Abstract
Introduction
The success of pulmonary vein isolation (PVI) for atrial fibrillation (AF) may be improved if stable AF sources identified by Focal Impulse and Rotor Mapping (FIRM) are also eliminated. The long-term results of this approach are unclear outside the centers where FIRM was developed; thus, we assessed outcomes of FIRM-guided AF ablation in the first cases at 10 experienced centers.
Methods
We prospectively enrolled n = 78 consecutive patients (61 ± 10 years) undergoing FIRM guided ablation for persistent (n = 48), longstanding persistent (n = 7), or paroxysmal (n = 23) AF. AF recordings from both atria with a 64-pole basket catheter were analyzed using a novel mapping system (Rhythm View™; Topera Inc., CA, USA). Identified rotors/focal sources were ablated, followed by PVI.
Results
Each institution recruited a median of 6 patients, each of whom showed 2.3 ± 0.9 AF rotors/focal sources in diverse locations. 25.3% of all sources were right atrial (RA), and 50.0% of patients had ≥1 RA source. Ablation of all sources required a total of 16.6 ± 11.7 minutes, followed by PVI. On >1 year follow-up with a 3-month blanking period, 1 patient lost to follow-up (median time to 1st recurrence: 245 days, IQR 145–354), single-procedure freedom from AF was 87.5% (patients without prior ablation; 35/40) and 80.5% (all patients; 62/77) and similar for persistent and paroxysmal AF (P = 0.89).
Conclusions
Elimination of patient-specific AF rotors/focal sources produced freedom-from-AF of ≈80% at 1 year at centers new to FIRM. FIRM-guided ablation has a rapid learning curve, yielding similar results to original FIRM reports in each center’s first cases.
Keywords: atrial fibrillation, catheter ablation, FIRM-guided ablation, rotors, focal impulse
Introduction
Atrial fibrillation (AF) is a major public health problem that causes morbidity from palpitations, reduced exercise tolerance, stroke, and even death.1 However, medical therapy to limit ventricular rate in AF2 and to maintain sinus rhythm3,4 has been disappointing. Catheter ablation to eliminate arrhythmogenic tissue shows promise, but its success in AF has been limited in part by mechanistic uncertainty due to difficulties in mapping AF.5–7 AF ablation thus currently isolates potential triggers in the pulmonary veins (PV) and other regions,8 or targets unclearly defined electrical substrates using lines or ablation of complex fragmented atrial electrograms, with a single procedure 1-year success of ≈50–60% for paroxysmal AF9–12 and lower success for persistent AF.7
It has recently been shown13,14 that human AF, after it has been triggered,15,16 is sustained by the electrical substrates of spiral waves (rotors) and focal sources that are sufficiently stable to be eliminated by localized ablation (Focal Impulse and Rotor Modulation, FIRM). In the CONFIRM trial (CONventional ablation for AF with or without FIRM), elimination of sources and conventional ablation improved freedom from AF to 82.4% from 44.9% by conventional ablation alone in patients with persistent and paroxysmal AF13 that has recently been confirmed at 3 years.17 However, the efficacy of FIRM-guided ablation is unclear outside these initial centers.
We performed a prospective registry study of FIRM mapping and ablation at 10 centers new to the technique. We set out to define, acutely, whether AF rotors and focal sources on FIRM-mapping lay near or remote from conventional PV targets and, long-term, to establish the outcome from FIRM-guided ablation in patients with paroxysmal and persistent AF by centers early in their learning experience.
Methods
Patient Enrollment
We report 78 consecutive patients undergoing FIRM-guided AF mapping and ablation in the 1-year period from November 30, 2011 to November 29, 2012. These were the first FIRM cases at 10 experienced U.S. ablation centers: Indiana University School of Medicine; Heart Place, Baylor University Medical Center, Dallas, TX; Arizona Heart Institute, Phoenix, AZ; Duke University Medical Center, Durham, NC; Ohio State University, Columbus, OH; Intermountain Heart Institute, Murray, UT; Medical College of Virginia, Richmond, VA; Mount Sinai Medical Center, NY; UCLA Cardiac Arrhythmia Center, Los Angeles, CA; and Valley Health System, Ridgewood, NJ. Patients from the Veterans’ Affairs and University of California Medical Centers, San Diego (where FIRM was developed), were intentionally excluded from this report. The acute response to FIRM-only ablation in the first n = 14 patients has been reported.14
We enrolled patients with paroxysmal, persistent, and longstanding persistent AF referred for standard indications.7 Enrollment was performed in consecutive patients studied during the initial learning-phase availability of FIRM mapping and ablation, many of whom had failed conventional ablation (n = 38). Left atrial diameter was assessed by intracardiac echocardiography or preprocedural imaging and, although diameter <55 mm was suggested since the largest basket has diameter 55–60 mm when fully deployed, patients’ atria were often larger (Table 1). All patients provided written informed consent, with data inclusion approved by the IRB at each center. The registry includes data recorded at each FIRM-procedure, with follow-up data provided by each site to the writing group (JMM, RCK, SMN, KS).
TABLE 1.
