Abstract
Different with other fishes, the guppies (Poecilia reticulata) is ovoviviparity, which retain their fertilized eggs within the follicle throughout gestation. The synchronously growing diplotene oocytes store nutrients in droplets and yolk, before their maturation and fertilization. The lecithotrophic strategy of development entails the provisioning of embryos with resources from the maternal yolk deposit rather than from a placenta, it allows the extracorporeal culture of guppy embryo. Studies on their early development of live bearers like the guppy including lineage tracing and genetic manipulations, have been limited. Therefore, to optimize conditions of embryo in vitro culture, explanted embryos from pregnant females were incubated in embryo medium (L-15 medium, supplemented with 5, 10, 15, 20% fetal bovine serum, respectively). We investigated whether the contents of FBS in vitro culture medium impact the development of embryos, and whether they would hatch in vitro. Our study found that in 5% of FBS of the medium, although embryos developed significantly slower in vitro than in the ovary, it was impossible to exactly quantify the developmental delay in culture, due to the obvious spread in developmental stage within each batch of eggs, and embryos can only be maintained until the early-eyed. And although in culture with 20% FBS the embryos can sustain rapid development of early stage, but cannot be cultured for the entire period of their embryonic development and ultimately died. In the medium with 10% and 15% FBS, the embryos seems well developed, even some can continue to grow after follicle ruptures until it can be fed. We also observed that embryonic in these two culture conditions were significantly different in development speed, in 15% it is faster than 10%. But 10% FBS appears to be more optimizing condition than 15% one on development process of embryos and survival rate to larvae stage.
Keywords: Poecilia reticulate, In vitro embryo culture, Ovoviviparous fish
INTRODUCTION
Small aquarium fish, such as the Zebrafish, Medaka and Guppy, are popular model organisms for study of vertebrate development and toxicology (Powers, 1989; Rossant & Hopkins, 1992). And, the guppy (Poecilia reticulata) has become a model organism for behavioral traits such as courtship and mate choice as well as genetics and breeding studies (Evans et al., 2003; Magurran & Henderson, 2003). Guppy was one of the first vertebrates in which sex-linked inheritance of color loci was demonstrated (Winge, 1927). The rich variations in adult color patterns of male guppies have attracted the attention of geneticists and ecologists for almost a century.
Different with other fishes, guppies are ovoviviparity, which retain their fertilized eggs within the follicle throughout gestation (Turner, 1940; Lambert, 1970). The synchronously growing diplotene oocytes store nutrients in oil droplets and yolk, before their maturation and fertilization. In guppies, the interface between the embryonic yolk portal system and the maternal follicle allows for efficient gas exchange and waste disposal, while maternal food provisioning does not seem to be required after fertilization (Turner, 1940; Thibault & Schultz, 1978).
The study of development and differentiation is most advanced in those organisms of the fish kingdom that allow the application of a broad spectrum of experimental tools. Unfortunately, studying the early development of live bearers is more complicated than that of oviparous species because of the inaccessibility of developing embryos for experimental manipulation. Therefore, despite a wealth of classic genetic studies on the male color polymorphism found in guppies, knowledge of both the ontogeny is virtually nonexistent. Observations on embryos during early gestation require the optimizing condition for in vitro embryo culture, which allow the continuous observation of early development.
The lecithotrophic strategy of development entails the provisioning of embryos with resources from the maternal yolk deposit rather than from a placenta (Reznick et al., 2002). It allows the extracorporal culture of guppy embryos. Ulrike et al. (2006) showed that guppy embryos can continue development in culture. But the embryos could not be cultured for the entire period of their embryonic development. They also mentioned that some individuals survived in vitro for longer than the normal gestational period suggests that survival in vitro is not the limiting factor. To optimize in vitro culture of guppy embryos, we established culture conditions according to the concentration of fetal bovine serum in the medium impacting on the embryonic development.
MATERIALS AND METHODS
1. Fish culture and observation
Guppies were maintained at 25°C in a 14-hr light and 10-hr dark cycle. The following strains (Full Red) were used: guppies from an aquarium (our lab). Embryos were staged according to Haynes (1995) and observed on a Nikon optical microscope (type104) connected to camera.
2. Explantation
Adult female guppies were anaesthetized in 0.035% MS-222 (Ethyl 3-aminobenzoate methanesulfonate) and decapitated. Using scissors and fine forceps, the peritoneum was opened from the abdomen, and then ovaries were explanted under a dissecting microscope and transferred into 90% phosphate buffered saline (PBS). The ovarian tissue was removed to isolate individual embryos.
