Abstract
Background
In routine practice, irritable bowel syndrome (IBS) symptoms are often difficult to be relieved and impair significantly patients’ quality of life (QoL). A randomised, double-blind, placebo-controlled study has shown the efficacy of alverine citrate/simeticone (ACS) combination for IBS symptom relief.
Aim
As IBS symptoms are often intermittent, this pragmatic study was designed to compare the efficacy of an on-demand ACS treatment vs. that of usual treatments.
Methods
Rome III IBS patients were enrolled by 87 general practitioners who were randomly allocated to one of two therapeutic strategies: on-demand ACS or usual treatment chosen by the physician. The primary outcome measure was the improvement of the IBSQoL score between inclusion and month 6.
Results
A total of 436 patients (mean age: 54.4 years; women: 73.4%) were included, 222 in the ACS arm and 214 patients in the usual treatment arm, which was mainly antispasmodics. At 6 months, improvement of IBSQoL was greater with ACS than with the usual treatment group (13.8 vs. 8.4; p < 0.0008). The IBS-severity symptom score (IBS-SSS) was lower with ACS than in the usual treatment arm with a mean (SE) decrease of 170.0 (6.6) vs. 110.7 (6.7), respectively (p = 0.0001). An IBS-SSS < 75 was more frequent in the ACS group (37.7% vs. 16.0%; p < 0.0001). Improvement of both abdominal pain and bloating severity was also greater with the on-demand ACS treatment, which was associated with both lower direct and indirect costs.
Conclusions
After 6 months, on-demand ACS treatment led to a greater improvement of QoL, reduced the burden of the disease and was more effective for IBS symptom relief than usual treatments.
What's known.
IBS symptoms are often intermittent. A combination of alverine citrate/simeticone has been shown to be effective for the relief of IBS symptoms.
What's new.
Alverine citrate/simeticone combination can be given as an on-demand treatment in IBS patients with moderate symptoms.
Introduction
Irritable bowel syndrome (IBS) is a chronic gastrointestinal disorder, which is defined by the Rome III criteria as recurrent abdominal pain or discomfort with marked changes in bowel habits without any evidence of anatomic, metabolic, inflammatory or neoplastic process 1,2. A large community survey in eight European countries has shown an overall prevalence of 11.5% 3 and the disease is one of the more common reasons for visiting general practitioners or gastroenterologists 1,4. In addition, IBS has a well-demonstrated negative impact on quality of life (QoL) and is associated with a significant burden related to both direct and indirect (inability to work) costs 5.
The effective relief of abdominal pain or discomfort remains a clinical challenge. Different drugs from various pharmacological classes have been proposed to relieve IBS symptoms 6–13. Antispasmodics, low-dose tricyclic antidepressants (amitriptyline, desipramine) or selective serotonin reuptake inhibitors (fluoxetine, paroxetine) in association with laxatives or antidiarrhoeal agents, are recommended, whereas the efficacy of probiotics or antibiotics warrant further studies to be definitely assessed. However, a systematic review has underlined the limited evidence for the efficacy of currently available therapies for IBS in Europe 14.
Preclinical pharmacological studies have shown the beneficial effects of alverine citrate on both intestinal motility and sensitivity 15–18. As a selective 5-HT1A receptor subtype antagonist, alverine citrate inhibits the rectal hypersensitivity induced by serotonin, a mediator involved in IBS hyperalgesia 17. Simeticone is an antifoaming agent potentially able to reduce gas-related abdominal symptoms 19 and, when combined with alverine citrate, to potentiate the anti-nociceptive effect of the latter drug 20. A randomised double-blind placebo-controlled study, designed with the latest recommendations of the Rome Committee, has demonstrated that an alverine citrate/simeticone (ACS) combination was significantly more effective than placebo for the relief of abdominal pain or discomfort in IBS patients 21,22.
In Hungin's survey, 69% of the IBS subjects reported that their IBS symptoms occurred twice daily, for 7 days a month, IBS symptoms being present in 23% of the days 3. Another 12-week study examining symptom frequency, duration and severity, as well as episodes patterns in IBS, confirmed this intermittent onset with pain/discomfort on 33% of days, bloating on 28%, and altered stool form or stool passage on 25% and 18% of the days, respectively 23. If IBS symptoms occur often cyclically, an on-demand treatment during the symptomatic periods could be logical, with the additional benefit of lowering the treatment cost. This therapeutic option has not been extensively studied, particularly in patients seen in primary care.
Controlled clinical trials are either explanatory or pragmatic. Pragmatic trials measure effectiveness and the degree of beneficial effect in the real clinical setting 24,25.
Therefore, to assess the effectiveness of an on-demand treatment with ACS, we designed a 6-month randomised pragmatic clinical study to compare the efficacy and effectiveness of two treatment strategies in IBS patients, on-demand long-term treatment in one arm vs. usual treatment in the other.
Methods
Patients selection
Men and women aged more than 18 years who met Rome III criteria 2 for any subtype of IBS, lasting from 1 to 10 years were eligible for the study if they consulted for moderate or severe IBS symptoms, defined by an IBS-severity symptom score (IBS-SSS) between 175 and 400 26. IBS was defined according to the Rome III criteria 27. To rule out any organic cause for symptoms, patients could undergo other investigations, including colonoscopy especially for patients aged more than 50 years.
