Abstract
Aim
The goal of this study was to determine if long-term testosterone (T) therapy in men with hypogonadism, henceforth referred to as testosterone deficiency (TD), ameliorates or improves metabolic syndrome (MetS) components.
Methods
We performed a cumulative registry study of 255 men, aged between 33 and 69 years (mean 58.02 ± 6.30) with subnormal plasma total T levels (mean: 9.93 ± 1.38; range: 5.89–12.13 nmol/l) as well as at least mild symptoms of TD assessed by the Aging Males' symptoms scale. All men received treatment with parenteral T undecanoate 1000 mg (Nebido®, Bayer Pharma, Berlin, Germany), administered at baseline and 6 weeks and thereafter every 12 weeks for up to 60 months. Lipids, glucose, liver enzymes and haemoglobin A1c analyses were carried out in a commercial laboratory. Anthropometric measurements were also made throughout the study period.
Results
Testosterone therapy restored physiological T levels and resulted in reductions in total cholesterol (TC) [7.29 ± 1.03 to 4.87 ± 0.29 mmol/l (281.58 ± 39.8 to 188.12 ± 11.31 mg/dl)], low-density lipoprotein cholesterol [4.24 ± 1.07 to 2.84 ± 0.92 mmol/l (163.79 ± 41.44 to 109.84 ± 35.41 mg/dl)], triglycerides [3.14 ± 0.58 to 2.16 ± 0.13 mmol/l (276.16 ± 51.32 to 189.78 ± 11.33 mg/dl)] and increased high-density lipoprotein levels [1.45 ± 0.46 to 1.52 ± 0.45 mmol/l (56.17 ± 17.79 to 58.85 ± 17.51 mg/dl)] (p < 0.0001 for all). There were marked reductions in systolic and diastolic blood pressure, blood glucose, haemoglobin A1c, C-reactive protein (6.29 ± 7.96 to 1.03 ± 1.87 U/l), alanine aminotransferase and aspartate aminotransferase (p < 0.0001 for all).
Conclusions
Long-term T therapy, at physiological levels, ameliorates MetS components. These findings strongly suggest that T therapy in hypogonadal men may prove useful in reducing the risk of cardiometabolic diseases.
What's known.
Metabolic syndrome (MetS) is associated with increased risk for cardiovascular disease and diabetes mellitus. There is a strong association between MetS and testosterone (T) deficiency. Clinical interventions with diet, exercise and behavioural therapy (lifestyle changes) and use of statins and antidiabetic agents to normalise lipid profiles, control hypertension, improve insulin sensitivity and reduce abdominal obesity are among the steps taken to ameliorate this disorder and reduce cardio-metabolic risk.
What's new.
Testosterone therapy significantly reduced total cholesterol, low-density lipoprotein cholesterol, triglycerides and increased HDL cholesterol levels. Furthermore, T treatment significantly reduced blood glucose and HbA1c levels and improved systolic and diastolic blood pressure. These findings strongly suggest that T therapy ameliorates MetS components and this may prove useful in reducing the risk of cardio-metabolic diseases.
Introduction
It has been recognised for quite some time that testosterone (T) is a metabolic and vascular hormone and plays a key role in regulation of functional metabolism and is a relevant therapeutic hormone in hypogonadism 1,2. T exerts a wide range of beneficial physiological effects critical to men's health 3–6. T deficiency (TD; hypogonadism) is known to alter functional metabolism and significantly contributes to changes in body anthropometric parameters and body composition [reviewed in 7]. TD also increases vascular disease risk factors, such as type 2 diabetes mellitus (T2DM), MetS and obesity, thus contributing to cardiovascular risk 3,8–24. It has been suggested that changes in functional metabolism lead to development and/or progression of MetS, a disorder characterised by a cluster of cardiovascular risk factors including increased abdominal obesity, elevated triglycerides (TGs), reduced high-density lipoprotein (HDL), high blood pressure, increased fasting glucose and hyperinsulinaemia 4–7,9–11,25–34.
Testosterone regulates body composition (fat and muscle mass), and androgen deficiency produces impaired glucose metabolism and higher levels of TGs and cholesterol concomitant with reduced HDL cholesterol 4–7,13,34. Brand et al. 25 evaluated a number of studies investigating the relationship between T and MetS and concluded that reduced T levels (TD) are associated with MetS. Similarly, Li et al. 35 showed that the prevalence of MetS in 1226 men is associated with reduced levels of total T, free T and bioavailable T and sex hormone-binding globulin (SHBG). Corona et al. 36 demonstrated a higher prevalence of MetS, waist circumference (WC) and TGs as a function of reduced T and age, suggesting that a strong association exists between TD and MetS. Corona also reported increased components of MetS such as elevated BP, hyperglycaemia, WC, high TGs and low HDL cholesterol in 1491 men attending an andrology unit for sexual dysfunction 36. In the SHIP study, Haring et al. 33 suggested that the components of the MetS at baseline predicted low total T in all age groups investigated. We have previously pointed out that the relationship between TD and MetS is bidirectional 9–11, and we believe that restoring physiological T ameliorates the components of MetS and appropriate clinical management of MetS may normalise or increase T levels. This premise has recently been investigated and support for this contention is presented in recent studies 22,37.
Metabolic syndrome and TD are closely linked 38. Epidemiological studies suggested that TD is associated with obesity, insulin resistance (IR) and an adverse lipid profile in men. Conversely, men with MetS and type 2 diabetes have a high prevalence of TD. MetS and TD are both independently associated with increased all-cause and cardiovascular mortality. Observational and experimental data suggest that physiological replacement of T produces improvement in IR, obesity, dyslipidaemia and sexual dysfunction along with improved quality of life. However, there are no prospective long-term interventional studies to assess the effect of T replacement on mortality in men with low T levels.
As MetS is a risk factor for cardiovascular disease (CVD), it is likely that TD contributes to the onset and/or progression of CVD by altering endothelial function, lipid profiles, inflammatory responses, vascular smooth muscle reactivity and other critical cellular signalling pathways in the vascular beds 4–6,9–11. T therapy has been shown to ameliorate IR and improve glycaemic control 23,39–44 with objective measures of reduced body fat mass and increased lean muscle mass 7,34. T therapy is also associated with reduced cholesterol and TGs levels 9–11. A number of studies investigated the effects of T on body composition, muscle mass and lipid profiles, and a strong positive association was demonstrated between T therapy and improved insulin sensitivity, increased muscle mass and reduced fat mass and potential amelioration of some of the MetS elements 7,45–48. However, many of these studies had small sample size and/or were of short duration and therefore were subjected to criticism that the long-term effects of T therapy remained unknown. Using a registry 49, we have examined the effects of T therapy in 255 hypogonadal men over a 5-year period on the functional metabolic profiles as it relates to MetS. Here, we report that long-term T therapy ameliorates elements of the MetS.
Methods and procedures
We performed a cumulative registry study of 255 mainly elderly men, aged between 33 and 69 years (mean 58.02 ± 6.30). All subjects had sought urological consultation in a single urologist's office for various medical conditions, e.g. erectile dysfunction, decreased libido, questions about their T status or a variety of urological complaints. A number of subjects, for instance, men with osteoporosis, had been referred by other specialists with a suspicion of TD. Upon clinical and laboratory investigation, the subjects were found to have subnormal plasma total T levels (mean: 9.93 ± 1.37; range: 5.89–12.13 nmol/l) as well as at least mild symptoms of hypogonadism assessed by the Aging Males' symptoms scale. All men received treatment with parenteral T undecanoate 1000 mg (Nebido®, Bayer Pharma, Berlin, Germany), administered at baseline and 6 weeks and thereafter every 12 weeks for up to 60 months.
Although there is no international consensus as to the normal range of T, clinical data suggest that the normal range of T in adult men is between 12 and 40 nmol/l 50. A threshold of 12.1 nmol/l was recently confirmed by Bhasin et al. in an analysis of a number of well-known studies such as Framingham Heart Study generations 2 and 3, European Male Aging Study and the Osteoporotic Fractures in Men Study 51.
Measurements of anthropometric parameters were performed at baseline (height, weight, WC) and at each visit (weight, WC) and blood samples drawn at each visit, prior to the next injection of T. Therefore, T levels were trough levels at the end of an injection interval. WC was measured midway between the last rib and the uppermost border of the right iliac crest. T was measured by standard laboratory measurement as described previously 49. Because of the cumulative registry design of the study, the number of subjects decreased over time. New subjects are entered into the database once they have received 1 year of treatment with T. All 255 subjects were followed up for at least 1 year, 215 for at least 2 years, 182 for 3 years, 148 for 4 years and 116 for 5 years. The declining number of patients reflects duration of treatment but not drop-out rates. On the contrary, adherence to treatment was excellent, and T was only discontinued in three men.
Total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, HDL cholesterol, plasma TGs, fasting glucose, haemoglobin A1c, C-reactive protein (CRP) and liver function tests were carried out by a commercial laboratory using standard test methods. Measurements of systolic and diastolic blood pressure were performed via a sphygmomanometer.