Clinical Characteristics of Population
| Characteristic | All Patients | First Ablation | Prior Failed PVI | P value |
|---|---|---|---|---|
| Number of patients | 78 | 40 | 38 | |
| AF type (N [%]) | 0.10 | |||
| Paroxysmal | 24 (29.5%) | 8 (20.0%) | 16 (39.5%) | |
| Persistent | 47 (61.5%) | 28 (70.0%) | 19 (52.6%) | |
| Longstanding persistent | 7 (9.0%) | 4 (10.0%) | 3 (7.9%) | |
| Age (years) | 61.3 ± 10.1 | 61.6 ± 10.9 | 60.9 ± 9.4 | 0.77 |
| Gender (Male/female) | 55/23 | 28/12 | 27/11 | 0.55 |
| Left atrial diameter (mm) | 56 ± 9 | 56 ± 9 | 57 ± 10 | 0.78 |
| No. with LA >55 mm (%) | 38 (50.4%) | 18 (45%) | 20 (52.6%) | 0.43 |
| LVEF (%) | 55 ± 8 | 54 ± 8 | 56 ± 8 | 0.28 |
| Remote amiodarone use (N [%]) | 12 (15.4%) | 5 (12.5%) | 7 (18.4%) | 0.47 |
AF = atrial fibrillation; PVI = pulmonary vein isolation; LA = left atrium; LVEF = left ventricular ejection fraction; Remote amiodarone = prior treatment with amiodarone. P value for AF type was assessed by Fisher’s test with Freeman–Halton extension.
Procedural Details
Procedures were performed >5 half-lives after discontinuing antiarrhythmic medications except amiodarone (Table 1). Via femoral venous access, a multipolar catheter was placed in the coronary sinus and a 64-pole basket catheter (Constellation, Boston Scientific Inc., Natick, MA, USA, 48 or 60 mm diameter) was advanced to right then left atrium via 8.5 F sheaths.13 Fluoroscopy, electrography, and intracardiac echocardiography (according to investigator preference) were used to achieve optimal contact.18 Heparin was administered by infusion routinely to maintain ACT >300 seconds. Mapping could not be performed in 2 patients due to technical issues with cabling, so acute data are reported in 76 patients with intention-to-treat follow-up reported in all 78 patients.
We mapped spontaneous AF, or AF induced by rapid atrial pacing (e.g., cycle lengths [CL]: 500, 450, 400, 350, 300 milliseconds, then in 10 milliseconds steps to AF) with isoproterenol in a minority of cases as described.13 Sustained AF was achieved in all n = 76 mapped patients, and analyzed after >5 minutes for stabilization of AF. Unipolar electrograms were filtered at 0.05–500 Hz and exported digitally from electrophysiological recorders at each site for analysis on RhythmView™ (Topera, Palo Alto, CA, USA).
AF Mapping and Definition of Stable AF Sources
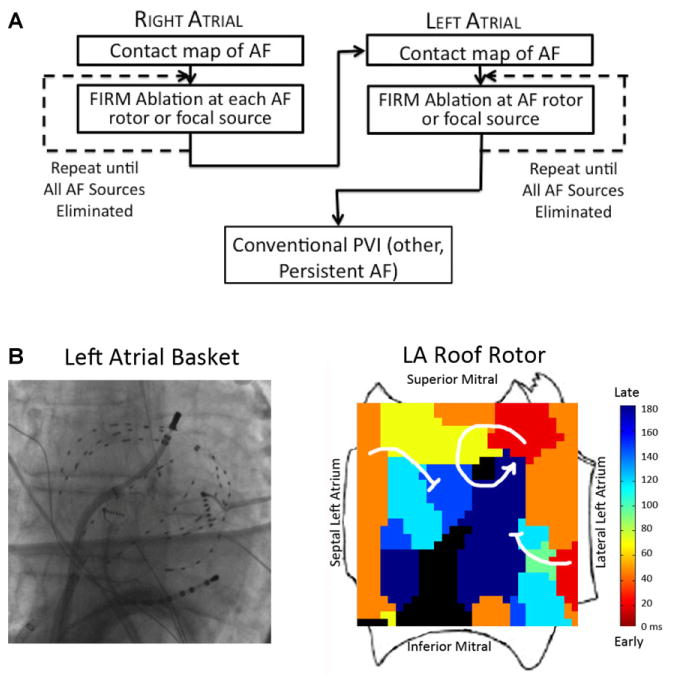
The FIRM-mapping and ablation workflow was consistent for all sites in the registry, and is shown in Figure 1A. Three-dimensional basket catheters were placed successively in each atrium (Fig. 1B) to record AF electrograms that were exported for analysis. The FDA-approved RhythmView™ console (Topera Inc.) comprises a workstation with analytic software that applies computational approaches18,19 to create an activation trail that can identify AF rotors or focal sources within the atria.18–20 The system performs an assessment of electrogram quality based primarily upon atrial signal amplitude above noise, and represents lack of signal as black. Basket repositioning was recommended to optimize basket contact in cases with very large atria or if regions of interest had no signals. AF propagation maps from contact recordings were projected onto 2D grids aligned to atrial anatomy (Fig. 1B).
Figure 1.
(A) Workflow for FIRM-guided ablation of AF. (B) Typical basket placement and results from FIRM-guided ablation. Basket placed in left atrium, with resulting FIRM map showing AF rotor at roof with surrounding spiral arms disorganizing and fusing with the fibrillatory milieu (blocked arrows) and rotor precession within a limited area on successive cycles (not shown).
