Abstract
NG2 cells (polydendrocytes) are the fourth major non-neuronal cell type in the central nervous system parenchyma. They exhibit diverse properties, ranging from their well-established role as oligodendrocyte precursors to their ability to respond to neurotransmitters released by synaptic and non-synaptic mechanisms. The functional diversity of NG2 cells has prompted the question of whether they represent a single cellular entity or multiple distinct cell populations. This review first summarizes recent findings on the nature and mechanism underlying the diversity of NG2 cells with regard to their proliferative and differentiation behavior. This will be followed by a synopsis of observations on how their microenvironment, particularly neuronal activity, influences their dynamic behavior, and how these changes in NG2 cells could in turn influence neural function and animal behavior. GLIA 2014;62:1195–1210
Keywords: oligodendrocyte, oligodendrocyte precursor cell, polydendrocyte, myelin, demyelination, plasticity, white matter, PDGF
Introduction
NG2 cells, also called polydendrocytes, are a resident population of glial cells in the central nervous system (CNS) with distinct morphological and physiological characteristics (Dawson et al., 2000; Nishiyama, 2012; Nishiyama et al., 2009; Richardson et al., 2011). Currently they are defined as cells in the CNS parenchyma that express the NG2 chondroitin sulfate proteoglycan (CSPG4) and the alpha receptor for platelet-derived growth factor (PDGFRα) (Nishiyama, 2007; Nishiyama et al., 1996). In the mature CNS, NG2 cells are uniformly distributed in gray and white matter and are the major proliferative cell population outside the neurogenic regions of the subventricular zone (SVZ) and the hippocampus (Dawson et al., 2003). Their most well established role is to produce oligodendrocytes (Zhu et al., 2008a,b), and thus they are also commonly referred to as oligodendrocyte precursor cells (OPCs). While this is likely their primary function, other properties that are seemingly unrelated to their oligodendrogliogenic function have been described. These include their ability to generate astrocytes in some regions (Zhu et al., 2008a,b) and the intriguing observation that they receive neuronal synaptic inputs (Bergles et al., 2000). Furthermore, some NG2 cells are likely to remain as NG2 cells without differentiating into oligodendrocytes. Consensus on the name and definition of the NG2 cell population has not been reached, and this could be partly due to the conceptual difficulty in attributing these diverse functions to a single cellular entity. Therefore, it is important to determine how divergent or related their seemingly disparate properties are in order to clearly define this population and understand their role in the CNS under physiological and pathological conditions.
NG2 cells maintain a resident population by self-renewal while continuously producing oligodendrocytes throughout life. Their proliferative rates change across development and different brain regions (Dawson et al., 2003; Psachoulia et al., 2009; Simon et al., 2011; Young et al., 2013) and correlate with the rate of oligodendrocyte production (Dimou et al., 2008; Kang et al., 2010; Rivers et al., 2008; Zhu et al., 2011). Myelinating oligodendrocytes that are generated from NG2 cells provide support and maintenance for axons in addition to dramatically increasing the speed of action potential conduction (Fields, 2008; Nave, 2010). Therefore differences in NG2 cell function are intimately associated with differences in neuronal function, connectivity and survival. Deviation from the normal density of myelinating oligodendrocytes can result in severe functional deficit, and oligodendrocyte density is maintained constant under exquisitely tight control. Exactly how an NG2 cell makes the decision to self-renew or terminally differentiate into an oligodendrocyte at the appropriate time and place remains unknown. This article will first examine recent findings on the heterogeneity of the origin of NG2 cells and the age- and region-dependent differences in their proliferation and differentiation. This will be followed by a discussion on how neuronal activity and behavioral changes dynamically affect NG2 cells, oligodendrocytes, and myelin.
Heterogeneity in the Origin of NG2 Cells
In the rodent CNS, NG2 cells first arise from discrete loci in the ventral germinal zone during mid-gestation. In the spinal cord, they emerge between E12.5 and E14.5 from the pMN domain defined by the expression of the basic helix-loop-helix transcription factor Olig2 induced by the ventral morphogen Sonic hedgehog as well as from the ventrally adjacent p3 domain defined by the homeodomain transcription factor Nkx2.2. A few days later, additional NG2 cells are generated from the dorsal germinal zone and contribute to approximately 10% of NG2 cells in the myelencephalon (Huang et al., 2013; Rowitch, 2004; Woodruff et al., 2001). In the developing forebrain, NG2 cells also appear sequentially from different domains, initially from the ventral germinal zones of the medial and lateral ganglionic eminences defined by the transcription factors Nkx2.1 and Gsh2, respectively (Fig. 1, purple and blue), followed by the dorsal ventricular zone defined by Emx1 that begins to generate NG2 cells perinatally (Fig. 1, green; Kessaris et al., 2006; Rowitch and Kriegstein, 2010). In addition to dorsoventral patterning, the rostrocaudal identity of the cellular source is defined by the Hox genes and is transmitted to the progeny by epigenetically established chromatin domains (Mazzoni et al., 2013; Philippidou and Dasen, 2013).
Figure 1.
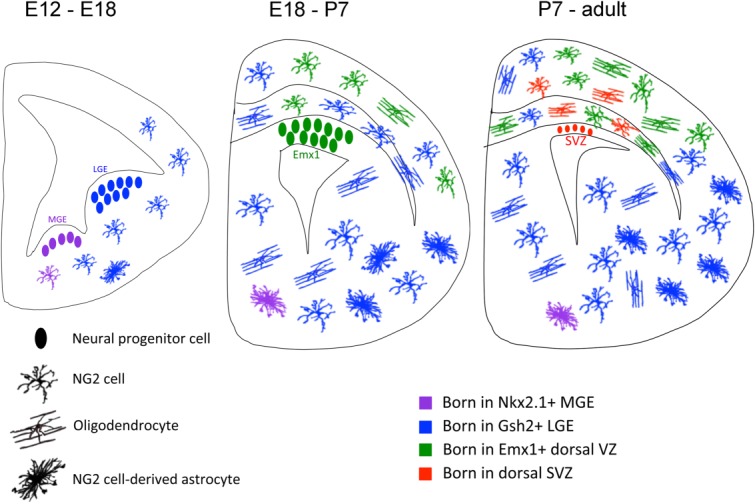
Heterogeneous origin and fate of NG2 cells in the forebrain. During mid-gestation, NG2 cells in the forebrain are generated from the ventral germinal zones of medial and lateral ganglionic eminences (MGE (purple) and LGE (blue), respectively) defined by the transcriptional factors Nkx2.1 and Gsh2, respectively. The LGE-derived cells expand and constitute the majority of NG2 cells in the subpallial forebrain but also migrate dorsally to populate the corpus callosum and to a lesser extent the neocortex. Subsequently, from late embryonic stages to early postnatal period, additional NG2 cells are generated from the dorsal VZ defined by the Emx1 transcriptional factor (Emx1, green). After the VZ has ceased to produce new neurons and glia, a minor population of NG2 cells is generated from the SVZ (red). NG2 cells produced from all of these sources are capable of generating oligodendrocytes. In addition, those from the ventral germinal zones differentiate into protoplasmic astrocytes, constituting up to 40% of the protoplasmic astrocytes in the ventral posterior gray matter of the forebrain. It is not yet known whether the astrogliogenic NG2 cells originate in MGE or LGE or both. As soon as NG2 cells exit the germinal zone, they acquire one or more processes, thus already exhibiting the polydendrocytic morphology from as early as mid-embryonic stages.
