Abstract
Dual antiplatelet therapy, composed of aspirin plus a P2Y12-receptor antagonist, is the cornerstone of treatment for patients with acute coronary syndrome (ACS). A number of U.S. Food and Drug Administration–approved P2Y12-receptor antagonists are available for treating patients with ACS, including the thienopyridine compounds clopidogrel and prasugrel. Ticagrelor, the first of a new class of antiplatelet agents, is a noncompetitive, direct-acting P2Y12-receptor antagonist. Unlike the thienopyridine compounds, ticagrelor does not require metabolism for activity. Also, whereas clopidogrel and prasugrel are irreversible inhibitors of the P2Y12 receptor, ticagrelor binds reversibly to inhibit receptor signaling and subsequent platelet activation. In pharmacodynamic studies, ticagrelor demonstrated faster onset and more potent inhibition of platelet aggregation than clopidogrel. These properties of ticagrelor may contribute to reduced rates of thrombotic outcomes compared with clopidogrel, as demonstrated in a phase III clinical trial. However, in addition to bleeding, distinctive adverse effects of this new chemical entity have not been reported with the thienopyridine P2Y12-receptor inhibitors. Although ticagrelor represents an advancement in P2Y12-receptor inhibition therapy, a thorough understanding of this compound as an antiplatelet therapy remains to be elucidated.
Keywords: antiplatelets, coronary artery disease, CAD, ticagrelor, P2Y12 inhibitors, acute coronary syndrome, ACS
In the United States, over a million people are diagnosed annually with acute coronary syndrome (ACS).1 ACS consists of non–ST-segment elevation (NSTE) ACS and ST-segment elevation myocardial infarction (STEMI); NSTE ACS consists of unstable angina or non–ST-segment elevation myocardial infarction. Due to the central role of platelets in the pathophysiology of arterial thrombosis, antiplatelet therapy is critical for the acute and chronic treatment of patients with ACS.
Dual antiplatelet therapy with aspirin and the P2Y12-receptor antagonist clopidogrel demonstrated significant benefit over aspirin alone in patients with NSTE ACS in the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial in 2001.2 Since that time, dual antiplatelet therapy has been considered the standard of care for patients with ACS and has been incorporated into current treatment guidelines.3–5
Despite the widespread use of clopidogrel, there is a rate of recurrent cardiovascular (CV) events of at least 10% within 1 year of an ACS event.2 These events are potentially explained by issues related to clopidogrel including variability in antiplatelet response, pharmacogenomic influences, and drug interactions.6–8 The P2Y12-receptor inhibitor prasugrel overcame a number of limitations of clopidogrel but has a similar chemical structure (thienopyridine). Prasugrel must be activated through metabolism to provide antiplatelet effects, although the clinical relevance of this process remains unknown.9 However, ticagrelor is the first U.S. Food and Drug Administration–approved agent of a new class of antiplatelet agents—cyclopentyltriazolopyrimidines—and has distinct pharmacologic properties compared with those of the thienopyridines.
Mechanism of Action and Chemical Properties
Ticagrelor is an orally administered direct-acting P2Y12-receptor antagonist.10,11 In vitro studies have demonstrated that ticagrelor binds reversibly and noncompetitively to the P2Y12 receptor at a site distinct from that of the endogenous agonist adenosine diphosphate (ADP).10 In contrast, the thienopyridine compounds clopidogrel and prasugrel (Figure1) bind irreversibly to the P2Y12 receptor for the life of the platelet.12
Figure 1.
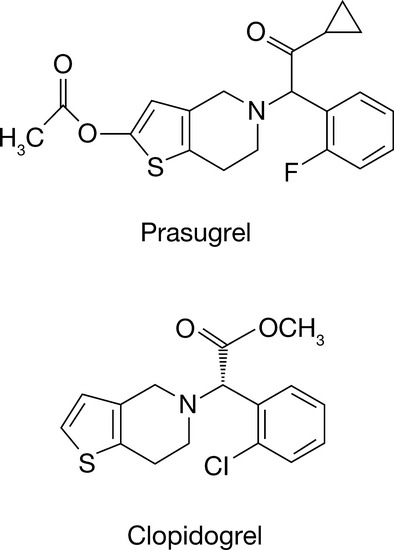
Chemical structures of the thienopyridine compounds prasugrel and clopidogrel.
The development of ticagrelor began by leveraging the structure of adenosine triphosphate, which is an endogenous antagonist of the P2Y12 receptor (Figure2). Prior to the development of ticagrelor, cangrelor was identified as a potent and selective P2Y12-receptor antagonist (Figure2). Currently, cangrelor is being developed for intravenous administration. To identify orally active derivatives, the structure of cangrelor was altered by replacing the purine with a triazolopyrimidine heterocycle as well as substitutions at other key locations.13,14 As a result, the compound AZD6140 (ticagrelor) was identified to possess acceptable affinity and metabolic stability.14
Figure 2.
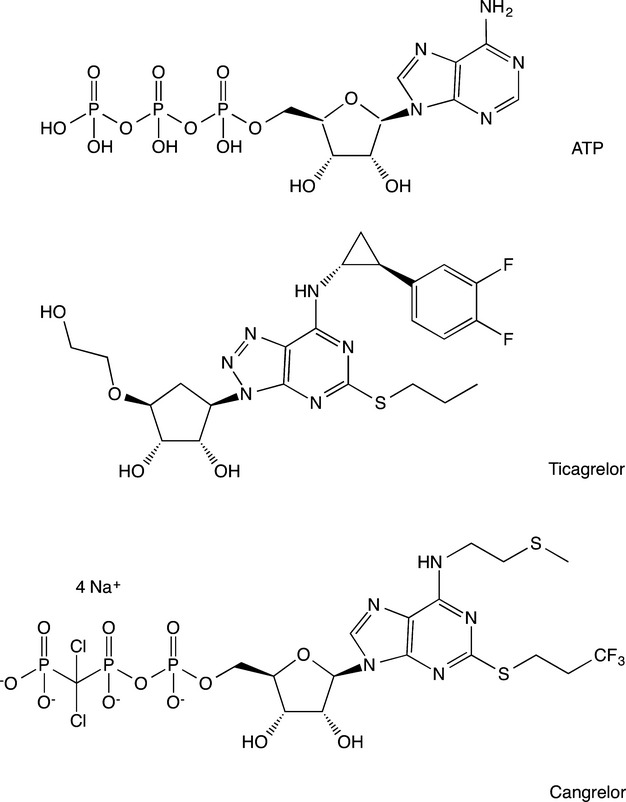
Chemical structures of adenosine triphosphate (ATP), ticagrelor, and cangrelor.
