Abstract
Background
There is a paucity of data on biophysical parameters during radiofrequency ablation of scar-mediated ventricular tachycardia (VT).
Methods and Results
Data was collected from consecutive patients undergoing VT ablation with open-irrigation. Complete data was available for 372 lesions in 21 patients. The frequency of biophysical parameter changes were: >10Ω reduction (80%), bipolar EGM reduction (69%), while loss of capture was uncommon (32%). Unipolar injury current was seen in 72% of radiofrequency applications. Both EGM reduction and impedance drop were seen in 57% and a change in all 3 parameters was seen in only 20% of lesions. Late potentials were eliminated in 33%, reduced/modified in 56%, and remained after ablation in 11%. Epicardial lesions exhibited an impedance drop (90% vs 76%, p=0.002) and loss of capture (46% vs 27%, p<0.001) more frequently than endocardial lesions. Lesions delivered manually exhibited a >10Ω impedance drop (83% vs 71%, p=0.02) and an EGM reduction (71% vs 40%, p< 0.001) more frequently than lesions applied using magnetic navigation, although loss of capture, elimination of LPs, and a change in all 3 parameters were similarly observed.
Conclusions
VT ablation is inefficient as the majority of radiofrequency lesions do not achieve more than one targeted biophysical parameter. Only one-third of RF applications targeted at LPs result in complete elimination. Epicardial ablation within scar may be more effective than endocardial lesions and lesions applied manually may be more effective than lesions applied using magnetic navigation. New technologies directed at identifying and optimizing ablation effectiveness in scar are clinically warranted.
Keywords: ventricular tachycardia, radiofrequency, ablation, biophysical, scar, endocardial, epicardial
Radiofrequency (RF) ablation of ventricular tachycardia (VT) has been shown to reduce symptomatic arrhythmias and defibrillator therapies, in both preemptive and palliative settings.1-3 Despite advances in electroanatomic mapping, imaging integration, and catheter technology, recurrence rates for scar-mediated VT remain significant (~50% at 6-12 months).4, 5 Recurrence may be attributable to an evolving complex substrate, a site of origin at an unmapped or inaccessible location (epicardial or intramural), incomplete substrate modification, or ineffective lesion formation.
The effectiveness of lesion formation during catheter ablation of scar-mediated ventricular tachycardia (VT) has not been systematically analyzed. The clinical efficacy of ablation is likely to be improved if lesion formation can be reliably monitored and confirmed. Currently, contact force sensing technology is not widely available. As tissue temperature cannot be directly measured, standard parameters frequently used as indicators of effective RF delivery include a drop in tissue impedance, local electrogram (EGM) reduction, and loss of tissue excitability during pacing (electrically unexcitable scar). A unipolar current of injury (UIC) seen with pacing leads has been associated with adequate active endomyocardial fixation 6 and when a significant contact force is present on a catheter electrode.
The aims of the current study were: 1) to report the frequency of targeted biophysical parameters during ablation of scar-mediated VT 2) to evaluate the frequency of late potential (LP) elimination during an RF application 3) to compare the relative effectiveness of RF delivered at endocardial versus epicardial sites 4) to compare the effectiveness of RF applications delivered with manual versus magnetic navigation.
METHODS
Biophysical parameters of RF lesions were collected during consecutive scar-mediated VT ablations at UCLA between Nov 2010 and Aug 2011 and retrospectively analyzed. The diagnosis of NICM was based on the absence of coronary artery disease (>75% stenosis), prior myocardial infarction, or significant valvular disease. The diagnosis of ICM was established by prior history of infarction with Q waves, focal wall motion abnormality, or fixed perfusion defect correlated with coronary stenosis or prior intervention. All studies were performed under general anesthesia after pre-procedural transesophageal echocardiography excluded intracardiac thrombus. Epicardial mapping was performed as clinically indicated at the discretion of the operator. The Institutional Review Board at UCLA approved review of the retrospective data.
Ablation targets were chosen using activation and entrainment mapping for hemodynamically tolerated VT, and a strategy to abolish abnormal electrograms and LPs for patients with untolerated scar-mediated VT, as previously described.7, 8 LPs were defined as electrograms with a component after the offset of the QRS.
All patients underwent ablation using the CARTO (Biosense Webster, Diamond Bar, CA) mapping system, with open-irrigation 3.5mm RF ablation (30-50W, 30cc flow rate, temp limit 45°C endocardium, 50°C epicardium). Power was titrated with the goal of reducing impedance by 10 Ohms, reducing the targeted electrogram voltage, and eliminating LPs, when present. (FIGURE 1) Bipolar pacing was performed before and after each RF application from the distal electrode pair of the ablation catheter to assess for loss of myocardial capture at 10mA at 2ms pulse width (FIGURE 2). Cases performed with remote magnetic navigation (Stereotaxis) with 3.5mm irrigated (Celsius RMT, Biosense Webster, Diamond Bar, CA) were included. Catheter contact was confirmed by the operator with fluoroscopy, intracardiac echocardiography, and electroanatomic mapping.
