Abstract
Manganese (Mn2+) is a trace element that is essential for normal physiology, and is predominantly obtained from food. Several lines of evidence, however, demonstrated that overexposure to MnCl2 exerts serious neurotoxicity, immunotoxicity and developmental toxicity, particularly in male. The present study aimed to evaluate the effect of 0, 1.0, 3.3, and 10 mg/kg/day doses of MnCl2 on the reproductive organs in the immature female rats. Rats (PND 22; S.D. strain) were exposed to MnCl2 (MnCl2 ∙ 4H2O) dissolved in drinking water for 2 weeks. The animals were sacrificed on PND 35, then the tissues were immediately removed and weighed. Histological studies were performed using the uteri tissue samples. Serum LH and FSH levels were measured with the specific ELISA kits. Body weights of the experimental group animals were not significantly different from those of control group animals. However, ovarian tissue weights in 1 mg and 3.3 mg MnCl2 dose groups were significantly lower than those of control animals (p<0.05 and p<0.01, respectively). Uterine tissue weights of 3.3 mg dose MnCl2 groups were significantly lower than those of control animals (p<0.01), while the 1 mg MnCl2 dose and 10 mg MnCl2 dose failed to induce any change in uterine weight. Similarly, only 3.3 mg MnCl2 dose could induce the significant decrease in the oviduct weight compared to the control group (p<0.05). Non-reproductive tissues such as adrenal and kidney failed to respond to all doses of MnCl2 exposure. The uterine histology revealed that the MnCl2 exposure could affect the myometrial cell proliferation particularly in 3.3 mg dose and 10mg dose group. Serum FSH levels were significantly decreased in 1mg MnCl2 dose and 10 MnCl2 mg groups (p<0.05 and p<0.01, respectively). In contrast, treatment with 1 mg MnCl2 dose induced a significant increment of serum LH level (p<0.05). The present study demonstrated that MnCl2 exposure is capable of inducing abnormal development of reproductive tissues, at least to some extent, and altered gonadotropin secretions in immature female rats. Combined with the well-defined actions of this metal on GnRH and prolactin secretion, one can suggest the Mn2+ might be a potential environmental mediator which is involved in the female pubertal process.
Keywords: Manganese (Mn2+), Immature female rats, Ovary, Uterus, Gonadotropins, Pubertal process
INTRODUCTION
Manganese (Mn2+) is a trace metal and is essential element that is required for normal mammalian physiology. Several enzyme systems have been reported to interact with or depend on Mn2+ for their optimal catalytic or regulatory function (Gunter et al., 2006). On the other hand, excessive exposure to Mn2+ seems to cause serious neurotoxicity, immunotoxicity and developmental toxicity, particularly in male (Michalke et al., 2007).
Concerning the reproductive toxicity of Mn2+, studies have been focused on the mammalian testicular dysfunction. Chronic exposure to Mn3O4 (1,050 ppm) retarded the sexual development shown significantly smaller testis, seminal vesicle, and preputial gland weights in mice (Gray & Laskey, 1980; Webster & Valois, 1987). In human, occupational exposure to Mn2+ decreased libido and impotency (Emara et al., 1971; Mena et al., 1967), and may result in lowered sperm count and semen quality (Hjollund et al., 1998).
So far, however, research on the effect of Mn2+ exposure on female reproductive physiology has been conducted poorly. The present study aimed to evaluate the effect of 0, 1.0, 3.3, and 10 mg/kg/day doses of MnCl2 on the reproductive organs in the immature female rats.
MATERIALS & METHODS
1. Animals
Timely pregnant Sprague-Dawley rats were obtained from DBL (Chungcheongbuk-do, Korea) and reared in sangmyung university animal facility under conditions of 12-h light/dark cycle (lights on at 07:00 h) and constant temperature of 22±1 °C. During pregnancy and lactation, the mothers had free access to normal chow and tap water. All procedures used were approved by the Animal Care and Use Committee at Sangmyung University.