Pharmacokinetics
In healthy subjects, ticagrelor is rapidly absorbed, with a median time to peak concentration (Tmax) of 2–3 hours after multiple twice/day oral dosing. Similarly, the median Tmax for one active metabolite of ticagrelor, AR-C124910XX, is ∼2.5–4 hours.15 After absorption, ticagrelor and AR-C124910XX are highly bound to plasma proteins (more than 99.8%) and largely restricted to the plasma space.16,17 The absolute bioavailability of ticagrelor is estimated at 36%, and the steady-state volume of distribution of ticagrelor is 88 L.18
Unlike clopidogrel and prasugrel, ticagrelor is not a prodrug and does not require metabolic activation for antiplatelet activity.19 Still, ticagrelor is extensively metabolized, with ticagrelor and its active and approximately equipotent metabolite composing the major circulating components in the plasma.17 Exposure to the active metabolite AR-C124910XX, formed through cytochrome P450 (CYP) 3A4- and 3A5-mediated metabolism, is approximately a third that of ticagrelor.15,17,21,22
Ticagrelor and AR-C124910XX exhibit predictable linear pharmacokinetics in healthy volunteers as well as in patients with atherosclerosis, stable coronary artery disease (CAD), and ACS.15,21–24 After multiple twice/day doses of ticagrelor in healthy volunteers, the mean elimination half-lives for ticagrelor and AR-C124910XX were 6.7–9.1 hours and 7.5–12.4 hours, respectively.15 After administration of radiolabelled ticagrelor in healthy subjects, the mean radioactivity recovery was 58% in feces and 27% in urine.17 Levels of unchanged ticagrelor and AR-C124910XX in urine were less than 0.05%, suggesting that renal excretion plays a relatively minor role in the elimination of ticagrelor and AR-C124910XX.17
As expected, severe renal impairment does not significantly affect the pharmacokinetics, pharmacodynamics, or safety of ticagrelor and AR-C124910XX.25 However, exposure to ticagrelor and AR-C124910XX was modestly increased in patients with mild hepatic impairment, although there were no subsequent effects on pharmacodynamics or tolerability.26 Based on these findings, no dosage adjustment is considered necessary in either of these patient groups. However, there are no pharmacodynamic data on the use of ticagrelor in patients with moderate or severe hepatic impairment; therefore, use should be avoided in these patients. It is also important to note that data are not available on the clinical response to ticagrelor in patients with hepatic impairment and ACS. Notably, a phase II study demonstrated that prior clopidogrel dosing or responder status does not affect the pharmacokinetics of ticagrelor.23
Pharmacodynamics
Inhibition of platelet aggregation (IPA) stimulated by ADP is a commonly used pharmacodynamic parameter for P2Y12-receptor antagonists. In this section, we refer to final platelet aggregation observed at the end of platelet response (i.e., 6 min), rather than maximal extent of platelet aggregation.27 The IPA by ticagrelor has been examined in several populations. For example, in healthy volunteers, single doses of ticagrelor (100–400 mg) were associated with a rapid (2 hrs), dose-dependent, and near-complete inhibition of 20-μM ADP-induced platelet aggregation.22 In a multiple-dose study in healthy volunteers, IPA gradually decreased with declining plasma concentrations from ∼12 hours after dosing, indicating that ticagrelor-associated IPA is concentration dependent and slowly reversible. Ticagrelor is only approved for twice/day dosing because this strategy showed greater and more consistent IPA than once/day dosing (50–600 mg) (Table1).15 In another study of healthy volunteers, the effect of age and sex on the pharmacodynamics of ticagrelor was assessed.28 Elderly and younger volunteers of both sexes received a single dose of ticagrelor 200 mg. Notably, elderly subjects had higher ticagrelor exposure compared with younger subjects, and women had higher exposure than men. The maximal concentration was significantly increased by 61% in young women, 73% in elderly men, and 148% in elderly women compared with young men. Interestingly, the elderly subjects with the highest ticagrelor exposure also had the lowest IPA, suggesting that platelets are less sensitive in the elderly. Despite these differences, no adjustment in ticagrelor dose is recommended based on age or sex because IPA was substantial in all groups examined (higher than 90% at 4 and 8 hrs after dosing).
Table 1.
Final Percentage of Mean Inhibition of Platelet Aggregation over Time in Healthy Subjects Receiving Multiple Doses of Ticagrelor15
| Treatment regimen | Final percentage of inhibition of platelet aggregation (%) | |||
|---|---|---|---|---|
| 4 hrs | 8 hrs | 12 hrs | 24 hrs | |
| Ticagrelor once/day | ||||
| 50 mg (n=7) | 94.0 ± 6.0 | 72.0 ± 31.5 | 62.0 ± 33.2 | 14.0 ± 13.2 |
| 100 mg (n=7) | 99.0 ± 2.5 | 95.0 ± 5.0 | 89.0 ± 9.3 | 57.0 ± 28.9 |
| 200 mg (n=14) | 99.0 ± 1.2 | 95.0 ± 6.3 | 95.0 ± 8.2 | 76.0 ± 26.7 |
| 300 mg (n=7) | 100.0 ± 0.2 | 97.0 ± 5.1 | 93.0 ± 8.6 | 82.0 ± 13.2 |
| 400 mg (n=6) | 97.0 ± 4.2 | 95.0 ± 6.8 | 91.0 ± 10.8 | 90.0 ± 11.8 |
| 600 mg (n=6) | 99.0 ± 1.9 | 97.0 ± 4.9 | 96.0 ± 4.7 | 91.0 ± 11.0 |
| Ticagrelor twice/day | ||||
| 50 mg (n=14) | 95.0 ± 10.8 | 90.0 ± 20.0 | 87.0 ± 23.9 | 79.0 ± 29.6 |
| 100 mg (n=13) | 97.0 ± 7.9 | 95.0 ± 10.9 | 93.0 ± 15.8 | 93.0 ± 10.8 |
| 200 mg (n=13) | 98.0 ± 4.8 | 98.0 ± 3.7 | 96.0 ± 6.4 | 97.0 ± 6.8 |
| 300 mg (n=7) | 100.0 ± 0.0 | 100.0 ± 0.0 | 99.0 ± 1.1 | 100.0 ± 0.0 |
| Clopidogrel once/day | ||||
| 75 mg (n=14) | 90.0 ± 21.1 | 82.0 ± 26.4 | 83.0 ± 22.5 | 77.0 ± 27.4 |
| Placebo once/day | ||||
| (n=23–39) | 7 ± 7.4 | 8 ± 8.8 | 8 ± 12.0 | 5 ± 6.5 |
Data are mean ± SD percentages.
Key Pharmacokinetic and Pharmacodynamic Trials
Ticagrelor was first evaluated in patients with stable CAD in the Dose Confirmation Study Assessing Anti-Platelet Effects of AZD6140 versus Clopidogrel in Non–ST-Segment Elevation Myocardial Infarction (DISPERSE) trial.21 In this randomized, double-blind, parallel-group study, 200 patients with stable CAD who were currently taking aspirin were administered either ticagrelor or clopidogrel for 28 days. The pharmacokinetics, pharmacodynamics, and safety of ticagrelor were evaluated for four different dose regimens—50 mg twice/day, 100 mg twice/day, 200 mg twice/day, or 400 mg/day—compared with clopidogrel 75 mg/day. The highest doses of ticagrelor (100 mg and 200 mg twice/day, and 400 mg/day) achieved peak IPA within 2–4 hours and comparable IPA at steady state (∼90–95%) (Table2). Conversely, ticagrelor 50 mg twice/day and clopidogrel 75 mg/day (without a loading dose) resulted in a slower onset and reduced IPA (68% for clopidogrel). Plasma concentrations for ticagrelor and AR-C124910XX were linear and dose proportional, and corresponded with IPA. The 100 mg twice/day regimen demonstrated peak blood levels of 800 ng/ml. Higher doses resulted in proportional increases in plasma concentrations without major changes in IPA (Table2).
Table 2.
Pharmacokinetic and Pharmacodynamic Parameters of Ticagrelor at Day 14 of the DISPERSE Trial21
| Parameter | Ticagrelor 50 mg twice/day | Ticagrelor 100 mg twice/day | Ticagrelor 200 mg twice/day | Ticagrelor 400 mg/day | Clopidogrel 75 mg/day |
|---|---|---|---|---|---|
| Tmax, hrs | 2.5 (56) | 2.8 (74) | 2.6 (69) | 2.4 (149) | – |
| Cmax, ng/ml | 375 (50) | 810 (41) | 2278 (31) | 3653 (41) | – |
| IPA, % | 75 (55–84) | 88 (82–95) | 95 (86–100) | 98 (88–100) | 68 (44–81) |
Cmax = maximum concentration; DISPERSE = Dose Confirmation Study Assessing Anti-Platelet Effects of AZD6140 versus Clopidogrel in Non–ST-Segment Elevation Myocardial Infarction; IPA = inhibition of platelet aggregation; Tmax = time to reach maximum concentration. Data are mean (% coefficient of variation) for Tmax and Cmax, and median (interquartile range) for IPA. IPA refers to an estimation of final percentage of inhibition of platelet aggregation at 4 hours after dosing on day 14.
From a safety perspective, bleeding times increased in all ticagrelor groups (14.8–23 min) compared with clopidogrel (10.5 min). Consistent with these findings, minor bleeding was more common with the three highest ticagrelor doses (44–51%) compared with either ticagrelor 50 mg twice/day (29%) or clopidogrel (32%). Also, one patient in the ticagrelor 400 mg/day group had a major bleeding event. The DISPERSE trial also identified an increased rate of dyspnea (10–20%) and elevated uric acid levels (by 5–10%) in the ticagrelor groups. Based on the superior antiplatelet effect compared with the 50 mg twice/day dose and the improved safety and tolerability compared with the 400 mg/day dose, the 100 mg and 200 mg twice/day doses were targeted for future study. After the DISPERSE trial findings, the formulation of ticagrelor was changed (the 100-mg tablet became 90 mg); therefore, the new corresponding doses of 90 mg and 180 mg twice/day were targeted in future studies.
