Abstract
Globally, spatial distributions of fish stocks are shifting but although the role of climate change in range shifts is increasingly appreciated, little remains known of the likely additional impact that high levels of fishing pressure might have on distribution. For North Sea cod, we show for the first time and in great spatial detail how the stock has shifted its distribution over the past 100 years. We digitized extensive historical fisheries data from paper charts in UK government archives and combined these with contemporary data to a time-series spanning 1913–2012 (excluding both World Wars). New analysis of old data revealed that the current distribution pattern of cod – mostly in the deeper, northern- and north-easternmost parts of the North Sea – is almost opposite to that during most of the Twentieth Century – mainly concentrated in the west, off England and Scotland. Statistical analysis revealed that the deepening, northward shift is likely attributable to warming; however, the eastward shift is best explained by fishing pressure, suggestive of significant depletion of the stock from its previous stronghold, off the coasts of England and Scotland. These spatial patterns were confirmed for the most recent 3½ decades by data from fisheries-independent surveys, which go back to the 1970s. Our results demonstrate the fundamental importance of both climate change and fishing pressure for our understanding of changing distributions of commercially exploited fish.
Keywords: Atlantic cod, distribution shift, fisheries, fishing pressure, Gadus morhua, marine climate change, North Sea
Introduction
Globally, spatial distributions of fish stocks are shifting (e.g. Cheung et al., 2013; Poloczanska et al., 2013), and this has been attributed to recent climate change, but also intensive fishing pressure, with many stocks having been reduced to historically low levels over the past half century. Atlantic cod Gadus morhua has been viewed as emblematic of these dramatic changes (Brander, 2010): on the one hand, strong relationships have been observed between cod reproductive output and climate variables such as sea surface temperature (e.g. O'Brien et al., 2000; Drinkwater, 2005), while on the other hand, cod has been almost completely extirpated in some regions such as off eastern North America (Hutchings & Myers, 1994; Rose, 2007) as a result of sustained exploitation, combined with unfavourable climatic conditions (Lilly et al., 2013).
Arguably cod has been among the most important of commercial fish species internationally. Its fishery has profoundly influenced the economical and political development of Europe and North America (e.g. Kurlansky, 1997). Disputes over cod fishing grounds have led to well-known conflicts such as the British/Icelandic ‘Cod Wars’ of the 1950s–1970s (Jónsson, 1982). Atlantic cod remains one of the world's most important fish species, and in 2010 ranked 12th among all fish species in terms of global landings (FAO, 2011), in spite of significant declines of many cod stocks in recent decades (Mieszkowska et al., 2009). At the northernmost extent of its range, for example, in the Barents Sea, cod seem to respond to warmer temperatures in a positive fashion, and in 2012/2013 populations are witnessing their highest levels since the late 1940s/early 1950s. By contrast, further south near the southern limit of distribution in the North and Celtic Seas, recruitment success (the number of juveniles entering the system) has been very low in recent years and there exists a strong negative correlation between cod recruitment and the prevailing sea surface temperature (O'Brien et al., 2000; Drinkwater, 2005) that may be related to change in the distribution of key zooplankton prey animals for fish larvae (Beaugrand & Kirby, 2010). Many of the basic tenets of climatic influences on fish and fisheries were established through working on cod, particularly as a result of the recent ‘ICES/GLOBEC’ initiative on ‘Cod and Climate Change’, that yielded many peer-reviewed research papers (Brander, 2010). However, it is important to separate the influence of long-term trends in global and local temperature from the effects of sustained fishing pressure, because fishing is also known to have exerted a strong selective pressure on populations, with fish now maturing earlier, at smaller sizes and perhaps distinct subpopulations having disappeared (Harrald et al., 2010) completely along with their local adaptations and traits.
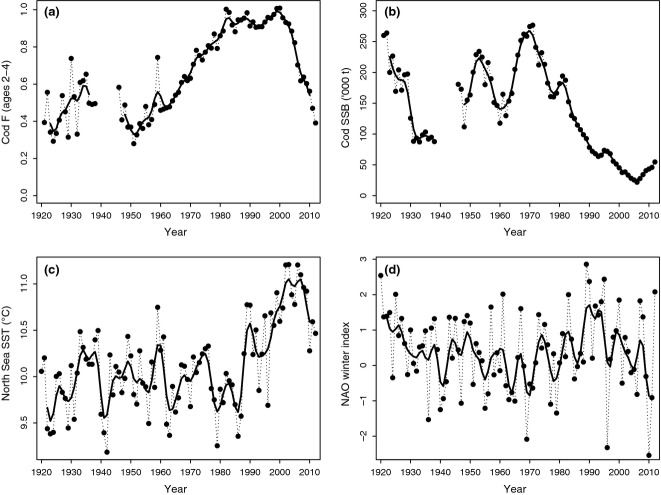
North Sea cod has also shown a marked decline, noteworthy because this followed a highly productive period, the ‘gadoid outburst’ (late 1960s to mid-1980s: Daan, 1978; Hislop, 1996). During the gadoid outburst, not only cod but also haddock Melanogrammus aeglefinus, whiting Merlangius merlangus, and saithe Pollachius virens produced some of the largest year-classes on record (Jones & Hislop, 1978). While previously, cod landings had remained fairly stable throughout the period 1900–1950, survey indices and stock assessment have indicated that the high landings of cod during the gadoid outburst were not only due to increasing fishing effort, but primarily the result of enhanced recruitment and stock biomass (Pope & Macer, 1996; and see Fig.1a and b), possibly associated with improved climatic conditions or removal of larval predators (herring Clupea harengus: Cushing, 1980). Unfortunately, cod recruitment dropped to much lower levels in the mid-1980s and especially 1990s, while fishing effort remained high until the Millennium (ICES, 2013c). In consequence, the spawning stock biomass (SSB) of cod has been at historically low levels in the North Sea for more than two decades now (Fig.1a and b). The North Sea cod stock is thought to be composed of several distinct subunits that differ both genetically and phenotypically (Harrald et al., 2010). A potential cause for such spatial variability is adaptive divergence, which may be linked to differences in thermal environment and (or) historical fishing pressure. Genetic analysis of archived cod otoliths (ear bones) has suggested that one distinct substock may have disappeared completely between 1970 and 1981, probably as a result of over-exploitation (Hutchinson et al., 2003), and this may result in future difficulties in recovering populations at certain localities.
Fig 1.
Time-series of (a) North Sea cod fishing mortality, averaged over ages 2–4 years; (b) North Sea cod spawning stock biomass (SSB); (c) Hadley interpolated sea surface temperature (SST) for the North Sea; and (d) North Atlantic oscillation (NAO) winter index. Long-term variability is illustrated by heavy solid lines, representing values smoothed with a low-pass filter with five weights (1, 3, 4, 3, and 1) to remove fluctuations with periods <3 years (following Hurrell, 1995). Data sources: see Methods.