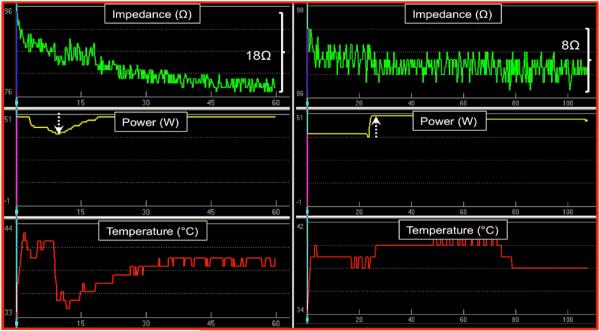
FIGURE 1.
Examples of effective and ineffective RF energy delivery in the epicardial space by impedance parameters. Left panel shows an effective lesion with 18Ω drop in impedance. Power was downtitrated transiently due to initial increase in temperature and rapid impedance drop to prevent steam pop. Right panel demonstrates uptitration of power after 25 seconds with failure to achieve a >10Ω impedance drop.
FIGURE 2.
Loss of capture achieved after ablation. Evidence of capture in the basal inferior left ventricle during bipolar pacing at 10mA with 2 ms pulse width. After one minute of RF application, the local tissue is unexcitable. AV sequential pacing is the baseline rhythm.
For each RF application, the following data were collected:
Presence of capture pre- and post-ablation (10mA, 2 ms)
Initial and lowest recorded impedance
Bipolar EGM voltage pre- and post-ablation
The presence of unipolar injury pre-, during, and post-ablation
Power setting
Maximum and average temperature achieved
Lesion location- endocardial or epicardial
Application-manual or magnetic navigation
Lesions where any of the abovementioned data was incomplete were excluded from analysis. Lesions in which catheter instability was suspected or seen were also excluded. RF was delivered for 60 seconds and those that were prematurely terminated due to catheter movement or reaching the temperature limit were excluded. Additional RF application at a site not satisfactorily ablated by an initial lesion was not included for analysis.
Unipolar injury current (UIC) was defined as any ST elevation from baseline (TP segment) on the distal ablation electrode visualized on the CARTO system (Wilson central terminal as indifferent electrode, FIGURE 3). Injury during ablation was defined as persistent if ST elevation was present throughout the duration of RF application or transient if there was ST elevation with periods of normalization to baseline during RF. If pre-ablation UIC was seen, an increase in ST elevation was required to categorize the RF application as having UIC during ablation. Consensus between the operator and two observers was reached for determination of the presence of injury.
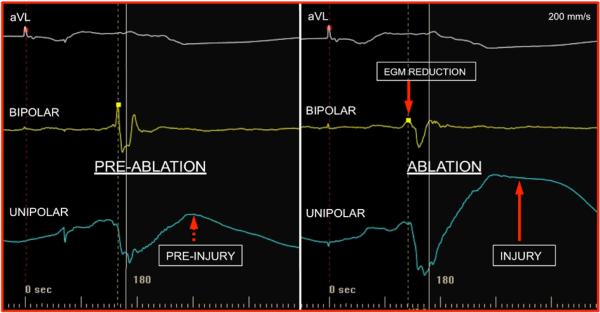
FIGURE 3.
Unipolar injury current before and during ablation. Slight ST segment elevation is seen pre-ablation. The bipolar EGM amplitude is reduced during RF delivery and injury is more pronounced on the unipolar EGM with dramatic elevation of ST segment.
STATISTICAL ANALYSIS
All symmetric continuous data were summarized with mean± SD and compared using unpaired student t-test. Asymmetric continuous data were summarized with medians and ranges. Comparison of proportions and categorical variables was performed using the Fisher exact test. Sets of quantitative data were compared using a paired t-test. Test characteristics of sensitivity, specificity, positive predictive value, and negative predictive values were calculated in relation to the gold standard chosen. Since there is no gold standard for an effective RF lesion, a change in all 3 standard parameters (impedance drop, EGM reduction, and loss of pacing capture) was chosen as the the most ideally effective RF lesion. A p-value of <0.05 was considered statistically significant.
RESULTS
Three hundred seventy-two RF applications were recorded and analyzed during 22 ablation procedures, on 21 patients, by the same operator. The etiology of scar-mediated VT was as follows: ICM n=10, NICM n=8, ARVC n=2, HCM n=1. (TABLE 1) One patient with ICM underwent a repeat ablation for recurrent VT. The mean age was 62±12 years and 86% were male. An average of 16.9±10.5 (range 5-38) RF applications were analyzed per patient (mean EGM amplitude 0.56±0.47mV). The mean power delivered was 43.2 ± 6.0 W and the mean maximum temperature was 38.6 ± 4.3°C.
TABLE 1.