Main exclusion criteria were digestive disorders related to organic intestinal disease (e.g. intestinal tumour, complicated diverticulosis, inflammatory bowel disease), bowel habit disorders related to faecal impaction, laxative abuse, a history of digestive surgery in the last 18 months (excluding appendectomy and hernia surgery), untreated endocrine disorders (thyroid disorders, hyperparathyroidism, non-insulin-dependent diabetes) and neurological disease. Pregnant or breastfeeding women were also excluded. The final exclusion criterion was treatment with ACS combination during the previous 6 months.
Study design
This randomised controlled pragmatic study was performed in two parallel cohorts of IBS patients enrolled by 87 general practitioners from December 2009 to May 2011 in France. Written informed consent was obtained from each patient. The protocol was conducted in accordance with the Declaration of Helsinki and European GCP for biomedical research, and was approved by the local Ethics Committee (‘Comité de Protection des Personnes’ Sud-Méditerranée I’) of Marseille University Hospital (France). The study was registered with the EudraCT identifier 2009-013049-27 and Clinicaltrials.gov Identifier: NCT01404923.
General practitioners were randomised to one of the two treatment strategy arms: either an on-demand treatment with ACS or the other arm, where they were allowed to decide which usual treatment was needed by the patients.
Drug treatment and prohibited treatment
In the ACS arm, patients were directed, when pain episodes occurred, to take one soft capsule containing alverine citrate (60 mg) and simeticone (300 mg) orally, three times a day prior to meals until the end of the pain episode. In this arm, the treatment duration was determined by the patient himself. In the usual treatment group, investigators had to prescribe what they considered the most appropriate treatment for the patient (drug, treatment duration, dosage and administration). In this arm, only the prescription of ACS was not allowed. In both groups, irritant laxatives were prohibited for IBS patients with constipation.
Randomisation
To avoid possible investigator bias in the assessment of treatment outcomes for patients treated with usual treatments (chosen by each physician) or the on-demand strategy (imposed by the study protocol), the investigators (and not patients) were randomised into the two treatment strategies. They were equally allocated to the on-demand strategy or to the usual care strategy by block randomisation, with a block size of four. The randomisation list was generated using a SAS program.
Data collection
After the inclusion visit, follow-up visits were scheduled at months 1 and 3 prior to a final visit at month 6. Self-administered questionnaires for QoL assessment (IBSQoL scale, SF-36 scale) 28,29, depression/anxiety (HAD, Hospital Anxiety and Depression scale) 30 and sleep quality (Epworth scale) 31 were completed at baseline, then at months 3 and 6. IBS-SSS was filled in prior to inclusion, then at months 1, 3 and 6. Concomitant treatments and adverse events were collected at months 1, 3 and 6 visits. The delivery of treatment units (on-demand ACS group) or the drug prescriptions (usual treatment group) were renewed at months 1 and 3.
End-points
The primary end-point was the difference in the magnitude of change in the total score of IBSQoL from baseline to month 6 between treatment groups. IBSQoL is a health-related QoL disease-specific scale, which has been adapted for French patients 32,28.
Secondary end-points were the changes in different dimensions of the IBSQoL, in the IBS-SSS, the percentage of responders, defined as patients reporting a decrease ≥ 50% of the IBS-SSS baseline value, the improvement of abdominal pain and bloating intensity. Moreover, SF-36, HAD and Epworth scores and the concomitant consumption of analgesics and psychotropic drugs were also analysed.
The IBS treatment consumption could be checked only for the on-demand ACS group, with the analysis of the patient's diary and accountability of returned study treatment units.
Safety assessments
All adverse events reported during the study for all patients were collected.
Statistical analysis
Assuming a standard deviation of 20 according to the study of Brun-Strang et al. 33, it was estimated that a sample size of 234 patients per group would provide 0.90 power to detect a difference of 6 points or more for IBSQoL score using two-sided test with a 0.05 type I error. Finally, a total of 250 patients per group was planned to account for a 10% discontinuation rate.
The primary analysis was an intention-to-treat (ITT) analysis. The ITT population included all patients who had at least one postbaseline evaluation. The safety population included all patients for whom at least one safety datum was available.
For the primary criterion ‘IBSQoL total score change from baseline to month 6’, comparison between the two groups was performed using an analysis of covariance with group as fixed factor and baseline value of total score as covariate (adjustment for baseline value). This analysis was conducted after imputing missing values by last-observation-carried-forward. The same method was used for all quantitative criteria (change in scores and subscores for the different scales).
In addition for the primary criterion, to measure the magnitude of the difference between the two groups, we calculate the Cohen's d effect size: a ‘small’ effect size being 0.20, a ‘medium’ effect size 0.50 and a ‘large’ effect size 0.80.
For qualitative criteria, χ2 test was performed to compare the two groups at the last follow-up visit.
Direct costs were calculated based on the following official French documents: the ‘Nomenclature Générale des Actes Professionnels’ for procedures and health professional fees, the ‘Nomenclature Générale des Actes de Biologie’ for biological tests, Vidal 2011 dictionary for price of the refunded medications.
The hospitalisation costs were calculated according to the duration of stay and based on the mean hospitalisation daily price at the ‘Assistance Publique des Hopitaux de Marseille’ (Marseille University Hospital). Indirect costs (days off work) were calculated based on the mean net salary quoted from the French National Institute of Economics and Statistics (INSEE).