The past three decades have also seen an apparent shift in the mean distribution of cod within the North Sea, to more northerly and on average deeper waters; this has been reported by studies based on fisheries-independent International Bottom Trawl Surveys (IBTS; see Hedger et al., 2004; Perry et al., 2005; Rindorf & Lewy, 2006; Dulvy et al., 2008). As this shift coincided with the decline in stock size, it is important to understand its possible causes, not only because of predicted links between distribution and abundance (Blanchard et al., 2005) but also because changes in fish distributions may have knock-on effects upon fisheries (Cheung et al., 2013).
Two main hypotheses have been put forward to explain the changes in cod distribution in the North Sea (Engelhard et al., 2010). Firstly, climate change is expected to result in contractions, expansions, or shifts in fish distribution (Rijnsdorp et al., 2009). In the North Sea, a distinct warming trend has occurred over the past 30 years (Fig.1c), and the area has been identified as a ‘hotspot’ of maritime climate change (Holt et al., 2012). This has coincided with a northward shift in many, but not all, North Sea fish species (Beare et al., 2004; Perry et al., 2005), and an average deepening shift of −3.6 m per decade since the 1980s (Dulvy et al., 2008). Secondly, fishing pressure has, over the same period, been consistently higher in the southern compared to northern part of the North Sea (Jennings et al., 1999). Thus, there may have been a greater rate of fishery-induced depletion in the south, and hence, an apparent northward shift in population distribution is to be expected.
Disentangling these two possibly inter-related drivers of changes in cod distribution is difficult, because consistent fishery-independent survey data are limited to the past 35 years. Here, we examine long-term changes in North Sea cod distribution, based on a unique data set of British fisheries landings per unit effort (lpue) records spanning a far longer timespan, 1913–2012, excluding both World Wars – covering warming and cooling periods, and including periods of contrasting levels of fishing effort. The data were largely digitized from historical paper charts recovered from UK government archives. For the recent period only (1977–2012), these data could be augmented with fishery-independent, scientific survey data on catch per unit effort (cpue). The aims of the present paper are to:
Describe long-term distribution shifts of cod within the North Sea over the period 1913–2012, based on commercial lpue data;
For the most recent period (1977–2012) assess if cod distribution based on commercial lpue and survey cpue reveal the same spatial patterns;
Examine the extent to which distribution shifts of North Sea cod can be attributed to climate change and/or fishing pressure.
Materials and methods
Data and modelling of cod distribution
For the period 1913–1980, cod data were obtained from historical fisheries ‘statistical charts’ (catalogued in Engelhard, 2005; http://www.cefas.defra.gov.uk/publications/techrep/tech128.pdf) that were produced by the UK Ministry of Agriculture, Fisheries and Food (MAFF), now the UK Department for Environment, Food and Rural Affairs (Defra). These charts show fishing effort (number of hours fished) and fish landings (cwt, converted into kg) by British otter trawlers (either steam- or motor-driven) for each ICES rectangle (0.5° latitude, by 1° longitude) in the North Sea. These data record all fish that were landed by the otter trawl fleet into England and Wales (1913, 1968–1980) or into England, Scotland and Wales (1920–1967). For the period 1968–2012, data on otter trawler landings into Scotland were obtained from the Fisheries Management Database of Marine Scotland. For 1982–2012, data on otter trawler landings into England and Wales were obtained from the Fisheries Activity Database of Defra/Cefas. Landings and effort data by rectangle were converted into landings per unit effort (lpue, kg h−1) to describe the spatial distribution of cod biomass within the North Sea.
Over the past century, the fishing power of otter trawlers to catch cod has steadily improved (Engelhard, 2008). Owing to this ‘technological creep’, lpue cannot be directly compared between decades to describe abundance change. Here, we do not use lpue to analyse temporal change in cod abundance, but only to look at trends in spatial distribution. To account for the confusing effect of increasing fishing power of trawlers over time, we first normalized the lpue values in any given year by dividing by the annual mean. For each rectangle i in year y, normalized lpue (lpue′i,y) was calculated as follows:
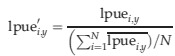 |
(1) |
where lpuei,y represents the raw lpue values in rectangle i and year y, and N is the total number of rectangles in the study area. It was assumed that relative lpue values across the statistical grid indicate the spatial distribution of cod. Some rectangles within the study grid were not fished by UK otter trawers in all years, leading to missing values of cod lpue in some years at these locations. To exclude data from rectangles that were not adequately fished by the otter trawlers in any given year, any rectangles comprising <50 h effort were removed from the analysis. Instead, lpue′i,y in the given rectangle was assumed to be equal to the long-term mean of lpue′i,y for the respective rectangle.
Next, when mapping cod spatial distribution we corrected for temporal change in overall cod biomass, using published estimates of North Sea cod SSB [1921–1962 from Pope & Macer, 1996; and 1963–2012 from the offical stock assessment by ICES (2013c)]. For each rectangle i in year y, SSB-scaled lpue (lpue″i,y) was calculated, as follows:
| (2) |
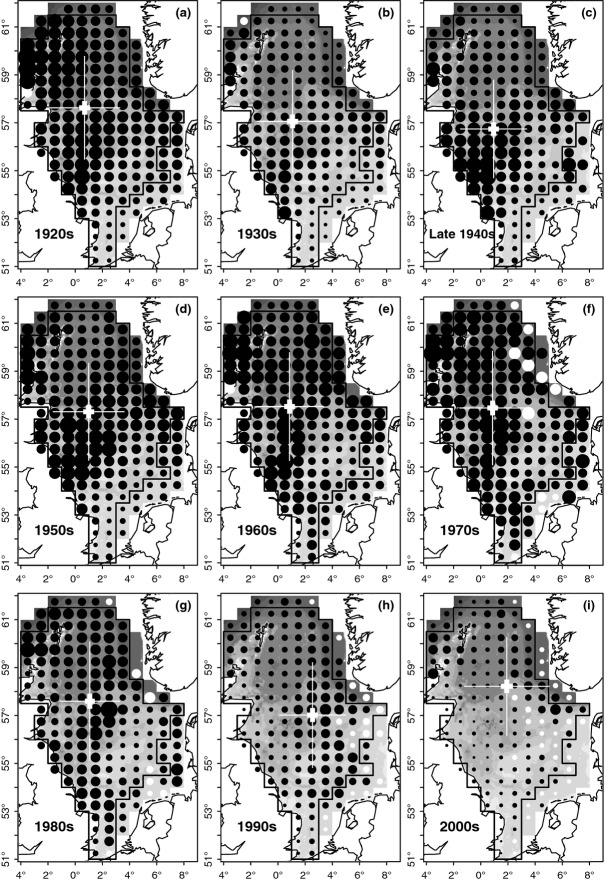
where SSBy is the estimate of SSB in year y and  is the long-term mean SSB. By decade, we calculated spatial distribution of lpue by rectangle for a large area encompassing the majority of the North Sea (shaded in Fig.2).
is the long-term mean SSB. By decade, we calculated spatial distribution of lpue by rectangle for a large area encompassing the majority of the North Sea (shaded in Fig.2).
