Abstract
Aims
To determine clinicopathological criteria and molecular markers helpful in distinguishing adrenocortical carcinomas (ACCs) from adrenocortical adenomas (ACAs).
Methods and results
We analysed retrospectively the clinical and pathological features of 50 adrenal cortical tumours, and tested the expression of miR483-3p by in-situ hybridization as well as the expression of IGF2 and Smad4 by immunohistochemistry. We found that tumour size, tumour weight, hormonal function and the Weiss system are all high-efficacy criteria for differentiating malignant from benign tumours (P < 0.001). MiR483-3p was overexpressed in 68% (17 of 25) of ACCs compared to 12% (three of 25) of ACAs (P < 0.05). Using a combination of miR483-3p and Smad4 improved diagnostic accuracy. Molecular markers were then tested in an independent set of 15 borderline tumours. We confirmed that the combined use of miR483-3p and Smad4 immunochemistry can complement the Weiss score in the diagnosis of ACC in cases that display borderline histology.
Conclusions
Tumour size, tumour weight, hormonal function and the Weiss system are useful clinicopathological criteria that can result in accurate diagnosis of most ACCs and ACAs. In challenging cases, miR483-3p and Smad4 expression may help in distinguishing these two entities.
Keywords: adrenal cortical tumours, clinicopathological features, differentiation, diagnosis, hsa-mir-483-3p, in-situ hybridization
Introduction
Adrenal masses are among the most frequent tumours in humans.1 A vast majority of these tumours are benign, and only a small subset of adrenal masses are malignant adrenocortical carcinomas (ACCs). ACC is a rare but very aggressive cancer. It affects one to two people per million per year and accounts for 0.02–0.2% of all cancer deaths.2–4 An accurate diagnosis is critical, because the prognosis, follow-up and therapeutic strategy for ACC are very different to those for a benign tumour. However, it is difficult in some cases to distinguish malignant from benign cortical tumours accurately through clinical characteristics or histological criteria. The Weiss system, including nine-point histopathological criteria, is currently the most commonly used method for assessing malignancy. The original threshold for malignancy, established in 1984, was the presence of at least four criteria5; but this was reduced to three or more criteria in 1989 after it was observed that some tumours with a score of 3 had recurred.6 In 2002, Aubert et al.7 proposed a modification of the Weiss system; the two systems correlated significantly, and in both the threshold for malignancy is a total score of 3. However, the Weiss system was found to be less specific, as several studies noted that some tumours with a Weiss score of 3 had a clinical benign course,7–9 and that individual tumours may behave in a malignant manner despite initially receiving a Weiss score of 2.10,11 Therefore, there is a need for a comprehensive understanding of the clinical and morphological characteristics of adrenocortical tumours, and the identification of reliable biomarkers as an adjunct to routine pathological analysis.
MicroRNAs (miRNAs) are endogenous, non-coding RNA of approximately 22 nucleotides that regulate gene expression by controlling target mRNA translation or degradation.12 Angelo et al.13 reported that miR483-3p is overexpressed in human breast, colon and liver cancers, suggesting a wide involvement of this miRNA in human tumorigenesis. However, its expression and the mechanisms underlying this expression in adrenocortical tumours are unclear.
The gene encoding Smad4/DPC4, a critical effector in the TGF-β signalling pathway,14 has been reported to be a target for miR483-3p in some studies,15 but the role of this effector in adrenal cortical tumours has not been established definitively. MiR483 is expressed from intron 2 of the IGF2 gene, and several studies have identified that up-regulation of IGF2 expression is the dominant change in malignant adrenocortical tumours.16,17
In this study, we analysed clinical and pathological features of adrenal cortical tumours and investigated the expression of miR483-3p and its association with IGF2 and Smad4 in ACCs, in order to develop a theoretical basis for differential diagnosis, prognostic evaluation and molecular target therapy.
Materials and methods
Patients and Tumour Samples
Samples of adrenal tumour were collected from patients undergoing adrenalectomy at Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, from July 2001 to July 2012. This study was approved by the Peking Union Medical College Hospital Ethics Committee. Twenty-five malignant tumours were available and used as experimental samples, and 25 benign tumours selected randomly from 1564 patients with adrenocortical adenomas (ACAs) were used as control samples. All 50 tumours had full clinical and pathological data. Diagnoses were based on clinical, biochemical and morphological data. The follow-up period was 6–123 months.