Statistical analyses
For continuous variables, the mean, median, standard deviation, range, minimum, maximum, and sample size for the overall sample and various groups were reported at each time point. For categorical variables, the frequency distribution was reported. We tested the hypotheses regarding change in outcome scores across the study period by fitting a linear mixed effects model to the data. Time (to indicate follow-up interviews) was included as fixed effect in the model. A random effect was included in the model for the intercept. Estimation and test of change in scores were determined by computing the differences in least square means at baseline vs. the score at each follow-up interview. For the correlation study, Pearson correlation was calculated between baseline changes in outcomes at various time points. The significance of each correlation was tested using Fisher's exact test.
Results
A host of comorbidities was reported in the patients in this registry (Table1). We have considered a number of specific concerns that pertains to MetS such as use of antihypertensive medications, which may not be associated with the respective diagnosis. Also, we examined blood pressure measured at the baseline visit as well as use of statins as lipid-lowering medications. We further recorded the use of antidiabetic medication in these men. The distribution of MetS components at baseline was as follows
Table 1.
Baseline characteristics of patients (mean age in years: 58.02 ± 6.30) in the registry
| Comorbidities relevant to metabolic syndrome at baseline | ||||
|---|---|---|---|---|
| Patient-reported | Investigator-assessed | |||
| n | Proportion (%) | n | Proportion (%) | |
| Elevated waist circumference | 244 | 96 | ||
| Hypertension | 101 | 40 | 237* | 93 |
| Dyslipidaemia | 47 | 18 | ||
| Elevated triglycerides | 255* | 100 | ||
| Reduced HDL cholesterol | 57* | 22 | ||
| Type 2 diabetes | 80 | 31 | 81 | 32 |
| Elevated fasting glucose | 46* | 18 | ||
| Coronary artery disease | 40 | 16 | ||
| Postmyocardial infarction | 39 | 15 | ||
| Erectile dysfunction | 145 | 57 | 173† | 68 |
Fulfilling metabolic syndrome criteria according to criteria discussed in reference 52.
At least mild ED according to IIEF-EF (questions 1–15 plus 15).
244 (96%) men had WC ≥ 94 cm and 174 men (68%) had WC ≥ 102 cm. 237 men (93%) had hypertension (diastolic BP ≥ 130 and/or systolic BP ≥ 85) and 255 men (100%) had dyslipidaemia (TGs ≥ 150 mg/dl and/or HDL ≤ 40 mg/dl). 127 men (50%) had IR (T2D, antidiabetic medication or fasting glucose ≥ 100 mg/dl). Only 11 patients did not fulfil three or more MetS criteria as described in the ‘reconciled’ definition by the International Diabetes Federation and the American Heart Association/National Heart, Lung and Blood Institute 52.
Total plasma T levels in men with TD treated with TU for 5 years
Total T levels increased significantly from 9.9 nmol/l at the beginning of therapy to about 18 nmol/l within the first 12 months of therapy (p < 0.0001), then reached a plateau at physiological levels and remained constant at this level throughout the course of treatment approaching 5 years (Figure1).
Figure 1.
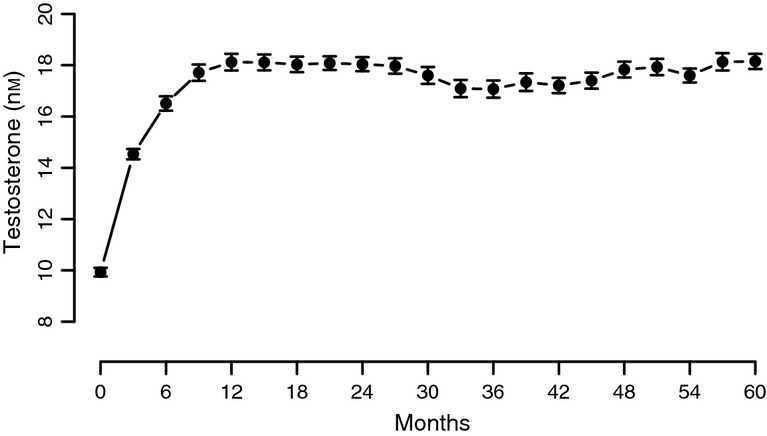
Mean total plasma testosterone levels in men with TD undergoing T therapy for a period of 5 years
Effects of T therapy on obesity
The effects of T on anthropometric parameters have been reported elsewhere 49. In short, we observed reductions of WC by 8.5 ± 0.17 cm and body weight by 15.35 ± 0.43 kg (p < 0.0001 for both) 49.
Effects of T therapy on MetS components
Metabolic syndrome is a cluster of cardiovascular risk factors including increased central abdominal obesity, elevated TGs, reduced HDL, elevated blood pressure and elevated fasting glucose or accordant treatment [reviewed in 9–11]. To determine if T therapy ameliorates the components of the MetS, we analysed TC, LDL cholesterol, HDL cholesterol, TGs, systolic and diastolic blood pressure, glucose, haemoglobin A1c and CRP.
Effects of T therapy on lipid profiles
Testosterone treatment in hypogonadal men resulted in gradual and consistent decrease in TC levels. The decrease was statistically significant as early as 12 months (p < 0.0001) and reached a plateau at 24 months (p < 0.0001 vs. 12 months, thereafter non-significant). The level of cholesterol at baseline was approximately 7.3 mmol/l (282 mg/dl). This concentration was reduced to about 4.9 mmol/l (188 mg/dl) and remained low throughout the 5-year period of the study (Figure2A). Similarly, T treatment resulted in marked and significant gradual and consistent decrease in LDL cholesterol levels from approximately 4.2 mmol/l (164 mg/dl) to approximately 2.8 mmol/l (110 mg/dl). The reduction in LDL levels was significant within the first year of treatment (p < 0.0001), significant at 24 months (p < 0.0001 vs. 12 months) and stable thereafter (Figure2B). Remarkably, the LDL cholesterol levels remained low over the course of 5-year period of T treatment. HDL cholesterol levels slightly but significantly increased and remained elevated over the 5-year period of treatment (Figure3A). The increase was gradual and significant within the first year of treatment (p < 0.0001). The TC/HDL ratio is thought to predict the risk of CVD, in particular, ischaemic heart disease. The ratio of TC/HDL in these treated patients improved considerably from 5.44 ± 1.61 to 3.49 ± 1.09 (p < 0.0001) (Figure3B), suggesting a favourable change in the lipid profile and a potential reduction in CVD risk.
Figure 2.
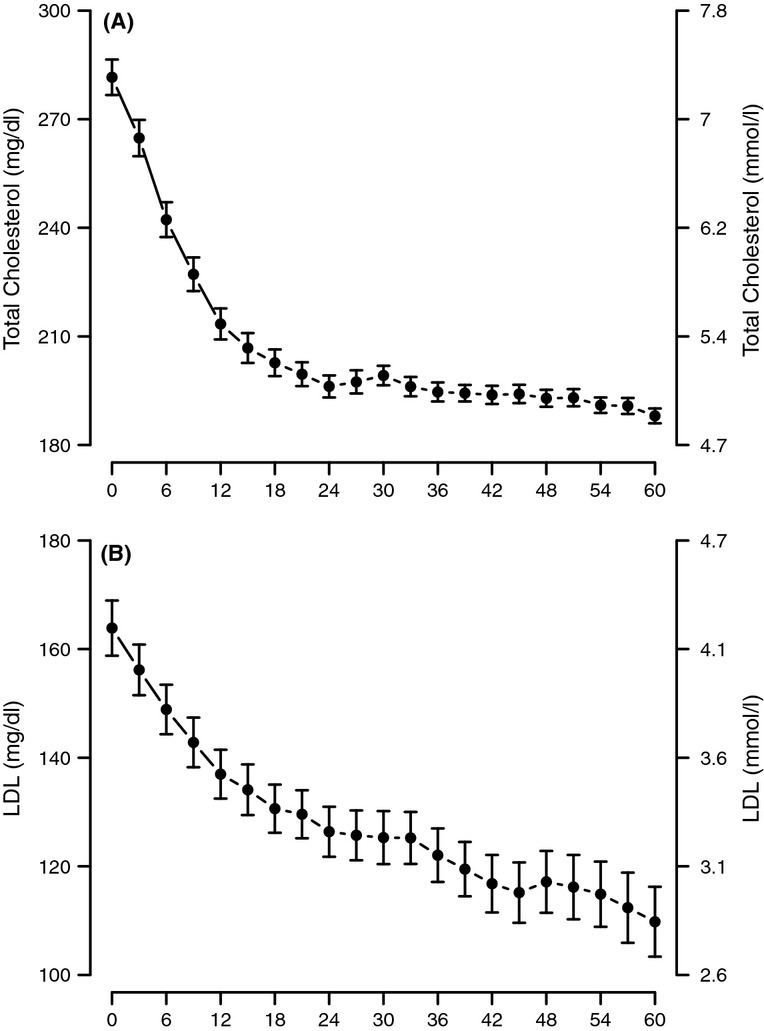
Total cholesterol (A) and LDL cholesterol levels (B) in men with TD undergoing T therapy for 5 years
Figure 3.