Direct costs were calculated for each treatment group as the sum of the treatments, physician's visits, investigations and hospitalisations costs related to IBS. Total cost was the sum of indirect (days off work) and direct costs. Costs were compared between treatment groups (Mann–Whitney test).
All statistical tests were bilateral at the 0.05 level. Analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Baseline characteristics
From December 2009 to May 2011, 87 general practitioners enrolled 436 patients: 222 were included in the on-demand ACS group and 214 in the other arm. The flow chart of the study is given in Figure1. Forty patients discontinued the study and the main reasons for study discontinuation were poor protocol observance (n = 11) or consent withdrawal (n = 11). No patient had major deviation from protocol.
Figure 1.
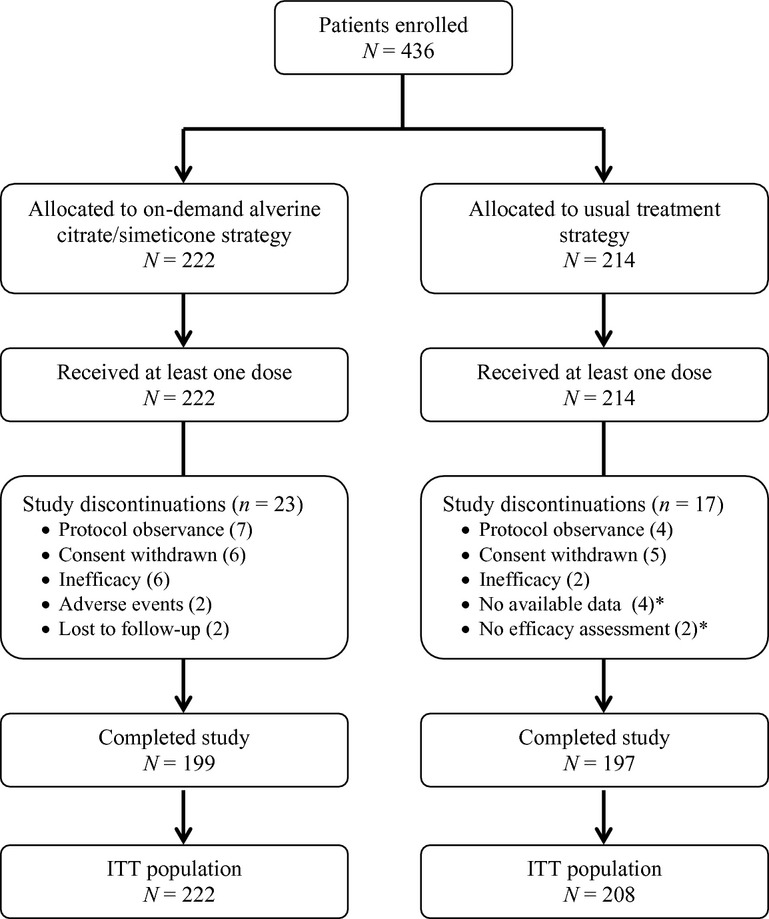
Flow chart. *These patients were excluded of the ITT population
Patient characteristics are shown in Table1. Mean age was 54.4 years and 73.4% were women. The mean duration of the IBS was 6.3 years with a median of five abdominal pain episodes per year (median free interval of 42.5 days). IBS was the reason for a mean of 3.5 visits to general practitioners and 0.6 to specialists during the year prior to the study. A specific treatment for IBS was taken in the last 12 months by 76.8% of patients. Most patients (77.8%) previously underwent colon exploration, mainly colonoscopy (94.9%), with normal results in 75.1% of cases. When present, anomalies were diverticulosis and colonic polyps.
Table 1.
Patients characteristics at baseline
| On-demand ACS strategy N = 222 | Usual treatment strategyN = 214 | |
|---|---|---|
| Age, mean (SD), years | 53.8 (14.9) | 55.1 (16.2) |
| Females, n (%) | 160 (72.1) | 160 (74.8) |
| Body mass index, mean (SD) (kg/m2) | 26.0 (4.8) | 24.8 (5.1) |
| Time since diagnosis, mean (SD), years | 6.3 (3.8) | 6.2 (4.2) |
| Abdominal pain episodes by year, mean (SD) | 8.3 (10.8) | 7.7 (8.9) |
| Predominant bowel habit, n (%)* | ||
| Constipation | 71 (32.3) | 59 (27.6) |
| Diarrhoea | 59 (26.8) | 51 (23.8) |
| Mixed | 50 (22.7) | 64 (29.9) |
| Unclassified | 40 (18.2) | 40 (18.7) |
| IBS QoL mean (SD) | 63.1 (15.4) | 62.8 (15.8) |
| IBS-SSS, mean (SD) | 292.3 (51.9) | 290.0 (48.4) |
| Moderate-to-severe abdominal pain, n (%) | 209 (94.2) | 200 (93.5) |
| Moderate-to-severe bloating at clinical examination, n (%) | 200 (90.1) | 186 (86.9) |
IBS-SSS, IBS-severity symptom score.
Missing data for two patients in on-demand ACS group.
At inclusion, 93.8% of patients reported moderate-to-severe abdominal pain. Moderate-to-severe bloating was observed at clinical examination in 88.5% of patients. According to the Rome III criteria, IBS subtypes were as follows: 30.0% IBS Constipation, 26.3% IBS Mixed, 25.3% IBS Diarrhoea and 18.4% IBS Unspecified.