Following the DISPERSE trial, researchers initiated a follow-up study termed DISPERSE-2.29 The DISPERSE-2 trial was a randomized double-blind study conducted in 990 patients with NSTE-ACS. The primary end point of this trial was protocol-defined major or minor bleeding at 4 weeks. This end point was not significantly different between the ticagrelor 90 mg twice/day (9.8%), 180 mg twice/day (8.0%), and clopidogrel 75 mg/day (8.1%) groups (p=0.43 and p=0.96, respectively, vs clopidogrel). The rates of major bleeding were also not significantly different between groups. However, there was a trend for increased minor bleeding with ticagrelor 180 mg twice/day compared with clopidogrel (3.8% vs 1.3%, p=0.0504) at 4 weeks that was significant at 12 weeks (6.1% vs 1.3%, p=0.01). Furthermore, two fatal bleeds occurred in the ticagrelor 90-mg group. The rates of death or CV death were not significantly different among groups, but a trend for lower rates of myocardial infarction (MI) was identified among the ticagrelor groups compared with clopidogrel (3.8% for ticagrelor 90 mg vs 5.6% for clopidogrel, p=0.41; 2.5% for ticagrelor 180 mg vs 5.6% for clopidogrel, p=0.06).
A substudy of DISPERSE-2 examined the pharmacodynamics of ticagrelor at doses of 90 mg and 180 mg twice/day compared with clopidogrel 75 mg/day for 12 weeks in clopidogrel-naive patients (clopidogrel 300-mg loading dose given; n=45) and clopidogrel pretreated patients (no clopidogrel loading dose given; n=44).24 Ticagrelor yielded greater and more consistent IPA than clopidogrel at 4 weeks and was associated with further suppression of platelet aggregation in patients pretreated with clopidogrel.
As reported in the DISPERSE trial, the rates of dyspnea were higher in both ticagrelor groups of the DISPERSE-2 trial (10.5–15.8%) compared with clopidogrel (6.4%, p=0.07 and p<0.0002 for the ticagrelor 90 mg and 180 mg twice/day groups, respectively, vs clopidogrel).29 Of the patients reporting dyspnea, 27% had resolution of symptoms within 24 hours while continuing therapy, and another 25% had relief after 24 hours, whereas 48% experienced symptoms during treatment lasting longer than 15 days. Diarrhea was also significantly more common in the ticagrelor 180-mg group compared with clopidogrel (7.4% vs 3.4%, p=0.02), and hypotension occurred more frequently in both ticagrelor groups (3.7% for the 180-mg group and 1.2% for the 90-mg group) compared with clopidogrel (0.6%; p=0.01 and p=0.004 for the ticagrelor 180-mg and 90-mg groups, respectively, vs clopidogrel). Patients treated with ticagrelor had higher rates of ventricular pauses greater than 2.5 seconds, but no corresponding significant difference in the rates of ventricular tachycardias; the ventricular pauses were significantly different in the ticagrelor 180-mg group. There were 4.9% of patients who experienced more than three episodes of ventricular pauses compared with 0.3% for clopidogrel (p<0.001). Due to the increased episodes of dyspnea and ventricular pauses in the ticagrelor 180-mg group, the 90-mg twice/day dose was selected for further investigation.
To assess the pharmacodynamic response of ticagrelor, investigators designed a multicenter, randomized, double-blind assessment of the onset and offset of antiplatelet effects of ticagrelor compared with clopidogrel in patients with stable CAD: the ONSET/OFFSET trial.31 In this study, ticagrelor, at a loading dose of 180 mg followed by 90 mg twice/day, was compared with clopidogrel, at a loading dose of 600 mg followed by the standard maintenance dose of 75 mg/day, or placebo, for 6 weeks. Patients with stable CAD (n=123) achieved significantly greater IPA when treated with ticagrelor plus aspirin compared with clopidogrel plus aspirin, at 0.5, 1, 2, 4, 8, and 24 hours and 6 weeks after loading dose administration (p<0.0001 for all time points; Figure3).31 Near maximal platelet inhibition (∼80%) was achieved within 1 hour in the ticagrelor group, and peak IPA was achieved at 2 hours with ticagrelor compared with peak IPA at 7.8 hours with clopidogrel.
Figure 3.
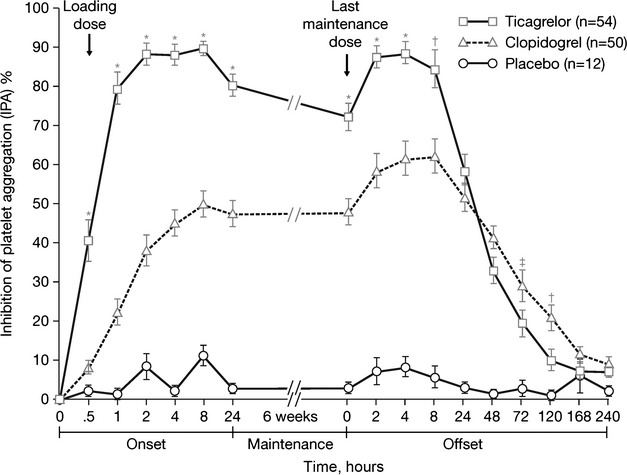
Final inhibition of platelet aggregation in patients with stable coronary artery disease who received ticagrelor (180-mg loading dose followed by a 90 mg twice/day maintenance dose), clopidogrel (600-mg loading dose followed by a 75-mg/day maintenance dose), or placebo for 6 weeks in the ONSET/OFFSET study. *p<0.0001; †p<0.005; ‡p<0.05.31
Consistent with its noncompetitive and reversible pharmacology, the rate of offset with ticagrelor was significantly faster compared with clopidogrel (p<0.0001; Figure3). However, 4–5 days were still required for platelet reactivity to return to normal with ticagrelor. Although mean IPA was higher with ticagrelor than clopidogrel during the 6-week treatment period, mean IPA for both antiplatelet agents was similar at 24 hours after the last maintenance dose (58% for ticagrelor vs 52% for clopidogrel) when measured by using light transmittance aggregometry (Figure3) and at 48 hours when measured by using the VerifyNow P2Y12 assay or the vasodilator-stimulated phosphoprotein phosphorylation assay. These findings suggest that patients treated with ticagrelor who are at steady state and miss a dose would have platelet inhibition similar to maximal pharmacodynamic response when using clopidogrel. Likewise, the risk of bleeding for ticagrelor is expected to remain higher than that for clopidogrel for at least 24–48 hours after the last dose.
Bleeding events occurred more frequently in the ticagrelor group (28.1%) compared with the clopidogrel (13.0%) and placebo groups (8.3%) in the ONSET/OFFSET trial, but no major bleeding events were identified. One patient in the placebo group and four patients in the ticagrelor group discontinued study treatment due to adverse events. Three patients in the ticagrelor group withdrew from the study due to dyspnea. Likewise, dyspnea was significantly more common in patients treated with ticagrelor compared with clopidogrel (25% vs 4%, p<0.01).
The pharmacodynamic response of ticagrelor in clopidogrel nonresponders with stable CAD was assessed in a randomized, double-blind, crossover trial: the Response to Ticagrelor in Clopidogrel Nonresponders and Responders and Effect of Switching Therapies (RESPOND) trial.32 This study assessed the absolute change in maximal 20-μM ADP-induced platelet aggregation in nonresponders (absolute change 10% or lower; n=41) and responders (absolute change more than 10%; n=57). The effect of switching therapy was also examined. As expected, IPA significantly increased in clopidogrel nonresponders treated with a 180-mg loading dose of ticagrelor followed by 90 mg twice/day, compared with a 600-mg loading dose of clopidogrel followed by 75 mg/day (p=0.005; Figure4A). Moreover, platelet aggregation decreased from 59% to 35% in patients who switched from clopidogrel to ticagrelor and increased from 36% to 56% in patients switched from ticagrelor to clopidogrel (p<0.0001 for both; Figure4). Similar findings were identified in clopidogrel responders (Figure4B). Therefore, the RESPOND trial helped confirm that patients can be switched from clopidogrel to ticagrelor without interruption of pharmacodynamic antiplatelet effect to obtain higher levels of platelet inhibition.
Figure 4.