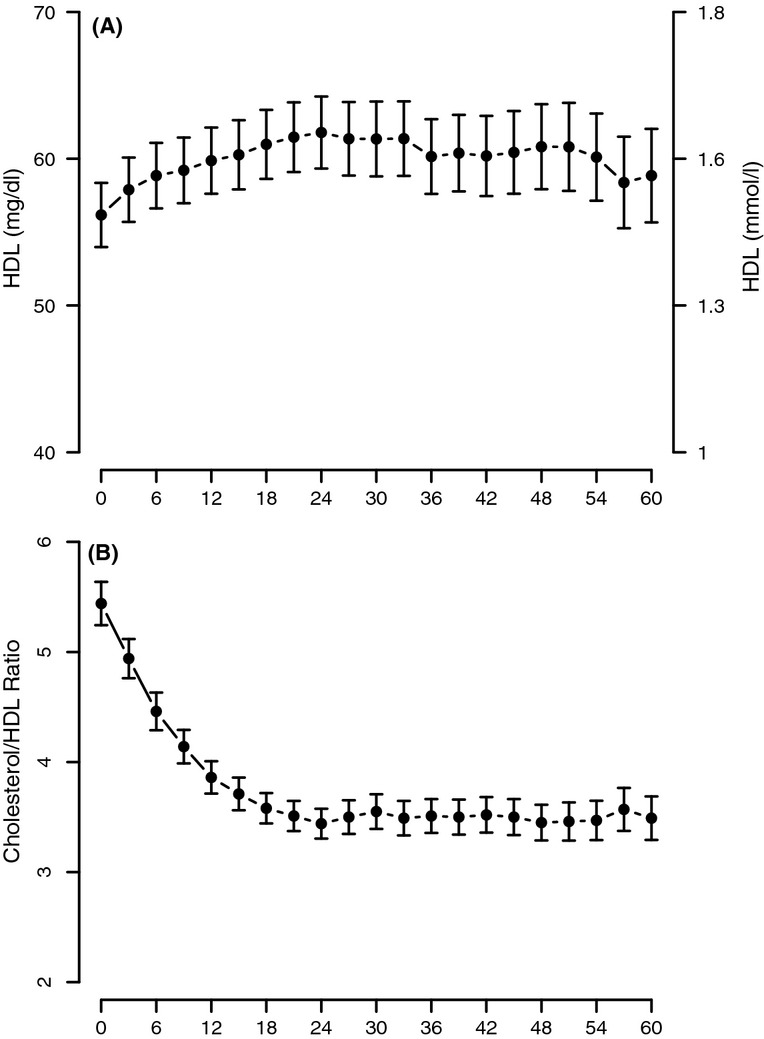
HDL cholesterol (A) and total cholesterol/HDL-C ratio (B) in men with TD undergoing therapy for 5 years
With T treatment, we also observed gradual and consistent decrease in TG levels from approximately 3.1 mmol/l (276 mg/dl) to 2.2 mmol/l (190 mg/dl) and they remained low throughout the 5-year period of the study (Figure4). The decrease was statistically significant within the first year (p < 0.0001), again significant at 24 months vs. 12 months (p < 0.0001) and thereafter remained low over the entire treatment period.
Figure 4.
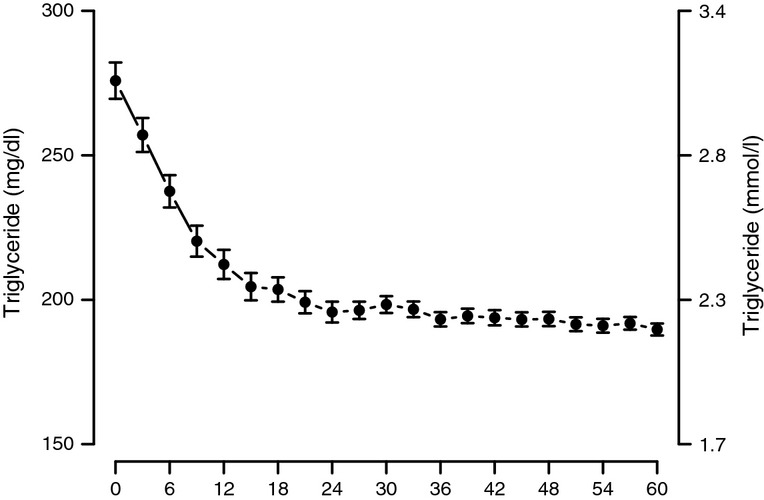
Triglyceride levels in men with TD undergoing T therapy for 5 years
Effects of T treatment on systolic and diastolic blood pressure
Testosterone treatment of men with TD produced marked and sustained gradual decrease in systolic blood pressure (SBP) from 153.55 ± 17.6 to 137.72 ± 10.9 mmHg (p < 0.0001) (Figure5A). The decrease was significant and gradual over the first 2 years and remained low over the entire course of the 5 years of treatment. Similar results were recorded with the diastolic blood pressure, which decreased from 93.49 ± 11.32 to 79.59 ± 7.36 mmHg (p < 0.0001) (Figure5B), in that a marked and rapid decrease was noted over the first 2 years of treatment and then remained low over the entire 5 years of treatment.
Figure 5.
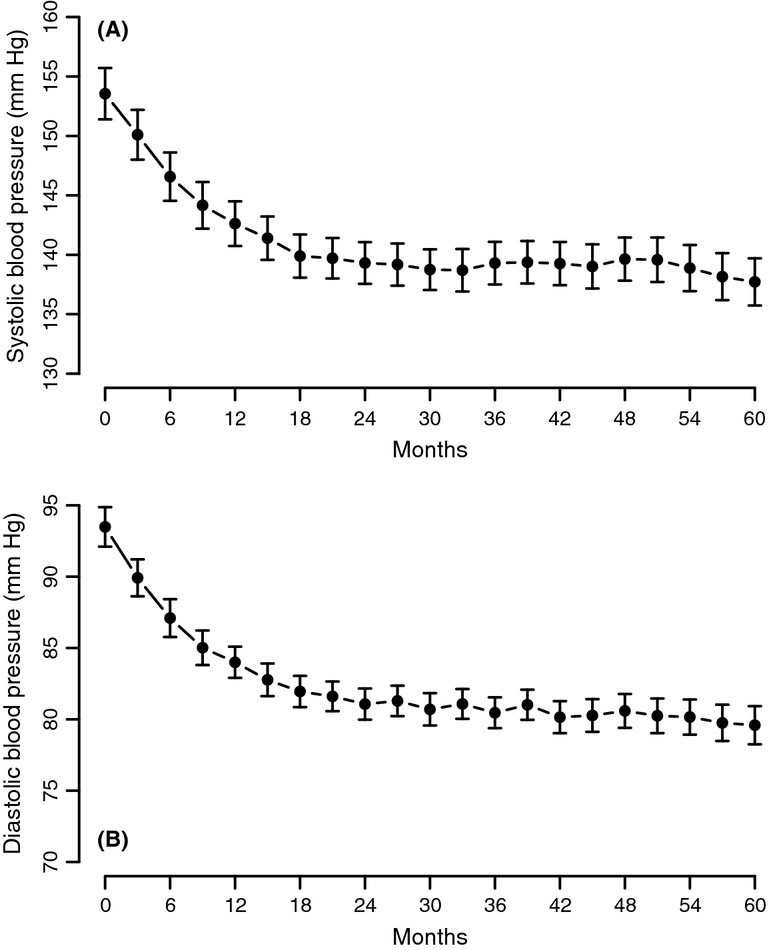
Systolic blood pressure (A) and diastolic blood pressure (B) in men with TD undergoing T therapy for 5 years
Effects of T treatment on blood glucose and haemoglobin A1c levels
Testosterone treatment of men with TD resulted in a significant gradual decrease in fasting blood glucose from 5.74 ± 0.8 mmol/l (103.35 ± 14.42 mg/dl) to 5.41 ± 0.8 mmol/l (97.56 ± 2.35 mg/dl) (Figure 6A). The decrease was significant after 12 months (p < 0.0001), further declined after 24 months (p = 0.012 vs. 12 months) and then reached a plateau. The decrease in fasting blood glucose was paralleled by a marked decrease in haemoglobin A1c from 7.06 ± 1.54% to 6.16 ± 1.35%. In contrast to fasting glucose, the decrease in HbA1c was statistically significant after 12 months (p < 0.0001), between 24 and 12 months (p < 0.0001), between 36 and 24 months (p = 0.0036), between 48 and 36 months (p = 0.0049), and between 60 and 48 months (p = 0.0149) (Figure6B).
Figure 6.