The Weiss system was applied to all 50 adrenal cortical tumours separately by two independent observers (H.W. and J.Z.) blinded to the clinical courses of the patients. A consensual Weiss score was established on a multiheaded light microscope. The nine Weiss histopathological criteria of malignancy were defined according to Aubert et al.7
An independent set of 15 borderline adrenal cortical tumours (Weiss score = 2 or 3) were used to validate further the diagnostic accuracy of the molecular markers. The morphological features of the 15 cases are summarized in Table S1.
In-situ Hybridization
A miRCURY LNA detection probe for human mature miR483-3p (5′-AAGACGGGAGGAGAGGAGTGA-3′), U6-positive control and the scrambled negative control, which were all 5′-digoxigenin- and 3′-digoxigenin-labelled, were purchased from Exiqon (Vedbaek, Denmark). Detection of miR483-3p by in-situ hybridization using the oligonucleotide probes was performed according to the manufacturer's instructions. Briefly, human tissues were deparaffinized, treated with proteinase K, washed in sterile diethylpyrocarbonate-treated phosphate-buffered saline (PBS), and subsequently fixed with 4% paraformaldehyde. After prehybridization at room temperature followed by incubation at 61°C separately for 1 h, hybridization was performed at 61°C for 21 h, followed by a wash in ×2 standard saline citrate and 3 × 30-min washes at 61°C in ×1 standard saline citrate and 50% formamide. After blocking (2% sheep serum and 2 mg/ml bovine serum albumin in PBS with Tween-20) at room temperature for 1 h, the blocking buffer was replaced with blocking buffer containing anti-digoxigenin-alkaline phosphatase (Cat. no. 11 093274910,150U, 200 μl, 1:500; Roche, Basel, Switzerland), and all slides were then placed into a ddH2O box and incubated at 4°C overnight. The next day, the slides were washed in PBS with Tween-20 for 7 × 5 min at room temperature, equilibrated, NBT/BCIP added (Cat. no. 11 681 451 001, 8 ml, 1:500, Roche), and incubated for 3 h in the dark at room temperature for visualization.
The slides were then scored independently by two pathologists (H.W. and Y.S.) as negative (–), weak or focally positive (1+) or strongly positive (2+).18
Immunohistochemistry
Immunohistochemical detection of IGF2, Smad4 and MIB-1 was performed on standard sections of paraffin-embedded tissue, which were cut at a 4-μm thickness and mounted on positively charged slides (SuperFrost Plus; Fisher Scientific, Loughborough, UK). After being conventionally deparaffinized and rehydrated, the sections were treated separately in 10 mm citrate buffer (pH 6.0) for 5 min for IGF2 and MIB-1, and in EDTA buffer (pH 8.0) for 10 min for Smad4, at 121°C in an autoclave for antigen retrieval. The sections were then immersed in ddH2O containing 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity, followed by incubation with 10% goat serum to block non-specific antibody binding sites. Sections were incubated at 4°C overnight with anti-IGF2 goat polyclonal antibody (sc-1415; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) diluted at 1:100, anti-MIB-1 monoclonal antibody working liquid (ZM-0167, ZSGB-BIO, China), or anti-Smad4 rabbit monoclonal antibody (ab40759; Abcam, Cambridge, MA, USA) diluted at 1:100. Appropriate positive and negative controls were employed. Scoring of IGF2 staining was graded semiquantitatively from 0 to 4,19,20 and Smad4 staining assessed as ‘diffusely positive’, ‘focally positive’ or ‘negative’, independently by two observers (H.W. and Y.S.).21 Discordant cases were reviewed on a multiheaded light microscope for discussion and consensus.
Scoring for In-situ Hybridization and Immunohistochemistry
For the purposes of our analysis, we dichotomized staining scores as negative/low versus elevated. For miR483-3p and Smad4, elevated included only ‘strongly positive’ or ‘diffusely positive’ cases. For IGF2, elevated was regarded as an immunoscore ≥3.
Statistical Analyses
Comparisons of frequencies and correlations between clinicopathological features, cellular proliferative activity and miR483-3p, IGF2 or Smad4 expression were undertaken using Pearson's χ2 test or Fisher's exact test. Comparisons of the means were performed using Wilcoxon's rank sum tests. Survival curves were plotted using the Kaplan–Meier method and P-values were calculated using the log-rank test. The length of overall survival (OS) was defined as the period from the date of surgery to the date of death as a result of the tumour or the latest follow-up. All statistical analyses were performed with spss software (spss version 17.0; SPSS, Inc., Chicago, IL, USA). A P-value < 0.05 was considered statistically significant.
