mice treated with ATG via the IP route whether high or low dose.
Discussion
OKT3 (for depletion of T cells during transplantation), Campath-1H (for treatment of B-cell chronic lymphocytic leukemia) and ATG (for depletion of lymphocytes during transplantation) are examples of Abs that are used successfully in human medicine.8, 11, 12, 13 All three have been associated with toxicity and CRS in patients. Therefore, we tested these mAbs in the hu-SCID model to determine if it would recapitulate these adverse effects seen clinically and be a useful preclinical tool for the detection of CRS.
There are many humanized mouse models but they are all based on a severely immunodeficient host (for example, neonatal mice, RAG or SCID; IL-2Rγ deficiency) usually with the NOD SIRPα allele (which recognizes hCD47).14 Human cells used for transfer include PBMCs, human CD34+ ‘stem' cells (for example, from cord blood) and a combination of bone marrow cells and thymus graft; the latter arguably produces the most comprehensive human immune system. We found our model of human PBMCs into NOD-SCID IL2rγnull mice to be quite robust and that despite variation in final numbers of human lymphocytes (especially between the experiments), the pattern of response elicited was consistently reproduced. It is probably the least labor intensive and inexpensive. As such, it lends itself to assess doses according to body weight or body surface area facilely. Indeed, experiments to determine ‘minimal anticipated biological effect level' and ‘no observed adverse effect level' would be practicable logistically and economically.
The use of anti-CTLA-4 and anti-PD-1 for cancer immunotherapy has brought new hope to patients with even very advanced cancers.15 Some anticancer mAbs can cause tumor lysis syndrome, as well as other adverse effects.16 It would be interesting to determine whether hu-SCID mice transplanted with human cancer xenografts could be used to test for such side effects.
The various preparations we tested have different isotypes (subclass and from different species). OKT3 is a mouse IgG2a, Campath-1H is a human IgG1, ATG is rabbit IgG (rabbits have only one IgG isotype). All the three isotypes have the LLGG motif in their hinge region and therefore bind Fcγ receptors well.17 All the three activate complement well. Interestingly, TGN1412 is a human IgG4 isotype, which is the isotype least effective at binding Fcγ receptors and unable to fix complement;18 this should not be confused with its ability to bind the neonatal FcR found on endothelial cells.19 Given the marked effects of TGN1412, it would seem that Fc binding and complement fixation are not the necessary properties to induce toxicity.
Multiple mechanisms are likely to govern cytokine production from different cell types, depending on the mAb target. NK cells, monocytes/macrophages, T cells, B cells, direct binding to cognate antigen, FcR-dependent clustering, activation of FcR and activation of complement may all be involved.2, 20 The hu-SCID system allows the investigation of multiple cell types with multiple readouts from the one system and appears to recapitulate many of the features that occur in patients experiencing a cytokine storm. The kinetics of cytokine released in the hu-SCID after OKT3 administration appear typical of those reported in humans during cytokine release syndrome.21, 22 Thus, plasma TNF-α increases early within 1 h followed by increases in other pro-inflammatory cytokines including IFN-γ, IL-1β, IL-2 and IL-6 over 5 h. One of the key features of the hu-SCID system is that in addition to detecting a cytokine storm it permits detection of a variety of other adverse effects. For example, even when cytokine concentrations had not (yet) increased hu-SCID mice showed marked signs of illness such as piloerection and hypomotility, as well as hypothermia. Such information would provide an alert as a prelude to human trials, whether or not cytokines were detected. Whether these signs are a prelude to a cytokine storm or are a different effect altogether can be debated. Acute reactions following infusion of mAbs can be caused by various mechanisms besides CRS,23 including acute anaphylactic reactions against the mAbs, serum sickness or complement activation by IgG aggregates.