2. Experimental design
The day after weaning (postnatal day 22, PND 22), female dams were randomly assigned to the following exposure groups (n=8 dams/group) from PND 22 until PND 35: (1) oral administration of 0.9% Saline (300 μℓ /100 g BW/day, Control group), (2) oral administration of 1.0 mg/kg BW/day MnCl2 ∙ 4H2O (Sigma-Aldrich), (3) oral administration of 3.3 mg/kg BW/day MnCl2 ∙ 4H2O, and (4) oral administration of 10 mg/kg BW/day MnCl2 ∙ 4H2O. Both saline and Mn2+ solution were supplied daily for 2 weeks. At PND 35, animals were sacrificed and the tissues(ovary, uterus, oviduct, adrenal, and kidney) were immediately removed and weighed at 1,800 hour.
3. Histology
Uterine tissue specimens were fixed 4% paraformaldehyde then were serially dehydrated in graded ethanol and xylene. Specimens were paraffin embedded and sectioned at 5 μm thickness. Sections were stained with Hematoxylin-Eosin (H & E) stain and examined under light microscope.
4. Measurement of serum gonadotropin levels
The trunk blood samples were collected and centrifuged at 3,000×g for 15 min. The measurements of the serum FSH and LH were carried out according to the commercial instructions for the specific ELISA kits (USCN, China). The sensitivities of the FSH and LH assay were 0.55 ng/ml and 122.5 pg/ml, respectively. The intra-assay and inter-assay coefficients of variation were <10% and <12% for both hormones, respectively.
5. Statistical analysis
All values are expressed as the means (±S.E.). Differences between control and treatment groups were analysed by Student’s t-test. P values less than 0.05 were considered significant. The IBM PC programs INSTAT and PRISM 3.0 (GraphPad, San Diego, CA, USA) were used to calculate and plot the results.
RESULTS
1. Tissue weights
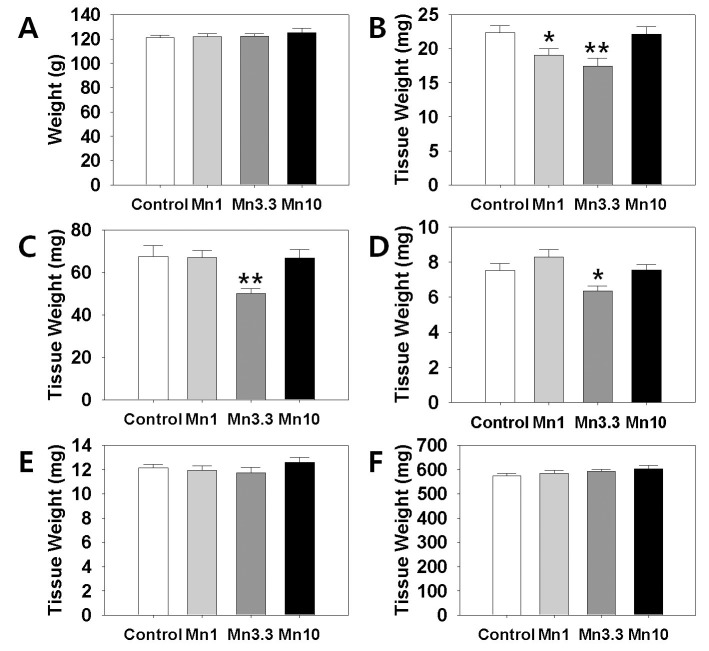