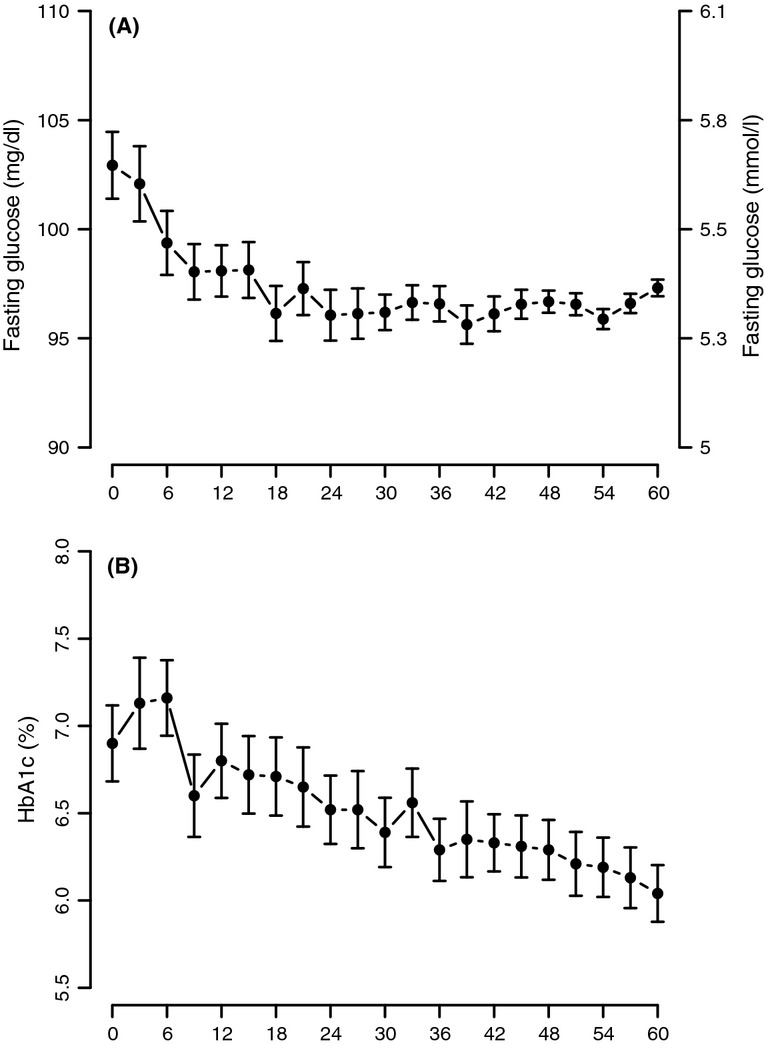
Glucose concentration (Panel A) and HbA1c levels (Panel B) in men with TD undergoing T therapy for 5 years
Effects of T treatment on CRP levels and markers of liver function
We have noted a marked and significant decrease in the levels of CRP (from 6.29 ± 7.96 to 1.03 ± 1.89 U/l) (p < 0.0001 with a plateau after 36 months), aspartate transaminase (AST) from 43.05 ± 17.29 to 20.18 ± 3.22 U/L) (p < 0.0001 with a plateau after 24 months) and alanine transaminase (ALT) from 43.89 ± 18.11 to 20.55 ± 3.92 U/l (p < 0.0001 with a plateau after 36 months) suggesting a reduced inflammatory response and improvement in liver function. (Figures7, 8A,B).
Figure 7.
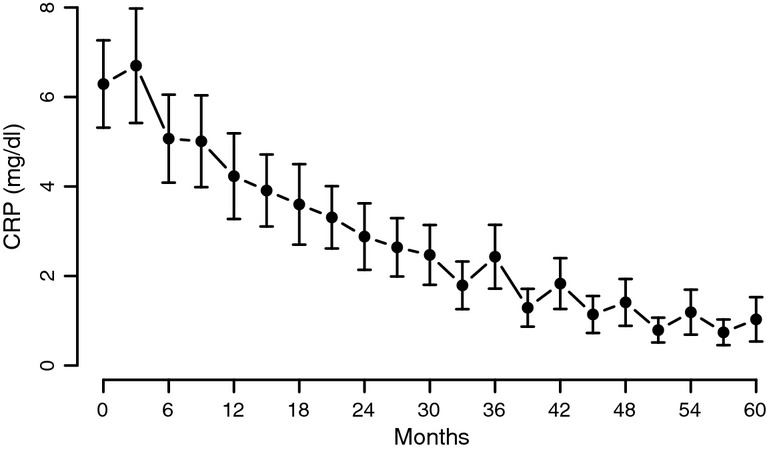
C-reactive protein (CRP) levels in men with TD undergoing T therapy for 5 years
Figure 8.
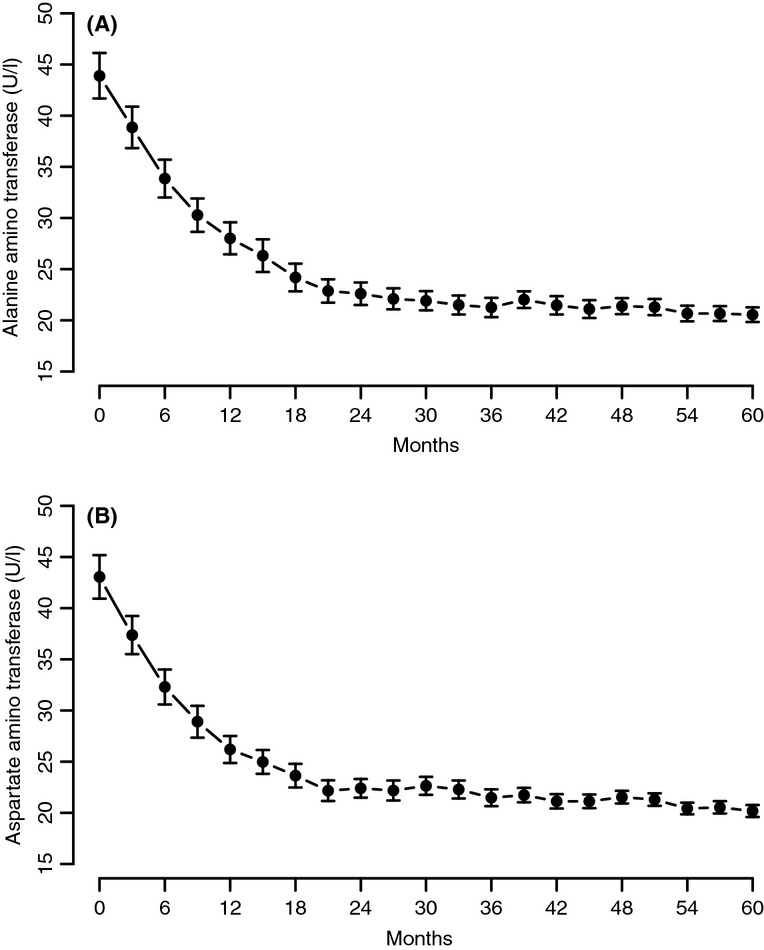
Alanine aminotransferase (A) and aspartate aminotransferase (B) in men with TD undergoing T therapy for 5 years
Testosterone therapy and prostate safety
In this observational study, mean prostate volume increased from 28.51 ± 11.2 ml to 30.04 ± 12.35 ml (p < 0.0001), reaching a plateau after 3 years. Mean prostate specific antigen increased from 1.77 ± 0.97 to 1.83 ± 0.95 ng/ml (p < 0.0001) with a plateau after 2 years. There were no occurrences of urinary retention or other problems related to benign prostatic hyperplasia (BPH). In addition, few subjects had increased haematocrit values > 52%, which were all resolved without intervention. With regard to prostate cancer (PCa), only three patients were diagnosed with PCa. This represents an incidence of 1.2% [95% CI (0.24–3.4%)] and an incidence of 30.3 [95% CI (0.9783–9.4052)] per 10,000 person-years, as previously reported 49. In the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial in which 38,345 men ages 55–74 years in the control arm were followed up to 13 years, Andriole et al., 53 showed that 3815 men were diagnosed with PCa representing an incidence of 97.1 per 10,000 person-years. In the European Randomized Study of Screening for Prostate Cancer Patients, Schröder et al. 54 reported on 72,891 patients, with mean ages 55–69 year and a follow up of 11 years. The authors demonstrated that 6963 patients were diagnosed with PCa (9.6%) and an incidence of 96.6 per 10,000 person-years 54. If one carefully examines the data from such extensive screening trials, it becomes clear that the incidence of PCa reported in our cohort remained far lower than expected. As discussed by Saad et al. 49 in their recent report on long-term T treatment, this incidence is far lower than reported in previous studies. To date, there is no compelling evidence that T is the driving factor in the development or progression of PCa 55. Recent guidelines developed for monitoring T treatment need be adhered to in order to ensure a safe therapy in men without suspicion of PCa. Recent reports have placed fears regarding T therapy and on PCa in a more rational perspective 55–57.
Discussion
In this long-term observational registry study, we investigated the effects of T therapy in 255 men with TD on MetS components. T therapy restored physiological T levels within the first 12 months and these levels were maintained with T therapy throughout the entire 5-year period.
One of the key findings of this study is that T therapy markedly and significantly reduced TC levels. This reduction in TC was pronounced and sustained over the entire treatment period. Interestingly, the reduction in TC was in a magnitude of 25–40% of TC at baseline, suggesting that T therapy influences both synthesis and disposal of TC. This confirms observations reported in previous studies 58–60. To our knowledge, this is the first study to report on 5-year long-term T therapy on cholesterol levels in men with TD.