AF maps were used to diagnose sustained rotors if observed on multiple epochs (1-minute recording segments) spanning several minutes (typically >10 minutes). The core regions of AF sources are spatially reproducible within precession areas of 1–2 cm2 for prolonged periods of time as described20,21 (i.e., bounded by ≈2 electrodes in each axis) with spiral arms that emanate and disorganize (fibrillatory conduction). AF focal sources were diagnosed if they showed centrifugal activation from an origin to surrounding atrium, also with peripheral disorganization and also in multiple recordings typically for >10 minutes.13 The ablation target was defined by the source precession area. Map interpretation and ablation were performed by the operator at each site.
Focal Impulse and Rotor Modulation (FIRM) Ablation
Ablation commenced with FIRM at sources identified from AF maps at all centers. Ablation was performed per investigator preference using various energy sources: 3.5-mm tip irrigated radiofrequency catheter (ThermoCool™, Biosense-Webster, Diamond Bar, CA, USA; Safire Blu™, St. Jude Medical, Minnetonka, MN, USA) at 25–40 W, a cryoballoon at sources near the PV antra in n = 2 cases (Arctic Front, Medtronic, MN, USA) or by an 8-mm nonirrigated radiofrequency catheter (Blazer, Boston Scientific, Sunnyvale, CA, USA) at 40–50 W, 52 °C target temperature. Ablation was applied to the basket grid coordinates of the center of rotation or focal impulse origin (≈2 cm2 areas20), identified either from fluoroscopy or from electrode positions on electroanatomic shells. Ablation continued until the source area was covered and sources were eliminated on repeat FIRM maps.
Conventional ablation for all patients in this registry followed guidelines,7 was performed after FIRM ablation was completed and comprised wide-area antral PV isolation verified using a circular catheter (Lasso™; Biosense-Webster or Optima™, St. Jude Medical). Additional ablation included a left atrial roof line in persistent AF patients, and ablation of observed clinically relevant atrial tachycardias or flutters. Ablation power, temperatures, and duration were as noted above. If AF persisted after completion of the ablation protocol, cardioversion was used to restore sinus rhythm.
Postprocedure Clinical Management
Follow-up for arrhythmia recurrence followed guidelines22 at each center. In the first 3 months postablation, antiarrhythmic medications were permitted but repeat ablation was not. Subjects were followed for >1 year, with no repeat procedures. The follow-up time censored at first recurrence was 245 days (IQR 145–354). Arrhythmia recurrence in this registry was detected by symptoms and event monitors to coincide with clinic visits, and also event monitoring at interim intervals at the time of symptoms as in other recent studies.
Study Endpoints
The primary long-term efficacy endpoint was freedom from AF, defined as AF >30 seconds on intermittent monitors.22 Secondary efficacy measures included outcomes in patients at first ablation, freedom from all atrial arrhythmias, and freedom from AF in patients with paroxysmal versus persistent AF. Patients were followed for ≥1 year after the single ablation procedure with a 3-month blanking period and no repeat procedures. Follow-up was censored at the first arrhythmia recurrence or last arrhythmia-free clinic visit, whichever came first (median time 245 days, IQR 145–354), with 1 patient lost to follow-up.
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD). The Student’s t-test was used to compare continuous variables between 2 groups, such as ablation time. Paired continuous variables were compared using linear regression and the paired t-test. The chi-square test was applied to contingency tables for categorical variables. Log-rank test was used to compare survival distributions. A P value of < 0.05 was considered statistically significant.
Results
Clinical characteristics of our population are summarized in Table 2, representing the first 6 (median) FIRM-guided cases in each institution. There was no significant difference in the characteristics of patients undergoing repeat versus first AF ablation. In Table 2, patients with persistent AF had lower LVEF, a higher remote use of amiodarone prior to ablation and a trend for larger left atria than those with paroxysmal AF. For technical connectivity issues, FIRM mapping was not completed in the first FIRM-guided ablation case at each of 2 institutions (n = 2 cases), who were followed on an intention-to-treat basis.
TABLE 2.
Clinical Characteristics of Patients with Persistent and Paroxysmal AF
| Characteristic | All Patients | Paroxysmal AF | Persistent AF | P value |
|---|---|---|---|---|
| Number of patients | 78 | 24 | 54 | |
| Age (years) | 61.3 ± 10.1 | 60.6 ± 10.4 | 61.6 ± 10.1 | 0.71 |
| Gender (male/female) | 55/23 | 16/8 | 39/15 | 0.79 |
| Left atrial diameter (mm) (intracardiac echocardiogram) | 56 ± 9 | 53 ± 7 | 58 ± 10 | 0.06 |
| No. with LA >55 mm (%) | 38 (50.4%) | 8 (33%) | 30 (55.6%) | 0.09 |
| LVEF (%) | 55 ± 8 | 58 ± 6 | 54 ± 8 | 0.04 |
| Remote amiodarone use (N [%]) | 12 (15.4%) | 0 (0.0%) | 12 (22.2%) | 0.01 |
Acute Results from FIRM Mapping and Ablation
AF sources were detected in 100% of patients in whom FIRM mapping was performed (76/76), or 97.4% (76/78) by intention-to-treat (2 patients were not mapped). Sources were spatially stable across multiple FIRM maps (Table 3).