Fig 2.
Decadal changes in North Sea cod distribution, 1920s–2000s, based on fisheries lpue (landings per unit effort by British trawlers). The area sizes of the black circles are proportional to cod lpue, normalized by decade (Eqn 1) and corrected for the average spawning stock biomass (SSB) in each decade (Eqn 2, to visualize the stock's long-term biomass dynamics. In rectangles where no lpue data were available in a given decade (no effort by British trawlers), white circles represent the long-term average lpue for the given rectangle (again corrected for mean decadal SSB). For each map, the white cross indicates the centre of gravity of cod distribution, with its standard error (shorter, thick white lines) and standard deviation (longer, thin white lines) in the longitudinal and latitudinal directions. The black-lined polygon encompasses those rectangles included in the analyses on centres of gravity of distribution. Bathymetry is indicated by light to dark grey shading (from shallow to deep).
To cross-validate the usefulness of commercial lpue to describe cod spatial patterns, a separate data set on cod distribution for 1977–2012 was collated from fisheries-independent International Bottom Trawl Surveys (IBTS). Survey catch per unit effort (cpue) data were limited to the winter IBTS, as consistent data for the spring, summer and autumn IBTS do not date back further than 1991 (ter Hofstede & Daan, 2008). The survey samples both juvenile and adult cod, whereas commercial landings will normally consist of fish above minimum landing size. To improve consistency between the two data sets, survey data were limited to fish >29 cm body length. Numbers per hour fishing were converted to cpue in kg h−1 using a standard length–weight relationship for North Sea cod (Coull et al., 1989).
As an approach to quantify shifts in population distribution, we calculated the ‘centres of gravity’ of the latitudinal, longitudinal, and depth distributions of North Sea cod (as in Engelhard et al., 2011). Centres of gravity were calculated for both cod lpue (1913–2012) and cpue (1977–2012) distribution. This analysis was based on a slightly less extensive area within the North Sea that included only those rectangles with cpue and lpue data available for the majority of years in the time series (see polygon line in Fig.2). To reveal distribution shifts over 1913–2012, lpue data were used to calculate the centre of gravity (COG) of latitudinal distribution for each year:
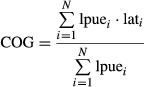 |
(3) |
where lpuei is the lpue for each rectangle i, lati is the latitudinal centre of each rectangle i, and N is the total number of rectangles. Weighted standard deviations and standard errors of the weighted mean latitudes were calculated (Engelhard et al., 2011). Analogously, the longitudinal and depth centres of gravity of distribution were calculated by using the longitude of the rectangle's centre or the mean depth, respectively. In addition, for cross-validation purposes, we used IBTS data for the period 1977–2012 to calculate latitudinal, longitudinal and depth centres of gravity of cod cpue distribution.
Modelling distribution shifts in relation to climate change and fishing pressure
We examined cod lpue distribution in relation to: (i) climatic variables; and (ii) cod fishing pressure and abundance. As an indicator of sea temperature variations within the North Sea, the Hadley interpolated sea surface temperature (HadISST) time-series was used (Fig.1c). Annual means of sea surface temperatures, interpolated to 1° latitude by 1° longitude, were used (Rayner et al., 2005; with updated values provided online by the UK Meteorological Office), within the area between 3°W and 7°E, and 51°N and 61°N. As a broad-scale climate indicator, the North Atlantic oscillation (NAO) winter index (December of the previous year to March of the focal year) for 1913–2012 was taken (Jones et al., 1997), with updated values provided online by the Climatic Research Unit, University of East Anglia, Norwich, UK (www.cru.uea.ac.uk/∼timo/datapages/naoi.htm; see Fig.1d). The NAO is associated with speed and direction of westerly winds across the North Atlantic and is particularly important in winter when it exerts a strong influence on European weather patterns, and on Atlantic water inflow into the North Sea; a positive NAO is generally linked with strong wind circulation and higher atmospheric and sea temperatures in western Europe (Hurrell, 1995; Jones et al., 1997).
To describe the effects of fishing pressure, estimates of fishing mortality (F) on 2–4 years old cod for the years 1921–1938 and 1946–1962 were taken from Pope & Macer's (1996) long-term study on North Sea cod population dynamics, and for 1963–2012 from the stock assessment (ICES, 2013c). From the same sources, we used estimates of cod SSB, as descriptors for the population abundance of North Sea cod. The fishing mortality and SSB time series are shown in Fig.1a and b.
We used correlations as a first approach to explore which environmental, abundance, and/or fishing pressure variables might be associated with descriptors of cod distribution (latitudinal, longitudinal and depth). Pearson cross-moment correlations (rp) were used, as the variables did not show distributions significantly different from normality (one-sample Kolmogorov–Smirnov tests, P > 0.05). There was, however, moderate to weak autocorrelation within several time-series variables. To account for this, the test procedure for significance of correlation was adjusted using Pyper and Peterman's (1998) method, by reducing the effective degrees of freedom (and therefore increasing the P-values) according to the degree of autocorrelation (adjusted P-values are referred to here as Padj).
We used a linear mixed-effects approach to examine the relative importance of climatic variables (SST and NAO), cod fishing pressure (F) and abundance (SSB) as determinants of cod distribution (longitudinal, latitudinal and depth centres of gravity). The models included year as a random factor, in an effort to account for temporal autocorrelation. A backward selection procedure was used, starting with a full model that included all fixed effects and their interactions (SST*NAO, F*SSB). For example, the longitudinal centre of gravity of distribution the starting model was
|
|
(1) |
The best-fitting models were then established by removing insignificant terms successively and based on lowest Akaike information criterion (AIC), to reach the minimum adequate model. The models were fitted and compared using maximum likelihood, and the final model was refitted using restricted maximum likelihood (REML).
Results
Shifts in cod distribution, 1913–2012, based on fisheries lpue
Long-term landings per unit effort data (lpue) suggest that the spatial distribution of cod within the North Sea did shift during the Twentieth Century but especially markedly during its last decade, moreover that the distribution during the first decade of the Twenty-first Century has been almost opposite to that during most of the Twentieth Century. Cod now appear to be distributed mainly in the northern- and northeasternmost parts of the North Sea, whereas historically, high abundances were to be found in the western/central North Sea – but the cod stock in this region has been reduced considerably since the 1980s (Fig.2).