Our study demonstrates that long-term T therapy progressively and significantly reduced LDL and this reduction was sustained throughout the treatment cycle. Remarkably, total LDL levels significantly reduced over the entire course of treatment period. This finding is of clinical importance since reduction in LDL is thought to correlate with reduced CVD risk. Furthermore, the reduction in LDL suggests that T therapy plays an important role in improving overall lipid profiles in men with TD and represents an important metabolic function in attenuating CVD risk and may ameliorate this pathology in men with TD. Our data confirm previous observations in which LDL reduction in response to T therapy was reported 13,58–60. In cross-sectional and prospective observational studies, TD was found to be associated with increased LDL and reduced HDL levels 61. Furthermore, T therapy in men with TD is associated with reduced levels of LDL and TC coupled with beneficial increase in HDL 13. Thus, we believe that the effects of T contribute to reduced CVD risk 62 and increased benefits, such as improved lipid profile 61.
It is worthy to note that we observed a marked and significant increase in HDL in response to T therapy over the entire course of 5 years of follow up. This is of clinical importance, as HDL is thought to play a critical role in reducing CVD risk. Furthermore, these data suggest that the increase in HDL levels may be attributed to restoration of physiological T levels. It should be noted that other studies have shown increase 58, decrease 63 or no changes 48 in HDL levels in men with TD treated with T [reviewed in 13]. We suggest that the discrepancies in the various studies may relate to use of varying formulations of T, dosage administered, duration of the studies, methods of assessment of the various end-points and age and comorbidities of subjects enrolled in the various studies. Isidori et al. 64 noted that the decrease in HDL in response to T therapy was seen mostly in studies with supraphysiological levels of T.
It should be noted that HDL is not a single particle but rather a composite of heterogeneous particles differing in size and apolipoprotein composition between individuals 65. It was shown that non-denaturing 2D electrophoresis analysis of HDL revealed the presence of distinct HDL subpopulations from the same individual, suggesting that alterations in HDL subpopulations and their distribution may correlate with CHD 65. T therapy has been shown to reduce HDL3-C subfraction and LpA-I:A-II particles but not the more anti-atherogenic HDL2-c and LpA-I particles 66. Rubinow et al. showed that androgen deprivation increased cholesterol efflux from macrophages and demonstrated that sex steroid manipulation modified the HDL proteome 67. Furthermore, T therapy in older hypogonadal men altered HDL protein and lipid composition but did not significantly change serum HDL-mediated cholesterol efflux 68.
The TC/HDL ratio is thought to predict the risk of CVD because it may indicate a cluster of abnormalities 69. It should be noted that variations in TC/HDL-C ratio may be associated with changes in metabolic function and this ratio may be predictive of IR and ischaemic heart disease risk 69. Modification in lipid metabolism by pharmacotherapy to lower the TC/HDL ratio is believed to reduce the risk of CVD. It is thought that the TC/HDL ratio is a better lipid predictor of CVD in T2DM patients 70.
Recent studies have also suggested that the non-HDL-C/HDL-C ratio is a better marker than the apoB/apoA1 ratio for identifying IR and MetS in Koreans 71. A recent study had demonstrated that patients with peripheral artery disease treated with atorvastatin showed improvement in endothelial function and this was associated with decreased TC/HDL ratio, suggesting that this ratio may relate to endothelial damage 72.
Another critical finding of this study was the marked and significant reduction in total TGs levels in response to T therapy in men with TD. It is important to note that the observed reduction represented normalisation of lipid profiles in men with TD in response to T therapy. As visceral fat storage is dependent on accumulation of TGs, these data are congruent with our previous report that T therapy in men with TD, who are overweight or obese, resulted in marked reduction in body weight, WC and body mass index (BMI) 49. Also, these results are in concordance with a number of studies demonstrating increased lean body mass and reduced fat mass in response to T therapy 7,34,49. Agledahl et al. 73 reported that a linear increase in serum TGs levels was found in men with total T levels below the 50th percentile, while serum TGs levels did not change in men with T levels above the 50th percentile. Total T and SHBG were inversely and independently associated with TGs, and positively and independently associated with HDL. Men with an unfavourable lipid profile had significantly lower levels of total T and SHBG in age- and BMI-adjusted analyses, compared with men with a normal lipid profile. In a high-fat diet-induced rabbit model of MetS, Maneschi et al. 74 showed that T treatment of these animals ameliorated the metabolic profile and reduced visceral adipose tissue.
The relationship between T and lipids was assessed in a large group of men 75. The data showed that (i) TG levels were negatively associated with quartile levels of T and the magnitudes of associations were greater for postprandial TGs than for fasting TGs; (ii) HDL cholesterol (HDL-C) levels were positively related to quartile levels of T, but the associations became insignificant after further control of TGs; and (iii) the calculated LDL cholesterol (LDL-C) levels were positively associated with quartile levels of T. The favourable association of T with HDL-C counterbalances the unfavourable association of it with LDL-C, while the net influence of T on plasma lipids for cardiovascular system was still in the beneficial direction because of its negative association with postprandial plasma TG levels 75. Data from clinical trials have shown that the efficacy of statins in reducing TC and LDL cholesterol ranges from 17% to 53% 76,77. Interestingly, T therapy produced marked and significant reductions in TC and LDL cholesterol. These observations suggest that T therapy modifies lipid metabolism resulting in favourable lipid profiles. This is an important finding and needs be further investigated.
Men with TD may be at a high risk of altered lipid profiles and MetS. T therapy may normalise lipid profiles and reduce the risk of CVD 62. Some studies suggested that T therapy does not reduce LDL cholesterol levels while reducing TC levels 64. One must consider the number of confounding factors that may have contributed to data in the various reported studies, such as T formulation, dosages and duration of T therapy. A marked reduction in TC and LDL cholesterol was reported in response to T treatment 78. Similar findings were reported in patients with T2DM and MetS treated with T 43,79,80.
Although TC and LDL cholesterol are not considered in the definitions of MetS, the reduced levels of HDL cholesterol and increased levels of TGs are considered among the key components of MetS. Clearly, T therapy has been shown to improve lipid profiles; however, a number of studies produced discrepant levels of HDL cholesterol in response to T treatment [reviewed in 13]. It is not surprising that endogenous T levels were shown to inversely correlate with TC and LDL cholesterol 81–87 but positively with HDL cholesterol 88.
We have previously reviewed the relationship between T and lipid profiles and have reported that the relationship between T and HDL levels is controversial 13 with a number of studies suggesting that HDL cholesterol levels increased in men in response to T therapy 13,88,89. The discrepancies in many of these studies are attributed to differences in age of men in the studies, route of administration and the preparation, as well as dose and duration of treatment. It is also possible that in studies where supraphysiological levels of T were achieved, HDL levels were thought to show a decrease 64 but without taking into account the various changes in HDL sub-fractions.
Testosterone deficiency is associated with higher TGs levels 39,40,73,90. A marked decrease in TG levels was reported in men treated with T 91, but this was not verified in other studies 73,90. A number of studies have shown that T treatment ameliorated IR, reduced HOMA index and improved glycemic control 23–37,39–46,80,92,93. In the TriUS Registry with approximately 37% of men with TD having MetS at baseline 94, after 12 month of T treatment (n = 849), a marked decrease in fasting glucose, WC and blood pressure was noted in the men with MetS 94. In a recent study (IPASS) (n = 1438), men with TD were treated with T and followed up for up to 12 months. In a subgroup of 60 men with poorly controlled diabetes (mean HbA1c 7.9%), treatment with T resulted in a mean decrease in HbA1c of 1.1% after 12 months 91.
Of considerable interest is the marked reduction in systolic and diastolic blood pressure values noted in our study of men with TD in response to T therapy. It has been suggested that T therapy improved hypertension in patients with TD, but the data provided were limited. This 5-year long-term T therapy study provided important and consistent data on these parameters. However, the exact mechanism of how T therapy modulates blood pressure is incompletely understood. Potential mechanisms on the role of T as a vascular hormone have been postulated recently by Jones et al. 4–6. Testosterone modulates arterial blood pressure, through a host of mechanisms, such as direct effects on the heart, the kidney and the vessels, as well as the endothelium 95,96. In elderly men with isolated systolic hypertension, it was shown that the plasma T levels were lower than those of the normotensive subjects and a strong inverse relationship appears to exist between T levels and SBP, suggesting that low T contributes to the increased arterial stiffness 97. Svartberg et al. 98 proposed that lower endogenous T levels are associated with higher blood pressure. The authors reported that in 1548 men aged 25–84 years, total T was inversely associated with SBP. Men with categorical hypertension had lower levels of total and free T before and after adjusting for BMI. Furthermore, data from TRiUS (Testim®, Auxilium Pharmaceuticals, Malvern, PA, USA) Registry study showed that MetS patients demonstrated significant decreases in WC, fasting blood glucose levels and blood pressure in response to T therapy after 12 months 94. Also, in a case–control study, hypogonadism was shown to be associated with higher SBP 82. Furthermore, in obese, hypogonadal and diabetic men treated with oral T, blood pressure was shown to decrease favourably 92,99. In contrast, men treated with androgen deprivation therapy for PCa have been shown to have increased arterial stiffness 100,101.
Among the key components of MetS is IR and hyperglycaemia with concomitant increase in the surrogate marker haemoglobin A1c (HbA1c). As noted in this study, T therapy of men with TD demonstrated progressive and sustained reduction in blood glucose and the fraction of measurable HbA1c, suggesting that T therapy improved glucose utilisation and increased insulin sensitivity, thus ameliorating this MetS component. The mechanisms by which T restores physiological glucose transport are discussed in recent reviews by Jones et al. 4–6.