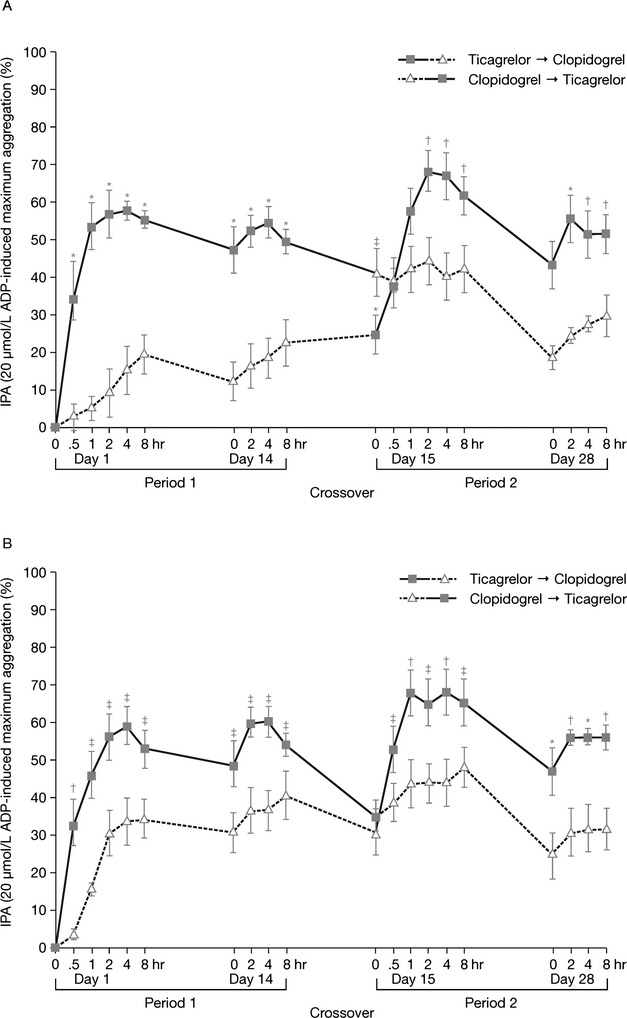
Maximum inhibition of platelet aggregation (IPA) in patients with stable coronary artery disease who were clopidogrel nonresponders (41 patients; panel A) and clopidogrel responders (57 patients; panel B) and were randomized to either ticagrelor (180-mg loading dose followed by 90 mg twice/day) or clopidogrel (600-mg loading dose followed by 75 mg once/day) for 14 days (period 1), then switched treatments (period 2; all nonresponders; half of responders). ADP = adenosine diphosphate. *p<0.0001; †p<0.001; ‡p<0.05.32
Clinical Efficacy
The phase III clinical trial evaluating the efficacy and safety of ticagrelor in patients with ACS was the Platelet Inhibition and Patient Outcomes (PLATO) trial.30 Patients in this trial (n=18,624) who presented within 24 hours of an ACS event (NSTE ACS or STEMI) were randomized in a double-blinded fashion to a clopidogrel loading dose of 300 mg followed by 75 mg/day, or a loading dose of ticagrelor 180 mg followed by 90 mg twice/day, for at least 6 months and up to 12 months. An additional loading dose of clopidogrel 300 mg at the time of PCI was allowed at the investigator's discretion, as well as an additional loading dose of ticagrelor 90 mg if PCI occurred more than 24 hours after randomization. All patients also received aspirin unless tolerance to aspirin prevented its use. The primary efficacy end point of the trial was the composite of CV death, MI, and stroke.
Patients randomized to ticagrelor demonstrated a significant 16% relative reduction in the primary end point compared with clopidogrel (hazard ratio [HR] 0.84, 95% confidence interval [CI] 0.77–0.92; Table3). The benefit of ticagrelor over clopidogrel was evident within the first 30 days of treatment (4.8% vs 5.4%, p=0.045) and continued to increase from day 31 to day 360 (5.3% vs 6.6%, p<0.001). Therefore, the benefits demonstrated with ticagrelor in the PLATO trial were not just due to early potent antiplatelet therapy but also presumably due to maintained potent antiplatelet therapy.
Table 3.
Efficacy and Safety of Ticagrelor in the Phase III PLATO trial30
| Outcome | Ticagrelor group | Clopidogrel group | p value |
|---|---|---|---|
| Primary efficacy outcome, % | |||
| Cardiovascular death, myocardial infarction, and stroke | 9.8 | 11.7 | < 0.001 |
| Secondary efficacy outcomes, % | |||
| Cardiovascular death | 4.0 | 5.1 | 0.001 |
| Myocardial infarction | 5.8 | 6.9 | 0.005 |
| Stroke | 1.5 | 1.3 | 0.22 |
| Death from any cause | 4.5 | 5.9 | < 0.001 |
| Stent thrombosis, definite | 1.3 | 1.9 | 0.009 |
| Stent thrombosis, probable or definite | 2.2 | 2.9 | 0.02 |
| Primary safety outcomes, % | |||
| Total major bleeding, PLATO criteria | 11.6 | 11.2 | 0.43 |
| Total major bleeding, TIMI criteriaa | 7.9 | 7.7 | 0.57 |
| Total life-threatening or fatal bleeding, PLATO criteria | 5.8 | 5.8 | 0.70 |
| Secondary safety outcomes, % | |||
| Non–CABG-related major bleeding, PLATO criteria | 4.5 | 3.8 | 0.03 |
| Non–CABG-related major bleeding, TIMI criteria | 2.8 | 2.2 | 0.03 |
| CABG-related major bleeding, PLATO criteria | 7.4 | 7.9 | 0.32 |
| CABG-related major bleeding, TIMI criteria | 5.3 | 5.8 | 0.32 |
| Major or minor bleeding, PLATO criteria | 16.1 | 14.6 | 0.008 |
| Major or minor bleeding, TIMI criteria | 11.4 | 10.9 | 0.33 |
CABG = coronary artery bypass grafting; PLATO = Platelet Inhibition and Patient Outcomes; TIMI = Thrombolysis in Myocardial Infarction.
Major bleeding and major or minor bleeding according to TIMI criteria62 refer to nonadjudicated events analyzed with the use of a statistically programmed analysis in accordance with previously used definitions.
The composite end point of CV death, MI, and stroke is a common end point in trials evaluating P2Y12 inhibitor therapy. However, a reduction in nonfatal MI is typically the driver of the overall benefit. Although the incidence of MI was significantly reduced by 16% with the use of ticagrelor in the PLATO trial (HR 0.84, 95% CI 0.75–0.95), there was also a significant 21% reduction in the incidence of CV death (HR 0.79, 95% CI 0.69–0.91; Table3). Although the PLATO trial was not designed specifically as a mortality trial, a reduction in CV death has rarely been demonstrated with an oral antiplatelet agent.33 It is unknown if reduced CV death while taking ticagrelor is due to its more potent antiplatelet effect compared with clopidogrel or if it the result of an additional pharmacologic property of ticagrelor.
Preclinical studies have demonstrated that ticagrelor can inhibit adenosine uptake by human erythrocytes and that ticagrelor can augment both endogenous and exogenous adenosine-induced coronary blood flow in a canine model.34 These findings have been confirmed in healthy volunteers, where ticagrelor augmented exogenous adenosine-induced coronary blood flow increases.35 Therefore, the mortality benefit demonstrated with the use of ticagrelor in the PLATO trial may be due to sustained and elevated adenosine levels in ischemic tissues leading to improved perfusion.