After the cells exit the germinal zones, they acquire NG2 expression and process-bearing morphology (and hence become bona fide polydendrocytes) as they proliferate and migrate to fill the entire parenchyma. The extent to which NG2 cells from different sources become intermingled varies in different regions (Tripathi et al., 2011). In the forebrain, NG2 cells in the corpus callosum comprise a mixed population of cells derived from the ventral dorsal domains, while the majority of NG2 cells in the neocortex are derived from the dorsal domain. However, even in the neocortex the NG2 cell population is not entirely homogeneous, and a significant number of ventrally derived cells coexist with the dorsally derived cells (Fig. 1; see also Fig. 2 in Tripathi et al., 2011). Later in postnatal life, additional NG2 cells are also generated from the SVZ of the lateral ventricles (Fig. 1, red), which contains neural stem cells as well as transit amplifying progenitor cells (Menn et al., 2006). There appears to be heterogeneity within the SVZ, where NG2 cells are generated primarily from the dorsal wall and do not share the lineage with neuronal cells (Ortega et al., 2013). The SVZ-derived cells may constitute a minority of NG2 cells in the dorsal forebrain, where the population is mostly maintained by local proliferation of existing NG2 cells (Hughes et al., 2013).
Figure 2.

Different functional outcomes from heterogeneous properties of NG2 cells between gray and white matter. Top: Cell intrinsic differences between gray and white matter NG2 cells. The more oxidized state of white matter NG2 cells could lead to increased PI3K signal or PDGFRα activation (left). White matter NG2 cells are also reported to be more depolarized and thus may have higher intracellular calcium concentrations. Middle: Differences in the ability of gray and white matter NG2 cells to sense their environment. Even in the presence of similar amounts of extracellular PDGF and cell surface PDGFRα, NG2 cells in white matter may undergo greater PDGFRα activation due to differences in receptor activation mechanisms. Bottom: The cell intrinsic and cell surface mechanisms shown above will define the specific outcomes that are detected as differences in the rate of proliferation and oligodendrocyte differentiation between gray and white matter NG2 cells.
Functional differences have not yet been detected postnatally among NG2 cells and oligodendrocytes that originate from different developmental sources. NG2 cells or mature oligodendrocytes derived from ventral and dorsal germinal zones exhibited similar basal proliferation rates, cell cycle times, and passive membrane properties, and there were no statistically significant differences in their response to GABA and glutamate (Psachoulia et al., 2009; Tripathi et al., 2011). However, there seemed to be tract preferences in the dorsal funiculus of the spinal cord for myelination by dorsally and ventrally derived oligodendrocytes (Tripathi et al., 2011). Whether these differences reflect the timing of their birth or genetic differences that arise from the site of origin remains unknown. Interestingly, a recent study on the development of GABAergic neurons in the forebrain revealed differences in glutamate receptor subunit composition and calcium permeation between interneurons derived from medial and caudal ganglionic eminences (Matta et al., 2013), suggesting that embryonic cellular origin defined by specific transcription factor codes could similarly affect the behavior of NG2 cells such as their calcium response to glutamate. Additional studies may uncover subtle but functionally significant differences in NG2 cells originating from different germinal zones in the telencephalon or between NG2 cells in different rostrocaudal locations.
Heterogeneity in Proliferation of NG2 Cells
Age- and Region-Dependent Differences in Cell Cycle Times
Reports on regional differences in NG2 cell proliferation generally show greater basal proliferation rates in white matter compared with gray matter, and proliferation rates decline with age. For example, 2-hour pulse labeling of 3-month-old rats with 5-bromo-2'-deoxyuridine (BrdU) revealed that the BrdU labeling index of NG2 cells was greater in the corpus callosum (4%) than in the neocortex (∼1%) (Dawson et al., 2003), although the BrdU labeling indices in such pulse-labeling studies are influenced by differences in the duration of S-phase or cell cycle time. Using cumulative BrdU labeling in mice, the growth fraction of NG2 cells was initially calculated to be around 50% (Psachoulia et al., 2009), while more recent reports suggest that it is greater than 98% (Kang et al., 2010; Young et al., 2013). Contrary to the previous speculation that NG2 cells represent a heterogeneous population comprised of proliferative and postmitotic cells (Hermann et al., 2010), these findings suggest that NG2 cells are equivalent in that none have irreversibly exited the cell cycle, although caution is needed when interpreting the results of these long-term BrdU labeling studies, which could be skewed by events such as cell death, differentiation and cell cycle re-entry. Nevertheless, there are irrefutable regional and age-dependent differences in the cell cycle times of NG2 cells. The cell cycle time lengthens dramatically in the developing CNS, from approximately 6 h at E13.5 immediately after their emergence from the myelencephalic ventricular zone to about 24 h around the time of birth (Calver et al., 1998). It continues to increase during postnatal development, reaching 10 to 36 days by postnatal day 60 (P60), depending on the anatomical region (Young et al., 2013) and can be as long as 170 days in the neocortex of 18-month-old mice (Psachoulia et al., 2009).
The regional difference in the cell cycle times of NG2 cells becomes more pronounced with postnatal age (Psachoulia et al., 2009; Simon et al., 2011; Young et al., 2013). For example, the cell cycle time in P21 mice is 2.7 days in the corpus callosum and 18.6 days in the neocortex (Young et al., 2013), while in younger mice at P6, prior to the onset of myelination, the cell cycle times are similar at 1.7 days in the corpus callosum and 2 days in the neocortex (Psachoulia et al., 2009). The Young study also shows that cell cycle times can vary in different white matter tracts such as the optic nerve (7.6 days at P21) and spinal cord (4.4 days at P21). An earlier study by Horner et al. (2000) showed that within the white matter of the spinal cord, the outermost subpial region exhibited greater NG2 cell proliferation than the more central white matter regions. While these estimates represent average cell cycle times, it is likely that within a given anatomical region at a given age, there is a high degree of variability of cell cycle times among individual NG2 cells. This is suggested by in vivo cluster analysis in which a low level of Cre induced in NG2creER or PDGFRα-creER mice crossed to Cre reporter mice led to a significant variability in the size of the reporter+ clones after a survival period of 60 to 80 days (Kang et al., 2010; Zhu et al., 2011), as well as in earlier analyses of clonal size after retroviral labeling of progenitor cells in the SVZ (Levison and Goldman, 1993; Levison et al., 1999), which demonstrated that while the majority of the clones in the rat neocortex underwent expansion during the first month after birth, a few clones continued to expand beyond 3 months of age. Furthermore, a recent study suggested that in addition to variability in the size of single NG2 cell clones, there is massive clonal expansion of NG2 cells in adult brain, providing further evidence for age-dependent differences in cell cycle and proliferation rates (Garcia-Marques et al., 2014). It will be interesting to determine whether slowly proliferative stem cell-like NG2 cells co-exist with more rapidly cycling amplifying cells within the same micro-region and how the local microenvironment might influence these properties.
Extracellular Mechanisms of Regional Heterogeneity in NG2 Cell Proliferation
Numerous extrinsic signals have been identified that can influence NG2 cell proliferation. These include secreted paracrine factors such as growth factors (reviewed in Franklin, 2002) and neurotransmitters; cell surface and extracellular matrix molecules such as laminin on axonal surface (Baron et al., 2002,2005; Colognato et al., 2002); and biophysical mechanisms resulting from axon-NG2 cell interactions (Lee et al., 2012; Rosenberg et al., 2008). Platelet-derived growth factor (PDGF) is one of the best characterized molecules that is secreted from neurons and astrocytes and stimulates NG2 cell proliferation (Noble et al., 1988; Raff et al., 1988; Richardson et al., 1988). The AA homodimer of PDGF (PDGF-AA) is used as the standard supplement in the proliferative medium for dissociated cultures of NG2 cells. The importance of this growth factor in vivo was demonstrated by severe depletion of NG2 cells and subsequent hypomyelination in mice that lack the gene encoding PDGF A subunit (PDGF-A) but not PDGF-B (Fruttiger et al., 1999). Conversely, transgenic overexpression of PDGF-A caused an increase in NG2 cell proliferation and density throughout the embryonic and early postnatal spinal cord (Calver et al., 1998).