Patient and ablation characteristics
| Age | Sex | Etiology | EF | Prior RFA | Ablation location | Manual/MNS | # of lesions |
|---|---|---|---|---|---|---|---|
| 69 | M | ICM | 20% | N | Endo | MNS | 32 |
| Y | Epi/Endo | Manual | 27/10 | ||||
| 57 | M | ICM | 20% | N | Endo | Manual | 11 |
| 77 | M | ICM | 20% | N | Endo | Manual | 4 |
| 81 | M | ICM | 23% | N | Epi/endo | MNS | 17/8 |
| 78 | M | ICM | 35% | N | Endo | MNS | 5 |
| 64 | M | ICM | 35% | Y | Epi/endo | Manual | 5/15 |
| 58 | F | ICM | 30% | N | Endo | Manual | 22 |
| 65 | M | ICM | 25% | N | Endo | Manual | 19 |
| 66 | M | ICM | 35% | N | Endo | Manual | 12 |
| 40 | M | ICM | 50% | N | Endo | Manual | 38 |
| 79 | M | NICM | 20% | N | Endo | Manual | 7 |
| 50 | M | NICM | 30% | N | Epi/endo | Manual | 21/9 |
| 64 | M | NICM | 45% | N | Epi | Manual | 11 |
| 51 | M | NICM | 15% | N | Epi | MNS | 6 |
| 61 | M | NICM | 20% | N | Endo | MNS | 5 |
| 51 | M | NICM | 20% | N | Endo | Manual | 10 |
| 58 | M | NICM | 25% | N | Epi | Manual | 22 |
| 61 | F | NICM | 25% | N | Endo | Manual | 7 |
| 65 | M | HCM | 60% | N | Endo | Manual | 17 |
| 65 | M | ARVD | 55% | N | Epi/endo | MNS | 7/11 |
| 32 | F | ARVD | 60% | N | Endo | Manual | 14 |
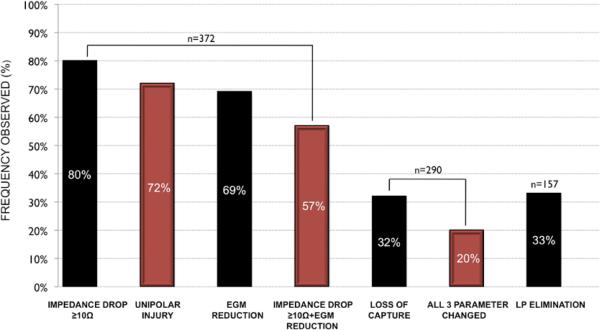
A 10 Ohm impedance drop was the parameter most commonly seen (80%) followed by reduction in EGM (69%), with loss of tissue capture least commonly seen (32%). (FIGURE 4) At 82 sites, there was no capture prior to ablation; these were subtracted from the denominator for reporting loss of capture (n=290). A change in all 3 standard targeted parameters was seen in 20%, any 2 parameters in 47%, only 1 parameter in 29%, and no parameters changed in 3%.
FIGURE 4.
Frequency of achieving targeted biophysical parameters after RF delivery displayed in order of prevalence.
Among the total RF applications analyzed, 42% (n=157) were targeted at LPs. LPs were eliminated in 33%, reduced/modified in 56%, and remained after ablation in 11%. In comparison to RF applications that did not have an effect on LP, RF applications that resulted in elimination of LP exhibited a greater frequency in a 2 parameter change (59.3% vs 23.5%, p=0.01) but not a 3 parameter change (38% vs 27.3%, p=0.73). Biophysical parameter changes at sites where LPs were modified or eliminated compared to RF applications that failed to effect LPs are summarized in TABLE 2. The presence of UIC during ablation had a sensitivity of 83%, specificity of 38%, positive predictive value of 39%, and negative predictive value of 82% for LP elimination. UIC was seen more frequently in patients with complete LP elimination compared to those with modified or persistent LP (83% vs 62%, p=0.01)
TABLE 2.
Frequency of biophysical parameter changes in RF application with LP modification or elimination versus no effect.
| No effect | LP modified | p value | LP eliminated | p value (vs no effect) | |
|---|---|---|---|---|---|
| Impedance drop (>10Ω) | 64.7% | 73.9% | 0.55 | 87.0% | 0.07 |
| EGM reduction | 41.2% | 69.3% | 0.05 | 67.3% | 0.09 |
| Loss of capture | 45.5% | 52.6% | 0.75 | 59.5% | 0.50 |
| Unipolar injury | 41.2% | 65.9% | 0.06 | 82.7% | 0.002 |
| EGM+imp drop | 23.5% | 54.5% | 0.03 | 59.6% | 0.01 |
| EGM+imp drop+loss of capture | 27.3% | 33.3% | 1.0 | 38% | 0.73 |
Two ablation lesions that resulted in termination of VT were seen in 2 patients (30W+titration, open-irrigated catheter with magnetic navigation). One termination occurred within anterior epicardial scar with a maximum temperature achieved of 38°C, where all parameters of EGM reduction, persistent injury, impedance drop, and elimination of LP were achieved except loss of capture. The second termination occurred in a patient with RV cardiomyopathy from the endocardial RV outflow tract at a site without a LP. With a maximum temperature achieved of 37°C, persistent injury was observed throughout the lesion as well as an impedance drop, although EGM reduction and loss of capture were not achieved.