Low circulating levels of TT, FT and SHBG were shown to be independently associated with an increase in HbA1c in middle-aged and older men, even across its normal range. Clarifying the nature of this relationship may provide new insights into determinants of glucose and sex hormone metabolism and how these may contribute to risk of CVD 102 Interestingly, statins have been shown to increase HDL-C, but did not show any differential effect on glucose metabolism 103. In contrast, T therapy had improved HDL-C and also reduced glucose levels and reduced HbA1c.
It is important to note that the levels of CRP, a non-specific marker of inflammation, were markedly reduced over the course of T therapy in men with TD. We have previously reported that T therapy results in significant and sustained weight loss (WL) 49. As WL significantly reduces plasma CRP concentrations 104, it is likely that the reduced weight re-establishes a new equilibrium with reduced inflammatory responses and reduced CRP levels. Interestingly, in the Jackson Heart Study, it was reported that CRP concentration was significantly and directly associated with change in SBP and WC but inversely associated with HDL cholesterol. The authors concluded that higher circulating CRP concentrations predict incident MetS 105. Furthermore, in longitudinal studies, elevated levels of hs-CRP predict future MetS independently of age, sex and smoking in apparently healthy Koreans 106. Ridker et al. 107 proposed that hs-CRP should be added to clinical criteria for MetS.
In addition, we noted a reduction in the activities of several liver enzymes, used as markers of liver function, suggesting that T therapy attenuates the inflammatory response and improves various physiological functions. Liver ALT levels are commonly used as markers in screening for liver disease with the upper-normal limit of 40 IU/l 108,109. The incidence of non-alcoholic fatty liver disease (NAFLD), a phenotype of MetS of the liver, is increasing 110,111. Epidemiological studies suggested that increased activities of liver enzymes such as ALT and AST may be associated with MetS and CVD and increased levels of ALT are associated with long-term development of multiple metabolic disorders 112. Even after age- and gender-adjusted analyses, increased ALT levels were significantly associated with an increased risk of T2DM and CVD. These findings are also supported by the Western Australian Health Department data linkage system, in which a strong association was demonstrated between levels of ALT and MetS, independent of IR 113. Hoyos et al. were first to demonstrate a significant reduction in liver fat content in response to short-term T therapy 114. Taken together, these findings strongly suggest that normalising T levels in men with TD ameliorates a host of MetS components and reduces inflammation, which may include NAFLD, thus reducing the risk of cardiometabolic diseases.
A number of studies reported a strong association of increased cardiovascular and metabolic disorders in men with low T 22,62,115,116. Increased all-cause and CVD mortality in men with TD has recently been reported 117. T therapy in men with TD reduced CVD risk factors with concomitant improvement in insulin sensitivity and glycaemic control, even in studies of shorter duration 45,46. Furthermore, a marked decrease in visceral fat mass and circulating inflammatory cytokines concomitant with improvement in endothelial cell function have been reported 43,69.
Testosterone is well known to regulate a host of metabolic functions in liver, adipose tissue, muscles, coronary arteries and the heart. Thus, it is not surprising that T therapy reduces the risk of CVD. An inverse relationship exists between T and obesity and TD is associated with dyslipidaemia, atherosclerosis, CVDs, MetS and diabetes. T therapy of men with TD improves lipid profile and lowers cholesterol, blood sugar and IR 118. Conditions such as androgen deficiency or androgen deprivation are recognised to promote adipogenesis, which contributes to MetS and obesity. Androgens strongly inhibit differentiation of pluripotent cells into adipocytes and promote myogenesis 119,120. In MetS, the increase in circulating glucocorticoids may inhibit the androgen function, via increased activity of aldo-keto reductase 1C enzymes, which contribute to androgen inactivation and increased adipogenesis 121,122. Visceral fat accumulation and weight gain has been linked with metabolic alterations, and the modulation of body fat distribution is likely a significant implication of TD in men. Clinically, the relevance of T therapy is the improvement in body composition/fat distribution, reduced inflammation and amelioration of the MetS components 7,34. In a recent randomised, double-blind, placebo-controlled and parallel group trial, T therapy, Hoyos et al. 114 demonstrated increased insulin sensitivity, reduced liver fat and increased muscle mass to a greater extent than placebo. T treatment decreased arterial stiffness and decreased the respiratory quotient compared with placebo. It was suggested that in obese men, T therapy improved cardiometabolic parameters but did not differentially reduce overall weight or the MetS and suggested that longer term studies are required 114. In a recent meta-analysis, Corona et al. 22 showed that WL is associated with an increase in both bound and unbound T levels. The normalisation of sex hormones induced by body WL is a possible mechanism contributing to the beneficial effects of surgery in morbid obesity. This finding is supported by data published recently on body weight fluctuations and sex hormones in two epidemiological studies 32,115. Androgen deprivation therapy in men with PCa produced accumulation of visceral and subcutaneous abdominal fat and increased visceral fat area appears more closely linked to T than estradiol deficiency. This was also associated with increased IR 123.
Recently, it has been shown that reduced levels of TT, free T and SHBG are associated with adverse plasma levels of TGs, insulin and HDL cholesterol in a young male population. T and SHBG are also correlated inversely with SBP. The effect of T and SHBG on CVD risk factors in men was attributed in part to the higher risk for type 2 diabetes, MetS and CVD 124. TD is associated with a pro-atherogenic lipid profile as observed in relation to the MetS. Studies using depot injections of intramuscular T undecanoate have shown reductions in TG, TC and LDL cholesterol and increases in HDL cholesterol 89.
The link between the various comorbidities underlying the MetS such as overweight, obesity, T2DM, hypertension and unfavourable lipid profile is commensurate with cardiometabolic dysfunction and CVD. Appropriate management and treatments of T2DM, hypertension and dyslipidaemia represent a challenging paradigm, and to date, there has been no single pharmaco-therapeutic agent that could ameliorate or improve most of these conditions. A host of agents is needed to treat such comorbidities 125. T therapy appears to improve many of such comorbidities and this is attributed to common mechanisms of action linking the pathophysiology of such conditions.
We have recently discussed the potential adverse side effects of T therapy in patients enrolled in this registry 49. There were no occurrences of urinary retention or other problems related to BPH. In addition, few subjects had increased haematocrit values > 52%, which all resolved without intervention. Our data are congruent with a recent study showing that T supplementation was well tolerated and there were fewer cardiovascular events in the T-treated groups compared with placebo 126.
A limitation of the study is its observational nature. The study was not designed to specifically investigate the effects of T on the MetS, and patients were not selected for specific comorbidities. On the contrary, patients represented a cohort in a real-life setting with various symptoms, comorbidities and conditions. Another limitation of this study was that a number of plasma hormones such as estradiol and gonadotrophins were not measured in all patients, in part because of financial constraints.
In this study, we demonstrate that long-term treatment with T to restore physiological levels ameliorates a number of the components of the MetS. T treatment significantly reduced TC, LDL cholesterol, TGs and increased HDL cholesterol levels. Furthermore, T treatment significantly reduced blood glucose and HbA1c levels and improved systolic and diastolic blood pressure. These findings strongly suggest that T therapy ameliorates MetS components and this may prove useful in reducing the risk of CVD. These findings suggest that long-term treatment of men with TD restoring physiological levels of T produces important clinical benefits. This study differs from previous studies in that it followed men with TD for a period of 5 years, which is the longest reported duration of treatment to date.
Author contributions
Dr Traish was involved in the concept and design of the study, as well as data analysis and interpretation and drafting of the manuscript. Dr Haider was responsible for data collection and also study concept and design. Dr Doros was responsible for performing all the statistical analyses and participated in data analyses and interpretations. Dr Saad was responsible for concept and design of the study as well as data analysis and interpretation and had a critical role in the revision of the manuscript. All authors contributed to the analysis and interpretation of study data and critically reviewed and approved the manuscript before submission.