A new study using organotypic slice cultures demonstrated that the proliferative response of NG2 cells to PDGF is significantly greater in the white matter tracts of the corpus callosum and cerebellum compared with that in adjacent gray matter regions (Fig. 2; Hill et al., 2013). While NG2 cells in white matter proliferated in a dose-dependent manner to PDGF-AA, NG2 cells in gray matter did not proliferate even in the presence of>50 ng/mL of PDGF-AA. This was rather surprising, given that PDGF-AA is used in proliferative medium, even for culturing “neocortical NG2 cells,” and that PDGFRα is widely known to be expressed by NG2 cells in both gray and white matter. Heterotopic cross-transplantation in slice cultures or isolated explant cultures of 300 µm3 pieces of gray or white matter tissue suggested that the differential proliferative response to PDGF was intrinsic to the tissue of origin. Since no significant difference in the intracellular signal transduction pathways was found between gray and white matter NG2 cells, the difference might be attributed to the immediate pericellular microenvironment. One possibility is that gray matter expresses saturating amounts of PDGF, thereby desensitizing the receptor. It is interesting to note that an earlier in situ hybridization study revealed a greater signal for PDGF-A transcript in the gray matter of E15.5 spinal cord than in the white matter (Calver et al., 1998), although overexpression of PDGF-A in embryonic neurons led to a generalized increase in NG2 cells throughout the spinal cord. Since there are no reports showing detectable differences in PDGFRα expression between gray and white matter NG2 cells (Hill et al., 2013; Nishiyama et al., 1996; Pringle et al., 1992), it is likely that the difference stems from differences in the mechanisms of receptor activation (Fig. 2), possibly mediated by extracellular matrix (Baron et al., 2002) or soluble paracrine factors such as the astrocyte-derived chemokine CXCL1 (GRO1), which has been shown to potentiate the effect of PDGF on NG2 cells from the spinal cord (Robinson et al., 1998; Wu et al., 2000). These pericellular factors could provide a precise local regulation of NG2 cell proliferation.
It is possible that in white matter regions, activity-dependent release of molecules such as PDGF (from neurons and/or astrocytes) instruct white matter NG2 cells to proliferate and differentiate into oligodendrocytes to myelinate electrically active axons (see Discussion below) (Barres and Raff, 1993; Demerens et al., 1996). In gray matter regions, however, PDGF may not play the same role in instructing gray matter NG2 cells to proliferate and differentiate. The difference in the proliferative behavior of NG2 cells in different CNS regions could also reflect regional differences in astrocyte function, such as their ability to sense neuronal activity (see below) or their general secretory mechanism. In addition to astrocytes, regional differences in microglia may lead to divergent behaviors in NG2 cells (see below). Finally, the newly emerging concept of the neurovascular niche has been broadened to include interactions between cerebrovascular endothelial cells and oligodendrocyte lineage cells (Miyamoto et al., 2014). Thus the proximity to and association with vascular components may promote their proliferative behavior, as seen for juxtavascular astrocytes in response to injury (Bardehle et al., 2013).
Cell Intrinsic Mechanisms of Regional Heterogeneity in NG2 Cell Proliferation
Among the intracellular signaling pathways activated by receptor tyrosine kinases such as PDGFRα, the Ras-Mitogen-activated kinase (MAPK) pathway plays a major role in cell proliferation (Andrae et al., 2008). Surprisingly, slice culture studies and in vivo studies using mice lacking the extracellular receptor kinases 1 and 2 (ERK1/2) indicate that the MAPK pathway is not the primary signal transduction pathway that mediates proliferation of NG2 cells (Fyffe-Maricich et al., 2011; Hill et al., 2013; Ishii et al., 2012), although this pathway is critical for oligodendrocyte differentiation. The slice culture studies suggest that both basal and PDGF-induced NG2 cell proliferation is mediated by a combination of phosphatidylinositol-3-kinase (PI3K) and the canonical Wnt signaling pathways (Hill et al., 2013), consistent with previous reports on NG2 cell proliferation in dissociated culture (Baron et al., 2002; Chew et al., 2011; Ebner et al., 2000). There is no evidence to suggest that these intracellular signal transduction pathways are differentially activated in NG2 cells in different CNS regions. While the exact nature of the cell intrinsic differences that endow NG2 cells in white matter with greater proliferative ability remains unknown, we describe here the resting membrane potential and redox state as two possible cell intrinsic mechanisms that could mediate regional differences in NG2 cell proliferation (Fig. 2).
Resting Membrane Potential
Reports have suggested that potassium channel currents (both voltage-gated Kv1 and inward rectifying Kir) and resting membrane potentials differ between gray and white matter NG2 cells Fig. 2, (Chittajallu et al., 2004). PDGF exposure can stimulate the expression of voltage gated Kv1.3 potassium channels by NG2 cells and over-expressing Kv1.3 and Kv1.4 subunits results in increased NG2 cell proliferation even in the absence of mitogens such as PDGF (Chittajallu et al., 2002; Vautier et al., 2004). If white matter NG2 cells express more Kv1.3 or Kv1.4 channels or if these channels are more active, then the ability of white matter NG2 cells to proliferate may be enhanced.
The inwardly rectifying potassium channel Kir4.1 is important for setting resting membrane potential in a variety of cells types and plays a role in potassium buffering by astrocytes (Djukic et al., 2007; Neusch et al., 2006). Kir channels are expressed by oligodendrocyte lineage cells, and Chittajallu et al. (2004) reported larger Kir currents and more hyperpolarized resting membrane potentials in neocortical NG2 cells compared with those in the corpus callosum. By contrast, De Biase et al. (2010) found no differences in membrane potential between gray and white matter regions. The differences may reflect slightly different stages of differentiation of the cells from which recordings were obtained. Regardless, Kir4.1 knockout animals display a marked hypomyelinating phenotype and do not survive past the third or fourth postnatal week (Djukic et al., 2007; Neusch et al., 2001). Furthermore, NG2 cells isolated from these animals exhibit depolarized membrane potentials and immature morphology while neurons appear relatively normal (Neusch et al., 2001). These data suggest that Kir4.1 channels might regulate the differentiation of NG2 cells into myelinating oligodendrocytes. If there are indeed differential Kir currents between NG2 cells in the neocortex and corpus callosum these variations could account for the differences in functional properties. Although it is not exactly clear how Kir currents could influence NG2 cell proliferation and differentiation, a correlation between membrane potential and the proliferative status has been seen in various progenitor populations (Blackiston et al., 2009; Sundelacruz et al., 2009).
Redox State
Intracellular redox state has been shown to influence the balance between self-renewal and differentiation in stem cell populations (Wang et al., 2013a). In neural stem cells (NSCs) in the SVZ, elevated reactive oxygen species (ROS) generated by NADPH-oxidase (NOX) favored self-renewal and enhanced neurosphere formation (Le Belle et al., 2011). In this study, elevated ROS levels in NSCs stimulated the PI3K-Akt pathway by oxidizing and inhibiting the function of PTEN (phosphatase and tensin homolog) protein, which dephosphorylates phosphatidylinositol-triphosphates and counters the PI3K-Akt pathway. Interestingly, isolated NG2 cells from the white matter are more oxidized, similar to proliferative NSCs (Power et al., 2002). Thus, as in NSCs, higher ROS in white matter NG2 cells could promote their proliferation via PI3K (Fig. 2) Furthermore, higher H2O2 levels in white matter NG2 cells could increase transcription of PDGFRα, as reported for vascular smooth muscle cells (Bonello et al., 2005), thereby making them more sensitive to PDGF (Fig. 2). However, this is not consistent with the negative correlation observed between oxidized state and self-renewal in dissociated NG2 cell culture. In these studies, oxidized NG2 cells were shown to be more quiescent, and neocortical gray matter cells were shown to be more reduced and thus more self-renewing, while NG2 cells from the optic nerve were more oxidized and tended to undergo terminal differentiation (Li et al., 2007; Power et al., 2002). This is contrary to the recent observation that white matter NG2 cells proliferate more in response to PDGF (Hill et al., 2013), although white matter NG2 cells also differentiate into oligodendrocytes more readily than gray matter cells. These differences could arise from the cellular microenvironment created by the experimental conditions. The proliferative behavior of NG2 cells can be context dependent. For example, fibroblast growth factor 2 (FGF2), which causes proliferation of dissociated NG2 cells, does not alter the proliferative behavior of NG2 cells in a more intact environment of slice culture or in vivo (Furusho et al., 2012; Hill et al., 2013). Further comparison of the redox state of NG2 cells in different regions and with different physiological preparations is necessary to resolve these differences. Overall these studies suggest that redox state may be an important physiological regulator of NG2 cell proliferation and fate during normal development and in the adult.