Pre-ablation UIC was present in 10% of RF applications (n=41) and persisted after ablation in 8%. UIC during ablation was seen in 88% of lesions when pre-ablation injury was present. UIC was observed during 72% of RF applications (n=269), the second most frequently observed parameter. Among the lesions that resulted in UIC, the injury was persistent in 58% and transient in 42%. UIC was seen more frequently with higher power settings (>35 W) when compared to standard settings (≤35W) (73% vs 47%, p<0.001) and UIC occurred more commonly with higher mean maximum temperatures (38.7±4.5C vs 38±3.9C, p=0.03).
The presence of any UIC during ablation predicted changes in all three targeted parameters with a PPV 20%, NPV 80% with a sensitivity of 75%, and specificity of 26%. The presence of persistent UIC had a PPV 19%, NPV 78%, sensitivity 44% and specificity of 53% for a three-parameter change.
In lesions in which a two-parameter change of impedance and EGM reduction occurred (57%), without requiring loss of capture, the presence of any UIC had a PPV 84%, NPV 26%, sensitivity 77% and specificity of 36%, while the presence of persistent UIC had a PPV 83%, NPV 19%, sensitivity 46% and specificity of 56% for a two-parameter change.
EPICARDIAL VERSUS ENDOCARDIAL RF APPLICATION
Sixty-nine percent of RF applications were delivered from the endocardium (n=256) and 31% were delivered on the epicardium (n=116). The power applied in the epicardial space was statistically higher than power applied from the endocardium (46.3±4.1W vs 41.6±6.7W, p<0.0001). No statistical difference was seen in the maximum temperature achieved in the epicardium vs endocardium (38.1±3.3 vs 38.8±4.8, p=0.5). Lesions on the epicardium exhibited an impedance drop more frequently than those on the endocardium (90% vs 76%, p=0.002). No statistically significant difference was seen in EGM reduction between the epicardium and endocardium (66% vs 70%, p=0.5). Loss of capture was also achieved after ablation more frequently from the epicardium compared to the endocardium (46% vs 27%, p=0.005). Lesions applied from the epicardium were more likely to achieve a change in all three standard parameters when compared to endocardial lesions, (36% vs 15%, p=0.003).
UIC was seen more frequently with endocardial lesions compared to epicardial lesions, although this difference was not statistically significant (75% vs 66%, p=0.06). Among RF applications targeting LPs, complete elimination of LP was seen in 39% (32/83) from the endocardium and 27% (20/74) from the epicardium (p=0.13). (FIGURE 5)
FIGURE 5.
Frequency of achieving targeted biophysical parameters after RF delivery on the epicardium compared to endocardium.
MANUAL VERSUS MAGNETIC NAVIGATION
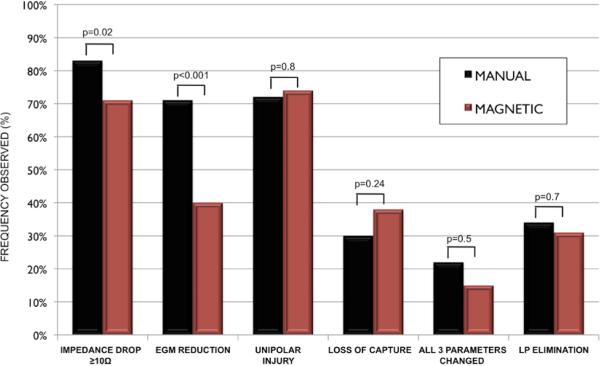
When comparing lesions delivered manually (n=281) and with remote magnetic navigation (n=91), an impedance drop occurred more frequently with manual application compared to magnetic (83% vs. 71%, p=0.02). An EGM reduction was also more frequently seen in manual lesions compared to magnetic (71% vs 40%, p<0.001), while loss of capture (30% vs 38%, p=0.24) was similar between manual and magnetic, respectively. There was no statistically significant difference between manual and magnetic navigation (22% vs 15%, p=0.5) for a change in all 3 standard parameters.
UIC was seen similarly with manual lesions compared to magnetic lesions (72% vs 74%, p=0.8). Among RF applications targeting LPs, no difference in the ability to completely eliminate LP was seen between manual and magnetic delivery (34% (35/102) vs. 31% (17/55), p=0.7). (FIGURE 6)
FIGURE 6.
Frequency of achieving targeted biophysical parameters after RF delivery manually compared to with magnetic navigation.
DISCUSSION
The major findings of the present study for patients undergoing VT ablation are: 1) The efficiency of ablation is suboptimal as the majority of RF applications do not achieve more than one targeted biophysical parameter; 2) Only one-third of RF applications targeting LPs resulted in complete elimination; 3) Epicardial ablation lesions demonstrate targeted biophysical parameters more frequently than endocardial lesions; 4) Lesions applied manually demonstrated targeted biophysical parameters more frequently than lesions applied using magnetic navigation. To our knowledge, this is the first systematic quantification of biophysical parameters in a real-world study of scar-mediated VT ablation. The majority of known biophysical changes during ablation from studies performed within normal myocardium, whereas RF applications were delivered in low voltage regions in the present study.