From the 1920s–1980s, cod were mainly distributed in the (especially western) central and northern North Sea (Fig.2a–g). Shifts within this 70-year period were comparatively minor; lpue tended to be highest around the Orkney and Shetland Islands in the 1920s, north-east of England from the 1930s (when overall abundance was lower) to 1950s, and in both these areas during the 1970s–1980s (coinciding with the gadoid outburst). This changed considerably during the last two decades, during which a significant local reduction of the cod population off the east coasts of England and Scotland became evident (Fig.2h). This was also evidenced by a marked eastward shift in lpue distribution in the 1990s, with a relative increase in the central-eastern North Sea. In the 2000s, cod abundance in the southern-central North Sea appears to have very much decreased, and high lpue values are now mainly concentrated in the northern- and north-easternmost North Sea (Fig.2i).
These spatial patterns are reflected in changes in the longitudinal, latitudinal and depth centres of gravity of lpue distribution, which were more marked during recent than earlier decades (Fig.3). This was most striking for the longitudinal centre of gravity (Fig.3a, black symbols): this remained almost constant at 1°E throughout the period 1913–1985, then suddenly shifted eastward to around 2.5°E in the 1990s, but again considerably westward in the 2000s. The latitudinal centre of gravity shifted southward during the 1920s–1930s (rp = −0.88, P < 0.0001), followed by a significant northward shift from the 1940s to the present (rp = 0.55, P < 0.0001; Fig.3b). The trend in depth distribution of cod lpue mirrored the latitudinal trend, reflecting the North–South depth gradient in the North Sea (Fig.3c): a shallowing shift in the 1920s–1930s (rp = 0.86, P < 0.0001), followed by a deepening shift from 1947–2012 (rp = −0.49, P < 0.0001). This deepening shift was interrupted for some years in the mid-1990s, when cod lpue distribution was particularly shallow (coinciding with the temporarily more south-easterly distribution). During the 2000s–2010s, the centre of gravity of cod lpue distribution was particularly deep (mean ± SD, 99.8 ± 4.5 m), and particularly far north (58.2 ± 0.2°N), compared with all earlier decades in the time-series (mean ± SD, depth 86.5 ± 6.8 m; latitude 57.3 ± 0.3°N).
Fig 3.
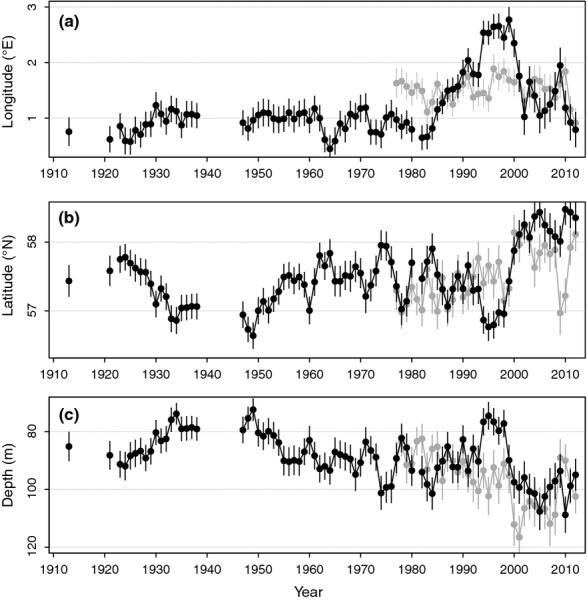
Long-term changes in (a) longitudinal and (b) latitudinal centre of gravity of North Sea cod distribution, and (c) long-term changes in the mean depth distribution, based on fisheries lpue (1913–2012, black symbols) and fisheries-independent survey cpue (1977–2012, grey symbols). Bars indicate the standard errors around the estimates.
Shifts in cod distribution, 1977–2012, based on survey cpue
For the most recent 3½ decades, distribution maps of cod based on cpue in winter IBTS surveys (see Supporting Information, Figure S1) were in broad agreement with the distribution maps based on lpue (Fig.2f–h), but different in some detail. Both cpue and lpue maps suggested a northward and deepening shift of cod during the 1990s–2000s as well as recent, significantly reduced cod abundance off eastern England and Scotland. However, the 1990s survey cpue map revealed less strong evidence for the temporary, exceptionally eastern distribution in the middle of this decade than was indicated by commercial lpue (compare Figure S1 with Fig.2h).
The longitudinal, latitudinal and depth centres of gravity of cod distribution, based on cpue, were each positively correlated with lpue-based centres of gravity (longitude: rp = 0.46, P < 0.001; latitude: rp = 0.52, P < 0.005; depth: rp = 0.36, P < 0.05). In accordance, cpue-based latitudinal and depth centres of gravity of cod distribution (Fig.3, grey symbols) showed significant changes over the study period 1977–2012 (latitude: rp = 0.62, P < 0.0001; depth: rp = 0.66, P < 0.0001), in the same direction as those observed for lpue-based centre of gravity: northward and deepening. There was no trend in longitudinal centre of gravity of cpue distribution over 1977–2012 (rp = −0.01, P = 0.94; Fig.3a). Still, the pattern of reduced presence of cod in the western-central North Sea in the 2000s was equally evident from the cpue-based and lpue-based distribution maps (compare Figure S1 with Fig.2i).
Distribution shifts in relation to climate and fishing
Correlation analyses and linear mixed-effects models suggested that distribution shifts during the past century were not straightforwardly related to any single climatic, or fisheries-related, variable, but best explained by a combination of climate and fishing pressure. Of both climatic variables, the Hadley index of North Sea SST was significantly correlated with the longitudinal (Padj = 0.0008), latitudinal (Padj = 0.006), and depth (Padj = 0.043) centres of gravity of cod distribution (see Supporting Information, Table S1), such that warmer temperatures were associated with more easterly, northerly and deeper distribution. There were no correlations between the NAO winter index and cod distribution metrics (Padj > 0.05; Table S1). The two fisheries-related variables, cod F and SSB, showed no correlations with the latitudinal or depth centres of gravity (Padj > 0.05), but both were highly significantly correlated with the longitudinal centre of gravity of cod distribution (both Padj = 0.0002); high F and low SSB were associated with more easterly distribution.
Linear mixed-effects models showed that the northward, and deepening, shifts in cod distribution could be explained by climatic variables alone (Table1). For both the latitudinal and depth responses, each of the fisheries-related variables were rejected from the full model (cod F and SSB; P > 0.05), with the final model (selection based on lowest AIC) retaining the North Sea SST and NAO index. The direction of responses was such that the northward, deepening shift was attributable to warmer sea temperatures and more negative phases of the NAO, with the temperature effect being strongest. By contrast, linear mixed-effects models also showed that the longitudinal distribution shift was explained most plausibly by the two fisheries-related variables, cod F (P < 0.00001) and SSB (P = 0.0006), and a significant interaction F × SSB (P < 0.00001). From a full model that included both climatic and fisheries-related variables, any climatic variables (SST, NAO, SST × NAO) were rejected. The direction of responses was such that high F, and a combination of high F and low SSB, were associated with more easterly distribution, lending support to the assertion that the particularly eastward cod distribution in recent decades was attributable to fishing pressure and reduced stock biomass.