TABLE 3.
Acute Characteristics of AF Sources
| Characteristic | All Patients N = 78 |
First Ablation N = 40 |
Prior Failed PVI N = 38 |
P value |
|---|---|---|---|---|
| Patients mapped | 76 | 38 | 38 | |
| Patients with detected sources | 76/76 (100%) | 38/38 (100%) | 38/38 (100%) | 1.0 |
| No. patients with sources in LA vs RA | 71/39 | 35/16 | 36/23 | – |
| No. concurrent biatrial AF sources/patient | 2.3 ± 0.9 | 2.1 ± 0.8 | 2.4 ± 0.9 | 0.08 |
| No. of LA sources/patient | 1.8 ± 0.7 | 1.8 ± 0.6 | 1.9 ± 0.8 | 0.49 |
| No. of RA source/patient | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.96 |
| LA/RA sources, as % of total sources | 74.7%/25.3% | 76.8%/23.2% | 73.8%/26.2% | 0.63 |
| Total FIRM-ablation time, all sources (min, Mean ± SD) | 16.6 ± 11.7 | 14.7 ± 11.3 | 18.5 ± 11.8 | 0.20 |
AF = atrial fibrillation; LA = left atrium; RA = right atrium.
Each patient had an average of 2.3 ± 0.9 concurrent rotors or focal sources (total of 174), with 1.8 ± 0.7 left atrial sources per patient and 1.1 ± 0.3 right atrial sources per patient. Notably, right atrial sources comprised 25.3% of the total, and 39/78 (50%) of patients showed at least 1 right atrial source (Table 3). Sources were predominantly rotors (172/174, 98.9%), with 1.1% focal sources (all of which lay in the left atrium). There was a nonsignificant trend towards more AF sources in those in whom a prior ablation had been done (P = 0.08) but no significant differences in numbers or right/left atrial distribution of sources.
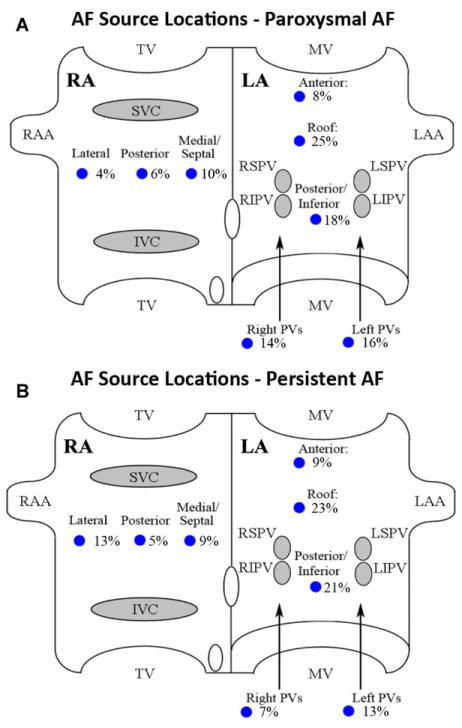
Figure 2 summarizes the distributions of AF sources within the atria in patients with paroxysmal AF (n = 24, 51 sources; Fig. 2A) and persistent AF (n = 54, 123 sources; Fig. 2B), as proportions of all detected sources. Sources were often remote from conventional PV ablation targets. From these source distributions, the estimated number of AF sources that may have been ablated coincidentally by wide area PVI with posterior wall ablation was ≈49% in paroxysmal AF, and ≈41% in persistent AF.
Figure 2.
AF source locations in both atria for patients with (A) paroxysmal and (B) persistent AF.
Total ablation time required to eliminate these sources was 16.6 ± 11.7 minutes, typically in both atria in each patient. There was no significant difference between the populations at first and repeat procedure in the ablation time required to eliminate right and left atrial sources in each patient.