3. Long-term in vitro culture of guppy embryos under different conditions
For in vitro culture, up to 15 embryos were incubated in 8 ml of sterile embryo medium (L-15 [Leibovitz] medium [GIBCO, cat. no. 11415-064], supplemented with 5, 10, 15, and 20% fetal bovine serum (Equitech-Bio, SFBU-0500) respectively, 20 units/mL penicillin, and 200 mg/mL streptomycin) in a dark incubator at 25°C. Remaining follicles detached from the embryos within the first 24 hr of culture and were removed. Embryos were inspected daily and medium was changed twice a week.
RESULTS
1. Survey of embryonic development
Fig. 1 shows embryos explanted at blastodisc stage were cultured. During stage 3, the mature ovum contains oil droplets that are evenly distributed over the yolk surface. After fertilization, the oil droplets coalesce underneath the embryo proper, which forms a blastdisc (stage 4; Fig. 1A). During stage 5 (Fig. 1B), the primitive streak is seen on top of the optic cup. During the early-eyed period, pigmentation of the eye, including the choroid, gradually increases, the pectoral fin buds emerge, and somatic as well as non-somatic muscles differentiate. Classification of the subsequent middle-eyed, late-eyed, and very late eyed stages (stages 8–10; Fig. 1C–E) is based on further differentiation of the eyes, which parallels an increase in the skin pigmentation of the head and trunk. The middle-eyed embryo has fully pigmented eyes (Fig. 1C), whereas, in the late-eyed embryo eyes (Fig. 1D), the choroid covers most of the retina, and rays of the dorsal, ventral, and caudal fins have differentiated. The myotome consists of approximately 30 somites (Fig. 1D). Simultaneous with the disappearance of the yolk, the almost rectangular flexure between the head and the trunk is gradually straightened (Fig. 1E). The mature embryo has resorbed completely its yolk and retracted the yolk sac. Finally, the follicle ruptures before birth (stage 11; Fig. 1F).
Fig. 1.
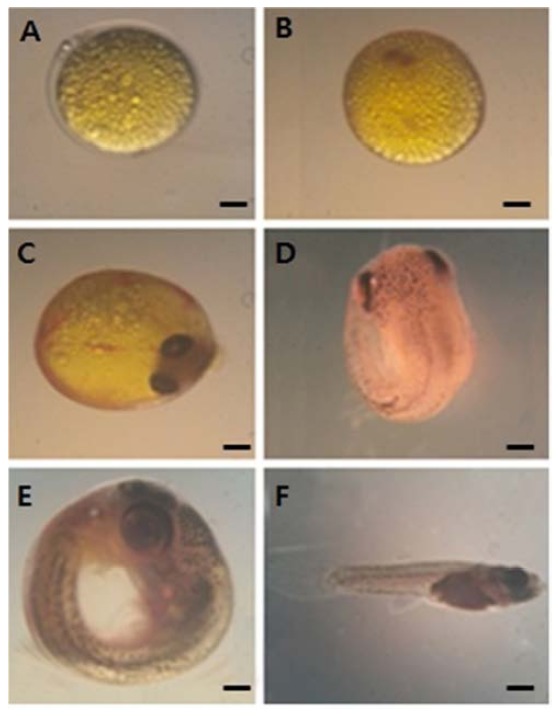
Embryonic development in vitro. A-F: Embryos explanted at blastodisc stage (A) were cultured as described in the Experimental Procedures section and photographed. A: Blastodisc. B: Optic cup. C: Middle eyed embryo. D: Late-eyed embryo. E: Very late-eyed embryo. F: Mature embryo. Scale bar = 500 μm in A–E, 1mm in F, respectively.
2. Long-term in vitro culture of guppy embryos
After showing that guppy embryos can continue development in culture (Fig. 1), we investigated whether the contents of FBS in vitro culture medium impact the development of embryos, and whether they would hatch in vitro. Our study found that in 5% of FBS of the medium, although embryos developed significantly slower in vitro than in the ovary, it was impossible to exactly quantify the developmental delay in culture, due to the obvious spread in developmental stage within each batch of eggs, and embryos can only be maintained until the early-eyed. In the medium with 10% and 15% FBS, the embryos seems well developed, Even some can continue to grow after follicle ruptures until it can be fed. We also observed that embryonic in these two culture conditions were significantly different in development speed, in 15% it is faster than 10% as shown in Fig. 2. Although in culture with 20% FBS the embryos can sustain rapid development of early stage, but cannot be cultured for the entire period of their embryonic development and ultimately died. Even in those individuals who can be fed with food, the eventually survived life after fed is not more than one month.