References
- 1.Aub JC. The use of testosterone. N Engl J Med. 1940;222:877–81. [Google Scholar]
- 2.Aub JC, Kety SS. Recent advances in testosterone therapy. N Engl J Med. 1943;228:338–43. [Google Scholar]
- 3.Traish AM, Kypreos KE. Testosterone and cardiovascular disease: an old idea with modern clinical implications. Atherosclerosis. 2011;214:244–8. doi: 10.1016/j.atherosclerosis.2010.08.078. [DOI] [PubMed] [Google Scholar]
- 4.Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217:R47–71. doi: 10.1530/JOE-12-0582. [DOI] [PubMed] [Google Scholar]
- 5.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217:R25–45. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- 6.Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and type 2 diabetes. Nat Rev Endocrinol. 2013;9:479–3. doi: 10.1038/nrendo.2013.122. [DOI] [PubMed] [Google Scholar]
- 7.Saad F, Aversa A, Isidori AM, Gooren LJ. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev. 2012;8:131–43. doi: 10.2174/157339912799424573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34:1669–75. doi: 10.2337/dc10-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009;30:10–22. doi: 10.2164/jandrol.108.005215. [DOI] [PubMed] [Google Scholar]
- 10.Traish AM, Saad F, Guay A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J Androl. 2009;30:23–32. doi: 10.2164/jandrol.108.005751. [DOI] [PubMed] [Google Scholar]
- 11.Traish AM, Saad F, Feeley RJ, Guay A. The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl. 2009;30:477–94. doi: 10.2164/jandrol.108.007245. [DOI] [PubMed] [Google Scholar]
- 12.Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med. 2011;124:578–87. doi: 10.1016/j.amjmed.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Traish AM, Abdou R, Kypreos KE. Androgen deficiency and atherosclerosis: the lipid link. Vascul Pharmacol. 2009;51:303–13. doi: 10.1016/j.vph.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–92. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462–8. doi: 10.1210/jc.2004-0804. [DOI] [PubMed] [Google Scholar]
- 16.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 17.Corona G, Mannucci E, Petrone L, et al. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res. 2006;18:190–7. doi: 10.1038/sj.ijir.3901391. [DOI] [PubMed] [Google Scholar]
- 18.Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 19.Corona G, Rastrelli G, Monami M, et al. Body mass index regulates hypogonadism-associated cv risk: results from a cohort of subjects with erectile dysfunction. J Sex Med. 2011;8:2098–105. doi: 10.1111/j.1743-6109.2011.02292.x. [DOI] [PubMed] [Google Scholar]
- 20.Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8:272–83. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 21.Corona G, Rastrelli G, Morelli A, Vignozzi L, Mannucci E, Maggi M. Hypogonadism and metabolic syndrome. J Endocrinol Invest. 2011;34:557–67. doi: 10.3275/7806. [DOI] [PubMed] [Google Scholar]
- 22.Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–43. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 23.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 24.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 25.Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol. 2011;40:189–207. doi: 10.1093/ije/dyq158. [DOI] [PubMed] [Google Scholar]
- 26.Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;143:889–97. doi: 10.1093/oxfordjournals.aje.a008832. [DOI] [PubMed] [Google Scholar]
- 27.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–4. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 28.Oh JY, Barrett-Connor E, Wedick NM, Wingard DL Rancho Bernardo Study. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 30.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–50. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez A, Muller DC, Metter EJ, et al. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92:3568–72. doi: 10.1210/jc.2006-2764. [DOI] [PubMed] [Google Scholar]
- 32.Selvin E, Feinleib M, Zhang L, et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–8. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 33.Haring R, Völzke H, Felix SB, et al. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes. 2009;58:2027–31. doi: 10.2337/db09-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad F. Androgen therapy in men with testosterone deficiency: can testosterone reduce the risk of cardiovascular disease? Diabetes Metab Res Rev. 2012;28(Suppl. 2):52–9. doi: 10.1002/dmrr.2354. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Ford ES, Li B, Giles WH, Liu S. Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care. 2010;33:1618–24. doi: 10.2337/dc09-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corona G, Mannucci E, Ricca V, et al. The age-related decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. Int J Androl. 2009;32:720–8. doi: 10.1111/j.1365-2605.2009.00952.x. [DOI] [PubMed] [Google Scholar]
- 37.Camacho EM, Huhtaniemi IT, O'Neill TW, et al. EMAS Group. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168:445–55. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 38.Muraleedharan V, Jones TH. Testosterone and the metabolic syndrome. Ther Adv Endocrinol Metab. 2010;1:207–23. doi: 10.1177/2042018810390258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mårin P. Testosterone and regional fat distribution. Obes Res. 1995;3(Suppl. 4):609S–12S. doi: 10.1002/j.1550-8528.1995.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 40.Mårin P, Odén B, Björntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab. 1995;80:239–43. doi: 10.1210/jcem.80.1.7829619. [DOI] [PubMed] [Google Scholar]
- 41.Simon D, Charles M-A, Lahlou N, et al. Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-month randomized placebo-controlled trial. Diabetes Care. 2001;24:2149–51. doi: 10.2337/diacare.24.12.2149. [DOI] [PubMed] [Google Scholar]
- 42.Naharci MI, Pinar M, Bolu E, Olgun A. Effect of testosterone on insulin sensitivity in men with idiopathic hypogonadotropic hypogonadism. Endocrine Pract. 2007;13:629–35. doi: 10.4158/EP.13.6.629. [DOI] [PubMed] [Google Scholar]
- 43.Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–37. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–9. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]
- 45.Aversa A, Bruzziches R, Francomano D, Spera G, Lenzi A. Efficacy and safety of two different testosterone undecanoate formulations in hypogonadal men with metabolic syndrome. J Endocrinol Invest. 2010;33:776–83. doi: 10.1007/BF03350341. [DOI] [PubMed] [Google Scholar]
- 46.Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7:3495–503. doi: 10.1111/j.1743-6109.2010.01931.x. [DOI] [PubMed] [Google Scholar]
- 47.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 48.Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in non-obese aging men. J Clin Endocrinol Metab. 2008;93:139–46. doi: 10.1210/jc.2007-1291. [DOI] [PubMed] [Google Scholar]
- 49.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20407. Advance online publication 22 Apr 2013, doi: 10.1002/oby.20407. [DOI] [PubMed] [Google Scholar]
- 50.Nieschlag E, Behre HM, editors. Andrology. Berlin, Heidelberg, New York: Springer; 1997. 1st edn. [Google Scholar]
- 51.Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–9. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 53.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schröder FH, Hugosson J, Roobol MJ, et al. ERSPC Investigators. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muller RL, Gerber L, Moreira DM, Andriole G, Castro-Santamaria R, Freedland SJ. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the reduction by dutasteride of prostate cancer events trial. Eur Urol. 2012;62:757–64. doi: 10.1016/j.eururo.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 56.Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–20. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 57.Morgentaler A. Goodbye androgen hypothesis, hello saturation model. Eur Urol. 2012;62:765–7. doi: 10.1016/j.eururo.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 58.Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92:3844–53. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- 59.Page ST, Herbst KL, Amory JK, et al. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26:85–92. [PubMed] [Google Scholar]
- 60.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 61.Monroe AK, Dobs AS. The effect of androgens on lipids. Curr Opin Endocrinol Diabetes Obes. 2013;20:132–9. doi: 10.1097/MED.0b013e32835edb71. [DOI] [PubMed] [Google Scholar]
- 62.Ohlsson C, Barrett-Connor E, Bhasin S, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J Am Coll Cardiol. 2011;58:1674–81. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 63.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 64.Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol. 2005;63:280–93. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 65.Kypreos K. HDL quality in atherosclerosis: can ratios between apolipoproteins of HDL be used effectively to indicate risk of premature myocardial infarction? Clin Lipidol. 2012;7:127–9. [Google Scholar]
- 66.Tan KC, Shiu SW, Pang RW, Kung AW. Effects of testosterone replacement on HDL subfractions and apolipoprotein A-I containing lipoproteins. Clin Endocrinol (Oxf) 1998;48:187–94. [PubMed] [Google Scholar]
- 67.Rubinow KB, Tang C, Hoofnagle AN, et al. Acute sex steroid withdrawal increases cholesterol efflux capacity and HDL-associated clusterin in men. Steroids. 2012;77:454–60. doi: 10.1016/j.steroids.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubinow KB, Vaisar T, Tang C, Matsumoto AM, Heinecke JW, Page ST. Testosterone replacement in hypogonadal men alters the HDL proteome but not HDL cholesterol efflux capacity. J Lipid Res. 2012;53:1376–83. doi: 10.1194/jlr.P026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lemieux I, Lamarche B, Couillard C, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med. 2001;161:2685–92. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 70.Gimeno-Orna JA, Faure-Nogueras E, Sancho-Serrano MA. [D] Usefulness of total cholesterol/HDL-cholesterol ratio in the management of diabetic dyslipidaemia. Diabet Med. 2005;22:26–31. doi: 10.1111/j.1464-5491.2004.01341.x. [DOI] [PubMed] [Google Scholar]