Reduction in EGM amplitude and tissue impedance are standard surrogates for RF lesion formation. As direct tissue temperature cannot be recorded with current technology, the catheter-tissue interface temperature has been used as an indirect measure of tissue heating.9 However, with the advent of irrigated catheter technology, tip temperatures readings are less useful due to large variability between surface and deep tissue temperature.10, 11 Loss of tissue capture has been shown to be an alternate marker of delineating electrically unexcitable myocardium or scar before and after ablation.12 The present study shows that achieving electrically unexcitable tissue from ablation is relatively uncommon but potentially the most specific marker of adequate RF delivery.
To our knowledge, this is the first comparison of biophysical ablation parameters between manual and magnetic navigation as well as epicardial and endocardial RF delivery. The clinical utility of magnetic navigation for VT ablation has been demonstrated in several reports.13-15 Comparison of clinical outcomes has not demonstrated any differences between the approaches across various substrates.16, 17 Our data suggests that lower contact force generated with magnetic navigation technology results in fewer reductions in electrogram amplitude and impedance compared to manual, although a similar incidence of a change in 3 parameters and loss of capture was observed. Further studies are needed to examine whether the diminished impedance changes are due to more inefficient ablation or as an artifact from lower contact force with more constant contact achieved with magnetic technology.
Epicardial ablation has become an important adjunctive technique for patients with failed endocardial ablation and as a primary approach in patients with NICM, HCM, ARVD, and Chagas disease, which tend to have a prominent epicardial substrate.18-21 Importantly, the present data suggests that ablation from the epicardium may be more efficient than from the endocardium, with epicardial lesions exhibiting an impedance reduction and loss of capture more frequently than endocardial lesions. Sacher et al demonstrated larger epicardial lesion size compared to endocardial ablation in an ovine model with contact force sensing technology.22 In that study, which used indirect markers including tactile feedback, fluoroscopy, and electrogram amplitude, a fifth of endocardial lesions did not result in lesion formation.
As the pericardial space contains physiologic fluid rather than a circulating blood pool, the milieu for the ablation is distinctively different from the endocardium. Stable parallel catheter contact may be promoted by the constraints of the pericardial space and lack of interfering structures, such as mitral valve leaflets, chordae tendinae, papillary muscles, trabeculations, and purkinje networks that are encountered with an endocardial approach. However, epicardial fat may insulate myocardium and limit the depth of RF delivery.23, 24 Temperature limit settings employed on the epicardium are more liberal than the endocardium, as char formation or development of a steam pop may have less catastrophic consequences in the pericardial space compared to the endocardial cavity. The extent to which the differences seen in the present study are attributable to a 4W difference in power settings, catheter orientation, and/or contact warrants further investigation.
CLINICAL SIGNIFICANCE
Procedural failures may be largely attributed to biophysical issues (poor contact or regions inaccessible for power delivery). Potential causes of ineffective RF application include inadequate catheter-tissue contact, insufficient power delivery into myocardium, or an intervening barrier between catheter and tissue, i.e., laminated thrombus, calcification, or fat. The relatively low success rate of VT ablation relative to surgical subendocardial resection25, 26 is likely due to the inability to created deep, extensive, and durable lesions. As transmural lesions have been proposed to optimize AF ablation results, the lesion formation in a thicker, fibrosed, and more trabeculated ventricle remains a challenge. Complete scar homogenization may not be achievable with current ablation technology. Alternative energy and ablative sources (HIFU, laser, alcohol injection, needle RF, microwave) are currently being evaluated for more complete and extensive tissue destruction.27-32
Contact force sensing has the ability to minimize energy delivery in regions of suboptimal contact, although this technology is unlikely to be widely implemented due to cost and access. A recent correlative study demonstrated a relationship between initial impedance drop with increasing contact force.33 While sufficient contact force is one part of the equation, it cannot assure or serve as an indicator of adequate power delivery into ventricular scar tissue.34, 35
Until tissue thermography and real-time MRI 36, 37 fully evolve, it is likely that a combination of indirect parameters will be required to assess the efficacy of an RF application. The integration of multiple biophysical parameter changes into electroanatomic mapping displays at ablation sites has the potential to improve acute and long-term clinical outcomes. At present, ablation tags are “all created equal” and there is no quick method to discern an effective ablation site from an ineffective site. Ablation tags systematically color-coded based on the number of parameter changes observed (Visitag, Biosense Webster, Diamond Bar, CA) may be helpful in directing further ablation toward areas where RF applications likely remain ineffective. Ablation for longer duration, higher power and temperature settings, or from across the wall may be more justifiable in regions with fewer parameter changes. Correlative studies with animal necropsy, real-time MRI, and contact force sensing technology are necessary to establish the optimal combination of parameters, and clinical studies are required to determine if VT ablation using this information results in improved freedom from recurrence.