No procedural complications occurred, and specifically neither thromboemboli nor perforations from use of the basket catheter, in agreement with prior reports.13
Long-Term Outcome After FIRM-Guided Ablation
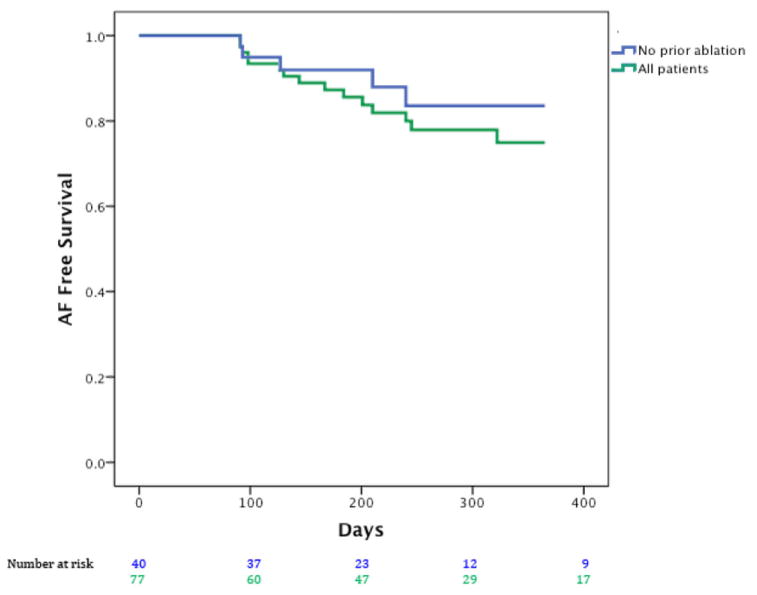
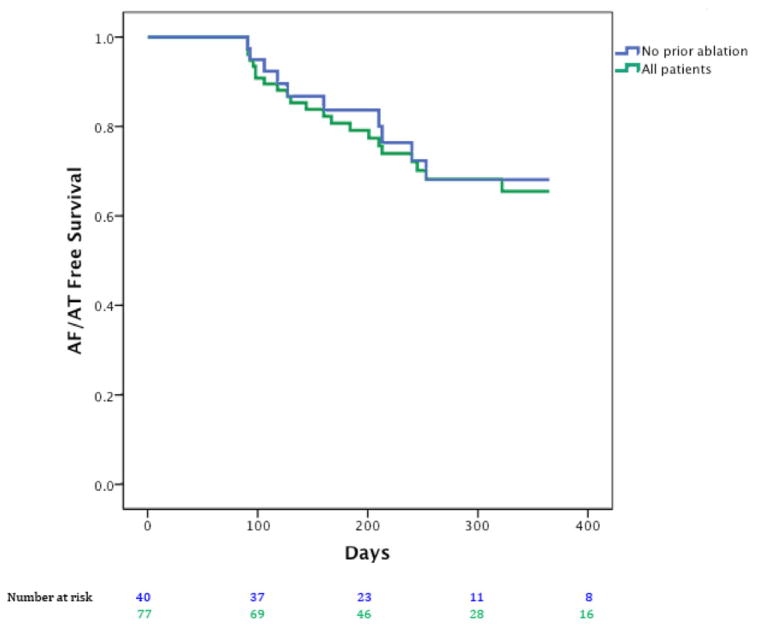
After 1-year follow-up, Figure 3 presents Kaplan–Meier curves showing that the single procedure freedom from AF was 80.5% (62/77) for all patients, and 87.5% in patients with no prior ablation (35/40; n = 32 persistent/longstanding persistent). In Figure 4, single procedural freedom from all atrial arrhythmias was 71.4% (55/77) for all cases and 75% (30/40) in patients undergoing their first ablation (n = 40; n = 32 persistent/longstanding persistent). Antiarrhythmic medications were continued at last follow-up due to physician or patient preference in n = 13 patients without arrhythmia recurrence and 7 with arrhythmia recurrence (i.e., counted as failures) for 20 overall. For the entire population, the proportions of patients who remained on antiarrhythmic drugs did not differ between persistent and paroxysmal AF (15/54, 27.8% vs. 5/24, 20.8%, P = 0.59, Fisher’s exact test) or between first ablation and redo cases (9/40, 22.5% vs. 11/38, 28.9%, P = 0.51 chi-square test), respectively.
Figure 3.
Freedom from atrial fibrillation after single (index) FIRM + PVI procedure for all cases (green) and patients at their first ablation (blue).
Figure 4.
Freedom from all atrial arrhythmias (atrial fibrillation and atrial tachycardia) after a single (index) FIRM + PVI procedure for all cases (green) and patients at their first ablation (blue).
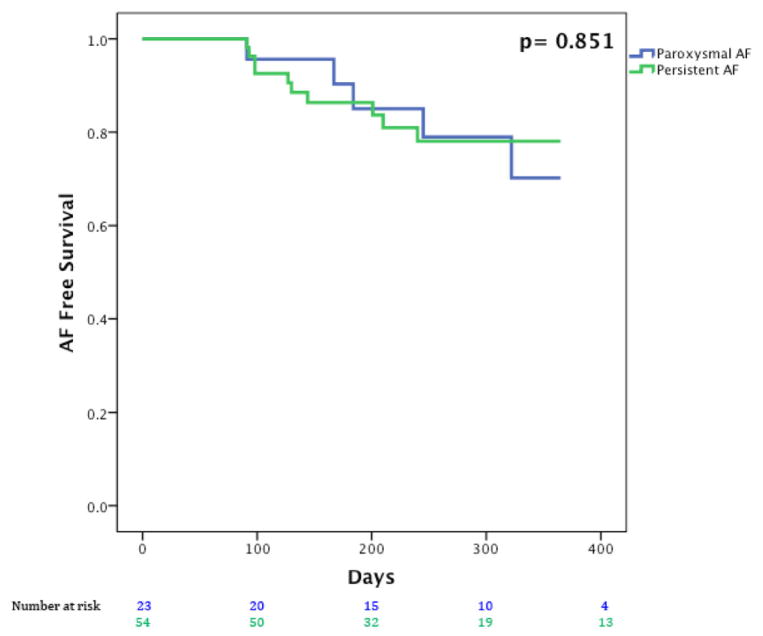
Figure 5 presents Kaplan–Meier curves showing that single procedural freedom from AF did not differ significantly between patients with persistent AF (44/54, 81.5%) and paroxysmal AF (18/23, 78.3%; P = 0.89).
Figure 5.
Freedom from atrial fibrillation after a single index FIRM + PVI procedure for patients with paroxysmal AF in blue and persistent AF (including longstanding persistent AF) in green.