Table 1.
Linear mixed-effects models on distribution responses of North Sea cod (latitudinal, longitudinal and depth centre of gravity of distribution) to variables related to climate and fishing pressure. All models included year as random effect to account for temporal autocorrelation. For each response variable, the final model is shown following a backward selection procedure, from a full model that included all main effects and interactions. Final models were selected by removing insignificant terms successively and based on lowest Akaike information criterion
| Response variable | Predictor | Estimate | SE | t | P |
|---|---|---|---|---|---|
| Longitudinal shift | F | 2.932 | 0.419 | 6.99 | <0.00001 |
| SSB | 0.007 | 0.002 | 3.56 | 0.0006 | |
| F × SSB | –0.015 | 0.003 | –5.26 | <0.00001 | |
| Latitudinal shift | SST | 0.374 | 0.102 | 3.64 | 0.0005 |
| NAO | –0.090 | 0.043 | –2.09 | 0.039 | |
| Depth shift | SST | 5.424 | 1.926 | 2.82 | 0.006 |
| NAO | –1.818 | 0.811 | –2.24 | 0.028 |
Discussion
This study shows that distribution shifts of North Sea cod have not been limited to the most recent three decades (as reported by Hedger et al., 2004; Perry et al., 2005; Rindorf & Lewy, 2006; Dulvy et al., 2008), but took place throughout the past 100 years. However, the distribution has shifted more markedly in the past 2–3 decades than in the entire 70-years period previously, so that the current distribution is very different from that during most of the Twentieth Century. Climate change alone was insufficient to account for the shifts; instead, these appeared attributable to both climate and fishing pressure.
Cod are distributed over a broad sweep of the North Atlantic (Drinkwater, 2005), and hence it is possible that patterns would be more clear-cut when the distribution is considered in its entirety. In the north, several cod stocks are currently at very high levels; the spawning stock in the Barents Sea is at a record high since 1946, the Icelandic stock is higher than has been observed over the last five decades, and also the Greenland stock is increasing (ICES, 2013a,b); by contrast, reduced catches are being observed further south. Thus, it is possible that the North Sea focus of our work is insufficient to adequately resolve broad-scale patterns of change. Simpson et al. (2011) looked at fish distribution changes over the whole northeast Atlantic region using combined survey datasets from multiple countries and found that 70% of the fish species seemed to have responded to warming by changing distribution and abundance. Similarly, Heath (2007) looked at patterns in international fisheries landings for the whole northeast Atlantic region. Densities of landings of each species (including cod) were summed by decade and expressed as a proportion of the total. Both northerly and southerly shifts were observed between decades for individual species, however more species shifted south than north between the 1970s and 1980s (a relatively cool period) and vice versa between the 1980s and 1990s (a relatively warm period). This seemed to parallel observed interdecadal changes in sea and air temperatures.
Our findings are partly based on commercial fisheries data, and the appropriateness of lpue to describe cod distribution could be challenged, given the well-known problems with fisheries data such as discarding and misreporting practices by fishers (Enever et al., 2009). However, for the period 1977–2012 where both lpue and cpue data were available, cod distribution maps based on the two sources showed broadly similar patterns (Fig.2, Figure S1) and agreed with published, survey-based studies (northward shift: Hedger et al., 2004; Perry et al., 2005; deepening shift: Dulvy et al., 2008; noting that no detailed distribution maps are provided in these studies). Still, for some specific years, centres of gravity of distribution based on cpue and lpue were quite different (e.g. mid-1990s). Differences might relate to lpue representing annual averages of cod distribution based on year-round trawling activity in any given rectangle, whereas cpue were limited to the winter (February–March) IBTS survey (consistent IBTS time-series for other seasons do not extend back further than 1991; ter Hofstede & Daan, 2008). Thus, our cpue-based maps provide a ‘snapshot’ of cod winter distribution, when cod are congregated on spawning grounds (Righton et al., 2007; Fox et al., 2008; Mieszkowska et al., 2009), whereas our lpue-based maps are more heavily weighted towards the distribution of cod while they are dispersed across feeding grounds across the North Sea during the months of April–December. We anticipate that data issues with lpue related to discarding and under-reporting practices will have been particularly prominent for the past two decades, owing to quota regulations that began in the 1980s and became increasingly strict in the 1990s (Bannister, 2004); fortunately, lpue can be cross-checked with cpue for this more recent period. Prior to the 1970s the British otter trawl fleet fishing for roundfish in the North Sea was fairly uniform, not subject to quota regulations, and there is evidence that its cod fishing power remained stable for many decades, especially during the long period when it was dominated by steam trawlers (i.e. 1900s–early 1960s: Gulland, 1964; Engelhard, 2008). This suggests that lpue data, along with survey cpue, are appropriate to describe the distribution dynamics of cod.
In the most recent, warm, decade, cod distribution was particularly northerly and deep compared to almost the entire earlier period, in line with Perry et al. (2005) and Dulvy et al. (2008) and with the general expectation with climate change of a poleward, deepening shift (Rijnsdorp et al., 2009). Indeed, cod's latitudinal and depth responses were strongly positively correlated with sea temperatures (Hadley index of North Sea SST). They were also, once the direct temperature effect was accounted for in regression analysis, significantly related with the NAO winter index but not with fisheries-related variables. Relationships of the latitudinal and depth responses with the NAO winter index were negative. This might relate to the reversed north–south temperature gradient in the North Sea during winter, and the strong, very cold easterly winds that often characterize winters during a negative phase of the NAO; this tends to result in severe winter conditions predominantly in the shallow south-eastern North Sea (Nye et al., 2014). Combined, this gives strong evidence that the northward, deepening shift of North Sea cod can be attributed to climate change.
The mechanism for climate-driven changes in distribution is likely to be complex. A simple explanation of ‘cod swimming north’ to seek out an optimal or specific temperature niche is unlikely because the thermal tolerance of cod is broad (Righton et al., 2010), and the metapopulation structure of cod in the North Sea is likely to provide a buffer through local adaptations (e.g., Perutz, 2007). Data storage tags have also revealed that individually tagged adult North Sea cod actively choose warmer areas in preference to cooler waters closer to laboratory defined optima, suggesting that warmer temperatures are not yet impairing cod physiologically (Neat & Righton, 2007). Instead, mechanisms for shifts are likely to include spatial differences in recruitment and survival, prey availability (Beaugrand & Kirby, 2010) and reproductive output, coupled with some influence on migrations (Rijnsdorp et al., 2009).