LIMITATIONS
The current patient population analyzed contains a mixture of ICM and NICM substrates and the sample size is small, limiting the ability to interpret the data for specific etiologies. The nature and extent of fibrosis is likely to have an impact on energy delivery into the tissue and a larger study would allow for analysis between various scar substrates. The major limitation of the present study is the lack of a gold standard for effective RF lesion formation, which reiterates and highlights the scope of the clinical problem. Direct visualization and histologic examination of the lesions with pathologic analysis would be the ideal to confirm adequate ablation, although this is not clinically feasible. Additionally, imaging with magnetic resonance currently lacks the necessary resolution and registration accuracy to show lesions, and many of the patients have ICDs. In this study, we use the achievement of three of the most commonly employed parameters in clinical practice as the most ideal gold standard, recognizing that all three may not be required for an effective lesion. For this reason, analysis of the data using a two-parameter change was also performed. As interventional MR-guided ablation continues to evolve, studies correlating these biophysical surrogate parameters with transmural lesions will provide additional insight.
An additional limitation of the present study is that data was not collected for every RF application delivered in patients, which introduces the potential for bias. The presence of UIC was recorded and analyzed in real-time, which added time to the procedure. Data of every RF application was not obtained in cases primarily due to prolonged procedural duration as a practical measure. The censoring of lesions where poor contact was suspected would be expected to provide a “best-case” representation. This, however, represents a small (<5%) proportion of RF applications as ablation is typically not performed until a stable catheter position on electroanatomic mapping and fluoroscopy is demonstrated at our institution. Although we do not have specific patient or clinical characteristics that indicate manual versus magnetic navigation, selection bias is possible in patient selection.
As contact force sensing was not available for clinical use at the time of the study, the correlation between all biophysical parameters recorded and contact force is not addressable in the current study. Comparative animal studies will be helpful to further clarify this relationship. Finally, we do not correlate biophysical parameters with clinical outcomes in this acute study. Since there may be tremendous heterogeneity in lesion effectiveness within the same patient, it would be challenging to determine which RF application accounted for clinical success or noninducibility. Multiple effective lesions could be delivered in a region not critical to reentry and, conversely, a single effective lesion amongst multiple ineffective lesions may render a patient free of VT recurrence.
CONCLUSION
Real-world ablation of scar-mediated VT is inefficient as the majority of RF applications fail to achieve more than one targeted biophysical parameter. Only one-third of RF applications targeted at LPs result in complete elimination. These findings may be an important contributing factor to procedural failure, incomplete substrate modification, and recurrence of VT. Epicardial ablation within scar may be more effective than endocardial lesions and leions applied manually may be more effective than lesions applied using magnetic navigation. New technologies directed at optimizing ablation effectiveness are warranted to improve clinical outcomes.
Acknowledgments
Supported by the NHLBI (R01HL084261) to K. Shivkumar.
List of abbreviations
- VT
ventricular tachycardia
- UIC
unipolar injury current
- EGM
electrogram
- RF
radiofrequency
- ICM
ischemic cardiomyopathy
- NICM
nonischemic cardiomyopathy
- LP
late potential
Footnotes
Dr. Shivkumar is an unpaid scientific advisor to Topera and is a corporate board member of BioSig. UCLA has received EP fellowship support from Biosense Webster, St. Jude Medical, Medtronic and Boston Scientific. Other authors: No disclosures.
REFERENCES
- 1.Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini G, Riva S, Moltrasio M, Cireddu M, Veglia F, Della Bella P. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: Short- and long-term outcomes in a prospective single-center study. Circulation. 2008;117:462–469. doi: 10.1161/CIRCULATIONAHA.106.686534. [DOI] [PubMed] [Google Scholar]