In order to evaluate a potential effect of Mn2+ on female reproductive organs, we measured the tissues weights of ovary, uterus and oviduct. Kidney and adrenal weight served as non-reproductive tissues. After 2 weeks of administration, body weights of the all Mn2+ exposure group animals were not significantly different from those of control group animals (Fig. 1, A). However, ovarian tissue weights (Fig. 1, B). in 1 mg MnCl2 dose group (19.06±0.98 mg, p<0.05) and 3.3 mg MnCl2 dose group (17.47±1.16 mg, p<0.01) were significantly lower than those of control animals (22.39±0.97 mg). Uterine tissue weights (Fig. 1, C). of 3.3 mg dose MnCl2 groups were significantly lower than those of control animals (Control : 3.3 mg dose MnCl2 = 67.52±5.15 : 50.04±2.41 mg, p<0.01), while the 1 mg MnCl2 dose (Control : 1 mg dose MnCl2=67.52±5.15 : 67.11±3.28 mg) and 10 mg MnCl2 dose (Control : 10 mg dose MnCl2 = 67.52±5.15 : 66.70±4.03 mg) failed to induce any change in uterine weight. Similarly, only 3.3 mg MnCl2 dose could induce the significant decrease in the oviduct weight (Fig. 1, D). compared to the control group (Control : 3.3 mg dose MnCl2 = 7.52±0.40 : 6.35±0.29 mg, p<0.05). Non-reproductive tissues such as adrenal (Control : 1 mg : 3.3 mg : 10 mg dose MnCl2 = 12.13±0.28 : 11.93±0.38 : 11.75±0.42 : 12.59±0.43 mg) and kidney (Control : 1 mg : 3.3 mg : 10 mg dose MnCl2 = 574.74±8.99 : 584.56±12.38 : 592.42±7.82 : 603.67±14.06 mg) failed to respond to all doses of MnCl2 exposure (Fig. 1, E & F, respectively).
Fig. 1.
Effect of MnCl2 exposure on changes in body weights and tissues weights. Animals were daily supplied 0.9% Saline (300 μℓ/100 g BW/day, Control) or MnCl2 ∙ 4H2O (1.0 mg/kg BW, 3.3 mg/kg BW, 10 mg/kg BW, respectively) for 2 weeks. A, body weight; B, ovary; C, uterus, D, oviduct, E, adrenal, and F, kidney. Values are expressed as mean±S.E. (n=8 per group). *, Significantly different from control group, p<0.05. **, Significantly different from control group, p<0.01.
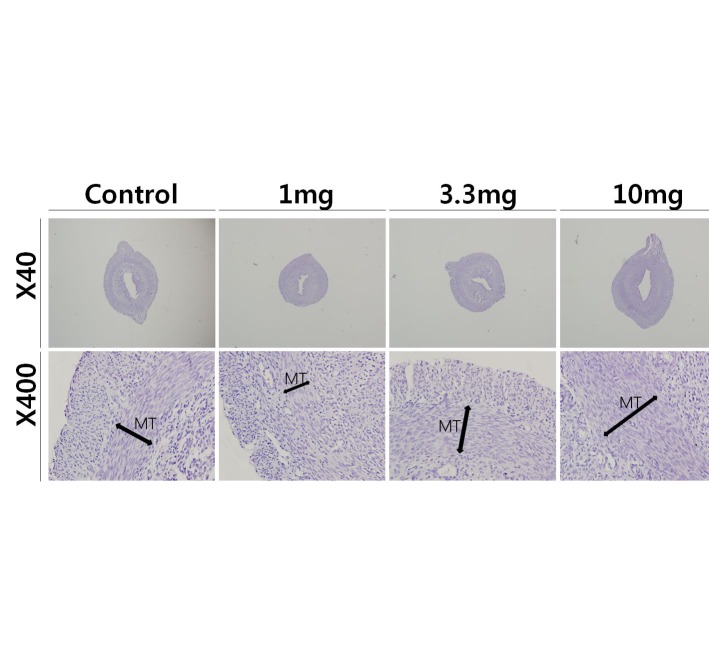
2. Uterine histology
To access the histological changes in MnCl2 exposured uteri, standard paraffin section and hematoxylin-eosin staining method were employed. 3.3 mg and 10 mg MnCl2 dose groups shown the thickend myometrial layer when compared to control (Fig. 2, Mn3.3 & Mn10). In contrast, 1 mg MnCl2 exposure reduced the thickness of myometrium (Fig. 2, Mn1).