Drug prescriptions and concomitant treatments
In the ACS group, the median frequency of treatment intake was 75.0% of the days during month 1, 54% during months 2–3 and then 45% for months 4–6. When patients took the treatment, a mean of 2.8 capsules/day was taken as recommended.
In the usual treatment group, medical prescription included one drug for 58.4% of the patients, a combination of two drugs for 30.8% and of at least three drugs for 8.9%. Prescription for IBS included at least one or more antispasmodics for 93.8% of patients. Antispasmodics were mainly trimebutine (37%), simeticone/phloroglucinol (31%), phloroglucinol/trimethyl phloroglucinol (24%), pinaverium (16%) and mebeverine (8%). Other prescribed drugs were laxatives in 20.2%, analgesics in 2.9% and bulking agents in 2.4%. Other drugs were prescribed in less than 3% of cases.
A concomitant treatment with an analgesic drug was given to 4.8% of the patients in the usual treatment group vs. only 1.4% in the ACS group (p = 0.04). Prescription of psychotropic agent was also more frequent in the usual treatment group in comparison with ACS (27.9% vs. 18.9%; p = 0.03).
Evolution of IBSQoL scores
The adjusted mean (SEM) change in the IBSQoL total score, from baseline to month 6, was significantly higher in the on-demand ACS group compared with the usual treatment group: 13.8 (1.1) vs. 8.4 (1.2) with a difference between groups of 5.4; (95% CI: 2.3–8.6; p = 0.0008). The percentage of improvement of the total IBSQoL score from baseline to month 6 was also greater in the on-demand ACS group than in the usual treatment group: 28.5% vs. 18.6% (p = 0.04). In addition, IBSQoL total scores at baseline, 3 and 6 months are shown in Table2.
Table 2.
Evolution of total score of Irritable Bowel Syndrome Quality of Life (IBSQoL)
| Total score of IBSQoL | |||||
|---|---|---|---|---|---|
| On-demand ACS strategy | Usual treatment strategy | Effect size | |||
| N | Mean (SD) | N | Mean (SD) | Cohen'sd | |
| Baseline | 187 | 63.5 (15.4) | 180 | 62.4 (15.3) | |
| Month 3 | 179 | 74.9 (16.6) | 169 | 68.8 (17.1) | 0.35 |
| Month 6 | 173 | 77.6 (16.4) | 167 | 71.2 (18.4) | 0.35 |
The IBSQoL dimensions changes from baseline to month 6 were more improved in the on-demand ACS group compared with usual treatments group (Figure2). The difference between groups was significant for six dimensions: Emotional health (17.6 vs. 12.9; p = 0.002), Mental health (11.1 vs. 5.9; p = 0.005), Sleep (14.3 vs. 6.7; p = 0.003), Energy (16.1 vs. 10.7; p = 0.01), Food (14.2 vs. 6.7; p = 0.0007) and Social life (10.8 vs. 6.3; p = 0.0009).
Figure 2.
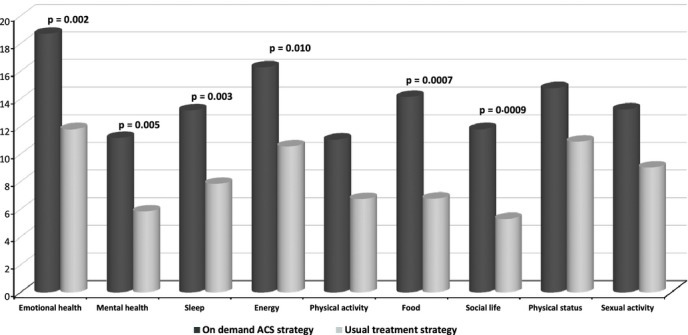
Mean changes in Irritable Bowel Syndrome Quality of Life (IBSQoL) questionnaire scores from baseline to month 6
Changes in IBS symptoms
Figure3 shows that IBS symptoms severity decreased significantly in both groups. However, the improvement was more pronounced in the ACS group than in the usual treatment group. The difference between groups was significant from month 1 to month 6. At 6 months, the adjusted mean (SEM) reduction in IBS-SSS was −170.0 (6.6) vs. −110.7 (6.7), respectively (difference of −59.3; 95% CI: −77.8 to −40.8; p = 0.0001). The decrease in the IBS-SSS was inversely correlated with the improvement of the total IBSQoL score (Pearson's coefficient: −0.56).
Figure 3.
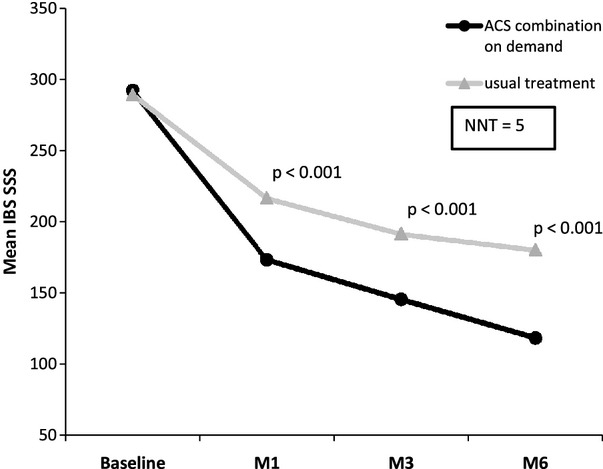
Evolution of IBS symptoms according to treatment strategy (mean IBS-SSS score)
The mean (SD) percentage of variation in IBS-SSS (from baseline to month 6) improvement was higher (p < 0.0001) in the ACS group than in the usual treatment group [57.8% (33.0) vs. 37.7% (33.5)]. Patient responders, defined by a reduction ≥ 50% of the IBS-SSS, were more numerous in the ACS group (58.6% vs. 35.9%; p < 0.0001). Therefore, a NNT (number needed to treat) of 5 was calculated. The percentage of patients who experienced no symptoms (pain or bloating) at the last follow-up visit was significantly higher in the on-demand ACS group (Table3). The percentage of patients with an IBS-SSS < 75 (disease remission) at 6 months was higher in the ACS group (37.7% vs. 16.0%; p < 0.0001) (Table3).