Cod has become emblematic of climate influences in fish and fisheries (Brander, 2010). Further evidence that North Sea cod is affected by climate change comes from the strong negative relationship of SST with cod recruitment and SSB, even after accounting for the reducing effect of fishing mortality (Horwood et al., 2006). All in all this suggests that the current, warm period is not favourable for North Sea cod (Drinkwater, 2005), affecting the stock in terms of distribution and stock productivity (Kell et al., 2005).
The recent eastward shift, however, was most plausibly explained by high F and low SSB, and climatic variables were not significant once fisheries-related variables were accounted for in linear mixed-effects models. It is noteworthy that this easterly distribution followed the period of most marked decline in cod SSB (Fig.1b), which has been generally attributed to reduced recruitment and sustained high fishing pressure (Bannister, 2004; Horwood et al., 2006), as well as the particularly marked reduction in cod in its previous stronghold, the western North Sea off England and Scotland (Fig.2, Figure S1). Fishing effort is not uniformly distributed, and international otter trawling effort during the 1980s and 1990s was higher close to England and Scotland than in the eastern North Sea (Jennings et al., 1999). Thus, heavier depletion in the western than eastern North Sea could be related to the apparent eastward shift of the 1990s. This is corroborated by molecular evidence for the disappearance of a former, local subpopulation of North Sea cod off Flamborough Head, northeast England, between the 1950s and 1970s (Hutchinson et al., 2003).
We conclude that North Sea cod distribution shifts are not solely attributable to climate change but rather to an interaction between climate and fishing. It is of note that North Sea cod distribution stayed fairly constant throughout the period 1920s–1980s in spite of variability in temperature and phase of the NAO and that the most significant shifts in the 1990s–2000s coincided with depletion and biomass decline. Since the Millennium, cod fishing mortality has been reduced as a result of strict management and a reduction of the fishing fleet (Horwood et al., 2006). There are some initial signs of stock recovery in the most recent few years (Fig.1b; ICES, 2013c). We are hopeful that if continued, reduced fishing mortality will allow the stock to rebuild, especially in the western-central North Sea where the species was formerly abundant. Under the current, warm climate regime, however, we regard it less likely that cod will substantially recolonize the most southerly regions.
Combined effects of fishing pressure and climate change on fish distribution patterns have been reported elsewhere in the world, including Australia (Last et al., 2011), California (Hsieh et al., 2008), Iceland and Norway (Holst et al., 2002), and for other species in the North Sea (sole Solea solea: Engelhard et al., 2011). Fishing can make populations more sensitive to climate change by reducing abundance and density, truncating the age distribution (Ottersen et al., 2006). A constriction of geographic distribution is often associated with intensive fishing pressure (Hsieh et al., 2008). At low abundance levels, populations may retreat to sites of optimal habitat only (Blanchard et al., 2005). In the Norwegian Sea, the very large herring Clupea harengus stock migrated annually between Iceland and Norway prior to its fishery-induced collapse in the 1960s, after which the tiny remaining stock migrated only along a narrow strip near the Norwegian coast; the stock, once rebuilt, again took up long-distance migrations (Holst et al., 2002). In the North Sea, turbot Scophthalmus maximus distribution contracted away from the north-west due to depletion from a ground off Scotland where it was formerly rather common but subject to intensive fishing; the ground still bears the name Turbot Bank – but turbot have only been caught sporadically here since the 1960s (Kerby et al., 2013). Studies have attempted to explicitly model these effects, using ideal free distribution theory (e.g. Blanchard et al., 2005).
The role of climate change in shifting fish distributions is now increasingly widely appreciated, through a large and growing body of literature (e.g., Beare et al., 2004; Perry et al., 2005; Rindorf & Lewy, 2006; Dulvy et al., 2008; Petitgas et al., 2012; Cheung et al., 2013; Poloczanska et al., 2013). This study highlights that fishing pressure, in addition, can be an important driver of distribution shifts (cf. Engelhard et al., 2011). Because globally, fish stocks are typically subject to the combined pressures of climate change and exploitation, and because fishing effort is often highly concentrated at specific localities within a species' overall range, there is need for wider recognition of the dual impact that both climate and fishing can have on fish distributions.
Acknowledgments
This study was supported by the Department for Food, Environment and Rural Affairs of the UK (Defra projects MF1108 ‘100 Years of Change in Fish and Fisheries’ and MF1228 ‘Physics to Fisheries’), Cefas (Seedcorn project ‘Trawling Through Time’) and the ICES Working Group on the History of Fish and Fisheries. Joyce Petrie, Bill Turrell, and Phil Kunzlik (Marine Scotland) provided Scottish fisheries data after 1968. Peter Robinson extracted post-1982 data from Defra's Fisheries Activity Database. The study benefited from discussions with Stephen Dye, Bernardo Garcia-Carreras, Laurence Kell, Christopher Lynam, Marta Söffker and Andy South, and from feedback by three anonymous referees.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Decadal changes in North Sea cod winter distribution, 1970s–2000s, based on fisheries-independent survey cpue (winter IBTS). For each decade, spatial distribution of cod cpue within the grey-shaded region is indicated by the area sizes of the black circles (proportional to cpue). In rectangles where no cpue data were available in a given decade, white circles represent the long-term average cpue. For each decade, the white cross indicates the centre of gravity of cod distribution, with its standard error (shorter, thick white lines) and standard deviation (longer, thin white lines) in the longitudinal and latitudinal directions. Bathymetry is indicated by light to dark grey shading (from shallow to deep).
Table S1. Correlations (rp) between cod distribution in the North Sea (longitude, latitude, and depth) and variables related to climate and cod fishing pressure and abundance.