Fig. 2.
Effect of MnCl2 exposure on histological changes in uteri from immature rats. Standard paraffin section and hematoxylin-eosin staining method were employed. Mn 1, 1.0 mg/kg BW/ day of MnCl2 ∙ 4H2O exposure; Mn 3.3, 3.3 mg/kg BW/day of MnCl2 ∙ 4H2O exposure; Mn 10, 10 mg/kg BW/day of MnCl2 ∙ 4H2O exposure, respectively.
3. ELISA
Serum LH and FSH levels were measured using specific ELISA kits. Fig. 3 (A) shows that the secretion of FSH was significantly decreased by treatment with 1 mg or 3.3 mg MnCl2 (Control:1 mg dose MnCl2 = 3.54±0.18 : 3.07±0.14 ng/ml, p<0.05; Control : 10 mg dose MnCl2 = 3.54±0.18:2.75±0.10 ng/ml, p<0.01). Serum LH level was significantly elevated by 1 mg MnCl2 exposure (Control : 1 mg dose MnCl2 = 39.85±8.93:76.71±12.36 ng/ml, p<0.05, Fig. 3, B). Higher doses of MnCl2 exposure failed to change the serum LH levels.
Fig. 3.
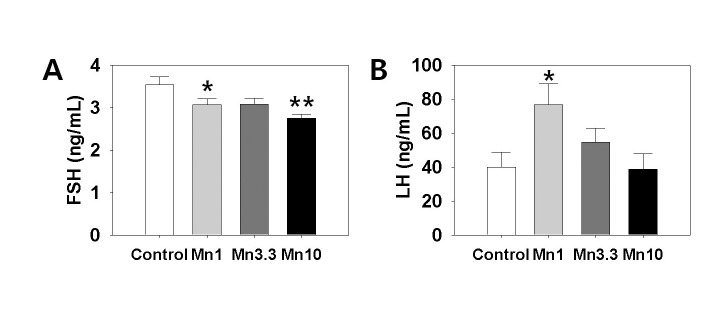
Measurement of serum FSH and LH levels in response to Mn2+ exposure. The trunk blood samples were collected and centrifuged at 3,000×g for 15 min. The measurements of the serum FSH and LH were carried out according to the commercial instructions for the specific ELISA kits (USCN, China). Values are expressed as mean±S.E. (n=6 per group). *, Significantly different from control group, p<0.05. **, Significantly different from control group, p<0.01.
DISCUSSION
In the present study we demonstrated that the Mn2+ exposure could change the weights of reproductive tissues in immature female rats. Although we failed to find the advance or delay of puberty onset in the Mn2+ exposured animals (data not shown), the potential reproductive toxicity of this metal in immature female rats cannot be ruled out. Mn2+ exposure, Indeed, not only affected the proliferative activity in myometrial layer, but induced significant changes in the serum FSH and LH levels.
More defined doses and exposure periods will be helpful to verify the physiological relevance of Mn2+ exposure in female reproduction.
Occupational exposure to Mn2+ could be occurred often at the workplaces such are mines and dried battery factories (Emara et al., 1971; Mena et al., 1967). In this respect, most studies on the toxicological effects of Mn2+ have been focused on the men. Furthermore, a relatively small portion of the studies dealt with reproductive toxicity of Mn2+ exposure using male animal models. Mn2+ exposure for 2 and 4 h inhibited rat primary Leydig cell steroidogenesis by decreasing StAR protein expression while 24 and 48 h exposure of MnCl2 caused adverse effects on both StAR protein and P450scc and 3b-HSD enzyme activity to reduce steroidogenesis (Cheng et al., 2003).