Table 3.
Improvement of symptoms according to on-demand ACS strategy and usual treatment strategy
| At the last follow-up visit | On-demand Strategy | Usual treatment strategy | p-value | NNT | |||
|---|---|---|---|---|---|---|---|
| % | n/N | % | n/N | Mean | 95% CI | ||
| Responder rate | 58.6 | 126/215 | 35.9 | 74/206 | 0.0001 | 5 | 4–8 |
| No abdominal pain | 36.9 | 82/222 | 15.5 | 32/206 | 0.0001 | 5 | 4–8 |
| No bloating | 32.9 | 73/222 | 10.6 | 22/207 | < 0.0001 | 5 | 4–7 |
| Patients in remission (IBS-SSS score < 75) | 37.7 | 81/215 | 16 | 33/206 | < 0.0001 | 5 | 4–8 |
n = number of patients fulfilling the criteria; N = total number of patients with available data.
Responder: decrease ≥ 50% of the IBS-SSS score.
Abdominal pain was improved in 76.1% of patients in the on-demand ACS group vs. 59.2% of patients in the usual treatment group (p = 0.0001). In patients reporting bloating at baseline, the symptom was improved in 76.6% of them in the ACS group vs. 57.0% in the usual treatment group (p < 0.0001).
Symptomatic efficacy according to IBS subtypes
Figure4 shows that IBS-SSS decreased in every IBS subgroup, but the difference between the treatment groups, in favour of the on-demand ACS group, is more pronounced and reached statistical significance in the IBS-C (p < 0.0001) and IBS-M (p = 0.0001) subgroups only. In IBS-D and IBS-U patients, the improvement is still in favour of the ACS group, but the difference did not reach statistical significance.
Figure 4.
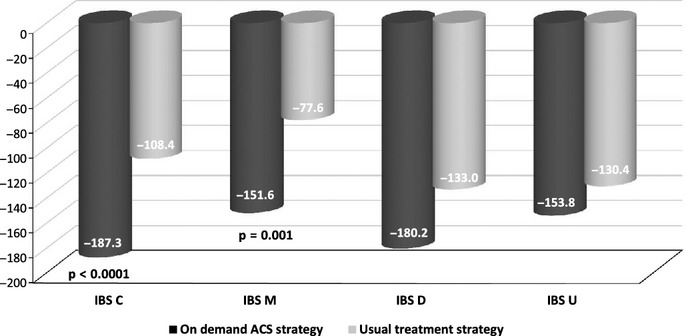
Improvement of IBS SSS score according to IBS subtypes
Evolution of global QoL, depression/anxiety, stress and sleep
At 6 months, SF-36 scores were greater in the on-demand ACS group compared with the usual treatment group, for most of the dimensions: Physical functioning (8.0 vs. 5.4; p = 0.0128), Role-physical (18.3 vs. 13.2; p = 0.0077), Bodily pain (16.0 vs. 13.1; p = 0.0055), General health (4.5 vs. 2.8; p = 0.0033), Vitality (9.4 vs. 6.2; p = 0.0139), Mental health (7.3 vs. 4.0; p = 0.0382). No significant difference was observed for social functioning (7.5 vs. 6.7) and Role-emotional (16.2 vs. 18.3).
At baseline, 13.6% and 16.4% of patients in the ACS and usual treatment groups, respectively, had a HAD-depression score > 10. At 6 months, the percentage of patients with an HAD-D score > 10 was lower in the ACS than in the usual treatment group (5.5% vs. 12.8%; p = 0.012). No difference between the two arms was observed in the percentage of patients with an HAD anxiety score > 10 at baseline and at 6 months between the two arms.
There was no significant difference in Epworth scale evolution between the treatment groups.
Estimation of costs related to IBS
The mean direct cost estimate for the 6-month period was significantly lower in the ACS group compared with usual treatment group (0.46 vs. 0.81 Euros/day, p < 0.0001). When indirect costs (days off work attributable to IBS) were included, the mean daily total costs were twofold lower in the ACS group (0.46 vs. 0.94 Euros/day, p < 0.0001).
Safety
The population of safety included 222 patients in the on-demand ACS group and 210 patients in the usual treatment group. At least one adverse event was reported by 90 patients (40.5%) in the on-demand ACS group and 86 patients (41.0%) in the usual treatment group.
No serious adverse event was drug-related. A total of 2.2% of patients experienced adverse effects considered as possibly related to ACS treatment, while no adverse event was designated as IBS drug-related in the usual treatment group.