A shift in the cellular balance toward a more oxidized state can occur not only under physiological conditions such as increased aerobic respiration (Wang et al., 2013a) but also in pathological states such as hypoxia and inflammation (Lo et al., 2003). Recently, evidence for oxidative changes was detected in scattered oligodendrocytes in the “initial preinflammatory lesion” between the edge of an active MS lesion and the perilesional white matter (Haider et al., 2011). NG2 cells undergo increased proliferation in response to a variety of insults such as demyelination and stroke, and increased ROS in NG2 cells could be a part of the mechanism for promoting their proliferation.
Mechanisms of Age-Dependent Changes in NG2 Cell Proliferation
During development, there is a need to populate the forming CNS with different types of neurons and glial cells. There could be a density-dependent mechanism that causes NG2 cells to stop proliferating as the density of NG2 cells and oligodendrocytes increases (Nakatsuji and Miller, 2001). Such a density-dependent signal could consist of a combination of paracrine secreted factors and contact-mediated mechanisms. As the animal matures and the normal density of NG2 cells and oligodendrocytes is reached, a higher tone of the inhibitory signal for NG2 cell proliferation could reduce the proliferative rates. This is consistent with increased proliferation of NG2 cells in dysmyelinating mutants as well as in demyelinated lesions in the adult (Bu et al., 2004; Di Bello et al., 1999; Keirstead et al., 1998; Wu et al., 2000) or after removal of single NG2 cells or oligodendrocytes (Hughes et al., 2013; Kirby et al., 2006). Another proposed mechanism for the gradual decline in NG2 cell proliferation is a cell-intrinsic timer that counts and restricts the number of divisions that an NG2 cell undergoes, possibly through accumulation of cell cycle inhibitors such as p27kip1 (Durand et al., 1997). This is consistent with the differences in cell cycle times observed for purified cultures of NG2 cells isolated from perinatal and adult optic nerves (Wolswijk and Noble, 1989). Epigenetic mechanisms such as age-dependent decline in histone acetylation (Shen et al., 2005) also contribute to the intrinsic mechanism and can be subject to regulation by the regional microenvironment. Such intrinsic age-dependent inhibitory effects on NG2 cell proliferation are reversible and can be overcome by changes in NG2 cell or oligodendrocyte density such as demyelination or ablation of NG2 cells.
Heterogeneity in Oligodendrocyte Differentiation from NG2 Cells
Regional Differences in Oligodendrocyte Differentiation from NG2 Cells
In tune with differences in rates of NG2 cell proliferation there are also differences in oligodendrocyte differentiation between regions and at different developmental stages. In white matter regions the requirement for oligodendrocyte production is greater given the number of densely packed large caliber axons that require myelination. In agreement with this, the density of NG2 cells is at least two-fold greater in the white matter than in the gray matter (Terai et al., 2003). Using a number of different inducible cre transgenic mouse lines, several groups have reported that oligodendrocyte production is faster in white matter regions during the first months of postnatal development (Fig. 2; Dimou et al., 2008; Guo et al., 2009; Kang et al., 2010; Rivers et al., 2008; Zhu et al., 2011). In both gray matter and white matter regions, at least a certain percentage of newly generated oligodendrocytes become stably integrated and form myelin internodes, which are shorter than existing ones (Young et al., 2013). Both cell-intrinsic and non-cell-autonomous mechanisms have been suggested to play a role in the regional differences in the rate of oligodendrocyte differentiation. Transplantation of NG2 cells from adult mouse white matter into adult mouse gray or white matter led to a similar extent of oligodendrocyte differentiation in both host locations, whereas transplantation of gray matter cells resulted in more oligodendrocytes in white matter than in gray matter, and those that differentiated in white matter looked more immature than oligodendrocytes from white matter NG2 cells (Viganò et al., 2013). These observations suggest that NG2 cells in adult gray matter have become programmed to differentiate more slowly into oligodendrocytes but this intrinsic property can be offset by the permissive environment of the white matter, although the environment in these studies constitutes both physiological differences mixed with injury response. Another example of the plasticity of NG2 cells from one region to adopt the fate of those in another region is revealed in an experiment in which NG2 cells from the optic nerve, which generate oligodendrocytes that myelinate uniformly sized small diameter axons, were shown to be capable of myelinating different sized axons including large diameter axons when grafted into the spinal cord (Fanarraga et al., 1998).
Age-Dependent Differences in Oligodendrocyte Differentiation from NG2 Cells
Oligodendrogliogenesis occurs rapidly from late embryonic stages through the second postnatal week, prior to peak myelination during the third postnatal week. Beyond this age, the rate of generation of new oligodendrocytes declines with age (Kang et al., 2010; Lasiene et al., 2009; Rivers et al., 2008; Zhu et al., 2011), and the balance between oligodendrocyte differentiation and self-renewal becomes shifted toward self-renewal with age. Both cell autonomous and non-autonomous mechanisms have been suggested. Intrinsically, histone deacetylases are required to initiate the oligodendrocyte differentiation program (Marin-Husstege et al., 2002; Ye et al., 2009), and level of acetylated histone H3 and H4 decreases during the first three weeks of postnatal development (Shen et al., 2005). With increasing age after the first month this epigenetic memory is lost, and histone acetylation increases, leading to transcriptional de-repression of genes that inhibit oligodendrocyte differentiation such as Hes5 and Id4 (Shen et al., 2008a). There are also cell non-autonomous changes in other glial cells in the microenvironment that occur with age and affect the ability of NG2 cells to differentiate into oligodendrocytes, as described below.
Region- and Age-Dependent Response of NG2 Cells to Demyelination
The majority of reports on remyelination of demyelinated lesions have focused on white matter (Franklin, 2002). However, multiple sclerosis (MS) also affects the gray matter (Kidd et al., 1999). Studies from biopsied material revealed that cortical MS lesions are common in early stages of the disease and are associated with a high frequency of meningeal inflammation and inflammatory T cells in the parenchyma (Lucchinetti et al., 2011), while other studies using autopsied material have reported a dearth of inflammatory cells in cortical lesions compared with white matter lesions (Albert et al., 2007; Peterson et al., 2001). One striking characteristic of cortical MS lesions is that they appear to undergo more extensive remyelination than white matter lesions (Albert et al., 2007; Chang et al., 2012). The reasons for this difference are not yet entirely clear, but differences in the extent of reactive astrogliosis and the composition of the extracellular matrix have been suggested. Cell intrinsically, the less proliferative gray matter NG2 cells may be less likely to undergo replicative senescence and retain the ability to be recruited for myelin repair.
Studies in rodents suggest that remyelination occurs but more slowly in older animals due to slower NG2 cell recruitment and slower maturation of oligodendrocytes (Sim et al., 2002). This could be caused by slower recruitment of histone deacetylases in older animals (Shen et al., 2008b). An elegant recent study using parabiosis revealed that exposure of old CNS to circulating macrophages from young animals after acute demyelination enhanced remyelination (Ruckh et al., 2012), due to more efficient clearance of myelin debris by macrophages from young mice. Furthermore, a switch from M1 to M2 macrophage phenotype and activin A production by the latter was associated with enhanced oligodendrocyte differentiation after demyelination mediated by the parabiotically introduced macrophages from young mice (Miron et al., 2013). These observations indicate that there may be cell intrinsic changes in NG2 cells and oligodendrocytes that occur with age, but that those changes can be reversed by the microenvironment created by young macrophages. The ability of subpial MS lesions in the gray matter to repair rapidly could be attributed to the extensive microglial reaction typically found in such lesions.