Discussion
The FIRM-registry provides the first prospective multi-center data on long-term outcomes from FIRM-guided ablation for AF. In this learning experience at each center, the single FIRM-guided procedural elimination of AF was 80.5% for all patients and 87.5% in patients at first ablation, in a population mostly of persistent AF. The number of stable concurrent AF sources in each patient and long-term results from FIRM-guided ablation were similar to those reported in the original CONFIRM trial although the proportion of rotors to focal sources was higher in this registry. Notably, unlike almost all prior AF ablation trials, FIRM-guided ablation had similar success in this registry for patients with persistent or paroxysmal AF. These data further support the predominant mechanistic role of rotors in sustaining AF across a wide range of presentations, and show that successful elimination of patient-specific sources can be achieved after a short learning curve to achieve a high level of arrhythmia freedom on follow-up.
Comparison of FIRM-Guided Outcomes with Previous Published Reports
This first independent validation of FIRM-guided AF ablation yielded similar results to the CONFIRM trial.13 Freedom from AF and atrial arrhythmias in patients at first ablation were actually slightly higher than in the CONFIRM trial, despite the fact that these were the first cases performed at each center.
A limitation of this study is the absence of a control group receiving conventional ablation alone, which will be addressed in recently started multicenter randomized controlled trials. However, the 1-year single FIRM-guided procedural AF freedom in this study (80.5%) is higher than the ≈50% single-procedure success of conventional ablation in recent reports of paroxysmal AF,9–12 <40–50% in persistent AF7 or 40–50% in mixed populations in CONFIRM13 and other experienced groups.23 Single FIRM-procedural freedom from all atrial arrhythmias was also higher than prior studies, although many prior trials did not report this endpoint for comparison.10,11 The mode of recurrence of AF or tachycardias after FIRM-guided ablation remain unclear at this point, since only a minority of patients have been repeat FIRM mapped (and none in this series), but this is under active investigation.
Comparison of Results with Prior Reports of Rotors
The numbers of AF sources in this study were similar to those reported in the CONFIRM trial,13 and were similar between patients at their first ablation and those with AF despite prior conventional ablation (Table 3). The predominance of rotors and lower prevalence of focal sources in this multicenter registry compared to the CONFIRM trial13 may reflect improved RhythmView™ software, or differences in interpretive experience or patients between centers, and should be tracked in ongoing studies of FIRM39 at other centers. The diverse locations of AF sources including the right atrium (Fig. 2) may explain why ablation success rises with extensive empirical ablation lesions, which have been shown to coincidentally ablate AF rotors and focal sources in prior studies.24
Several groups have recently investigated sources for human AF using diverse techniques including Shannon entropy25 or wave similarity analysis26 during conventional mapping, suggesting sufficient stability to be detected by detailed point-by-point mapping. Studies of virtual electrograms from the inverse solution initially reported very few and short-lived AF rotations,27 although newer analytic methods reveal rotors with this approach that are described as unstable yet remain in the same atrial area for days and are targeted by localized ablation.28 Studies are needed to define how these reports differ from FIRM, which uses specific physiologically directed algorithms to track spatially precessing rotors with spiral arm disorganization via fibrillatory conduction.18–21 To date, this study and the CONFIRM trial are the only reports of long-term outcomes from the FIRM-guided approach to rotor elimination.
Localized Sources as a General Mechanism for AF
FIRM-guided ablation produced highly unusual similar success for patients with paroxysmal and persistent AF, in contrast to nearly all prior studies showing lower success for persistent versus paroxysmal AF.7 Patients with paroxysmal and persistent AF differed in anticipated parameters (Table 2), with left atria sizes in both groups measured by intracardiac echocardiography or preprocedural MRI that may produce higher values than transthoracic echocardiography. These results support the concept that rotors and focal sources are a central AF-sustaining mechanism for a range of AF presentations, with the caveat that the study is relatively small. Further studies are required to test if outcomes remain similar between groups over longer follow-up periods.
The localized source model for AF has gained ground in recent years, based on elegant animal models in which spiral waves (rotors)29,30 or repetitive focal impulses31 cause extremely disordered surrounding activation (so-called “fibrillatory conduction”) that hampers their detection by simple activation or phase mapping. Stable AF sources readily explain clinical observations that AF exhibits conserved nonuniformities in rate and activation vector,32–34 and why ablation can in some cases rapidly terminate AF13,14,35 before PVs have been isolated.
Limitations
The first major limitation is the absence of a control group undergoing conventional ablation alone, although this information can be estimated by several recent trials of conventional ablation that produce lower success than by FIRM-guided ablation. Second, this study has the typical limitations of a registry design, including variations in guideline-driven conventional ablation between investigators. Follow-up was less rigorous than the CONFIRM trial, yet followed clinical guidelines and was analogous to many recent AF trials. As in any multicenter experience, some groups provided few patients. A resulting strength of these limitations, however, is that the study shows a rapid learning curve with FIRM-guided ablation results in a “real-world” experience similar to those of the CONFIRM trial. This contrasts with some approaches that have been difficult to extend beyond the originating center.36–38
The freedom from AF observed in cases of prior failed ablation in our study may theoretically reflect the impact of those lesions (“cleaning up” prior ablation). However, few series report success rates at this level, and were higher in patients undergoing their first ablation than those undergoing repeat ablation after a prior failed procedure.