Results
Clinical Features
The clinical data for both malignant and benign adrenocortical tumours are summarized in Table1. There was no statistical difference in sex, age and tumour location between the patients with malignant and benign tumours. However, malignant tumours were significantly larger and heavier than benign tumours (P < 0.001). Moreover, patients presenting with primary aldosterone syndrome were more likely to have a benign tumour (P < 0.001; Table1).
Table 1.
Clinical parameters of the 50 patients with adrenocortical tumors
| Malignant (n = 25) | Benign (n = 25) | P value | |
|---|---|---|---|
| Sex | |||
| Male | 11 | 11 | 1.000 |
| Female | 14 | 14 | |
| Tumor location | |||
| Right | 13 | 13 | 0.879 |
| Left | 12 | 11 | |
| Bilateral | 0 | 1 | |
| Endocrinic syndrome | |||
| Cushing syndrome | 7 | 3 | – |
| Primary aldosterone syndrome | 1 | 12 | <0.001* |
| Sex characteristics abnormality | 3 | 0 | – |
| Non-functioning | 14 | 10 | – |
| Age (years) | |||
| Mean | 44.4 | 48.6 | 0.229 |
| Range | 18–75 | 34–69 | |
| Tumor size (cm) | |||
| Mean | 11.5 | 2.4 | <0.001 |
| >6.5 | 17 | 0 | <0.001 |
| Tumor weight (g) | |||
| Mean | 558.0 | 6.7 | <0.001 |
| >50 | 21 | 0 | <0.001 |
P value for comparisons of frequencies between malignant and benign tumors presenting with primary aldosterone syndrome.
Among the patients with a malignant tumour, 13 recurred (local recurrence and/or metachronous distant metastasis) during follow-up. Moreover, 14 died from the cancer (mean OS: 23.1 months), two continue to live with the disease, three were alive and disease-free at their last follow-up and six were lost to follow up. Among patients with a benign tumour, eight were lost to follow up and 17 were alive and disease-free after their operations (mean follow-up: 57 months).
Pathological Features
The Weiss scores for the 25 benign tumours were all equal to or less than 2. No case of malignancy scored less than 4. The mean Weiss score was significantly higher for malignant tumours than benign tumours (P < 0.001). Each of the Weiss criteria was observed more frequently in malignant tumours (each P < 0.05). In our study, the diagnostic efficacy of Weiss scores ≥3 was high for malignancy, with 100% specificity and 100% sensitivity overall. The most sensitive criterion was ‘mitotic rate >5/50 HPF’ (100%), and the least sensitive criteria were ‘≤25% clear cells’ or ‘sinusoid invasion’ (36.0%). The most specific criteria were ‘mitotic rate >5/50 HPF’ ‘necrosis’, ‘venous invasion’, ‘sinusoid invasion’ and ‘capsular invasion’ (100%), and the least was ‘Führman nuclear grade III/IV’ (68%; Table2).
Table 2.
Weiss system for the 50 adrenocortical tumors
| Malignant (n = 25) | Benign (n = 25) | P value | |
|---|---|---|---|
| Nuclear grade Fu¨rhman III/IV | 21 | 8 | <0.001 |
| Mitotic rate >5/50 HPF | 25 | 0 | <0.001 |
| Abnormal mitoses | 23 | 2 | <0.001 |
| ≤25% clear cells | 9 | 3 | 0.047 |
| >1/3 diffuse architecture | 10 | 1 | 0.002 |
| Necrosis | 21 | 0 | <0.001 |
| Venous invasion | 11 | 0 | <0.001 |
| Sinusoid invasion | 9 | 0 | 0.002 |
| Capsular invasion | 18 | 0 | <0.001 |
| Total Weiss score (mean) | 5.76 | 0.56 | <0.001 |
Expression of miR483-3P, IGF2 and Smad4
MiR483-3p was overexpressed significantly in 68% (17 of 25) of ACCs compared to 12% (3 of 25) of ACAs (P < 0.05; Table3, Figure 1A,D). Moreover, there was a positive correlation between expression of miR483-3p and IGF2 (P < 0.05; Figure 1A,B,E, Table S2). Although Smad4 showed statistically significantly lower expression in ACCs than in ACAs (P < 0.05; Table3, Figure 1C,F), there was no negative relationship between expression of miR483-3p and Smad4 (P > 0.05; Table S2). Smad4 negative/low expression was sensitive in differentiating ACCs from ACAs (sensitivity = 92%), but with a low specificity of only 40%. However, the combination of negative/low Smad4 and elevated miR483-3p expression provided a diagnostic specificity of 92.8%.
Table 3.