Discussion
This 6-month pragmatic study in primary care showed that an on-demand treatment with ACS was effective in providing better QoL and a greater reduction in the intensity of IBS symptoms, mainly abdominal pain and bloating, when compared with usual prescriptions, mainly represented by antispasmodic given on a continuous basis. In addition to the symptomatic effect, the study demonstrated the effectiveness of the on-demand therapeutic strategy with both direct and indirect costs lowering and treatment requirement for the relief of IBS symptoms declining throughout the 6-month follow-up period. Compared with the usual treatment group, the effectiveness of the on-demand ACS strategy was observed in all IBS subgroups, but a greater difference was found in the IBS-C and IBS-M subgroups.
The efficacy of ACS was indirectly reinforced by the significantly lower consumption of pain-relieving drugs in this arm when with the usual treatment group. This efficacy of ACS and the rate of responders (abdominal pain VAS score decrease ≥ 50%) confirmed the data of the previous randomised placebo-controlled in which the rate of responders was 46.8% in the ACS group (n = 205) and 34.3% in the placebo group (n = 204) 21. The superiority of on-demand ACS over standard treatments was also evidenced on depression symptoms. It could be again hypothesised that the superiority of the on-demand ACS strategy on these parameters is at least partly related to the better control of abdominal pain and gas-related discomfort. This is consistent with the recently published pharmacological data on the ACS combination showing an antinociceptive and potentiating effect of both agents 20.
A pragmatic trial does not mean that the same treatment is offered to each patient 25. Moreover, pragmatic trials are not always blinded and a placebo is not generally used, as this type of clinical trials help clinicians to assess effectiveness and to decide between a new treatment and the best current treatment. In such trials, the treatment response is the total difference between the two treatments that includes the treatment effect and the associated placebo effects. This global response is the best reflect of the clinical therapeutic response in the real life 25. A hallmark of pragmatic trials is that participants reflect the population for which the treatment is intended 24. Our results were obtained in a population of more than 400 IBS patients referring to primary medical care for moderate-to-severe symptoms. A large majority of enrolled patients have previously been treated for IBS. The mean age in our study population (53 years) was higher than that reported in some recent epidemiological studies 34–36. This could be partly explained by the fact that these patients were included after a mean 6-year duration of symptoms. On the other hand, an age older than 50 was shown to be one of the determinant of healthcare-seeking behaviour because of the fear of the patients that their abdominal symptoms are related to cancer or other illness 37. The characteristics of this study population were comparable with those of IBS populations enrolled in other studies carried out in France, with a female predominance (3/1 ratio) 32,33,38. In addition, a European survey has shown that the IBS prevalence in the female general population was 22% in the 30–39, 15% in the 40–49 and 18% in the general population age groups 39. Moreover, in the Drossman's study, age was not a determinant factor influencing IBS severity 40. The choice to carry on the study in primary care may account for the relatively low percentage of patients with anxiety and depressive symptoms. Therefore, we can conclude that our trial was carried out in the target population.
The assessment of QoL is considered the best end-point for efficacy and effectiveness evaluation of a treatment given for several months 41. At baseline, scores of both QoL scales (SF-36 and IBSQoL) were comparable to those reported by Amouretti et al. 32.
In our study, the greater improvement of QoL was observed for most dimensions. Several years ago, the importance of all the dimensions in the QoL of IBS patients was highlighted 28. Several epidemiological surveys have reported the correlation between a poor QoL and the severity of abdominal pain in patients with IBS 32,38,41–45. Our results support these observations through a significant inverse correlation between the IBS-SSS and IBSQoL total scores.
In the controlled arm, treatments were mainly represented by antispasmodics. Indeed, several antispasmodics (phloroglucinol, mebeverine, pinaverium bromide, trimebutine but not hyoscine nor otilonium bromide) are available on the French market. According to published guidelines, antispasmodics remain the first-line treatment of IBS symptoms 46,47. No patient in this study was treated by low dose of antidepressants. However, the use of tricyclics or serotonin reuptake inhibitors for the relief of IBS pain is not recommended in France. Consequently, antidepressant agents are only prescribed by gastroenterologists especially in tertiary centres. The differences observed in this study between ACS and the usual treatment group cannot be related to antispasmodics effects lower than previously reported. In Poynard's meta-analysis of the placebo-controlled studies concerning antispasmodics, the mean percentage of patients with global improvement and pain improvement was 56% (n = 927) and 53% (n = 567), respectively, in the antispasmodics group 48.
If we referred to other pathological conditions where an on-demand treatment is discussed, such as gastro-oesophageal reflux disease, the symptomatic results between continuous and on-demand treatments were similar and the benefit of the on-demand treatment was mainly related to a significant reduction in drug intake leading to lower costs of treatment. In this study, we have been able to show differences concerning efficacy and even effectiveness between the two arms, but these results do not allow us to provide an explanation of the better symptomatic results of the on-demand arm. However, we may hypothesise that an active involvement of the patient in his own symptoms management may generate a positive effect on the symptoms control as it is reported in behavioural therapy. Also, our study does not allow us to explain why the magnitude of the difference observed on QoL and IBS-SSS in the on-demand treatment was greater in the IBS-C and IBS-M subgroups than in IBS-D or IBS-U patients. The symptomatic effect of usual treatment strategy was more pronounced in IBS-D and IBS-U patients than in the two others subgroups. One hypothesis could be that the impact of the transit disturbances, mainly the postmeal diarrhoea and urgency, is an important contributor for a poor QoL of IBS-D patients. Therefore, the prescription of antidiarrhoeal agents is probably a way to induce more significant effects on QoL.