We surmise that the IV and IP routes in mice reflect rapid and slow infusion, respectively. For example, etanercept (an IgG fusion protein) injected IP into mice reached 50% maximum blood levels in 2 h.24 We showed a marked difference in both adverse clinical and biochemical parameters when mAbs were administered via different routes. This suggests that the hu-SCID could guide therapeutic delivery route of new mAbs. The hu-SCID model should also allow testing of mAbs in combination with counteracting medications such as corticosteroids.21, 25
Currently, in vivo testing of mAbs in non-human primates (when the mAb cross-reacts) and in vitro testing on human cells are used to characterize mAb and predict cytokine storm. The experience with TGN1412, a mAb against human CD28, which triggered severe cytokine storm in a phase 1 trial in human volunteers highlights the limitations of these approaches. First, it appears that although TGN1412 bound CD28+ lymphocytes of Cynomolgus macaques, a cytokine storm was not detected.19 This ‘false' negative result questions the suitability and validity of existing preclinical non-human primate models for safety testing.4 Second, when first tested on human PBMCs in vitro, TGN1412 did not apparently induce a robust cytokine response.19, 26
Since the TGN1412 mishap, elaborate in vitro whole-blood27, 28 and PBMC4, 29 assays have been introduced to improve the prediction of a cytokine storm. Some assays have used different methods of immobilizing mAbs, for example, on polystyrene beads coated with protein A or anti-human IgG.1 Of the six different methods tested to present TGN1412 mAb to PBMCs or whole blood,19 only those in which TGN1412 was immobilized by drying onto wells captured by immobilized anti-Fc Ab or presented via an endothelial monolayer elicited substantial cytokine release. The relevance of these may be peculiar to TGN1412 and may or may not be useful generally. An advantage of the hu-SCID model is that even if cytokines were not detected, mice may still exhibit clinical signs of illness following mAb administration.
As different mAbs have different modes of action, predicting cytokine storms is not likely to be achieved with a single screening assay. However, the hu-SCID mouse, being an in vivo reconstituted human model, offers the potential advantages of not being limited by species or cell specificity. Although not comprehensively investigated here, we suggest that the hu-SCID model is a relatively facile and economical platform to determine the starting doses of mAbs and other agents in first-in-human trials.
Methods
Animals
NOD-SCID IL2rγnull mice30 were bred under specific pathogen-free conditions at the Walter and Eliza Hall Institute. The Institution's Animal Ethics Committee approved all the experimental procedures (#2011.015).
Antibodies
Flow cytometric Abs were purchased from BD Biosciences (San Jose, CA, USA), eBiosciences (San Diego, CA, USA) or Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Human IgG (Intragam) (CSL Ltd, Parkville, Victoria, Australia) was a gift from CSL. Purified anti-CD16/CD32 (clone 2.4G2), used for blocking of mouse Fc receptors before staining for analysis by flow cytometry, and OKT3 were produced at the Walter and Eliza Hall Institute's Antibody Facility. Campath-1H and ATG were purchased from Bayer HealthCare Pharmaceuticals Inc., Leverkusen, Germany and Fresenius Biotech GmbH, Gräfelfing, Germany, respectively. All Abs had endotoxin levels <0.5 IU per mg.
Isolation of PBMCs
Buffy coats were obtained from the Australian Red Cross Blood Service, Melbourne, Australia. Human PBMCs were isolated over Ficoll-Plaque Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and washed in phosphate-buffered saline. After lysis of red blood cells, nucleated cells were washed three times with phosphate-buffered saline. This study was approved by the Walter and Eliza Hall Institution's Human Research Ethics Committee (#92/03).
Hu-SCID, measurement of cytokines and activation markers
NOD-SCID IL2rγnull mice were injected with 2 × 107 PBMCs IV. Ten days later mice were injected with OKT3, Campath-1H or IgG (2 or 10 μg, IV or IP) or with ATG or IgG (30 or 150 μg, IV or IP). Mice were bled over a time course of 1, 2 and 5 h (for OKT3, ATG and controls) or 10, 20 and 60 min (for Campath-1H and controls). Heparinized plasma was collected and analyzed for cytokines using a multiplex immunoassay system (Precision Pro human cytokine assay panel; Bio-Rad, Hercules, CA, USA). Cytokines measured include human IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13, IFN-γ and TNF-α. Treated hu-SCID mice were killed after their last bleed (5 h or 60 min) and activation markers were analyzed by flow cytometry. Human PBMCs from mouse spleens were blocked with 2.4G2 before staining for fluorochrome-conjugated CD69, CD25, CD45 and CD3.