In contrast to these studies suggesting a decline in oligodendrogliogenic potential of NG2 cells with age, comparison of the myelinating potential of fetal and adult human NG2 cells suggests that those from the adult brain are more capable of generating myelin-forming cells when transplanted into the hypomyelinated shiverer brain (Windrem et al., 2004). Furthermore, in a follow up study the authors suggests that human induced pluripotent stem cells (hiPSCs) have a greater myelinating potential compared with NG2 cells isolated from fetal brain (Wang et al., 2013b). Although the cell intrinsic mechanisms underlying these differences in myelinating potential are not clear, these studies suggest that age-dependent cell intrinsic differences cannot always be reversed by the cellular microenvironment. It is not clear why these latter two studies show greater myelin-forming capacity of NG2 cells from more mature animals while the studies by Franklin and colleagues described above show greater remyelinating ability of NG2 cells from young animals. One possible explanation is that the microenvironment of a hypomyelinated brain differs significantly from that of an acute experimentally induced demyelination in adult mice, for example, in the degree of activation of astrocytes and microglia and breakdown of the blood-brain barrier.
Differentiation into Astrocytes: When and Where
In vitro NG2 cells generate astrocytes as well as oligodendrocytes (Raff et al., 1983). Additionally when exposed to certain factors in vitro, these cells also differentiate into neurons via an intermediate astrocytic neural stem cell-like phenotype (Kondo and Raff, 2000). Despite these early observations, unequivocal evidence for lineage multipotentiality of NG2 cells could not be obtained in vivo (Richardson et al., 2011). Among the Cre-loxP-mediated genetic fate mapping studies, only NG2-cre lines have shown that a subpopulation of protoplasmic astrocytes are generated from NG2 cells (Zhu et al., 2008a,b). Surprisingly, contrary to the expectations from optic nerve culture studies (Kondo and Raff, 2000; Raff et al., 1983), NG2 cells gave rise only to protoplasmic astrocytes in gray matter, and none of the fibrous astrocytes in the white matter of the forebrain, optic nerve, cerebellum, and spinal cord were derived from NG2 cells. Within the gray matter of the forebrain, NG2 cell-derived astrocytes were found predominantly in the posterior ventral gray matter, mostly in the entorhinal cortex, hypothalamus, and thalamus where NG2 cell-derived astrocytes comprised more than one-third of the total astrocytes and more than 40% of the reporter+ progeny of NG2 cells (Fig. 1). When Cre was induced postnatally, no reporter+ astrocytes were detected (Kang et al., 2010; Rivers et al., 2008; Zhu et al., 2011), suggesting that astrocytes are generated from NG2 cells that exist prenatally. Indeed, when Cre was induced at E16.5 and the brains examined postnatally (Zhu et al., 2011), reporter+ protoplasmic astrocytes were found similarly distributed in the ventral forebrain. Although single induced NG2 cells appeared to give rise to either astrocytes or oligodendrocytes but not both, NG2 cell-derived astrocytes and oligodendrocytes were seen intermingled within the same region of the ventral forebrain. Thus, there does not seem to be a niche for astrocyte differentiation from NG2 cells, but rather, astrogliogenic NG2 cells coexist with oligodendrogliogenic NG2 cells within the same ventral forebrain gray matter (Fig. 1).
What causes a subpopulation of prenatal NG2 cells in the ventral forebrain to follow an astrocyte fate? The astrogliogenic NG2 cells initially express the oligodendrocyte transcription factor Olig2, but they spontaneously downregulate Olig2 expression as they transition into astrocytes perinatally (Zhu et al., 2012). Since a given NG2 cell in the ventral forebrain could downregulate Olig2 and follow an astrocyte fate while an adjacent NG2 cell less than 50 µm away could retain Olig2 and remain an NG2 cell or differentiate into an oligodendrocyte, the switch to an astroglial fate is unlikely to be caused by a gradient of extracellular signal. Rather, the astrogliogenic fate of NG2 cells may be instructed by the location of the germinal zone from which the particular NG2 cell arises or the time of its birth. Deletion of Olig2 in NG2 cells using conditional Olig2 knockout mice resulted in an almost complete fate switch of neocortical NG2 cells to protoplasmic astrocytes at the expense of oligodendrocytes, and this was accompanied by severe hypomyelination (Zhu et al., 2012). In these mice, a minority of NG2 cells in the corpus callosum also became astrocytes. The astrocytes derived from Olig2-deleted NG2 cells were dye-coupled and displayed a linear current-voltage relationship indistinguishable from endogenous astrocytes (Zhu et al., 2012). Interestingly, the distribution of NG2 cells that were converted into astrocytes upon Olig2 deletion coincided with the distribution of NG2 cells that are derived from the dorsal Emx1-domain of the germinal zone (Fig. 1; Kessaris et al., 2006; Tripathi et al., 2011). NG2 cells that were distributed in the ventral forebrain where Gsh2+ LGE-derived cells are known to reside did not switch their fate (Zuo and Nishiyama, 2013). Deletion of Olig2 did not result in aberrant neuronal differentiation from NG2 cells, suggesting that in NG2 cells neuronal genes are repressed more tightly and permanently than astrocyte genes.
The ability of NG2 cells to generate astrocytes is not only region-specific but also age-dependent. With normal Olig2 gene dosage only prenatal NG2 cells generate astrocytes. When Olig2 is deleted, the ability of neocortical NG2 cells to switch their fate to astrocytes declines with postnatal age (Zhu et al., 2012; Zuo et al., unpublished observations). NG2 cells in the adult CNS do not generate astrocytes, even in response to a stab wound under normal or Olig2-deleted conditions (Dimou et al., 2008; Komitova et al., 2011). Although the mechanism underlying the age-dependent loss of lineage plasticity of NG2 cells is unknown, increased heterochromatin structure may cause more permanent repression of astrocyte-inducing genes as their chromosomal loci become targeted to the nuclear periphery, analogous to what has been described for age-dependent lineage restriction in Drosophila neuroblasts (Kohwi et al., 2013).
Neuronal Activity and NG2 Cell Behavior
Unlike any other glial cell population, NG2 cells receive distinct neuronal synaptic input in both gray and white matter and express glutamate and GABA receptors and voltage gated sodium channels (Bergles et al., 2000, 2010; De Biase et al., 2010; Kukley et al., 2007,2010; Ziskin et al., 2007), although functional consequences for these synapses are not known. It is appealing to propose that these synaptic inputs allow precise monitoring and control over oligodendrocyte production on the individual axon scale. However, direct evidence for this is lacking, and it is not known whether synaptic input to NG2 cells dynamically changes with age or in pathological states. Independently of synaptic inputs, neuronal activity can influence NG2 cell differentiation and oligodendrocyte myelination under culture conditions (Ishibashi et al., 2006; Stevens et al., 1998,2002). These studies suggest that NG2 cell differentiation and myelin formation likely play an important role during the development and refinement of neural networks and possibly in the adult nervous system (Fields, 2011; Zatorre et al., 2012).
Several studies have attempted to examine the effect of neuronal activity on NG2 cell behavior. One of the first such studies analyzed NG2 cell proliferation after injections of the voltage dependent sodium channel blocker tetrodotoxin (TTX) to the optic nerve (Barres and Raff, 1993). This study found that decreased neuronal activity resulted in decreased NG2 cell proliferation (Fig. 3), which could be rescued by injecting COS-7 cells that artificially released PDGF. These data suggested that the decrease in neuronal activity was limiting the amount of PDGF available to NG2 cells in the optic nerve and thus limiting their proliferation (Barres and Raff, 1993). The source of the PDGF was suspected to be astrocytes. In this model, activity-dependent changes in NG2 cell proliferation were independent of neuron-NG2 cell synapses and were instead a result of a change in activity-dependent release of PDGF from astrocytes. Another study demonstrated that intraocular injections of TTX decreased the number of myelinated axon segments but not the total number of MBP+ oligodendrocytes suggesting activity-dependent regulation of myelination (Demerens et al., 1996).
Figure 3.