Fig. 2.
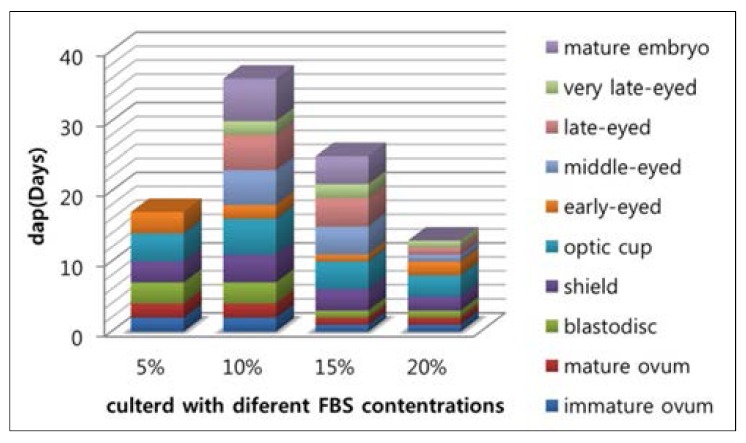
Daily changes of embryos in vitro in the different condition of FBS of L-15 medium.
DISCUSSION
Previously, Tavolga (1949) has published a more detailed description of platyfish (Platypoecilus) and swordtail (Xiphophorus hellerii) embryogenesis. And Haynes (1995) has described the embryonic development of Poeciliids using Specific developmental stages derived from Gambusia (Haynes, 1995). We briefly describe guppy embryonic development. We investigated whether the contents of FBS in vitro culture medium impact the development of embryos, and whether they would hatch in vitro. And we preliminarily staged them according to Haynes (1995), based on external criteria. Our results show that embryos explanted at blastodisc stage were cultured. And after fertilization, the oil droplets coalesce underneath the embryo proper, which forms a blastdisc. During the early-eyed period, pigmentation of the eye, including the choroid, gradually increases, the pectoral fin buds emerge, and somatic as well as non-somatic muscles differentiate. Classification of the subsequent middle-eyed, late-eyed, and very late eyed stages is based on further differentiation of the eyes, which parallels an increase in the skin pigmentation of the head and trunk. The middle-eyed embryo has fully pigmented eyes, whereas, in the late-eyed embryo eyes, The mature embryo has resorbed completely its yolk and retracted the yolk sac. Finally, the follicle ruptures before birth. These results showed similar developing patterns as like Ulrike et al. (2006), who showed that guppy embryos can continue development in culture in the culture medium (L-15 medium, 15%).
The lecithotrophic strategy of development entails the provisioning of embryos with resources from the maternal yolk deposit rather than from a placenta (Reznick et al., 2002). It allows the extracorporal culture of guppy embryos. After showing that guppy embryos can continue development in culture, we found that in the medium with 10%, embryos seems well developed. That some individuals which survived in vitro for longer than the normal gestation period suggests that survival in vitro is not the limiting factor. Although guppy embryos are considered as completely lecithotrophic (Thibaultand Schultz, 1978; Reznick et al., 2002), we cannot exclude the possibility that specific factors required for normal development are delivered by means of the maternal circulation and, therefore, that these would be lacking in vitro. Haas-Andela (1976) succeeded in rearing Xiphophorus embryos in vitro, starting at neurulation, and obtained fertile fish. Similar to guppies, Xiphophorus embryos develop more slowly in vitro than in the follicle, and often fail to retract the yolk sac. These findings suggest that retraction of the yolk sac may be most critical step of in vitro culture in both species and that it may require as yet unidentified low molecular weight factors from the mother, which could be contained in or substituted for by the aquarium water.
Our success in culturing guppy embryos in vitro demonstrated that some of the drawbacks of live bearing fish as objects of early developmental studies can be overcome. Ulrike et al. (2006) cultured guppy embryos with 15% L-15 medium, and can continue development. However, our results according to experimental data showed, both 10% and 15% FBS in medium can be used for in vitro culture, but the development in 10% FBS appears to be more optimizing condition than 15% FBS on development process of embryos and survival rate to larvae stage (Table 1).