- 71.Kim SW, Jee JH, Kim HJ, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013 doi: 10.1016/j.ijcard.2013.03.027. Advance online publication March 29 2013, doi: 10.1016/j.ijcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 72.Bleda S, de Haro J, Varela C, Esparza L, Rodriguez J, Acin F. Improving total-cholesterol/HDL-cholesterol ratio results in an endothelial dysfunction recovery in peripheral artery disease patients. Cholesterol. 2012;2012:895326. doi: 10.1155/2012/895326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agledahl I, Skjaerpe PA, Hansen JB, Svartberg J. Low serum testosterone in men is inversely associated with non-fasting serum triglycerides: the Tromsø study. Nutr Metab Cardiovasc Dis. 2008;18:256–62. doi: 10.1016/j.numecd.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 74.Maneschi E, Morelli A, Filippi S, et al. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J Endocrinol. 2012;215:347–62. doi: 10.1530/JOE-12-0333. [DOI] [PubMed] [Google Scholar]
- 75.Jiann BP, Hsieh JT, Liu SP, Hsu SH, Wu HC. Associations of endogenous testosterone and lipid profiles in middle-aged to older Taiwanese men. Int J Impot Res. 2011;23:62–9. doi: 10.1038/ijir.2011.5. [DOI] [PubMed] [Google Scholar]
- 76.Robinson JG, Wang S, Jacobson TA. Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol. 2012;110:1468–76. doi: 10.1016/j.amjcard.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 77.Adams SP, Tsang M, Wright JM. Lipid lowering efficacy of atorvastatin. Cochrane Database Syst Rev. 2012;12:CD008226. doi: 10.1002/14651858.CD008226.pub2. [DOI] [PubMed] [Google Scholar]
- 78.Permpongkosol S, Tantirangsee N, Ratana-olarn K. Treatment of 161 men with symptomatic late onset hypogonadism with long-acting parenteral testosterone undecanoate: effects on body composition, lipids, and psychosexual complaints. J Sex Med. 2010;7:3765–3674. doi: 10.1111/j.1743-6109.2010.01994.x. [DOI] [PubMed] [Google Scholar]
- 79.Jones TH. Effects of testosterone on type 2 diabetes and components of the metabolic syndrome. J Diabetes. 2010;2:146–56. doi: 10.1111/j.1753-0407.2010.00085.x. [DOI] [PubMed] [Google Scholar]
- 80.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJG, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol. 2010;73:602–12. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 81.Barud W, Palusiński R, Bełtowski J, Wójcicka G. Inverse relationship between total testosterone and anti-oxidized low density lipoprotein antibody levels in ageing males. Atherosclerosis. 2002;164:283–8. doi: 10.1016/s0021-9150(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 82.Simon D, Charles MA, Nahoul K, et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: the Telecom Study. J Clin Endocrinol Metab. 1997;82:682–5. doi: 10.1210/jcem.82.2.3766. [DOI] [PubMed] [Google Scholar]
- 83.Mäkinen JI, Perheentupa A, Irjala K, et al. Endogenous testosterone and serum lipids in middle-aged men. Atherosclerosis. 2008;197:688–93. doi: 10.1016/j.atherosclerosis.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78:539–45. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 85.Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1992;117:807–11. doi: 10.7326/0003-4819-117-10-807. [DOI] [PubMed] [Google Scholar]
- 86.Haffner SM, Valdez RA, Stern MP, Katz MS. Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disord. 1993;17:643–9. [PubMed] [Google Scholar]
- 87.Haffner SM, Mykkänen L, Valdez RA, Katz MS. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J Clin Endocrinol Metab. 1993;77:1610–5. doi: 10.1210/jcem.77.6.8263149. [DOI] [PubMed] [Google Scholar]
- 88.Van Pottelbergh I, Braeckman L, De Bacquer D, De Backer G, Kaufman JM. Differential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged men. Atherosclerosis. 2003;166:95–102. doi: 10.1016/s0021-9150(02)00308-8. [DOI] [PubMed] [Google Scholar]
- 89.Stanworth RD, Kapoor D, Channer KS, Jones TH. Dyslipidaemia is associated with testosterone, oestradiol and androgen receptor CAG repeat polymorphism in men with type 2 diabetes. Clin Endocrinol. 2011;74:624–30. doi: 10.1111/j.1365-2265.2011.03969.x. [DOI] [PubMed] [Google Scholar]
- 90.Agledahl I, Hansen JB, Svartberg J. Impact of testosterone treatment on postprandial triglyceride metabolism in elderly men with subnormal testosterone levels. Scand J Clin Lab Invest. 2008;68:641–8. doi: 10.1080/00365510801999068. [DOI] [PubMed] [Google Scholar]
- 91.Zitzmann M, Mattern A, Hanisch J, Gooren L, Jones H, Maggi M. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1,438 men. J Sex Med. 2013;10:579–88. doi: 10.1111/j.1743-6109.2012.02853.x. [DOI] [PubMed] [Google Scholar]
- 92.Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7. [PubMed] [Google Scholar]
- 93.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 94.Bhattacharya RK, Khera M, Blick G, Kushner H, Nguyen D, Miner MM. Effect of 12 months of testosterone replacement therapy on metabolic syndrome components in hypogonadal men: data from the Registry Testim in the US (TRiUS) BMC Endocr Disord. 2011;11:18–29. doi: 10.1186/1472-6823-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 96.Dubey RK, Oparil S, Imthum B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 97.Fogari R, Preti P, Zoppi A, et al. Serum testosterone levels and arterial blood pressure in the elderly. Hypertens Res. 2005;28:625–30. doi: 10.1291/hypres.28.625. [DOI] [PubMed] [Google Scholar]
- 98.Svartberg J, von Mühlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur J Endocrinol. 2004;150:65–71. doi: 10.1530/eje.0.1500065. [DOI] [PubMed] [Google Scholar]
- 99.Li JY, Zhu JC, Dou JT, et al. Effects of androgen supplementation therapy on partial androgen deficiency in the aging male: a preliminary study. Aging Male. 2002;5(1):47–51. [PubMed] [Google Scholar]
- 100.Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 101.Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci. 2003;104:195. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 102.Brand JS, Wareham NJ, Dowsett M, et al. Associations of endogenous testosterone and SHBG with glycated haemoglobin in middle-aged and older men. Clin Endocrinol (Oxf) 2011;74:572–8. doi: 10.1111/j.1365-2265.2010.03951.x. [DOI] [PubMed] [Google Scholar]
- 103.Sasaki J, Otonari T, Uchida Y, Ikeda Y, Biro S, Kono S. Effects of pravastatin and atorvastatin on HDL cholesterol and glucose metabolism in patients with dyslipidemia and glucose intolerance: the PRAT Study. J Atheroscler Thromb. 2013;20:368–79. doi: 10.5551/jat.13532. [DOI] [PubMed] [Google Scholar]
- 104.Richard C, Couture P, Desroches S, Lamarche B. Effect of the Mediterranean diet with and without weight loss on markers of inflammation in men with metabolic syndrome. Obesity (Silver Spring) 2013;21:51–7. doi: 10.1002/oby.20239. [DOI] [PubMed] [Google Scholar]
- 105.Musani SK, Vasan RS, Bidulescu A, et al. Aldosterone, C-reactive protein, and plasma b-type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: findings from the Jackson Heart Study. Diabetes Care. 2013;36:3084–92. doi: 10.2337/dc12-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jung CH, Lee WY, Kim SY, et al. The risk of metabolic syndrome according to the high-sensitivity C-reactive protein in apparently healthy Koreans. Int J Cardiol. 2008;129:266–71. doi: 10.1016/j.ijcard.2007.07.092. [DOI] [PubMed] [Google Scholar]
- 107.Ridker PM, Wilson PWF, Grandy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–25. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 108.Siest G, Schiele F, Galteau MM, et al. Aspartate aminotransferase and alanine aminotransferase activities in plasma: statistical distributions, individual variations, and reference values. Clin Chem. 1975;21:1077–87. [PubMed] [Google Scholar]
- 109.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;27:1266–71. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 110.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 111.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–50. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 112.Goessling W, Massaro JM, Vasan RS, D'Agostino RB, Ellison RC, Fox CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology. 2008;135:1935–44. doi: 10.1053/j.gastro.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olynyk JK, Knuiman MW, Divitini ML, Davis TM, Beilby J, Hung J. Serum alanine aminotransferase, metabolic syndrome, and cardiovascular disease in an Australian population. Am J Gastroenterol. 2009;104:1715–22. doi: 10.1038/ajg.2009.229. [DOI] [PubMed] [Google Scholar]
- 114.Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167:531–41. doi: 10.1530/EJE-12-0525. [DOI] [PubMed] [Google Scholar]
- 115.Gates MA, Mekary RA, Chiu GR, Ding EL, Wittert GA, Araujo AB. Sex steroid hormone levels and body composition in men. J Clin Endocrinol Metab. 2013;98(6):2442–50. doi: 10.1210/jc.2012-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–19. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–8. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 118.De Maddalena C, Vodo S, Petroni A, Aloisi AM. Impact of testosterone on body fat composition. J Cell Physiol. 2012;227:3744–8. doi: 10.1002/jcp.24096. [DOI] [PubMed] [Google Scholar]
- 119.Traish AM, Toselli P, Jeong SJ, Kim NN. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen deficiency. J Androl. 2005;26:242–8. doi: 10.1002/j.1939-4640.2005.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 120.Singh R, Artaza JN, Taylor WE, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147(1):141–54. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blouin K, Nadeau M, Perreault M, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf) 2010;72:176–88. doi: 10.1111/j.1365-2265.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- 122.Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol. 2009;301:97–103. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 123.Hamilton EJ, Gianatti E, Strauss BJ, et al. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf) 2011;74:377–83. doi: 10.1111/j.1365-2265.2010.03942.x. [DOI] [PubMed] [Google Scholar]
- 124.Firtser S, Juonala M, Magnussen CG, et al. Relation of total and free testosterone and sex hormone-binding globulin with cardiovascular risk factors in men aged 24-45 years. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2012;222:257–62. doi: 10.1016/j.atherosclerosis.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 125.Niswender K. Diabetes and obesity: therapeutic targeting and risk reduction – a complex interplay. Diabetes Obes Metab. 2010;12:267–87. doi: 10.1111/j.1463-1326.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 126.Hildreth KL, Barry DW, Moreau KL, et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab. 2013;98:1891–900. doi: 10.1210/jc.2012-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]