- 2.Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, Kralovec S, Sediva L, Ruskin JN, Josephson ME. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–2665. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacretaz E, Pitschner HF, Kautzner J, Schumacher B, Hansen PS. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (vtach): A multicentre randomised controlled trial. Lancet. 2010;375:31–40. doi: 10.1016/S0140-6736(09)61755-4. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson WG, Wilber DJ, Natale A, Jackman WM, Marchlinski FE, Talbert T, Gonzalez MD, Worley SJ, Daoud EG, Hwang C, Schuger C, Bump TE, Jazayeri M, Tomassoni GF, Kopelman HA, Soejima K, Nakagawa H. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: The multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 5.Tanner H, Hindricks G, Volkmer M, Furniss S, Kuhlkamp V, Lacroix D, C DEC, Almendral J, Caponi D, Kuck KH, Kottkamp H. Catheter ablation of recurrent scar-related ventricular tachycardia using electroanatomical mapping and irrigated ablation technology: Results of the prospective multicenter euro-vt-study. J Cardiovasc Electrophysiol. 2010;21:47–53. doi: 10.1111/j.1540-8167.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 6.Saxonhouse SJ, Conti JB, Curtis AB. Current of injury predicts adequate active lead fixation in permanent pacemaker/defibrillation leads. J Am Coll Cardiol. 2005;45:412–417. doi: 10.1016/j.jacc.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 7.Tung R, Nakahara S, Maccabelli G, Buch E, Wiener I, Boyle NG, Carbucicchio C, Bella PD, Shivkumar K. Ultra high-density multipolar mapping with double ventricular access: A novel technique for ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 2011;22:49–56. doi: 10.1111/j.1540-8167.2010.01859.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakahara S, Tung R, Ramirez RJ, Michowitz Y, Vaseghi M, Buch E, Gima J, Wiener I, Mahajan A, Boyle NG, Shivkumar K. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55:2355–2365. doi: 10.1016/j.jacc.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haines DE. The biophysics of radiofrequency catheter ablation in the heart: The importance of temperature monitoring. Pacing Clin Electrophysiol. 1993;16:586–591. doi: 10.1111/j.1540-8159.1993.tb01630.x. [DOI] [PubMed] [Google Scholar]
- 10.Bruce GK, Bunch TJ, Milton MA, Sarabanda A, Johnson SB, Packer DL. Discrepancies between catheter tip and tissue temperature in cooled-tip ablation: Relevance to guiding left atrial ablation. Circulation. 2005;112:954–960. doi: 10.1161/CIRCULATIONAHA.104.492439. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa H, Yamanashi WS, Pitha JV, Arruda M, Wang X, Ohtomo K, Beckman KJ, McClelland JH, Lazzara R, Jackman WM. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation. 1995;91:2264–2273. doi: 10.1161/01.cir.91.8.2264. [DOI] [PubMed] [Google Scholar]
- 12.Soejima K, Stevenson WG, Maisel WH, Sapp JL, Epstein LM. Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: Feasibility for guiding ventricular tachycardia ablation. Circulation. 2002;106:1678–1683. doi: 10.1161/01.cir.0000030187.39852.a7. [DOI] [PubMed] [Google Scholar]
- 13.Arya A, Eitel C, Bollmann A, Wetzel U, Sommer P, Gaspar T, Husser D, Piorkowski C, Hindricks G. Catheter ablation of scar-related ventricular tachycardia in patients with electrical storm using remote magnetic catheter navigation. Pacing Clin Electrophysiol. 2010;33:1312–1318. doi: 10.1111/j.1540-8159.2010.02818.x. [DOI] [PubMed] [Google Scholar]
- 14.Aryana A, d'Avila A, Heist EK, Mela T, Singh JP, Ruskin JN, Reddy VY. Remote magnetic navigation to guide endocardial and epicardial catheter mapping of scar-related ventricular tachycardia. Circulation. 2007;115:1191–1200. doi: 10.1161/CIRCULATIONAHA.106.672162. [DOI] [PubMed] [Google Scholar]
- 15.Di Biase L, Burkhardt JD, Lakkireddy D, Pillarisetti J, Baryun EN, Biria M, Horton R, Sanchez J, Gallinghouse GJ, Bailey S, Beheiry S, Hongo R, Hao S, Tomassoni G, Natale A. Mapping and ablation of ventricular arrhythmias with magnetic navigation: Comparison between 4- and 8-mm catheter tips. J Interv Card Electrophysiol. 2009;26:133–137. doi: 10.1007/s10840-009-9416-5. [DOI] [PubMed] [Google Scholar]
- 16.Bradfield J, Tung R, Mandapati R, Boyle NG, Shivkumar K. Catheter ablation utilizing remote magnetic navigation: A review of applications and outcomes. Pacing Clin Electrophysiol. 2012;35:1021–1034. doi: 10.1111/j.1540-8159.2012.03382.x. [DOI] [PubMed] [Google Scholar]
- 17.Akca F, Onsesveren I, Jordaens L, Szili-Torok T. Safety and efficacy of the remote magnetic navigation for ablation of ventricular tachycardias--a systematic review. J Interv Card Electrophysiol. 2012;34:65–71. doi: 10.1007/s10840-011-9645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, Riley M, Cooper J, Dixit S, Gerstenfeld E, Callans D, Marchlinski FE. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 19.d'Avila A. Epicardial catheter ablation of ventricular tachycardia. Heart Rhythm. 2008;5:S73–75. doi: 10.1016/j.hrthm.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Bai R, Di Biase L, Shivkumar K, Mohanty P, Tung R, Santangeli P, Saenz LC, Vacca M, Verma A, Khaykin Y, Mohanty S, Burkhardt JD, Hongo R, Beheiry S, Dello Russo A, Casella M, Pelargonio G, Santarelli P, Sanchez J, Tondo C, Natale A. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: Arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 4:478–485. doi: 10.1161/CIRCEP.111.963066. [DOI] [PubMed] [Google Scholar]