References
- Bannister RCA. The rise and fall of cod (Gadus morhua) in the North Sea. In: Payne AIL, O'Brien CM, Rogers SI, editors. Management of Shared Fish Stocks. Oxford: Blackwell; 2004. pp. 316–338. [Google Scholar]
- Beare DJ, Burns F, Greig A, et al. Long-term increases in prevalence of North Sea fishes having southern biogeographic affinities. Marine Ecology Progress Series. 2004;284:269–278. [Google Scholar]
- Beaugrand G, Kirby RR. Climate, plankton and cod. Global Change Biology. 2010;16:1268–1280. [Google Scholar]
- Blanchard JL, Mills C, Jennings S, Fox CJ, Rackham BD, Eastwood PD, O'Brien CM. Distribution–abundance relationships for North Sea Atlantic cod (Gadus morhua): observation versus theory. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:2001–2009. [Google Scholar]
- Brander K. Cod Gadus morhua and climate change: processes, productivity and prediction. Journal of Fish Biology. 2010;77:1899–1911. doi: 10.1111/j.1095-8649.2010.02782.x. [DOI] [PubMed] [Google Scholar]
- Cheung WWL, Watson R, Pauly D. Signature of ocean warming in global fisheries catch. Nature. 2013;497:365–368. doi: 10.1038/nature12156. [DOI] [PubMed] [Google Scholar]
- Coull KA, Jermyn AS, Newton AW, Henderson GI, Hall WB. Length–weight relationships for 88 species of fish encountered in the North East Atlantic. Scottish Fisheries Research Report. 1989;43:1–82. [Google Scholar]
- Cushing DH. The decline of the herring stocks and the gadoid outburst. Journal du Conseil International pour l'Exploration de la Mer. 1980;39:70–81. [Google Scholar]
- Daan N. Changes in cod stocks and cod fisheries in the North Sea. Rapports et Procès-Verbaux des Réunions du Conseil International pour l'Exploration de la Mer. 1978;172:39–57. [Google Scholar]
- Drinkwater KF. The response of cod (Gadus morhua) to future climate change. ICES Journal of Marine Science. 2005;62:1327–1337. [Google Scholar]
- Dulvy NK, Rogers SI, Jennings S, Stelzenmüller V, Dye SR, Skjoldal HR. Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. Journal of Applied Ecology. 2008;45:1029–1039. [Google Scholar]
- Enever R, Revill AS, Grant A. Discarding in the North Sea and on the historical efficacy of gear-based technical measures in reducing discards. Fisheries Research. 2009;95:40–46. [Google Scholar]
- Engelhard GH. Catalogue of Defra historical catch and effort charts: six decades of detailed spatial statistics for British fisheries. Cefas Science Series Technical Report. 2005;128:1–42. Available at: www.cefas.defra.gov.uk/publications/techrep/tech128.pdf (accessed 6 January 2014) [Google Scholar]
- Engelhard GH. One hundred and twenty years of change in fishing power of English North Sea trawlers. In: Payne A, Cotter J, Potter T, editors. Advances in Fisheries Science 50 Years on from Beverton and Holt. Oxford: Blackwell Publishing; 2008. pp. 1–25. [Google Scholar]
- Engelhard GH, Heath MR, Pinnegar JK. Cod. In: Rijnsdorp AD, Peck MA, Engelhard GH, Möllmann C, Pinnegar JK, editors. Resolving climate impacts on fish stocks. Copenhagen, Denmark: ICES Cooperative Research Reports, 301, International Council for the Exploration of the Sea; 2010. pp. 162–174. [Google Scholar]
- Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD. Nine decades of North Sea sole and plaice distribution. ICES Journal of Marine Science. 2011;68:1090–1104. [Google Scholar]
- FAO. Review of the State of World Marine Fishery Resources. Rome, Italy: FAO Fisheries and Aquaculture Technical Paper 569, Food and Agriculture Organization of the United Nations; 2011. [Google Scholar]
- Fox CJ, Taylor M, Dickey-Collas M, et al. Mapping the spawning grounds of North Sea cod (Gadus morhua) by direct and indirect means. Proceedings of the Royal Society B. 2008;275:1543–1548. doi: 10.1098/rspb.2008.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulland JA. The reliability of the catch per unit effort as a measure of abundance in North Sea trawl fisheries. Rapports et Procès-Verbaux des Réunions du Conseil International pour l'Exploration de la Mer. 1964;155:99–102. [Google Scholar]
- Harrald M, Wright PJ, Neat FC. Substock variation in reproductive traits in North Sea cod (Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences. 2010;67:866–876. [Google Scholar]
- Heath MR. Responses of fish to climate fluctuations in the Northeast Atlantic. In: Emery LE, editor. The Practicalities of Climate Change: Adaptation and Mitigation, Proceedings of the 24th Conference of the Institute of Ecology and Environmental Management, Cardiff, 14–16 November 2006. Winchester, Hampshire, UK: Institute of Ecology and Environmental Management; 2007. pp. 102–116. [Google Scholar]
- Hedger R, McKenzie E, Heath M, Wright P, Scott B, Gallego A, Bridson J. Analysis of the spatial distributions of mature cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) abundance in the North Sea (1980–1999) using Generalised Additive Models. Fisheries Research. 2004;70:17–25. [Google Scholar]
- Hislop JRG. Changes in North Sea gadoid stocks. ICES Journal of Marine Science. 1996;53:1146–1156. [Google Scholar]
- ter Hofstede R, Daan N. A proposal for a consistent use of the North Sea IBTS data. 2008. ICES CM 2008/R: 25.
- Holst JC, Dragesund O, Hamre J, Misund OA, Østvedt OJ. Fifty years of herring migrations in the Norwegian Sea. ICES Marine Science Symposia. 2002;215:352–360. [Google Scholar]
- Holt J, Hughes S, Hopkins S, et al. Multi-decadal variability and trends in the temperature of the northwest European continental shelf: a model-data synthesis. Progress in Oceanography. 2012;106:96–117. [Google Scholar]
- Horwood J, O'Brien CM, Darby C. North Sea cod recovery? ICES Journal of Marine Science. 2006;63:961–968. [Google Scholar]
- Hsieh CH, Reiss CS, Hewitt RP, Sugihara G. Spatial analysis shows that fishing enhances the climatic sensitivity of marine fishes. Canadian Journal of Fisheries and Aquatic Sciences. 2008;65:947–961. [Google Scholar]
- Hurrell JW. Decadal trends in the North Atlantic Oscillation: regional temperatures and precipitation. Science. 1995;269:676–679. doi: 10.1126/science.269.5224.676. [DOI] [PubMed] [Google Scholar]
- Hutchings JA, Myers RA. What can be learned from the collapse of a renewable resource? Atlantic cod, Gadus morhua, of Newfoundland and Labrador. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51:2126–2146. [Google Scholar]
- Hutchinson WF, van Oosterhout C, Rogers SI, Carvalho GR. Temporal analysis of archived samples indicates marked genetic changes in declining North sea cod (Gadus morhua. Proceedings of the Royal Society B. 2003;270:2125–2132. doi: 10.1098/rspb.2003.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICES. Copenhagen: ICES Headquarters; 2013a. Report of the Arctic Fisheries Working Group (AFWG), 18–24 April 2013. ICES CM 2013/ACOM:05. 726 pp. [Google Scholar]