Expression of miR483-3p, IGF2 and Smad4 in adrenocortical tumors
| Malignant (n = 25) | Benign (n = 25) | P value | |||
|---|---|---|---|---|---|
| Elevated | Negative/low | Elevated | Negative/low | ||
| miR483-3p | 17 | 8 | 3 | 22 | <0.001 |
| IGF2 | 16 | 9 | 7 | 18 | 0.011 |
| Smad4 | 2 | 23 | 10 | 15 | 0.008 |
Figure 1.
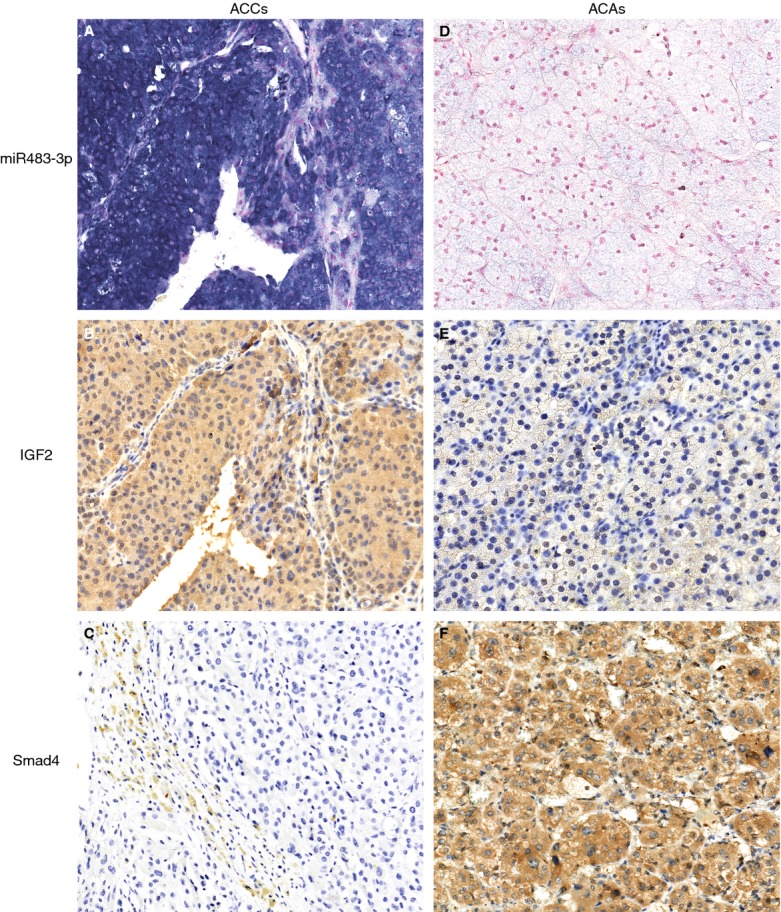
Representative staining for miR483-3p, IGF2 and Smad4 in adrenal cortical tumours. MiR483-3p was detected by in-situ hybridization (ISH), and IGF2 and Smad4 by immunochemistry. A, MiR483-3p in an adrenocortical carcinoma (ACC). The miR483-3p ISH signal is expressed mainly in the cytoplasm in all tumour cells of the ACC. Positive staining has a blue–violet colour. B, Perinuclear dot-like and homogeneous cytoplasmic staining for IGF2 in all or nearly all tumour cells in the same ACC correlating with the expression of miR483-3p (4+ staining). C, Smad4 in an ACC showing negative staining (score 0). Note positive labelling in the non-neoplastic fibrous tissue on the left. D, Negative miR483-3p ISH in an ACA. E, IGF2 in an ACA showing 1+ staining. Note intra-cytoplasmic specks only. F, An ACA displaying strong, diffuse cytoplasmic and occasional nuclear expression of Smad4.
Correlation between the Expression of miR483-3P, IGF2 OR Smad4 AND Clinicopathological Variables in Adrenocortical Carcinomas
We evaluated the relationship between the three markers and clinicopathological variables in ACCs. MiR483-3p overexpression was not associated with age, tumour size, tumour weight, hormonal function, recurrence, metastasis or Weiss scores, but there was a relationship with gender, as male patients were statistically more likely to overexpress miR483-3p than were females (P < 0.05; Table S3). For IGF2, there was no relationship with any clinicopathological variables (Table S3). Regarding Smad4, there were only two patients with elevated Smad4 expression, and there were no specific correlations with the clinicopathological features (Table S4).