Possible outcomes and routes for how changes in neuronal activity influence NG2 cells. A. Changes in neuronal activity (Δ neuronal activity) have been shown to alter NG2 cell proliferation (left), oligodendrocyte differentiation (middle), or survival of NG2 cells or newly differentiated oligodendrocytes (right). B. Two possible routes of neuron-to-NG2 cell communication. Left: Direct synaptic input to NG2 cells allows specificity of the NG2 cell response to the activity of the specific axon from which it receives synaptic input and can be frequency coded. Right: Nonsynaptically released neurotransmitters from axons or astrocytes can exert a more generalized effect on multiple NG2 cells in the micro-region.
Myelinating co-cultures of dissociated dorsal root ganglion (DRG) axons and NG2 cells were used to further investigate the mechanisms of activity-dependent changes in NG2 cell behavior. These studies revealed that activity-dependent release of adenosine from DRG axons inhibited NG2 cell proliferation and increased oligodendrocyte production (Fig. 3). These effects were not dependent on AMPA receptor activation (Stevens et al., 2002) and suggested a novel adenosine receptor-dependent mechanism for activity-dependent regulation of NG2 cell proliferation and oligodendrocyte differentiation that is distinct from previously hypothesized mechanisms involving PDGF or synaptically released glutamate (Fig. 3B, right). Subsequent studies from another group found that electrically induced axonal activity in optic nerve explants caused glutamate-dependent release of ATP from astrocytes that in turn increased intracellular calcium concentrations in NG2 cells (Hamilton et al., 2008,2010). Glutamate itself directly increased intracellular calcium in NG2 cells after bath application and the calcium response in NG2 cells was augmented when AMPA receptor desensitization and glutamate uptake were blocked (Ge et al., 2006; Hamilton et al., 2010). In these studies however it is not clear whether calcium increase is caused by entry through calcium permeable AMPA receptor on NG2 cells or through other secondary pathways. Furthermore, AMPA receptors and microregional calcium transients may play important roles at NG2 cell processes (Haberlandt et al., 2011).
Studies to determine the role of glutamate in mediating NG2 cell proliferation and/or differentiation have produced varying results. NG2 cells express both glutamatergic NMDA receptors and calcium permeable AMPA receptors. Glutamate application to dissociated NG2 cells and cerebellar slice cultures decreased NG2 cell proliferation and oligodendrocyte differentiation (Gallo et al., 1996; Yuan et al., 1998). By contrast, another study reported that the generation of oligodendrocytes from cultured SVZ-derived NSCs was increased by glutamate acting on NMDA receptors (Cavaliere et al., 2012). Furthermore glutamate application increased the levels of ROS in differentiating progenitor cells, presumably NG2 cells, providing an intriguing connection between region-dependent redox state discussed above and glutamate dependent control of oligodendrogliogenesis (Cavaliere et al., 2012). Pharmacological manipulation of NMDA receptor activation in vitro and in vivo altered oligodendrocyte differentiation and remyelination after cuprizone induced demyelination (Li et al., 2013), whereas NG2 cell-specific knockout of NMDA receptor NR1 subunit did not alter NG2 cell morphology, membrane properties, proliferation, or oligodendrocyte differentiation with the exception of altering the expression of calcium permeable AMPA receptors (De Biase et al., 2011). Regardless of the mechanism, changes in intracellular calcium is likely to play a vital role in regulating NG2 cell proliferation and/or differentiation (Barres et al., 1990; Boscia et al., 2012; Paez et al., 2009).
A few recent studies have attempted to obtain evidence for direct activity-dependent regulation of NG2 cell proliferation and/or differentiation under more physiological conditions. Electrical stimulation of corticospinal tracts in adult mice resulted in increased NG2 cell proliferation and oligodendrocyte differentiation (Li et al., 2010), while long-term wheel running reduced proliferation of NG2 cells and concomitantly increased oligodendrocyte differentiation in the motor cortex of adult mice (Simon et al., 2011). Whisker removal in newborn mice was reported to increase proliferation and alter the distribution of NG2 cells in one study (Mangin et al., 2012) but shown to have no effect on NG2 cell distribution in another (Hill et al., 2011) specifically in the somatosensory barrel cortex. The variable results from these latter studies may have been due to different methods for altering neuronal activity and characterizing the outcome. In addition it is not known whether there is age- or region-dependent heterogeneity in their responses to neuronal activity. Further investigation is necessary to explore more directly how direct neuronal activity influences NG2 cell proliferation, differentiation and survival in vivo.
From these observations, one could conceive of the following two routes for transduction of neuronal activity to NG2 cells (Fig. 3B). Direct synaptic input to NG2 cells allows a single cell to sense the firing pattern of an individual axon and thus decide to proliferate, differentiate into an oligodendrocyte, or die in response to changes in synaptic signaling. This type of signal would result in single axon specificity in addition to frequency dependent control. In contrast, non-synaptic release of neurotransmitters or growth factors could result in signal transduction to a larger population of cells spread over a particular region. This type of signaling may provide a more generalized and widespread signal to alter proliferation, differentiation, or survival but one might guess that frequency dependence could be lost with such diffuse signaling. It is possible that both types of signals work in parallel (in addition to unknown mechanisms), and each may contribute uniquely to modulating activity-dependent NG2 cell behavior. A developmental switch from synaptic to extrasynaptic GABAergic input onto NG2 cells (Balia et al., in press) is an example of such a switch from an axon-specific regulation to a more generalized effect. Regardless of the mechanism of communication, recent evidence suggests that NG2 cells can tune in and listen to the neural network and modulate their proliferation or differentiation. They have not yet been shown to communicate back to neurons via neurotransmitter signaling, but evidence suggests that they could modulate neural activity by dynamically altering myelination (see below).
Neuronal Activity, Myelin Formation, and Plasticity
Activity-dependent differentiation of oligodendrocytes from NG2 cells does not necessarily lead to activity-dependent myelin formation. It is likely that compact myelin formation requires signaling mechanisms between axons and mature oligodendrocytes that are distinct from signals instructing oligodendrocyte differentiation from NG2 cells. Experiments using co-cultures of oligodendrocytes and DRG neurons demonstrated that vesicular release of glutamate from neurons increased the local translation of MBP and the formation of myelin at specific contact sites between neurons and oligodendrocytes (Wake et al., 2011). The nature of the contact (sites) between neurons and mature oligodendrocytes in these cultures are somewhat of a mystery, for synaptic input is rapidly lost as NG2 cells differentiate into myelinating oligodendrocytes (De Biase et al., 2010; Kukley et al., 2010). Nonetheless these studies suggest that local myelin production may be regulated by neuronal activity.
There is limited in vivo evidence for neuronal activity dependent regulation of myelin formation and plasticity. Two recent studies investigated the effects of social isolation on developmental myelination and changes in myelin structure in the adult prefrontal cortex. Animals housed alone exhibited thinner myelin sheaths in addition to altered internode lengths (Liu et al., 2012; Makinodan et al., 2012). Mechanisms for these changes are not entirely clear, as one study suggested chromatin changes (Liu et al., 2012), while data from the other implicated altered Neuregulin1-ErbB3 signaling (Makinodan et al., 2012). The following are some possible signaling mechanisms that act on oligodendrocytes to direct their myelinating behavior (Fig. 4A): (1) individual oligodendrocyte processes could receive a signal that directs them to myelinate only active axons; (2) once a premyelinating oligodendrocyte receives a signal to myelinate, the actual process of myelination takes place on all axons of a particular diameter or other biophysical properties in that microregion regardless of their activity; or (3) myelin formation is not dependent on activity or axonal properties but is regulated by other cell intrinsic or extracellular mechanisms. In a recent live imaging study in the zebrafish, it was shown that once an oligodendrocyte starts to myelinate, it myelinates all the internodes within a finite period of time, suggesting that the signal and response are likely to be an all or none type of mechanism rather than a graded one (Czopka et al., 2013).
Figure 4.