Compared with clopidogrel, ticagrelor reduced thrombotic CV events in the PLATO trial, regardless of the management strategy. The magnitude of effect of ticagrelor was consistent between patients in whom an invasive strategy was planned (HR 0.84, 95% CI 0.75–0.94) and those assigned to a noninvasive medicinal strategy (HR 0.85, 95% CI 0.73–1.00).36,37 Similar results were also demonstrated in the subgroup of patients with STEMI in whom primary percutaneous coronary intervention (PCI) was planned (n=7544; HR 0.87, 95% CI 0.75–1.01, p=0.07) and in patients who underwent coronary artery bypass grafting (CABG) surgery during the trial (planned or not) and who received their last dose of study drug within 7 days before surgery (n=1899; HR 0.84, 95% CI 0.60–1.16, p=0.29).38,39
The PLATO trial allowed for a 300- or 600-mg loading dose of clopidogrel to be administered prior to PCI, with only 19.6% of patients in the clopidogrel group receiving the 600-mg dose.30 Therefore, there is concern that the clopidogrel group may have been at a disadvantage during the first few days of the trial because most patients did not receive the 600-mg clopidogrel loading dose. Although it will remain unknown how the outcomes may have been altered if all patients in the clopidogrel group would have received the 600-mg loading dose, two points should be considered. First, even with a 600-mg loading dose of clopidogrel, patients receiving ticagrelor 180 mg have significantly faster and more potent inhibition of platelet aggregation.31 Second, in the subgroup of patients receiving PCI, ticagrelor demonstrated similar efficacy in patients receiving less than a 600-mg loading dose of clopidogrel (HR 0.83, 95% CI 0.73–0.95) compared with those receiving 600 mg or more of clopidogrel (HR 0.87, 95% CI 0.69–1.10), with an interaction p value of 0.733.37 Therefore, the loading dose of clopidogrel did not appear to influence the outcomes of the PLATO trial.
The consistency of the effects of ticagrelor on efficacy and safety end points in PLATO was further explored in 25 prespecified subgroups and 8 post hoc subgroups, without adjustment for multiple comparisons.30 In these subanalyses, no significant heterogeneity was found regarding the primary end point, with three exceptions. The benefit of ticagrelor appeared to be attenuated in patients not taking lipid-lowering drugs at randomization, in those weighing less than the median weight for their sex, and in those enrolled in North America. Interactions for weight by sex and lipid-lowering therapy did not exhibit quantitative differences and were not considered clinically meaningful.40 However, the region interaction suggested that ticagrelor was less effective in North America, specifically the United States. In fact, patients in the United States enrolled in the PLATO trial actually had a numerical increase in the primary end point with the use of ticagrelor compared with clopidogrel (11.9% vs 9.5%, p=0.1459), as well as each of the individual components of the composite end point.
There are a number of possible explanations for this finding. This effect may have been due to chance from the basic risk of conducting multiple subgroup analyses. There were also a number of differences in baseline demographics in patients enrolled in the United States (n=1413) compared with the rest of the world, although this effect was maintained after later adjustment for these factors. This effect may also have been due to the higher maintenance dose of aspirin used in the United States, as more patients in the United States took a median maintenance dose of aspirin of 300 mg/day or more (53.6%) than the rest of the world (1.7%).40 Patients enrolled in the United States who received a maintenance dose of aspirin of 300 mg/day or more had an increase in risk of events with the use of ticagrelor compared with clopidogrel (HR 1.62, 95% CI 0.99–2.64), but a reduction in risk if a maintenance dose of aspirin of 100 mg or less was used (HR 0.73, 95% CI 0.40–1.33). Because only 284 patients in the United States received ticagrelor and a maintenance dose of aspirin of 100 mg or less, clinicians may be concerned over having this small group explain the geographic treatment discrepancy. It should be noted that the effect of aspirin dose was also demonstrated in the rest of the world, with a lower maintenance dose having a benefit with ticagrelor compared with clopidogrel (HR 0.78, 95% CI 0.69–0.87) that seems to be lost with a higher maintenance dose (HR 1.23, 95% CI 0.71–2.14). Therefore, it seems that a maintenance dose of aspirin 75–100 mg/day is needed to realize the benefits of ticagrelor over clopidogrel, and doses greater than 100 mg/day are not recommended (Table4). It should be also noted that the lowest event rates in both the ticagrelor and clopidogrel groups occurred in patients receiving low-dose aspirin.
Table 4.
Drug Interactions with Ticagrelor
| Basis for Interaction | Examples of interacting drugs | Recommendations for patients receiving ticagrelor therapy |
|---|---|---|
| CYP3A4 | Strong CYP3A4 inhibitors: ketoconazole, itraconazole, voriconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, atazanavir, telithromycin Potent CYP3A4 inducers: rifampin, dexamethasone, phenytoin, carbamazepine, phenobarbital |
Potent CYP3A4 inhibitors are contraindicated Potent CYP3A4 inducers should be avoided Coadministration with simvastatin or lovastatin doses > 40 mg/day should be avoided (ticagrelor increases serum concentrations of these drugs) |
| P-glycoprotein | Digoxin (P-glycoprotein substrate) | Monitor digoxin levels when initiating ticagrelor |
| Clinical effectiveness | Aspirin | Avoid maintenance doses of aspirin > 100 mg/day |
CYP = cytochrome P450.
No accepted biological rationale is currently available to explain the interaction between ticagrelor and higher aspirin doses. One potential hypothesis is based on the knowledge that aspirin not only inhibits platelet cyclooxygenase and subsequent thromboxane A2 production but also endothelial release of prostacyclin in a dose-dependent manner at doses of greater than 80 mg/day.41 Prostacyclin inhibits platelet activation and promotes vasodilation. In the setting of already high-level platelet inhibition from an agent such as ticagrelor, the ability of aspirin to provide added platelet inhibition through reducing thromboxane A2 is limited, and the inhibition of prostacyclin may attenuate the beneficial impact of potent P2Y12 inhibition.42,43 Although this hypothesis may have some pharmacologic merit, this aspirin effect was not seen with the high-level P2Y12 inhibition of prasugrel in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel—Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) trial.44 In the CURRENT-OASIS 7 trial, higher dose aspirin attenuated the effect of lower dose clopidogrel but not with higher dose clopidogrel.45
Safety
Bleeding Events
Bleeding is the major safety concern with any antiplatelet agent due to the significant detrimental impact on morbidity and mortality and cost of care when major bleeding events occur. Bleeding events are also important with antiplatelet agents because by inhibiting platelet activity, the ability to achieve hemostasis in the event of injury or surgery is also impaired. In the DISPERSE trials, there was an increase in the risk of minor bleeding with ticagrelor compared with clopidogrel, but very few major bleeding events were recorded.21,29 Total major bleeding was the primary safety end point in the PLATO trial and was assessed with a new PLATO definition of major bleeding,30 as well as the more standard Thrombolysis in Myocardial Infarction (TIMI) definition of major bleeding62 (Table5). The incidence of total major bleeding (using either the PLATO or TIMI definitions) was not significantly increased with the use of ticagrelor compared with clopidogrel (Table3).30 The use of total major bleeding as a primary safety end point is somewhat unique to the PLATO trial. Most antiplatelet therapy trials use non–CABG major bleeding as the primary safety outcome due to the high rate of major bleeding seen in CABG surgery. In the PLATO trial, 70% of the patients undergoing CABG surgery had sufficient blood loss to classify as a major bleeding event, representing two-thirds of all the major bleeding events in the study but only 10% of the study population.46 It is important to note that despite ticagrelor's faster offset of antiplatelet activity in pharmacodynamic trials, no significant difference in the incidence of CABG-related major bleeding was noted compared with clopidogrel in patients receiving therapy within 7 days of surgery.39 These bleeding events are more likely representative of routine operating room procedures and suggest that clinically meaningful bleeding events are diluted by the disproportionately high incidence of patients receiving transfusion for CABG surgery. When non–CABG-related major bleeding was evaluated, a significant increase was demonstrated in patients receiving ticagrelor compared with clopidogrel (Table3), but life-threatening bleeding and fatal bleeding were not significantly different. A significant increase in the rate of fatal intracranial bleeding was also noted with ticagrelor compared with clopidogrel, but the percentages were small (0.1% vs 0.01% for ticagrelor vs clopidogrel, p=0.02).
Table 5.
Bleeding Definitions
| PLATO definition30 | TIMI definition62 | ||
|---|---|---|---|
| Major bleeding | Fatal bleeding Intracranial hemorrhage Clinically overt or apparent bleeding with a drop in Hgb ≥ 3 g/dl Transfusion of ≥ 2 units of whole blood or PRBCs Severe hypotension requiring pressors or surgery; intrapericardial hemorrhage with tamponade; hypovolemic shock Substantially disabling |
Major bleeding | Fatal bleeding Leads to hypotension and requires i.v. inotropic agents Requires surgical intervention Transfusion of ≥ 4 units of whole blood or PRBCs Intracranial hemorrhage Clinically overt with a drop in Hgb > 5 g/dl or drop in HCT > 15% |
| Minor bleeding | Requires medical intervention to stop or treat bleeding | Minor bleeding | Clinically overt with a drop in Hgb of 3 to ≤ 5 g/dl or drop of HCT of 9 to ≤ 15% |
| Minimal bleeding | All other bleeding not requiring intervention | Minimal bleeding | Clinically overt with drop in Hgb < 3 g/dl or drop in HCT < 9% |
HCT = hematocrit; Hgb = hemoglobin; i.v. = intravenous; PLATO = Platelet Inhibition and Patient Outcomes; PRBCs = packed red blood cells; TIMI = Thrombolysis in Myocardial Infarction.