- 21.Santangeli P, Di Biase L, Lakkireddy D, Burkhardt JD, Pillarisetti J, Michowitz Y, Sanchez JE, Horton R, Mohanty P, Gallinghouse GJ, Dello Russo A, Casella M, Pelargonio G, Santarelli P, Verma A, Narasimhan C, Shivkumar K, Natale A. Radiofrequency catheter ablation of ventricular arrhythmias in patients with hypertrophic cardiomyopathy: Safety and feasibility. Heart Rhythm. 2010;7:1036–1042. doi: 10.1016/j.hrthm.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Sacher F, Wright M, Derval N, Denis A, Ramoul K, Roten L, Pascale P, Bordachar P, Ritter P, Hocini M, Dos Santos P, Haissaguerre M, Jais P. Endocardial versus epicardial ventricular radiofrequency ablation: Utility of in vivo contact force assessment. Circ Arrhythm Electrophysiol. 2013;6:144–150. doi: 10.1161/CIRCEP.111.974501. [DOI] [PubMed] [Google Scholar]
- 23.Tung R, Nakahara S, Ramirez R, Lai C, Fishbein MC, Shivkumar K. Distinguishing epicardial fat from scar: Analysis of electrograms using high-density electroanatomic mapping in a novel porcine infarct model. Heart Rhythm. 2010;7:389–395. doi: 10.1016/j.hrthm.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desjardins B, Morady F, Bogun F. Effect of epicardial fat on electroanatomical mapping and epicardial catheter ablation. J Am Coll Cardiol. 2010;56:1320–1327. doi: 10.1016/j.jacc.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 25.Josephson ME. Electrophysiology of ventricular tachycardia: An historical perspective. J Cardiovasc Electrophysiol. 2003;14:1134–1148. doi: 10.1046/j.1540-8167.2003.03322.x. [DOI] [PubMed] [Google Scholar]
- 26.Harken AH, Josephson ME, Horowitz LN. Surgical endocardial resection for the treatment of malignant ventricular tachycardia. Annals of surgery. 1979;190:456–460. doi: 10.1097/00000658-197910000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiagalingam A, Pouliopoulos J, Barry MA, Boyd AC, Eipper V, Yung T, Ross DL, Kovoor P. Cooled needle catheter ablation creates deeper and wider lesions than irrigated tip catheter ablation. J Cardiovasc Electrophysiol. 2005;16:508–515. doi: 10.1046/j.1540-8167.2005.40540.x. [DOI] [PubMed] [Google Scholar]
- 28.Sacher F, Sobieszczyk P, Tedrow U, Eisenhauer AC, Field ME, Selwyn A, Raymond JM, Koplan B, Epstein LM, Stevenson WG. Transcoronary ethanol ventricular tachycardia ablation in the modern electrophysiology era. Heart Rhythm. 2008;5:62–68. doi: 10.1016/j.hrthm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Tokuda M, Sobieszczyk P, Eisenhauer AC, Kojodjojo P, Inada K, Koplan BA, Michaud GF, John RM, Epstein LM, Sacher F, Stevenson WG, Tedrow UB. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: An update. Circ Arrhythm Electrophysiol. 2011;4:889–896. doi: 10.1161/CIRCEP.111.966283. [DOI] [PubMed] [Google Scholar]
- 30.Laughner JI, Sulkin MS, Wu Z, Deng CX, Efimov IR. Three potential mechanisms for failure of hifu ablation in cardiac tissue. Circ Arrhythm Electrophysiol. 2012;5:409–416. doi: 10.1161/CIRCEP.111.967216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heist EK, Barrett C, Perna F, Danik S, Ruskin JN, Mansour M. Direct visualization of epicardial structures and ablation utilizing a visually guided laser balloon catheter: Preliminary findings. J Cardiovasc Electrophysiol. 2011;22:808–812. doi: 10.1111/j.1540-8167.2010.02004.x. [DOI] [PubMed] [Google Scholar]
- 32.von Bary C, Mazzitelli D, Voss B, Kubler F, Schmeller ML, Ndrepepa G, Zrenner B. Evaluation of epicardial microwave lesions in the pig model using an electroanatomic mapping system. J Interv Card Electrophysiol. 2008;22:5–11. doi: 10.1007/s10840-008-9241-2. [DOI] [PubMed] [Google Scholar]
- 33.Reichlin T, Knecht S, Lane C, Kuhne M, Nof E, Chopra N, Tadros TM, Reddy VY, Schaer B, John RM, Osswald S, Stevenson WG, Sticherling C, Michaud GF. Initial impedance decrease as an indicator of good catheter contact: Insights from radiofrequency ablation with force sensing catheters. Heart Rhythm. 2014;11:194–201. doi: 10.1016/j.hrthm.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Kuck KH, Reddy VY, Schmidt B, Natale A, Neuzil P, Saoudi N, Kautzner J, Herrera C, Hindricks G, Jais P, Nakagawa H, Lambert H, Shah DC. A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm. 2012;9:18–23. doi: 10.1016/j.hrthm.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Thiagalingam A, D'Avila A, Foley L, Guerrero JL, Lambert H, Leo G, Ruskin JN, Reddy VY. Importance of catheter contact force during irrigated radiofrequency ablation: Evaluation in a porcine ex vivo model using a force-sensing catheter. J Cardiovasc Electrophysiol. 2010;21:806–811. doi: 10.1111/j.1540-8167.2009.01693.x. [DOI] [PubMed] [Google Scholar]
- 36.Halperin HR, Nazarian S. Damage assessment after ablation role of cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;52:1272–1273. doi: 10.1016/j.jacc.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Nazarian S, Kolandaivelu A, Zviman MM, Meininger GR, Kato R, Susil RC, Roguin A, Dickfeld TL, Ashikaga H, Calkins H, Berger RD, Bluemke DA, Lardo AC, Halperin HR. Feasibility of real-time magnetic resonance imaging for catheter guidance in electrophysiology studies. Circulation. 2008;118:223–229. doi: 10.1161/CIRCULATIONAHA.107.742452. [DOI] [PMC free article] [PubMed] [Google Scholar]