Measurement of body temperature
Rectal temperature of mice was measured before treatment and again immediately before each time-point bleed. Temperature was measured by the insertion of a rectal thermocouple probe (Kent Scientific Corp., Torrington, CT, USA) and waiting until a stable reading was obtained (~10 s). Mice with a temperature <32 °C were killed.
Measurement of clinical score
As advised by the Canadian Council of Animal Care (www.ccac.ca/en_/standards/guidelines), we performed pilot studies to determine the most appropriate clinical signs and the grading of scores. At each time point mice were observed and given a clinical score. Score: 0=normal activity; 1=normal activity, piloerection, tiptoe gait; 2=hunched, reduced activity but still mobile; 3=hypomotile but mobile when prompted; 4=moribund. Mice with a clinical score of 4 were killed.
Statistical analysis
Unpaired one-tailed t-test was used to calculate the significance of the difference between groups using Prism (GraphPad Software Inc., San Diego, CA, USA).
Acknowledgments
This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIIS. This work was supported by the Juvenile Diabetes Research Foundation (112613), Australian National Health and Medical Research Council (1037321, 1043414, 637303 and 1080321) and the Rebecca L Cooper Foundation.
The authors declare no conflict of interest.
References
- Walker MR, Makropoulos DA, Achuthanandam R, Van Arsdell S, Bugelski PJ. Development of a human whole blood assay for prediction of cytokine release similar to anti-CD28 superagonists using multiplex cytokine and hierarchical cluster analysis. Int Immunopharmacol. 2011;11:1697–1705. doi: 10.1016/j.intimp.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Bugelski PJ, Achuthanandam R, Capocasale RJ, Treacy G, Bouman-Thio E. Monoclonal antibody-induced cytokine-release syndrome. Expert Rev Clin Immunol. 2009;5:499–521. doi: 10.1586/eci.09.31. [DOI] [PubMed] [Google Scholar]
- Horvath C, Andrews L, Baumann A, Black L, Blanset D, Cavagnaro J, et al. Storm forecasting: additional lessons from the CD28 superagonist TGN1412 trial Nat Rev Immunol 201212740author reply 740. [DOI] [PubMed] [Google Scholar]
- Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol. 2010;161:512–526. doi: 10.1111/j.1476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier N, Mary C, Dilek N, Hervouet J, Minault D, Blancho G, et al. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab' antibody. Am J Transplant. 2012;12:2630–2640. doi: 10.1111/j.1600-6143.2012.04164.x. [DOI] [PubMed] [Google Scholar]
- Waldron-Lynch F, Henegariu O, Deng S, Preston-Hurlburt P, Tooley J, Flavell R, et al. Teplizumab induces human gut-tropic regulatory cells in humanized mice and patients. Sci Transl Med. 2012;4:118ra12. doi: 10.1126/scitranslmed.3003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vudattu NK, Waldron-Lynch F, Truman LA, Deng S, Preston-Hurlburt P, Torres R, et al. Humanized mice as a model for aberrant responses in human T cell immunotherapy. J Immunol. 2014;193:587–596. doi: 10.4049/jimmunol.1302455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardinger KL. Rabbit antithymocyte globulin induction therapy in adult renal transplantation. Pharmacotherapy. 2006;26:1771–1783. doi: 10.1592/phco.26.12.1771. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Brady JL, Sutherland RM, Hancock M, Kitsoulis S, Lahoud MH, Phillips PM, et al. Anti-CD2 producing pig xenografts effect localized depletion of human T cells in a huSCID model. Xenotransplantation. 2013;20:100–109. doi: 10.1111/xen.12025. [DOI] [PubMed] [Google Scholar]