Other Adverse Events
A number of distinctive adverse effects have been reported with ticagrelor, likely because ticagrelor has a different chemical structure compared with the traditional thienopyridine P2Y12 inhibitors. Dyspnea associated with ticagrelor was reported in the DISPERSE trials and the ONSET/OFFSET trial.21,29,31 As previously discussed, the higher rate of dyspnea in the DISPERSE-2 trial with the ticagrelor 180-mg dose compared with the 90-mg twice/day dose contributed to the lack of continued study of the higher dose. Dyspnea in the ONSET/OFFSET trial was thought to be due to ticagrelor in 24.6% of patients compared with 3.7% with clopidogrel.47 Of the 22 cases reported with ticagrelor, three were judged to be of moderate severity, and all others were mild. The dyspnea usually occurred early after ticagrelor initiation, with 8 of the 22 events occurring within 24 hours, and 17 of the 22 occurring within 1 week. This is in comparison with four of five events with clopidogrel that occurred beyond 1 week. No significant differences in cardiac or pulmonary function parameters were noted in patients with dyspnea.47 In the PLATO trial, patients randomized to ticagrelor also had a significantly higher rate of reported dyspnea compared with those receiving clopidogrel (13.8% vs 7.8%, p<0.001).30 Of the patients who reported dyspnea while receiving ticagrelor, 5.9% prematurely discontinued therapy compared with 1.6% of the patients receiving clopidogrel who reported dyspnea (p<0.001). The discontinuation rate due to dyspnea in the overall PLATO trial was 0.9% for ticagrelor and 0.1% for clopidogrel (p<0.001).48
Therefore, dyspnea is often self-limiting, and the need to discontinue therapy is rare. Patients with dyspnea in the PLATO trial had a similar rate of thrombotic events compared with patients without dyspnea, and therefore no loss of treatment effect was seen in patients who developed dyspnea.48 There was no change in pulmonary function demonstrated in a subset of patients who underwent pulmonary function testing in the PLATO trial (n=199) with ticagrelor or clopidogrel.48 Patients with prior history of congestive heart failure, chronic obstructive pulmonary disease (COPD), or other causes of dyspnea were not at higher risk of developing ticagrelor-related dyspnea. This finding has been confirmed in another evaluation in which healthy elderly individuals and patients with asthma or COPD were not at higher risk of developing dyspnea with ticagrelor.49
Another adverse effect noted with ticagrelor has been an increase in ventricular pauses longer than 2.5 seconds. This was first recognized in the DISPERSE-2 trial in patients who underwent continuous electrocardiographic monitoring.29 This finding led to a substudy of the PLATO study in which 2908 patients had a 7-day continuous electrocardiogram recorded starting at the time of randomization and repeated at 1 month.50 Based on the previous findings of the DISPERSE-2 trial, patients at increased risk for a bradycardic event (known as sick sinus syndrome, second- or third-degree atrioventricular conduction block, or previously documented syncope suspected to be due to bradycardia unless treated with a pacemaker) were excluded from the PLATO trial. Ventricular pauses of 3 seconds or longer occurred in a higher proportion of patients receiving ticagrelor than clopidogrel (5.8% vs 3.6%, p=0.006) during the first week after randomization but in a similar proportion of patients at 1 month (2.1% vs 1.7%, p=0.52). Most of the difference between the groups was in the incidence of sinoatrial (SA) node pauses. Importantly, there were no significant differences between the groups in the incidence of clinically reported bradycardia-related adverse events such as dizziness, syncope, pacemaker placement, or cardiac arrest.30
The mechanism behind these distinctive adverse effects of ticagrelor may be due to the drug's ability to delay adenosine metabolism. It is known that continuous infusions of adenosine can produce dyspnea without bronchospasm.51 Continuous infusions of adenosine have also demonstrated the ability to produce SA node pauses compared with the more common occurrence of atrioventricular block produced by bolus doses of adenosine.51 Data from a canine model and healthy volunteers have demonstrated an increase in the effect of adenosine when exposed to ticagrelor, which is reversed when theophylline is given (an adenosine antagonist).34,35,52 These studies have shown that ticagrelor can interfere with adenosine metabolism and increase adenosine concentrations through inhibition of adenosine uptake by erythrocytes, most likely through inhibition of the sodium-independent equilibrative nucleoside transporter (ENT)-1).34,35,52 Erythrocyte ENT-1 is responsible for uptake of adenosine into the cell, where it is metabolized by multiple mechanisms. The ability of ticagrelor to inhibit adenosine's uptake through ENT-1 is likely due to the similar chemical structure of the two molecules (Figure2). This interaction produces an increase in adenosine exposure. Therefore, the same effect that produces increased coronary blood flow also produces these distinctive adverse effects. Because there is an elevation of adenosine produced during the acute ischemic burden of an ACS event in local tissues, the adverse effects of dyspnea and ventricular pauses are typically seen early in ticagrelor therapy. When the ischemic stimulus is reduced over the course of the next 30 days, the rates of these adverse effects are reduced, and the need to discontinue therapy is rare. This most likely also explains the increase in uric acid concentration demonstrated with ticagrelor early in treatment because uric acid would be a breakdown product of purine (adenosine) metabolism. There was also an increase in serum creatinine in ticagrelor patients compared with clopidogrel patients (10% vs 8% change from baseline; p<0.001).30 The effect on serum creatinine concentration may also be explained by the impact of ticagrelor on adenosine uptake because increased adenosine could alter renal hemodynamics by decreasing tension in the afferent arteriole, thereby lowering the glomerular filtration pressure. Although this difference was statistically significant, the clinical significance of this finding has yet to be determined.