Table 1.
The survival ratio of embryos in vitro culture with different FBS concentration
| Embryonic |
No. of survival |
|||
|---|---|---|---|---|
| Culture medium | Blastodisc | Early-eyed | Very late-eyed | Larvae |
| L-15(5% FBS) | 30 | 0 | ||
| L-15(10% FBS) | 30 | 19 | 9 | 5 |
| L-15(15% FBS) | 30 | 17 | 7 | 3 |
| L-15(20% FBS) | 30 | 13 | 0 | |
No heartbeating or body-fluid exchanging embryos were considered as dead.
Extension of in vitro culture for the entire gestation period would allow for experimental procedures not normally possible in live bearers, including lineage tracing and genetic manipulations, such as RNA interference or application of morpholino oligonucleotides.
ACKNOWLEDGMENTS
This paper was supported by research funds (2013) of Kunsan National University. The authors thank to Coastal Research Center, Kunsan National University for supporting the research.
REFERENCES
- 1.Endler JA. Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vision Res. 1991;31:587–608. doi: 10.1016/0042-6989(91)90109-i. [DOI] [PubMed] [Google Scholar]
- 2.Endler JA. Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol Evol. 1995;10:22–29. doi: 10.1016/s0169-5347(00)88956-9. [DOI] [PubMed] [Google Scholar]
- 3.Evans JP, Zane L, Francescato S, Pilastro A. Directional postcopulatory sexual selection revealed by artificial insemination. Nature. 2003;421:360–363. doi: 10.1038/nature01367. [DOI] [PubMed] [Google Scholar]
- 4.Haas-Andela H. In vitro culture of embryos of the Xiphophorine fish and their raising to adulthood. Zool Anz Jena. 1976;197:1–5. [Google Scholar]
- 5.Lambert JGD. The ovary of the guppy Poecilia reticulata.The granulosa cells as sites of steroid biogenesis. Gen Comp Endocrinol. 1970;15:464–476. doi: 10.1016/0016-6480(70)90119-x. [DOI] [PubMed] [Google Scholar]
- 6.Magurran AE, Henderson PA. Explaining the excess of rare species in natural species abundance distributions. Nature. 2003;422:714–716. doi: 10.1038/nature01547. [DOI] [PubMed] [Google Scholar]
- 7.Powers DA. Fish as model systems. Science. 1989;246:352–357. doi: 10.1126/science.2678474. [DOI] [PubMed] [Google Scholar]
- 8.Reznick DN, Mateos M, Springer MS. Independent origins and rapid evolution of the placenta in the fish genus Poeciliopsis. Science. 2002;298:1018–1020. doi: 10.1126/science.1076018. [DOI] [PubMed] [Google Scholar]
- 9.Reznick DN. Life history evolution in guppies (Poecilia reticulata): guppies as a model for studying the evolutionary biology of aging. Exp Gerontol. 1997;32:245–258. doi: 10.1016/s0531-5565(96)00129-5. [DOI] [PubMed] [Google Scholar]
- 10.Rossant J, Hopkins N. Of fin and fur: mutational analysis of vertebrate embryonic development. Genes and Develop. 1992;6:1–13. doi: 10.1101/gad.6.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Thibault RB, Schultz RJ. Reproductive adaptations among viviparous fishes (Cyprinodontiformes: Poeciliidae) Evolution. 1978;32:320–333. doi: 10.1111/j.1558-5646.1978.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 12.Thibault RB, Schultz RJ. Reproductive adaptations among viviparous fishes (Cyprinodontiformes: Poeciliidae) Evolution. 1978;32:320–333. doi: 10.1111/j.1558-5646.1978.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 13.Turner CL. Pseudoamnion, pseudochorion, and follicular pseudoplacenta in poeciliid fishes. J Morphol. 1940;67:59–87. [Google Scholar]
- 14.Ulrike Martyn, Detlef Weigel, Christine Dreyer. In vitro culture of embryos of guppy, Poecilia reticulata. Developmental Dynamics. 2006;235:617–622. doi: 10.1002/dvdy.20664. [DOI] [PubMed] [Google Scholar]
- 15.Winge O¨. The location of eighteen genes in Lebistes reticulatus. J Genet. 1927;18:1–43. [Google Scholar]