Pine et al. (2005) reported that Mn2+ administered acutely into the third ventricle shown dose-dependently to stimulate LH release in prepubertal female rat, and this effect was due to a Mn2+-induced stimulation of GnRH. The authors demonstrated that Mn2+ can stimulate specific puberty-related hormones and suggested that it may facilitate the normal onset of puberty. According to them, Mn2+ may contribute to precocious puberty if an individual is exposed to elevated levels of Mn2+ too early in developmental process. This hypothesis was verified by same research group; Lee et al. (2006) demonstrated that Mn2+ is a direct stimulator of prepubertal GnRH/LH secretion and may facilitate the normal onset of male puberty. Their data that Mn2+ can cause GnRH release in adult males, suggested this action should be considered in relation to age, gender, as well as mechanistic and functional differences between adult and immature animals.
In the present study we failed to confirm the advanced puberty onset in Mn2+ exposed female rats (up to 10 mg/kg BW, data not shown). Pine et al. (2005) shown that same dose of Mn2+ exposure initiated a moderate but significant advancement in age at vaginal opening (VO) in terms of days (1.5 days). The only difference between the two studies was timing and duration of Mn2+ exposure; Our treatment regimen was PND 21-35 (14 days), and theirs was PND 12-29 (18 days). Though Mn2+ can acutely induce GnRH secretion in adult males, one should consider that additional action of Mn2+ to release GABA, a GnRH inhibitor, may ultimately contribute to suppressed reproductive function observed in adult animals following exposure to high chromic levels of Mn2+ (Prestifilippo et al., 2008). Because neurotransmitter secretory circuits in female during peripubertal period are remarkably unstable, the extent of GABA GnRH regulation, and probably more neuronal regulation, may vary responding to the minor difference. Studies indicate the more specific action mechanism of Mn2+ within the hypothalamus; Mn2+ activates soluble guanylate cyclase (sGC) directly and/or as a cofactor with available nitric oxide (NO), hence generating cGMP and resulting in prepubertal GnRH release (Lee et al., 2007). More recently, Mn2+, through the upregulation of IGF-1 and COX-2, may promote maturational events and glialneuronal communications facilitating the increased neurosecretory activity, including that of GnRH, resulting in precocious pubertal development (Hiney et al., 2011).
Special emphasis should be made on the action of Mn2+ in brain. Inhalation of the mixture of MnCl2 and Mn(OAc)3 for 5 months developed movement abnormalities, significant loss of substantia nigra compacta (SNc) dopaminergic neurons; these symptoms similar to those observed in Parkinson disease (PD) (Ordoñez-Librado et al., 2011). Dopamine (DA) depletion is closely related to pituitary prolactin biosynthesis. Male rats exposed to Mn2+ for 4 or 13 weeks showed a progressive and significant decrease in hypothalamic DA, whereas prolactin and Pit-1 mRNA levels increased in response to Mn2+ exposure (Kim et al., 2009). These results suggest that exposure to Mn2+ decreases hypothalamic DA and promotes the production of prolactin in the pituitary and that Pit-1 might be a regulator of DA and prolactin. Furthermore, such Mn2+ exposure induced significant increase in serum prolactin levels seemed to be highly correlated with the testis toxicity (Lee, 2009). In this context, relationship between Mn2+ exposure and DA/prolactin secretions in immature female rats will be helpful to understand the function of the metal during pubertal development.