- ICES. Copenhagen: ICES Headquarters; 2013b. Report of the North Western Working Group (NWWG), 25 April–02 May 2013. ICES CM 2013/ACOM:07. 1538 pp. [Google Scholar]
- ICES. Copenhagen: ICES Headquarters; 2013c. Report of the Working Group on the Assessment of Demersal Stocks in the North Sea and Skagerrak (WGNSSK), 24–30 April 2013. ICES CM 2013/ACOM:13. 1435 pp. [Google Scholar]
- Jennings S, Alvsvåg J, Cotter AJR, et al. Fishing effects in northeast Atlantic shelf seas: patterns in fishing effort, diversity and community structure. III. International trawling effort in the North Sea: an analysis of spatial and temporal trends. Fisheries Research. 1999;40:125–134. [Google Scholar]
- Jones R, Hislop JRG. Changes in North Sea haddock and whiting. Rapports et Procès-Verbaux des Réunions du Conseil International pour l'Exploration de la Mer. 1978;172:58–71. [Google Scholar]
- Jones PD, Jonsson T, Wheeler D. Extension to the North Atlantic Oscillation using early instrumental pressure observations from Gibraltar and South-West Iceland. International Journal of Climatology. 1997;17:1433–1450. [Google Scholar]
- Jónsson H. Friends in Conflict: The Anglo-Icelandic Cod Wars and the Law of the Sea. London: C. Hurst and Co; 1982. [Google Scholar]
- Kell LT, Pilling GM, O'Brien CM. Implications of climate change for the management of North Sea cod (Gadus morhua. ICES Journal of Marine Science. 2005;62:1483–1491. [Google Scholar]
- Kerby TK, Cheung WWL, van Oosterhout C, Engelhard GH. Entering uncharted waters: long-term dynamics of two data limited fish species, turbot and brill, in the North Sea. Journal of Sea Research. 2013;84:87–95. [Google Scholar]
- Kurlansky M. Cod: A Biography of the Fish that Changed the World. New York: Walker Publishing; 1997. [Google Scholar]
- Last PR, White WT, Gledhill D, Hobday AJ, Brown R, Edgar GJ, Pecl GT. Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Global Ecology and Biogeography. 2011;20:58–72. [Google Scholar]
- Lilly GR, Nakken O, Brattey J. A review of the contributions of fisheries and climate variability to contrasting dynamics in two Arcto-boreal Atlantic cod (Gadus morhua) stocks: persistent high productivity in the Barents Sea and collapse on the Newfoundland and Labrador Shelf. Progress in Oceanography. 2013;114:106–125. [Google Scholar]
- Mieszkowska N, Genner MJ, Hawkins SJ, Sims DW. Effects of climate change and commercial fishing on Atlantic cod Gadus morhua. Advances in Marine Biology. 2009;56:213–273. doi: 10.1016/S0065-2881(09)56003-8. [DOI] [PubMed] [Google Scholar]
- Neat F, Righton D. Warm water occupancy by North Sea cod. Proceedings of the Royal Society B. 2007;274:789–798. doi: 10.1098/rspb.2006.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye JA, Baker MR, Bell R, et al. Ecosystem effects of the Atlantic Multidecadal Oscillation. Journal of Marine Systems. 2014 doi: 10.1016/j.jmarsys.2013.02.006. [Google Scholar]
- O'Brien CM, Fox CJ, Planque B, Casey J. Climate variability and North Sea cod. Nature. 2000;404:142. doi: 10.1038/35004654. [DOI] [PubMed] [Google Scholar]
- Ottersen G, Hjermann DØ, Stenseth NC. Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fisheries Oceanography. 2006;15:230–243. [Google Scholar]
- Perry AL, Low PJ, Ellis JR, Reynolds JD. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. [DOI] [PubMed] [Google Scholar]
- Perutz M. Glasgow: University of Glasgow; 2007. Population variation in the life history traits and thermal responses of Atlantic cod, Gadus morhua L. PhD thesis. [Google Scholar]
- Petitgas P, Alheit J, Peck MA, et al. Anchovy population expansion in the North Sea. Marine Ecology Progress Series. 2012;444:1–13. [Google Scholar]
- Poloczanska ES, Brown CJ, Sydeman WJ, et al. Global imprint of climate change on marine life. Nature Climate Change. 2013;3:919–925. [Google Scholar]
- Pope JG, Macer CT. An evaluation of the stock structure of North Sea cod, haddock, and whiting since 1920, together with a consideration of the impacts of fisheries and predation effects on their biomass and recruitment. ICES Journal of Marine Science. 1996;53:1157–1169. [Google Scholar]
- Pyper BJ, Peterman RM. Comparison of methods to account for autocorrelation in correlation analyses of fish data. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:2127–2140. [Google Scholar]
- Rayner NA, Brohan P, Parker DE, et al. Improved analyses of changes and uncertainties in sea surface temperature measured in situ since the mid-nineteenth century: the HadSST2 dataset. Journal of Climate. 2005;19:446–469. [Google Scholar]
- Righton D, Quayle VA, Hetherington S, Burt G. Movements and distribution of cod (Gadus morhua) in the southern North Sea and English Channel: results from conventional and electronic tagging experiments. Journal of the Marine Biological Association of the United Kingdom. 2007;87:599–613. [Google Scholar]
- Righton DA, Andersen KH, Neat F, et al. Thermal niche of Atlantic cod Gadus morhua: limits, tolerance and optima. Marine Ecology Progress Series. 2010;420:1–13. [Google Scholar]
- Rijnsdorp AD, Peck MA, Engelhard GH, Möllmann C, Pinnegar JK. Resolving the effect of climate change on fish populations. ICES Journal of Marine Science. 2009;66:1570–1583. [Google Scholar]
- Rindorf A, Lewy P. Warm, windy winters drive cod north and homing of spawners keeps them there. Journal of Applied Ecology. 2006;43:445–453. [Google Scholar]
- Rose GA. Cod: The Ecological History of the North Atlantic Fisheries. John's, Newfoundland: Breakwater Books, St; 2007. [Google Scholar]
- Simpson SD, Jennings S, Johnson MP, Blanchard JL, Schön P-J, Sims DW, Genner MJ. Continental shelf-wide response of a fish assemblage to rapid warming of the sea. Current Biology. 2011;21:1565–1570. doi: 10.1016/j.cub.2011.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Decadal changes in North Sea cod winter distribution, 1970s–2000s, based on fisheries-independent survey cpue (winter IBTS). For each decade, spatial distribution of cod cpue within the grey-shaded region is indicated by the area sizes of the black circles (proportional to cpue). In rectangles where no cpue data were available in a given decade, white circles represent the long-term average cpue. For each decade, the white cross indicates the centre of gravity of cod distribution, with its standard error (shorter, thick white lines) and standard deviation (longer, thin white lines) in the longitudinal and latitudinal directions. Bathymetry is indicated by light to dark grey shading (from shallow to deep).
Table S1. Correlations (rp) between cod distribution in the North Sea (longitude, latitude, and depth) and variables related to climate and cod fishing pressure and abundance.