Drug Interactions
In vitro studies indicate that ticagrelor is a substrate and a weak inhibitor of CYP3A.20 Ticagrelor is largely metabolized by CYP3A4 and to a lesser extent by CYP3A5. Because strong CYP3A4 inhibitors increase exposure to ticagrelor, their combined use is not recommended (Table4). For example, concomitant administration of ketoconazole with ticagrelor increased the ticagrelor Cmax 2.4-fold and the area under the concentration-time curve (AUC) 7.3-fold.53 Moderate CYP3A4 inhibitors have a proportional effect on ticagrelor exposure and are not contraindicated. For example, diltiazem increased the ticagrelor Cmax by 69%, and the AUC was increased 2.7-fold.53 Conversely, potent inducers of CYP3A4 may decrease exposure to and hence reduce the efficacy of ticagrelor. The combined use of potent CYP3A4 inducers with ticagrelor should be avoided based on a healthy volunteer study in which coadministration of rifampin decreased the ticagrelor Cmax and AUC by 73% and 86%, respectively (Table4).54 Coadministration of ticagrelor with CYP3A4 substrates with a narrow therapeutic index (e.g., cisapride, ergot alkaloids) is also not recommended because ticagrelor is a weak inhibitor of CYP3A4 and could increase the exposure of these drugs.55
Statins are widely prescribed to patients with ACS, and several are metabolized by CYP3A4. In a dedicated interaction study in healthy volunteers, increases in simvastatin Cmax (81%) and AUC (56%) were observed with coadministration of ticagrelor.56 In some individuals, however, more than 2-fold increases in simvastatin AUC were observed, and ticagrelor may have similar effects on lovastatin pharmacokinetics. Therefore, coadministration of ticagrelor with simvastatin or lovastatin doses higher than 40 mg/day could potentially cause adverse statin effects and should be avoided (Table4).56
Ticagrelor and AR-C124910XX are also substrates and inhibitors of P-glycoprotein. Coadministration of ticagrelor and digoxin in healthy volunteers resulted in a 75% and 28% increase in digoxin Cmax and AUC, respectively.55 Furthermore, mean trough digoxin levels were increased by ∼30% with up to 2-fold increases observed in some individuals. Based on these findings, digoxin levels should be monitored when initiating ticagrelor (Table4). P-glycoprotein inhibitors such as cyclosporine increase the exposure of ticagrelor, but no dosage adjustment is needed.57
In addition, ticagrelor undergoes intestinal CYP3A4-mediated first-pass metabolism as demonstrated in a recent interaction study in which grapefruit juice (200 ml 3 times/day for 4 days) increased exposure to ticagrelor 2-fold but decreased exposure to AR-C124910XX.18
Pharmacogenomics
It is well established that the variability of clopidogrel is partially due to genetic polymorphisms of the CYP2C19 enzymes required for metabolism of the inactive parent compound to the active metabolite.58 Although there is no mechanistic basis for the pharmacokinetic and pharmacodynamic profiles of ticagrelor to be influenced by genotype, the lack of effect of genotype was confirmed in a pooled analysis of data from the RESPOND and ONSET/OFFSET trials. Compared with clopidogrel, ticagrelor resulted in a significantly lower platelet reactivity (p<0.01), irrespective of CYP2C19 carrier status (loss- or gain-of-function), ABCB1 polymorphisms, and metabolizer status.59 These findings are supported by a substudy of the PLATO trial, which demonstrated that the reduction in thrombotic end points with the use of ticagrelor compared with clopidogrel was independent of CYP2C19 and ABCB1 polymorphisms.60 Additionally, single nucleotide polymorphisms in platelet P2Y12, P2Y1, or integrin β3 receptors did not influence the effect of ticagrelor on IPA in patients with stable CAD or ACS enrolled in the DISPERSE and DISPERSE-2 trials.61
Conclusion
Inhibiting the P2Y12 platelet receptor has demonstrated an ability to reduce adverse CV outcomes significantly over the use of aspirin alone. Despite over a decade of use with clopidogrel, the agent has a number of limitations that need to be addressed. Ticagrelor is a P2Y12-receptor inhibitor that overcomes many of these limitations. The different chemical structure, which is not a prodrug, allows for rapid, potent, and consistent inhibition of platelet aggregation. These attractive pharmacologic, pharmacokinetic, and pharmacodynamic properties may have contributed to a significant reduction in thrombotic events in the phase III PLATO trial. Although there was an increase in non–CABG-related major bleeding, the absolute difference was not as great as the clinical benefit. The ability of ticagrelor to alter adenosine uptake by red blood cells may influence both the efficacy and safety of the agent. Comparisons to prasugrel are more difficult to evaluate because direct comparison trials have not been conducted. Unlike prasugrel, ticagrelor offers advantages in being able to be used regardless of the management strategy (medical and invasive) of the ACS event. Prasugrel is currently only indicated in patients receiving PCI. Ticagrelor can be given upstream before knowing the coronary anatomy, whereas prasugrel cannot. There are no limitations to the use of ticagrelor based on a patient's history of ischemic stroke, body weight, or age. However, prasugrel does possess an increased magnitude of benefit in patients with diabetes mellitus that ticagrelor has yet to demonstrate. The once/day dosing of prasugrel is certainly an advantage compared with twice/day dosing of ticagrelor. Until a head-to-head comparison trial is conducted, comparisons between these agents remain speculative.
Although ticagrelor has been studied in only one major clinical trial, the use of ticagrelor in patients with ACS has a number of advantages. As mentioned earlier, ticagrelor can be used regardless of the type of ACS event, except in the setting of fibrinolytics for reperfusion in STEMI. Patients receiving ticagrelor will require education on the potential for dyspnea as well as the importance of patient adherence with the twice/day dosing regimen. Since ticagrelor is a branded product, cost may be prohibitive for some patients, and generic clopidogrel may be the most financially feasible option. Ticagrelor is currently being studied in the long-term prevention of CV events in patients with a previous MI (in the past 1–3 yrs) in the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin (PEGASUS-TIMI 54) trial (ClinicalTrials.gov registry number NCT01225562). The study of ticagrelor is continuing in other vascular beds as well, such as in the treatment and secondary prevention of stroke in the Acute Stroke or Transient Ischaemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes (SOCRATES) trial (NCT01994720) and in patients with peripheral arterial disease in the Examining Use of Ticagrelor in PAD (EUCLID) trial (NCT01732822). As data continue to develop, the complete role of ticagrelor in patients with atherosclerotic disease will evolve.
Acknowledgments
The authors would like to thank Ms. Melody Montgomery at the University of Nebraska Medical Center for her professional editorial assistance in preparing this manuscript for publication from the authors' original work. The authors also acknowledge the professional editorial assistance in preparing this manuscript for publication from the authors' original work provided by Tara Miller and Lisa Michel at Gardiner Caldwell Communications, which has received funding from AstraZeneca.
Conflicts of Interest
Dr. Dobesh has served as a consultant and has received research support from AstraZeneca. Drs. Dobesh and Oestreich have received research support from the Daiichi Sankyo and Eli Lilly alliance. AstraZeneca provided funding to Gardiner Caldwell Communications, who assisted in the preparation of this manuscript.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta RS, Yusuf S, Peters JG, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–33. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 3.Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 4.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–55. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 6.O'Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989–97. doi: 10.1016/S0140-6736(09)61525-7. [DOI] [PubMed] [Google Scholar]
- 7.Freynhofer MK, Bruno V, Brozovic I, et al. Variability of on-treatment platelet reactivity in patients on clopidogrel. Platelets. 2014;25:328–36. doi: 10.3109/09537104.2013.827781. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Chang K, Koh YS, et al. CYP2C19 poor metabolizer is associated with clinical outcome of clopidogrel therapy in acute myocardial infarction but not stable angina. Circ Cardiovasc Genet. 2013;6:514–21. doi: 10.1161/CIRCGENETICS.113.000109. [DOI] [PubMed] [Google Scholar]
- 9.Dobesh PP. Pharmacokinetics and pharmacodynamics of prasugrel, a thienopyridine P2Y12 inhibitor. Pharmacotherapy. 2009;29:1089–102. doi: 10.1592/phco.29.9.1089. [DOI] [PubMed] [Google Scholar]
- 10.van Giezen JJ, Nilsson L, Berntsson P, et al. Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost. 2009;7:1556–65. doi: 10.1111/j.1538-7836.2009.03527.x. [DOI] [PubMed] [Google Scholar]