Our study has limitations. The aim of the study imposed an open-label design and only the investigators were randomised. In such conditions, efficacy assessment by the investigator may be influenced by the strategy he has to comply with: freely chosen treatment or specified study drug. However, to minimise the bias, questionnaires were self-administered, thus further limiting the investigator's bias. Investigators were all GPs. Therefore, we can suppose that they have a similar relationship with their patients who could have been different if we had mixed GPs and gastroenterologists among the investigators. However, the lack of more precise information on the profile of investigators participating to this trial, is a second limitation for this study. In addition, for practical reasons attributable to the large sample size of both investigators and patients, we were unable to control treatment observance in the usual treatment group with the calculation of the empty treatment boxes. Therefore, we cannot exclude the possibility that treatments prescribed on a continuous basis in this arm were in fact incompletely taken, leading to an efficacy different from expected with the treatment in the usual treatment group.
In conclusion, this pragmatic study in a large cohort of IBS patients provides evidence for the effectiveness of long-term on-demand strategy in routine clinical practice. The effectiveness of on-demand treatment with ACS administered over 6 months was superior to usual treatments on the improvement of QoL and IBS symptoms.
Funding
The study was sponsored and funded by Mayoly Spindler France.
References
- 1.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Hunging APS, Chang L, Locke GR, Dennis EH, Barghouth V. Irritable bowel syndrome in the United States: symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–75. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120:652–68. doi: 10.1053/gast.2001.21908. [DOI] [PubMed] [Google Scholar]
- 5.Jackson JL, O'Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome – results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329–41. doi: 10.1111/j.1365-2036.2008.03881.x. [DOI] [PubMed] [Google Scholar]
- 7.George AM, Meyers NL, Hickling RI. Clinical trial: renzapride therapy for constipation-predominant irritable bowel syndrome – multicentre, randomized, placebo-controlled, double-blind study in primary healthcare setting. Aliment Pharmacol Ther. 2008;27:830–7. doi: 10.1111/j.1365-2036.2008.03649.x. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrom PM, Hein J, Bytzer P, Bjornsson E, Kristensen J, Schambye H. Clinical trial: the glucagon-like peptide-1 analogue ROSE-010 for management of acute pain in patients with irritable bowel syndrome: a randomized, placebo-controlled, double-blind study. Aliment Pharmacol Ther. 2009;29:198–206. doi: 10.1111/j.1365-2036.2008.03870.x. [DOI] [PubMed] [Google Scholar]
- 9.Lembo AJ, Cremonini F, Meyers N, Hickling R. Clinical trial: renzapride treatment of women with irritable bowel syndrome and constipation – a double-blind, randomized, placebo-controlled, study. Aliment Pharmacol Ther. 2010;31:979–90. doi: 10.1111/j.1365-2036.2010.04265.x. [DOI] [PubMed] [Google Scholar]
- 10.Simren M, Ohman L, Olsson J, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome – a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–27. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 11.Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med. 2008;358:1692–9. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talley NJ. Irritable bowel syndrome. Intern Med J. 2006;36:724–8. doi: 10.1111/j.1445-5994.2006.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahedi H, Merat S, Momtahen S, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27:678–84. doi: 10.1111/j.1365-2036.2008.03633.x. [DOI] [PubMed] [Google Scholar]
- 14.Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review: the efficacy of treatments for irritable bowel syndrome – a European perspective. Aliment Pharmacol Ther. 2006;24:183–205. doi: 10.1111/j.1365-2036.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- 15.Abysique A, Lucchini S, Orsoni P, Mei N, Bouvier M. Effects of alverine citrate on cat intestinal mechanoreceptor responses to chemical and mechanical stimuli. Aliment Pharmacol Ther. 1999;13:561–6. doi: 10.1046/j.1365-2036.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- 16.Bouvier M, Grimaud JC, Abysique A, Chiarelli P. Effects of alverine on the spontaneous electrical activity and nervous control of the proximal colon of the rabbit. Gastroenterol Clin Biol. 1992;16:334–8. [PubMed] [Google Scholar]
- 17.Coelho AM, Jacob L, Fioramonti J, Bueno L. Rectal antinociceptive properties of alverine citrate are linked to antagonism at the 5-HT1A receptor subtype. J Pharm Pharmacol. 2001;53:1419–26. doi: 10.1211/0022357011777783. [DOI] [PubMed] [Google Scholar]
- 18.Hayase M, Hashitani H, Suzuki H, Kohri K, Brading AF. Evolving mechanisms of action of alverine citrate on phasic smooth muscles. Br J Pharmacol. 2007;152:1228–38. doi: 10.1038/sj.bjp.0707496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tongprasert S, Sobhonslidsuk A, Rattanasiri S. Improving quality of colonoscopy by adding simethicone to sodium phosphate bowel preparation. World J Gastroenterol. 2009;15(24):3032–7. doi: 10.3748/wjg.15.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bueno L, Beaufrand C, Theodorou V, Andro Delestrain MC. Influence of simethicone and alverine on stress induced alterations of colonic permeability and sensitivity in rats: beneficial effect of their association. J Pharm Pharmacol. 2013;65:567–73. doi: 10.1111/jphp.12021. [DOI] [PubMed] [Google Scholar]