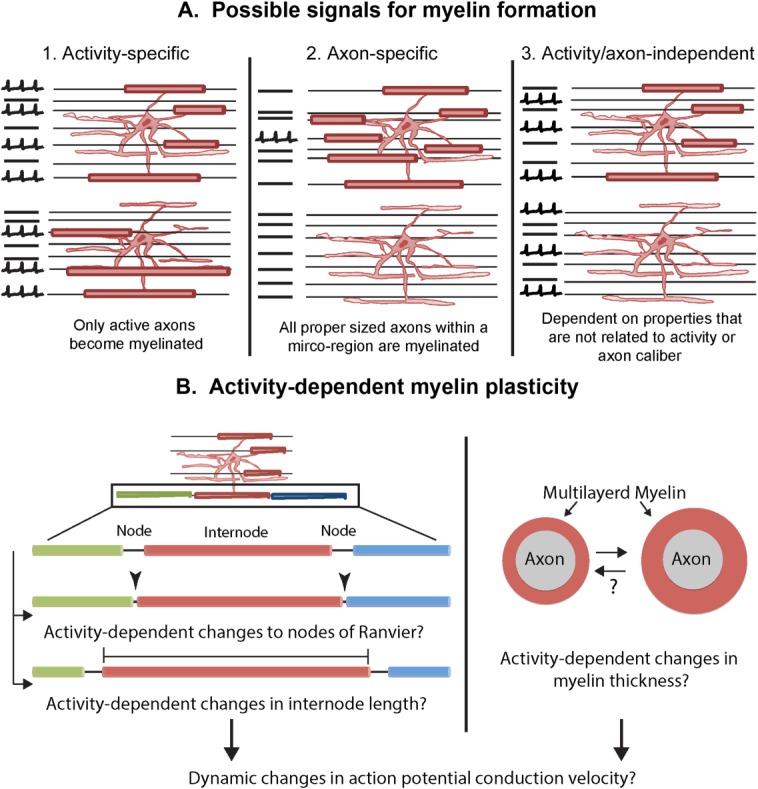
Possible mechanisms of activity-dependent myelination and myelin plasticity. A. Different ways in which oligodendrocytes can be signaled to myelinate. 1. The oligodendrocyte could receive activity-dependent signals and myelinate only active axons. 2. The myelination signal could consist of physical properties of the axon such as axon caliber. 3. The signal to myelinate could be an intrinsic or extracellular mechanism that is independent of axonal activity or caliber. B. Possible ways in which activity-dependent myelin plasticity could be achieved. Left: Neuronal activity could affect the release of neurotransmitters or other active substances from the nodes of Ranvier, signal to astrocyte, or NG2 cell processes at the node, or slightly shift the position of the node and change the internode length. Right: Neuronal activity could also result in changes in the thickness of myelin. Both of these mechanisms could potentially bring about dynamic changes in conduction.
Once myelin internodes are formed, it is possible that neuronal activity could induce fine changes in myelin structure that could in turn influence conduction velocity and neural network function (Fields, 2008). Activity-dependent changes in myelin thickness have been demonstrated and it is possible that small changes in internode length or alterations to nodes of Ranvier may occur as a result of neuronal activity (Fig. 4, bottom) (Wurtz and Ellisman, 1986), somewhat reminiscent of activity-dependent changes in the axon initial segment (Grubb and Burrone, 2010). A recent modeling study suggests that seemingly small changes in conduction velocity could result in dramatic alterations in synchrony and connectivity in neural networks (Pajevic et al., in press).
There is accumulating evidence from new imaging studies that learning-dependent changes occur in the brain's white matter structure in adult humans (Fields, 2010; Zatorre et al., 2012). The majority of this data is based on diffusion tensor imaging (DTI) which is a magnetic resonance imaging technique that reveals preferential water diffusion in tissues. These studies have revealed changes in white matter structure when adults acquire the ability to read, practice musical instruments, or learn a complex motor task like juggling (Bengtsson et al., 2005; Carreiras et al., 2009; Scholz et al., 2009). Rodent studies described above showing the generation of new myelinating oligodendrocytes in the adult brain suggest that myelination of previously unmyelinated axons and/or oligodendrocyte replacement by NG2 cells may occur during the process of learning new skills. Determining the mechanisms and the extent of activity-dependent control of NG2 cell proliferation and differentiation in vivo in specific brain regions will reveal how NG2 cells and oligodendrocytes influence neuronal communication and plasticity during development and in the adult. Refinement of imaging and other techniques to simultaneously manipulate and monitor changes in neural activity and glial cellular changes would facilitate the elucidation of the functional role for NG2 cells in the neural network.
References
- Albert M, Antel J, Bruck W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17:129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balia M, Vélez-Fort M, Passlick S, Schäfer C, Audinat E, Steinhäuser C, Seifert G, Angulo MC. Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb Cortex. 2013 doi: 10.1093/cercor/bht309. doi: 10.1093/cercor/bht309. [DOI] [PubMed] [Google Scholar]
- Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- Baron W, Colognato H, ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49:467–479. doi: 10.1002/glia.20132. [DOI] [PubMed] [Google Scholar]
- Baron W, Shattil SJ, ffrench-Constant C. The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of alpha(v)beta3 integrins. EMBO J. 2002;21:1957–1966. doi: 10.1093/emboj/21.8.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Koroshetz WJ, Chun LL, Corey DP. Ion channel expression by white matter glia: The type-1 astrocyte. Neuron. 1990;5:527–544. doi: 10.1016/0896-6273(90)90091-s. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bello IC, Dawson MR, Levine JM, Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol. 1999;28:365–381. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F, Ullén F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jabs R, Steinhauser C. Neuron-glia synapses in the brain. Brain Res Rev. 2010;63:130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci. 2011;31:12650–12662. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 2010;30:3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: Ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3519–3528. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscia F, D'Avanzo C, Pannaccione A, Secondo A, Casamassa A, Formisano L, Guida N, Annunziato L. Silencing or knocking out the Na(+)/Ca(2+) exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ. 2012;19:562–572. doi: 10.1038/cdd.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu J, Banki A, Wu Q, Nishiyama A. Increased NG2(+) glial cell proliferation and oligodendrocyte generation in the hypomyelinating mutant shiverer. Glia. 2004;48:51–63. doi: 10.1002/glia.20055. [DOI] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, Price CJ. An anatomical signature for literacy. Nature. 2009;461:983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Cavaliere F, Urra O, Alberdi E, Matute C. Oligodendrocyte differentiation from adult multipotent stem cells is modulated by glutamate. Cell Death Dis. 2012;3:e268. doi: 10.1038/cddis.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Chen Y, Wang H, Yuan X, Ghiani CA, Heckman T, McBain CJ, Gallo V. Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci USA. 2002;99:2350–2355. doi: 10.1073/pnas.042698399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Evercooren AB-V, Georges-Labouesse E, ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: Are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand B, Gao FB, Raff M. Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J. 1997;16:306–317. doi: 10.1093/emboj/16.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner S, Dunbar M, McKinnon RD. Distinct roles for PI3K in proliferation and survival of oligodendrocyte progenitor cells. J Neurosci Res. 2000;62:336–345. doi: 10.1002/1097-4547(20001101)62:3<336::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Fanarraga ML, Griffiths IR, Zhao M, Duncan ID. Oligodendrocytes are not inherently programmed to myelinate a specific size of axon. J Comp Neurol. 1998;399:94–100. [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Neuroscience. Change in the brain's white matter. Science. 2010;330:768–769. doi: 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Imaging learning: the search for a memory trace. Neuroscientist. 2011;17:185–196. doi: 10.1177/1073858410383696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJM. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betsholtz C, Richardson WD. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Furusho M, Dupree JL, Nave KA, Bansal R. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J Neurosci. 2012;32:6631–6641. doi: 10.1523/JNEUROSCI.6005-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Karlo JC, Landreth GE, Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J Neurosci. 2011;31:843–850. doi: 10.1523/JNEUROSCI.3239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marques J, Nunez-Llaves R, Lopez-Mascaraque L. NG2-Glia from pallial progenitors produce the largest clonal clusters of the brain: Time frame of clonal generation in cortex and olfactory bulb. J Neurosci. 2014;34:2305–2313. doi: 10.1523/JNEUROSCI.3060-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312:1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlandt C, Derouiche A, Wyczynski A, Haseleu J, Pohle J, Karram K, Trotter J, Seifert G, Frotscher M, Steinhauser C, Jabs R. Gray matter NG2 cells display multiple Ca2+-signaling pathways and highly motile processes. PLoS One. 2011;6:e17575. doi: 10.1371/journal.pone.0017575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Kirchhoff F, Verkhratsky A, Robbins J, Gorecki DC, Butt AM. Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia. 2008;56:734–749. doi: 10.1002/glia.20649. [DOI] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Wigley R, Butt AM. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia. 2010;58:66–79. doi: 10.1002/glia.20902. [DOI] [PubMed] [Google Scholar]