FIRM-guided ablation still presents several limitations. Many patients had atria larger than current basket catheters (55–60 mm diameter fully deployed), using accurate intraprocedural imaging, which almost certainly limited efficacy. Two patients were not mapped due to technical issues that were resolved with experience, and one patient was lost to long-term follow-up. Notably, despite the lack of prior experience with the basket catheter at many centers, no complications were reported. By limiting ablation within the atrium, it is reasonable to hope that FIRM mapping (if used as a primary approach) may increase the safety of ablation. This requires further testing.
Conclusions
In this multicenter learning curve experience of FIRM mapping, mapping and elimination of patient-specific rotors and focal sources with trigger isolation provided >80% single procedure AF elimination in patients predominantly with persistent AF. These acute and chronic results were similar to those originally reported from FIRM by the originating group. FIRM-guided ablation for AF thus has a rapid learning curve at experienced ablation centers, and may help to improve outcomes for ablation of this troublesome arrhythmia.
Acknowledgments
Dr. Miller reports consulting fees/honoraria from Topera, Stereotaxis, Biosense Webster, Biotronik, and Medtronic, and fellowship support from Medtronic, Boston Scientific, Biotronik, and Biosense Webster. Dr. Daubert has received consulting fees/honoraria from Medtronic, St. Jude Medical, Boston Scientific, Sorin Group, and CardioFocus. He has received research grants from Boston Scientific, Biosense Webster, Medtronic, and Gilead Sciences. He has also received fellowship support from Medtronic, Boston Scientific, Biotronik, St. Jude Medical, Biosense Webster, and Bard Electrophysiology. Dr. Ellenbogen reports consulting fees/honoraria from Medtronic and Boston Scientific, speaking honoraria from Medtronic, Boston Scientific, and Biotronik, and research grants from Medtronic, Boston Scientific, and Biosense Webster. Dr. Hummel reports consulting fees/honoraria from Medtronic and research grants from St. Jude Medical and Boston Scientific. Dr. Kowal has received consulting fees/honoraria from Medtronic. Dr. Krummen has received consulting fees from Insilicomed and research grants from the American Heart Association and NIH. He has also received fellowship support from Medtronic, St. Jude Medical, Biosense Webster, Boston Scientific, and Biotronik. Dr. Narayan was supported by NIH (HL83359, HL103800). Dr. Narayan is coauthor of intellectual property owned by the University of California Regents and licensed to Topera Inc. Topera does not sponsor any research, including that presented here. Dr. Narayan holds equity in Topera, and reports having received honoraria from Medtronic, St. Jude Medical, Biotronik, and Boston Scientific. He has received consulting fees from the American College of Cardiology Foundation and Topera and has received royalty income from UpToDate. His institution has received fellowship support from Medtronic, St. Jude Medical, Biosense Webster, Boston Scientific, and Biotronik. Dr. Reddy reports consulting fees/honoraria from Medtronic, Philips, CardioInsight Technologies, Biosense Webster, St. Jude Medical, Boston Scientific, CardioFocus, Voyage Medical, ACT, Endosense, and Vytronus, and research grants from Biosense Webster, Medtronic, St. Jude Medical, Boston Scientific, CardioFocus, Voyage Medical, Philips, ACT, Endosense, and Vytronus. Dr. Shivkumar is an unpaid scientific advisor to Topera and is a corporate board member of BioSig. UCLA has received EP fellowship support from Biosense Webster, St. Jude Medical, Medtronic, and Boston Scientific. Dr. Steinberg reports consulting fees/honoraria from Medtronic, St. Jude Medical, Boston Scientific, Philips, Biosense Webster, Pfizer, Bristol Meyers Squibb, and Janssen Pharmaceuticals; and research grants from Biosense Webster, Boston Scientific, and Medtronic. Dr. Swarup has received consulting fees/honoraria from Biosense Webster and research grants from Biosense Webster, Medtronic, Boston Scientific, St. Jude Medical, and Biotronik. Dr. Wheelan has received consulting fees/honoraria from Biosense Webster and Medtronic.