- 11.Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27:259–74. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Giezen JJ, Berntsson P, Zachrisson H, Björkman JA. Comparison of ticagrelor and thienopyridine P2Y(12) binding characteristics and antithrombotic and bleeding effects in rat and dog models of thrombosis/hemostasis. Thromb Res. 2009;124:565–71. doi: 10.1016/j.thromres.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Ingall AH, Dixon J, Bailey A, et al. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42:213–20. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- 14.Springthorpe B, Bailey A, Barton P, et al. From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett. 2007;17:6013–8. doi: 10.1016/j.bmcl.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 15.Butler K, Teng R. Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol. 2010;70:65–77. doi: 10.1111/j.1365-2125.2010.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sillén H, Cook M, Davis P. Determination of unbound ticagrelor and its active metabolite (AR-C124910XX) in human plasma by equilibrium dialysis and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2315–22. doi: 10.1016/j.jchromb.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514–21. doi: 10.1124/dmd.110.032250. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg MT, Tornio A, Joutsi-Korhonen L, et al. Grapefruit juice markedly increases the plasma concentrations and antiplatelet effects of ticagrelor in healthy subjects. Br J Clin Pharmacol. 2013;15:1488–96. doi: 10.1111/bcp.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giorgi MA, Cohen Arazi H, Gonzalez CD, Di Girolamo G. Beyond efficacy: pharmacokinetic differences between clopidogrel, prasugrel and ticagrelor. Expert Opin Pharmacother. 2011;12:1285–95. doi: 10.1517/14656566.2011.550573. [DOI] [PubMed] [Google Scholar]
- 20.Zhou D, Andersson TB, Grimm SW. In vitro evaluation of potential drug-drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction, and differential kinetics. Drug Metab Dispos. 2011;39:703–10. doi: 10.1124/dmd.110.037143. [DOI] [PubMed] [Google Scholar]
- 21.Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27:1038–47. doi: 10.1093/eurheartj/ehi754. [DOI] [PubMed] [Google Scholar]
- 22.Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y(12) receptor antagonist, in healthy subjects. Eur J Clin Pharmacol. 2010;66:487–96. doi: 10.1007/s00228-009-0778-5. [DOI] [PubMed] [Google Scholar]
- 23.Husted SE, Storey RF, Bliden K, et al. Pharmacokinetics and pharmacodynamics of ticagrelor in patients with stable coronary artery disease: results from the ONSET-OFFSET and RESPOND studies. Clin Pharmacokinet. 2012;51:397–409. doi: 10.2165/11599830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50:1852–6. doi: 10.1016/j.jacc.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 25.Butler K, Teng R. Pharmacokinetics, pharmacodynamics, and safety of ticagrelor in volunteers with severe renal impairment. J Clin Pharmacol. 2012;52:1388–98. doi: 10.1177/0091270011415526. [DOI] [PubMed] [Google Scholar]
- 26.Butler K, Teng R. Pharmacokinetics, pharmacodynamics, and safety of ticagrelor in volunteers with mild hepatic impairment. J Clin Pharmacol. 2011;51:978–87. doi: 10.1177/0091270010379409. [DOI] [PubMed] [Google Scholar]
- 27.Labarthe J, Théroux P, Angioï M, Ghitescu M. Matching the evaluation of the clinical efficacy of clopidogrel to platelet function tests relevant to the biological properties of the drug. J Am Coll Cardiol. 2005;46:638–45. doi: 10.1016/j.jacc.2005.02.092. [DOI] [PubMed] [Google Scholar]
- 28.Teng R, Mitchell P, Butler K. Effect of age and gender on pharmacokinetics and pharmacodynamics of a single ticagrelor dose in healthy individuals. Eur J Clin Pharmacol. 2012;68:1175–82. doi: 10.1007/s00228-012-1227-4. [DOI] [PubMed] [Google Scholar]
- 29.Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol. 2007;50:1844–51. doi: 10.1016/j.jacc.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 30.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 31.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–85. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 32.Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010;121:1188–99. doi: 10.1161/CIRCULATIONAHA.109.919456. [DOI] [PubMed] [Google Scholar]
- 33.Lewis HD, Davis JW, Archibald DG, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1983;309:396–403. doi: 10.1056/NEJM198308183090703. [DOI] [PubMed] [Google Scholar]
- 34.van Giezen JJ, Sidaway J, Glaves P, Kirk I, Björkman JA. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther. 2012;17:164–72. doi: 10.1177/1074248411410883. [DOI] [PubMed] [Google Scholar]
- 35.Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G, et al. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol. 2013;61:723–7. doi: 10.1016/j.jacc.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 36.James SK, Roe MT, Cannon CP, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ. 2011;342:d3527. doi: 10.1136/bmj.d3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannon CP, Harrington RA, James S, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375:283–93. doi: 10.1016/S0140-6736(09)62191-7. [DOI] [PubMed] [Google Scholar]
- 38.Steg PG, James S, Harrington RA, et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131–41. doi: 10.1161/CIRCULATIONAHA.109.927582. [DOI] [PubMed] [Google Scholar]
- 39.Held C, Asenblad N, Bassand JP, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol. 2011;57:672–84. doi: 10.1016/j.jacc.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544–54. doi: 10.1161/CIRCULATIONAHA.111.047498. [DOI] [PubMed] [Google Scholar]
- 41.FitzGerald GA, Oates JA, Hawiger J, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983;71:676–88. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storey RF, Sanderson HM, White AE, May JA, Cameron KE, Heptinstall S. The central role of the P2T receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br J Haematol. 2000;110:925–34. doi: 10.1046/j.1365-2141.2000.02208.x. [DOI] [PubMed] [Google Scholar]
- 43.Warner TD, Nylander S, Whatling C. Antiplatelet therapy: cyclooxygenase inhibition and the use of aspirin with particular regard to dual antiplatelet therapy. Br J Clin Pharmacol. 2011;72:619–33. doi: 10.1111/j.1365-2125.2011.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohli P, Udell JA, Murphy SA, et al. Discharge aspirin dose and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel. An analysis from the TRITION-TIMI 38 study (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction 38) J Am Coll Cardiol. 2014;63:225–32. doi: 10.1016/j.jacc.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Mehta SR, Tanguay J-F, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–43. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 46.May CH, Lincoff AM. Safety profile and bleeding risk of ticagrelor compared with clopidogrel. Expert Opin Drug Saf. 2012;11:959–67. doi: 10.1517/14740338.2012.720972. [DOI] [PubMed] [Google Scholar]
- 47.Storey RF, Bliden KP, Patil SB, et al. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J Am Coll Cardiol. 2010;56:185–93. doi: 10.1016/j.jacc.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 48.Storey RF, Becker RC, Harrington RA, et al. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011;32:2945–53. doi: 10.1093/eurheartj/ehr231. [DOI] [PubMed] [Google Scholar]
- 49.Butler K, Maya J, Teng R. Effect of ticagrelor on pulmonary function in healthy elderly volunteers and asthma or chronic obstructive pulmonary disease patients. Curr Med Res Opin. 2013;29:569–77. doi: 10.1185/03007995.2013.781502. [DOI] [PubMed] [Google Scholar]
- 50.Scirica BM, Cannon CP, Emanuelsson H, et al. The incidence of bradyarrhythmias and clinical bradyarrhythmic events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) trial: results of the continuous electrocardiographic assessment substudy. J Am Coll Cardiol. 2011;57:1908–16. doi: 10.1016/j.jacc.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 51.Camm AJ, Garratt CJ. Adenosine and supraventricular tachycardia. N Engl J Med. 1991;325:1621–9. doi: 10.1056/NEJM199112053252306. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014;19:209–19. doi: 10.1177/1074248413511693. [DOI] [PubMed] [Google Scholar]
- 53.Teng R, Butler K. Effect of the CYP3A inhibitors, diltiazem and ketoconazole, on ticagrelor pharmacokinetics in healthy volunteers. J Drug Assess. 2013;2:30–9. doi: 10.3109/21556660.2013.785413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teng R, Mitchell P, Butler K. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of ticagrelor in healthy subjects. Eur J Clin Pharmacol. 2013;69:877–83. doi: 10.1007/s00228-012-1436-x. [DOI] [PubMed] [Google Scholar]
- 55.Teng R, Butler K. A pharmacokinetic interaction study of ticagrelor and digoxin in healthy volunteers. Eur J Clin Pharmacol. 2013;69:1801–8. doi: 10.1007/s00228-013-1543-3. [DOI] [PubMed] [Google Scholar]
- 56.Teng R, Mitchell PD, Butler KA. Pharmacokinetic interaction studies of co-administration of ticagrelor and atorvastatin or simvastatin in healthy volunteers. Eur J Clin Pharmacol. 2013;69:477–87. doi: 10.1007/s00228-012-1369-4. [DOI] [PubMed] [Google Scholar]
- 57.Teng R, Kujacic M, Hsia J. Pharmacokinetic interaction study of ticagrelor and cyclosporine in healthy volunteers. Clin Drug Investig. 2014;34:529–36. doi: 10.1007/s40261-014-0205-2. [DOI] [PubMed] [Google Scholar]
- 58.Karaźniewicz-Łada M, Danielak D, Główka F. Genetic and non-genetic factors affecting the response to clopidogrel therapy. Expert Opin Pharmacother. 2012;13:663–83. doi: 10.1517/14656566.2012.666524. [DOI] [PubMed] [Google Scholar]
- 59.Tantry US, Bliden KP, Wei C, et al. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circ Cardiovasc Genet. 2010;3:556–66. doi: 10.1161/CIRCGENETICS.110.958561. [DOI] [PubMed] [Google Scholar]
- 60.Wallentin L, James S, Storey RF, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–8. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 61.Storey RF, Melissa Thornton S, Lawrance R, et al. Ticagrelor yields consistent dose-dependent inhibition of ADP-induced platelet aggregation in patients with atherosclerotic disease regardless of genotypic variations in P2RY12, P2RY1, and ITGB3. Platelets. 2009;20:341–8. doi: 10.1080/09537100903075324. [DOI] [PubMed] [Google Scholar]
- 62.Rao AK, Pratt C, Berke A, et al. Thrombolysis In Myocardial Infarction (TIMI) trial—phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11:1–11. doi: 10.1016/0735-1097(88)90158-1. [DOI] [PubMed] [Google Scholar]