- Hill RA, Natsume R, Sakimura K, Nishiyama A. NG2 cells are uniformly distributed and NG2 is not required for barrel formation in the somatosensory cortex. Mol Cell Neurosci. 2011;46:689–698. doi: 10.1016/j.mcn.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci. 2013;33:14558–14566. doi: 10.1523/JNEUROSCI.2001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhao X-F, Zheng K, Qiu M. Regulation of the timing of oligodendrocyte differentiation: Mechanisms and perspectives. Neurosci Bull. 2013;29:155–164. doi: 10.1007/s12264-013-1314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J Neurosci. 2012;32:8855–8864. doi: 10.1523/JNEUROSCI.0137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain 122(Pt. 1999;1):17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- Kohwi M, Lupton JR, Lai S-L, Miller MR, Doe CQ. Developmentally regulated subnuclear genome reorganization restricts neural progenitor competence in Drosophila. Cell. 2013;152:97–108. doi: 10.1016/j.cell.2012.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Serwanski DR, Lu QR, Nishiyama A. NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. Glia. 2011;59:800–809. doi: 10.1002/glia.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Kukley M, Nishiyama A, Dietrich D. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci. 2010;30:8320–8331. doi: 10.1523/JNEUROSCI.0854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiene J, Matsui A, Sawa Y, Wong F, Horner PJ. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell. 2009;8:201–213. doi: 10.1111/j.1474-9726.2009.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SYC, Mellon SH, Tuck SJ, Feng Z-Q, Corey JM, Chan JR. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C. A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia. 2013;61:732–749. doi: 10.1002/glia.22469. [DOI] [PubMed] [Google Scholar]
- Li Q, Brus-Ramer M, Martin JH, McDonald JW. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett. 2010;479:128–133. doi: 10.1016/j.neulet.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BFG, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Brück W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin J-M, Li P, Scafidi J, Gallo V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat Neurosci. 2012;15:1192–1194. doi: 10.1038/nn.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, McBain CJ. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci. 2013;16:1032–1041. doi: 10.1038/nn.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni EO, Mahony S, Peljto M, Patel T, Thornton SR, McCuine S, Reeder C, Boyer LA, Young RA, Gifford DK, Wichterle H. Saltatory remodeling of Hox chromatin in response to rostrocaudal patterning signals. Nat Neurosci. 2013;16:1191–1198. doi: 10.1038/nn.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Pham L-DD, Seo JH, Kim K-W, Lo EH, Arai K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol Life Sci. 2014;71:1055–1066. doi: 10.1007/s00018-013-1488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji Y, Miller RH. Control of oligodendrocyte precursor proliferation mediated by density-dependent cell cycle protein expression. Dev Neurosci. 2001;23:356–363. doi: 10.1159/000048719. [DOI] [PubMed] [Google Scholar]
- Nave K-A. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Neusch C, Papadopoulos N, Muller M, Maletzki I, Winter SM, Hirrlinger J, Handschuh M, Bahr M, Richter DW, Kirchhoff F, Hulsmann S. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: Impact on extracellular K+ regulation. J Neurophysiol. 2006;95:1843–1852. doi: 10.1152/jn.00996.2005. [DOI] [PubMed] [Google Scholar]
- Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci. 2001;21:5429–5438. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A. NG2 cells (Polydendrocytes). Chapter 10. In: Kettenmann H, Ransom BR, editors. Neuroglia. 3rd edition. Oxford University Press; 2012. doi: 10.1093/med/9780199794591.001.0001. [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Ortega F, Gascón S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol. 2013;15:602–613. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- Paez PM, Fulton D, Colwell CS, Campagnoni AT. Voltage-operated Ca(2+) and Na(+) channels in the oligodendrocyte lineage. J Neurosci Res. 2009;87:3259–3266. doi: 10.1002/jnr.21938. [DOI] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, Fields RD. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.11.007. [Epub ahead of print] doi: 10.1016/j.neuroscience.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidou P, Dasen JS. Hox genes: choreographers in neural development, architects of circuit organization. Neuron. 2013;80:12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J, Mayer-Proschel M, Smith J, Noble M. Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions. Dev Biol. 2002;245:362–375. doi: 10.1006/dbio.2002.0610. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: During late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: Fact or fantasy? Neuron. 2011;70:661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Tani M, Strieter RM, Ransohoff RM, Miller RH. The chemokine growth-regulated oncogene-alpha promotes spinal cord oligodendrocyte precursor proliferation. J Neurosci. 1998;18:10457–10463. doi: 10.1523/JNEUROSCI.18-24-10457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR, la Torre AR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci USA. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Ruckh JM, Zhao J-W, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJM. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169:577–589. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Liu A, Li J, Wolubah C, Casaccia-Bonnefil P. Epigenetic memory loss in aging oligodendrocytes in the corpus callosum. Neurobiol Aging. 2008a;29:452–463. doi: 10.1016/j.neurobiolaging.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJM, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008b;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJM. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Gotz M, Dimou L. Progenitors in the adult cerebral cortex: Cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59:869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci USA. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: A neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;18:9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. 2009;5:231–246. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, Okada M, Yamaguchi T. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116:1053–1062. doi: 10.1016/s0306-4522(02)00791-1. [DOI] [PubMed] [Google Scholar]
- Tripathi RB, Clarke LE, Burzomato V, Kessaris N, Anderson PN, Attwell D, Richardson WD. Dorsally and ventrally derived oligodendrocytes have similar electrical properties but myelinate preferred tracts. J Neurosci. 2011;31:6809–6819. doi: 10.1523/JNEUROSCI.6474-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautier F, Belachew S, Chittajallu R, Gallo V. Shaker-type potassium channel subunits differentially control oligodendrocyte progenitor proliferation. Glia. 2004;48:337–345. doi: 10.1002/glia.20088. [DOI] [PubMed] [Google Scholar]
- Viganò F, Möbius W, Götz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci. 2013;16:1370–1372. doi: 10.1038/nn.3503. [DOI] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang T, Dong Q, Nice EC, Huang C, Wei Y. Redox homeostasis: The linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013a;4:e537. doi: 10.1038/cddis.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013b;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Tekki-Kessaris N, Stiles CD, Rowitch DH, Richardson WD. Oligodendrocyte development in the spinal cord and telencephalon: common themes and new perspectives. Int J Dev Neurosci. 2001;19:379–385. doi: 10.1016/s0736-5748(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Wu Q, Miller RH, Ransohoff RM, Robinson S, Bu J, Nishiyama A. Elevated levels of the chemokine GRO-1 correlate with elevated oligodendrocyte progenitor proliferation in the Jimpy mutant. J Neurosci. 2000;20:2609–2617. doi: 10.1523/JNEUROSCI.20-07-02609.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz CC, Ellisman MH. Alterations in the ultrastructure of peripheral nodes of Ranvier associated with repetitive action potential propagation. J Neurosci. 1986;6:3133–3143. doi: 10.1523/JNEUROSCI.06-11-03133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao X, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn S-J, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008a;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008b;4:19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zuo H, Maher BJ, Serwanski DR, LoTurco JJ, Lu QR, Nishiyama A. Olig2-dependent developmental fate switch of NG2 cells. Development. 2012;139:2299–307. doi: 10.1242/dev.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo H, Nishiyama A. Polydendrocytes in development and myelin repair. Neurosci Bull. 2013;29:165–76. doi: 10.1007/s12264-013-1320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