Footnotes
Other authors: no disclosures.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 3.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O’Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 4.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A Comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 5.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 6.Jalife J. Deja vu in the theories of atrial fibrillation dynamics. Cardiovasc Res. 2011;89:766–775. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calkins CH, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS Expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–696. [Google Scholar]
- 8.Dixit S, Marchlinski FE, Lin D, Callans DJ, Bala R, Riley MP, Garcia FC, Hutchinson MD, Ratcliffe SJ, Cooper JM, Verdino RJ, Patel VV, Zado ES, Cash NR, Killian T, Tomson TT, Gerstenfeld EP. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol. 2012;5:287–294. doi: 10.1161/CIRCEP.111.966226. [DOI] [PubMed] [Google Scholar]
- 9.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, Reddy V, Augello G, Reynolds MR, Vinekar C, Liu CY, Berry SM, Berry DA. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. J Am Med Acad. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen JC, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 11.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, Lehmann JW, Ruskin JN. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: First results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 12.Morillo CA, Verma A, Connolly SJ, Champagne J, Nair G, Sterns L, Beresh H, Connolly SJ, Natale A. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): A randomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 13.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller J. Treatment of atrial fibrillation by the ablation of localized sources: The conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation: CONFIRM trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: First multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol. 2012;23:1277–1285. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt C, Ndrepepa G, Weber S, Schmieder S, Weyerbrock S, Schneider M, Karch MR, Deisenhofer I, Schreieck J, Zrenner B, Schomig A. Biatrial multisite mapping of atrial premature complexes triggering onset of atrial fibrillation. Am J Cardiol. 2002;89:1381–1387. doi: 10.1016/s0002-9149(02)02350-0. [DOI] [PubMed] [Google Scholar]
- 17.Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani G, Krummen DE, Shivkumar K, Miller JM. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared to trigger ablation alone: Extended follow-up of the CONFIRM (CONventional ablation with or without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2014;63:1761–1768. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayan SM, Krummen DE, Rappel W-J. Clinical mapping approach to identify rotors and focal beats in human atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayan SM, Krummen DE, Enyeart MW, Rappel W-J. Computational mapping approach identifies stable and long-lived electrical rotors and focal sources in human atrial fibrillation. PLos One. 2012;7:e46034. doi: 10.1371/journal.pone.0046034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayan SM, Shivkumar K, Krummen DE, Miller JM, Rappel W-J. Panoramic electrophysiological mapping but not individual electrogram morphology identifies sustaining sites for human atrial fibrillation (AF rotors and focal sources relate poorly to fractionated electrograms) Circ Arrhythm Electrophysiol. 2013;6:58–67. doi: 10.1161/CIRCEP.111.977264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rappel W-J, Narayan SM. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos. 2013;23:023113. doi: 10.1063/1.4807098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calkins H, Brugada J, Packer DH, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Scoiety (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS) Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haïssaguerre M, Jaïs P. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 24.Narayan SM, Clopton P, Krummen DE, Shivkumar K, Miller J. Direct or concidental ablation of localized sources may explain the success of atrial fibrillation ablation. On treatment analysis from the CONFIRM trial. J Am Coll Cardiol. 2013;62:138–147. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganesan AN, Kuklik P, Lau DH, Brooks AG, Baumert M, Lim WW, Thanigaimani S, Nayyar S, Mahajan R, Kalman JM, Roberts-Thomson KC, Sanders P. Bipolar electrogram shannon entropy at sites of rotational activation: Implications for ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:48–57. doi: 10.1161/CIRCEP.112.976654. [DOI] [PubMed] [Google Scholar]
- 26.Lin YJ, Lo MT, Lin C, Chang SL, Lo LW, Hu YF, Hsieh WH, Chang HY, Lin WY, Chung FP, Liao JN, Chen YY, Hanafy D, Huang NE, Chen SA. Prevalence, characteristics, mapping, and catheter ablation of potential rotors in nonparoxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:851–858. doi: 10.1161/CIRCEP.113.000318. [DOI] [PubMed] [Google Scholar]
- 27.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr, Li L, Rudy Y. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haïssaguerre M, Hocini M, Shah AJ, Derval N, Sacher F, Jaïs P, Dubois R. Noninvasive panoramic mapping of human atrial fibrillation mechanisms: A feasibility report. J Cardiovasc Electrophysiol. 2013;24:711–717. doi: 10.1111/jce.12075. [DOI] [PubMed] [Google Scholar]
- 29.Allessie MA, Bonke FIM, Schopman FJG. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III: the “leading circle” concept: A new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circulation Research. 1977;41:9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- 30.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 31.Ryu K, Shroff SC, Sahadevan J, Martovitz NL, Khrestian CM, Stambler BS. Mapping of atrial activation during sustained atrial fibrillation in dogs with rapid ventricular pacing induced heart failure: Evidence for a role of driver regions. J Cardiovasc Electrophysiol. 2005;16:1348–1358. doi: 10.1111/j.1540-8167.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 32.Gerstenfeld E, Sahakian A, Swiryn S. Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation. 1992;86:375–382. doi: 10.1161/01.cir.86.2.375. [DOI] [PubMed] [Google Scholar]
- 33.Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation. 2004;110:3181–3186. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 34.Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial mapping of chronic atrial fibrillation in patients: Preliminary observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 35.Haïssaguerre M, Sanders P, Hocini M, Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clementy J, Jaïs P. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 36.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004a;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 37.Oral H, Chugh A, Good E, Wimmer A, Dey S, Gadeela N, Sankaran S, Crawford T, Sarrazin JF, Kuhne M, Chalfoun N, Wells D, Frederick M, Fortino J, Benloucif-Moore S, Jongnarangsin K, Pelosi F, Jr, Bogun F, Morady F. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation. 2007;115:2606–2612. doi: 10.1161/CIRCULATIONAHA.107.691386. [DOI] [PubMed] [Google Scholar]
- 38.Oral H, Chugh A, Yoshida K, Sarrazin JF, Kuhne M, Crawford T, Chalfoun N, Wells D, Boonyapisit W, Veerareddy S, Billakanty S, Wong WS, Good E, Jongnarangsin K, Pelosi F, Bogun F, Morady F. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009;53:782–789. doi: 10.1016/j.jacc.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 39.Lin T, Kuck KH, Ouyang F, Tilz RR. First in-human robotic rotor ablation for atrial fibrillation. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu009. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]