REFERENCES
- 1.Cheng J, Fu J-L, Zhou Z-C. The inhibitory effects of manganese on steroidogenesis in rat primary Leydig cells by disrupting steroidogenic acute regulatory (StAR) protein expression. Toxicology. 2003;187:139–148. doi: 10.1016/s0300-483x(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 2.Emara AM, el-Ghawabi SH, Madkour OI, el-Samra GH. Chronic manganese poisoning in the dry battery industry. Br J Ind Med. 1971;28:78–82. doi: 10.1136/oem.28.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray LE, Jr, Laskey JW. Multivariate analysis of the effects of manganese on the reproductive physiology and behavior of the male house mouse. J Toxicol Environ Health. 1980;6:861–867. doi: 10.1080/15287398009529904. [DOI] [PubMed] [Google Scholar]
- 4.Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27:765–776. doi: 10.1016/j.neuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Hiney JK, Srivastava VK, Dees WL. Manganese induces IGF-1 and cyclooxygenase-2 gene expressions in the basal hypothalamus during prepubertal female development. Toxicol Sci. 2011;121:389–396. doi: 10.1093/toxsci/kfr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hjollund NH, Bonde JP, Jensen TK, Ernst E, Henriksen TB, Kolstad HA, Giwercman A, Skakkebaek NE, Olsen J. Semen quality and sex hormones with reference to metal welding. Reprod Toxicol. 1998;12:91–95. doi: 10.1016/s0890-6238(97)00156-1. [DOI] [PubMed] [Google Scholar]
- 7.Kim HY, Lee CK, Lee JT, Moon CS, Ha SC, Kang SG, Kim DH, Kim HD, Ahn JH, Lee SB, Kang MG. Effects of manganese exposure on dopamine and prolactin production in rat. Neuroreport. 2009;20:69–73. doi: 10.1097/WNR.0b013e328315cd35. [DOI] [PubMed] [Google Scholar]
- 8.Lee B, Pine M, Johnson L, Rettori V, Hiney JK, Dees WL. Manganese acts centrally to activate reproductive hormone secretion and pubertal development in male rats. Reprod Toxicol. 2006;22:580–585. doi: 10.1016/j.reprotox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Lee B, Hiney JK, Pine MD, Srivastava VK, Dees WL. Manganese stimulates luteinizing hormone releasing hormone secretion in prepubertal female rats: hypothalamic site and mechanism of action. J Physiol. 2007;578(Pt 3):765–772. doi: 10.1113/jphysiol.2006.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CK. Effects of manganese exposure on the testis function and serum prolactin concentration in rat. Devel Reprod. 2009;13:321–327. [Google Scholar]
- 11.Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology. 1967;17:128–136. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- 12.Michalke B, Halbach S, Nischwitz V. Speciation and toxicological relevance of manganese in humans. J Environ Monit. 2007;9:650–656. doi: 10.1039/b704173j. [DOI] [PubMed] [Google Scholar]
- 13.Ordoñez-Librado JL, Anaya-Martínez V, Gutierrez-Valdez AL, Colín-Barenque L, Montiel-Flores E, Avila-Costa MR. Manganese inhalation as a Parkinson disease model. Parkinsons. Dis doi: 10.4061/2011/6129892011. [DOI] [PMC free article] [PubMed]
- 14.Pine M, Lee B, Dearth R, Hiney JK, Dees WL. Manganese acts centrally to stimulate luteinizing hormone secretion: a potential influence on female pubertal development. Toxicol Sci. 2005;85:880–885. doi: 10.1093/toxsci/kfi134. [DOI] [PubMed] [Google Scholar]
- 15.Prestifilippo JP, Fernández-Solari J, Mohn C, De Laurentiis A, McCann SM, Dees WL, Rettori V. Effect of manganese on luteinizing hormone-releasing hormone secretion in adult male rats. Toxicol Sci. 2007;97:75–80. doi: 10.1093/toxsci/kfm015. [DOI] [PubMed] [Google Scholar]
- 16.Prestifilippo JP, Fernández-Solari J, De Laurentiis A, Mohn CE, de la Cal C, Reynoso R, Dees WL, Rettori V. Acute effect of manganese on hypothalamic luteinizing hormone releasing hormone secretion in adult male rats: involvement of specific eurotransmitter systems. Toxicol Sci. 2008;105:295–302. doi: 10.1093/toxsci/kfn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webster WS, Valois AA. Reproductive toxicology of manganese in rodents, including exposure during the postnatal period. Neurotoxicology. 1987;8:437–444. [PubMed] [Google Scholar]