- 21.Wittmann T, Paradowski L, Ducrotte P, Bueno L, Andro Delestrain MC. Clinical trial: the efficacy of alverine citrate/simeticone combination on abdominal pain/discomfort in irritable bowel syndrome – a randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2010;31:615–24. doi: 10.1111/j.1365-2036.2009.04216.x. [DOI] [PubMed] [Google Scholar]
- 22.Irvine EJ, Whitehead WE, Chey WD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–51. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 23.Hahn B, Watson M, Yan S, Gunput D, Heuijerjans J. Irritable bowel syndrome symptoms patterns: frequency, duration and severity. Dig Dis Sci. 1998;43:2715–8. doi: 10.1023/a:1026663613695. [DOI] [PubMed] [Google Scholar]
- 24.Godwin M, Ruhland L, Casson I, et al. Pragmatic controlled trials in primary care: the struggle between externalk and internal validity. BMC Med Res Methodol. 2003;3:28. doi: 10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin R, Torgeson J. Understanding controlled trials: what are pragmatic trials? Br Med J. 1998;316:285. [Google Scholar]
- 26.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 27.p. 889. Appendix A Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders http://www.romecriteria.org (accessed 26 January 2012)
- 28.Hahn BA, Kirchdoerfer LJ, Fullerton S, Mayer E. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547–52. doi: 10.1046/j.1365-2036.1997.00168.x. [DOI] [PubMed] [Google Scholar]
- 29.Perneger TV, Leplege A, Etter JF, Rougemont A. Validation of a French-language version of the MOS 36-Item Short Form Health Survey (SF-36) in young healthy adults. J Clin Epidemiol. 1995;48:1051–60. doi: 10.1016/0895-4356(94)00227-h. [DOI] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Amouretti M, Le Pen C, Gaudin AF, et al. Impact of irritable bowel syndrome (IBS) on health-related quality of life (HRQOL) Gastroenterol Clin Biol. 2006;30:241–6. doi: 10.1016/s0399-8320(06)73160-8. [DOI] [PubMed] [Google Scholar]
- 33.Brun-Strang C, Dapoigny M, Lafuma A, Wainsten JP, Fagnani F. Irritable bowel syndrome in France: quality of life, medical management, and costs: the Encoli study. Eur J Gastroenterol Hepatol. 2007;19:1097–103. doi: 10.1097/MEG.0b013e3282f1621b. [DOI] [PubMed] [Google Scholar]
- 34.Seon Choung R, Locke GR., III Epidemiology of IBS. Gastroenterol Clin N Am. 2011;40:1–10. doi: 10.1016/j.gtc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Lovell RM, Ford AC. Global prevalence of risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–21. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 36.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18(37):5151–63. doi: 10.3748/wjg.v18.i37.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams RE, Black CL, Kim H-Y, et al. Determinants of healthcare-seeking behavior among subjects with irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1667–75. doi: 10.1111/j.1365-2036.2006.02928.x. [DOI] [PubMed] [Google Scholar]
- 38.Dapoigny M, Bellanger J, Bonaz B, et al. Irritable bowel syndrome in France: a common, debilitating and costly disorder. Eur J Gastroenterol Hepatol. 2004;16:995–1001. doi: 10.1097/00042737-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Delvaux M. Functional bowel disorders and irritable bowel syndrome in Europe. Aliment Pharmacol Ther. 2003;18:75–9. doi: 10.1046/j.0953-0673.2003.01728.x. [DOI] [PubMed] [Google Scholar]
- 40.Drossmann DA, Whitehead WE, Toner B, et al. What determines severity among patients with painful functional bowel disorders? Am J Gastroenterol. 2000;95:974–80. doi: 10.1111/j.1572-0241.2000.01936.x. [DOI] [PubMed] [Google Scholar]
- 41.Dapoigny M, Dyard F, Grimaud JC, Guyot P, van Ganse E. Irritable bowel syndrome and healthcare consumption. An observational study in private gastroenterology. Gastroenterol Clin Biol. 2003;27:265–71. [PubMed] [Google Scholar]
- 42.Drossmann DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome. A Rome foundation working team report. Am J Gastroenterol. 2011;106:1749–59. doi: 10.1038/ajg.2011.201. [DOI] [PubMed] [Google Scholar]
- 43.Coffin B, Dapoigny M, Cloarec D, Comet D, Dyard F. Relationship between severity of symptoms and quality of life in 858 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2004;28:11–5. doi: 10.1016/s0399-8320(04)94834-8. [DOI] [PubMed] [Google Scholar]
- 44.Sabate JM, Veyrac M, Mion F, et al. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:484–90. doi: 10.1111/j.1365-2036.2008.03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koloski NA, Boyce PM, Jones MP, Talley NJ. What level of IBS symptoms drives impairment in health related quality of life in communitysubjects with irritable bowel syndrome. Qual Life Res. 2012;21:829–36. doi: 10.1007/s11136-011-9985-5. [DOI] [PubMed] [Google Scholar]
- 46.Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011:CD003460. doi: 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome mechanisms and practical management. Clinical Services Committee of The British Society of Gastroenterology. Gut. 2007;56(12):1770–98. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poynard T, Regimbeau C, Benhamou Y. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15:355–61. doi: 10.1046/j.1365-2036.2001.00937.x. [DOI] [PubMed] [Google Scholar]