- Wilde MI, Goa KL. Muromonab CD3: a reappraisal of its pharmacology and use as prophylaxis of solid organ transplant rejection. Drugs. 1996;51:865–894. doi: 10.2165/00003495-199651050-00010. [DOI] [PubMed] [Google Scholar]
- Pangalis GA, Dimopoulou MN, Angelopoulou MK, Tsekouras C, Vassilakopoulos TP, Vaiopoulos G, et al. Campath-1H (anti-CD52) monoclonal antibody therapy in lymphoproliferative disorders. Med Oncol. 2001;18:99–107. doi: 10.1385/mo:18:2:99. [DOI] [PubMed] [Google Scholar]
- Moreau T, Coles A, Wing M, Isaacs J, Hale G, Waldmann H, et al. Transient increase in symptoms associated with cytokine release in patients with multiple sclerosis. Brain. 1996;119:225–237. doi: 10.1093/brain/119.1.225. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology. 2013;2:e26333. doi: 10.4161/onci.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Woof JM. Human antibody effector function. Adv Immunol. 1992;51:1–84. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- Nezlin R, Ghetie V. Interactions of immunoglobulins outside the antigen-combining site. Adv Immunol. 2004;82:155–215. doi: 10.1016/S0065-2776(04)82004-2. [DOI] [PubMed] [Google Scholar]
- Stebbings R, Findlay L, Edwards C, Eastwood D, Bird C, North D, et al. "Cytokine storm" in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol. 2007;179:3325–3331. doi: 10.4049/jimmunol.179.5.3325. [DOI] [PubMed] [Google Scholar]
- Eastwood D, Bird C, Dilger P, Hockley J, Findlay L, Poole S, et al. Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release. Br J Clin Pharmacol. 2013;76:299–315. doi: 10.1111/bcp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L, Ferran C, Legendre C, Thouard I, Merite S, Reuter, et al. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation. 1990;49:697–702. doi: 10.1097/00007890-199004000-00009. [DOI] [PubMed] [Google Scholar]
- Abramowicz D, Schandene L, Goldman M, Crusiaux A, Vereerstraeten P, De Pauw L, et al. Release of tumor necrosis factor, interleukin-2, and gamma-interferon in serum after injection of OKT3 monoclonal antibody in kidney transplant recipients. Transplantation. 1989;47:606–608. doi: 10.1097/00007890-198904000-00008. [DOI] [PubMed] [Google Scholar]
- Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- Sumbria RK, Zhou QH, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Pharmacokinetics and brain uptake of an IgG-TNF decoy receptor fusion protein following intravenous, intraperitoneal, and subcutaneous administration in mice. Mol Pharm. 2013;10:1425–1431. doi: 10.1021/mp400004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegre ML, Vandenabeele P, Depierreux M, Florquin S, Deschodt-Lanckman M, Flamand V, et al. Cytokine release syndrome induced by the 145-2C11 anti-CD3 monoclonal antibody in mice: prevention by high doses of methylprednisolone. J Immunol. 1991;146:1184–1191. [PubMed] [Google Scholar]
- Hanke T.Lessons from TGN1412 Lancet 20063681569–1570.author reply 1570. [DOI] [PubMed] [Google Scholar]
- Coch C, Luck C, Schwickart A, Putschli B, Renn M, Holler T, et al. A human in vitro whole blood assay to predict the systemic cytokine response to therapeutic oligonucleotides including siRNA. PLoS One. 2013;8:e71057. doi: 10.1371/journal.pone.0071057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing MG, Waldmann H, Isaacs J, Compston DA, Hale G. Ex-vivo whole blood cultures for predicting cytokine-release syndrome: dependence on target antigen and antibody isotype. Ther Immunol. 1995;2:183–190. [PubMed] [Google Scholar]
- Findlay L, Eastwood D, Stebbings R, Sharp G, Mistry Y, Ball C, et al. Improved in vitro methods to predict the in vivo toxicity in man of therapeutic monoclonal antibodies including TGN1412. J Immunol Methods. 2010;352:1–12. doi: 10.1016/j.jim.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]