Correlation between miR483-3P, IGF2 or Smad4 and Cellular Proliferative Activity in Adrenocortical Carcinomas
Because the MIB-I labelling index has been shown to possess diagnostic and prognostic value in several studies,20,22–25 we tested MIB-1 expression and analysed its relationship with miR483-3p, IGF2 and Smad4 expression using the same study cohort. According to previous studies,24,25 a proliferation index <5% was considered to be low and a proliferation index >5% was considered to be high. Our results showed that MIB-1 was highly expressed in 64% (16 of 25) ACCs, compared to 4% (one of 25) of ACAs. However, the MIB-1 expression level was not associated with miR483-3p, IGF2 or Smad4 expression in ACCs. (Table4).
Table 4.
Correlation between miR483-3p, IGF2 or Smad4 expression and cellular proliferative activity
| miR483-3p | P | IGF2 | P | Smad4 | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Elevated | Negative/Low | Elevated | Negative/Low | Elevated | Negative/Low | ||||
| MIB1 | |||||||||
| >5% | 12 | 4 | 0.394 | 9 | 7 | 0.401 | 2 | 14 | 0.520 |
| <5% | 5 | 4 | 7 | 2 | 0 | 9 | |||
The Differential Diagnostic Efficacy of the Mollecular Markers in Borderline Tumours
To validate further the differential diagnostic efficacy of the molecular markers, their expression levels were evaluated in the 15 borderline tumours (Weiss score = 2 or 3). The results and the relationship with clinicopathological data are summarized in Table5. The follow-up period was 42–103 months. Among the 15 borderline cases, three relapsed (local recurrence and/or metastasis) during follow-up, four were lost to follow up and the other eight were all alive and disease-free after their surgery. We found that none of the four markers alone could predict the malignant potential accurately. However, there are only four cases with both elevated miR483-3p and negative/low Smad4 expression. Notably, among these four cases, three recurred and died of disease (mean survival: 29.7 months) and one patient was lost to follow-up. Together, these results indicated that the combination of miR483-3p and Smad4 is useful in assisting the Weiss system in establishing a diagnosis in borderline cases.
Table 5.
Clinical features and immunohistochemical results of the 15 borderline tumors
| Tu-mor no. | Sex | Age (years) | Tumor size (cm) | Tumor weight (g) | Tumor Lacation | Clinical symptom | Weiss score | MiR483-3p | IGF2 | Smad-4 | MIB-1 | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 55 | 6 | 82 | Left | No | 3 | E | N | N | L | Relapse, DOD after 47 m |
| 2 | M | 49 | 5 | 70 | Right | Cushing syndrome | 3 | E | E | E | L | NED after 90 m |
| 3 | F | 27 | 9 | 85 | Left | No | 3 | E | E | N | H | Metastases, DOD after 17 m |
| 4 | F | 26 | 5 | 33 | Right | No | 3 | E | E | E | L | NED after 58 m |
| 5 | F | 44 | 3 | 7 | Right | Cushing syndrome | 3 | E | E | N | L | Relapse, DOD after 25 m |
| 6 | F | 29 | 4 | 27 | Right | No | 3 | N | N | E | L | NED after 45 m |
| 7 | M | 40 | 2 | 2 | Right | No | 2 | N | E | N | L | NED after 100 m |
| 8 | M | 58 | 4.5 | 30 | Left | No | 2 | N | N | N | H | LTFU |
| 9 | M | 56 | 10 | 90 | Left | No | 2 | E | N | N | L | LTFU |
| 10 | F | 52 | 5 | 23 | Right | No | 2 | N | E | E | L | NED after 99 m |
| 11 | F | 23 | 3 | 11 | Left | Primary aldosterone sydrome | 2 | N | N | E | H | LTFU |
| 12 | F | 52 | 3 | 12 | Right | No | 2 | N | E | E | L | NED after 84 m |
| 13 | F | 59 | 7 | 125 | Left | No | 2 | N | N | N | L | NED after 61 m |
| 14 | F | 56 | 5 | 64 | Right | Primary aldosterone syndrome | 2 | E | N | E | H | LTFU |
| 15 | F | 45 | 3 | 20 | Left | No | 2 | N | N | E | L | NED after 53 m |
NED, No evidence of disease; DOD, dead of disease; LTFU, lost to follow-up; E, elevated; N, negative/low; H, high, >5%; L, low, <5%.
The Prognostic Value of miR483-3P AND Smad4 in ACCs
We further validated the prognostic value of the combined use of miR483-3p and Smad4 inmmunohistochemistry in adrenocortical carcinomas. Only patients with sufficient follow-up data were included (19 of 25). The trend of the OS indicated that ACC patients with tumours showing elevated expression of miR483-3p and negative/low Smad4 had a worse disease outcome than other patients, but the association was not statistically significant (P = 0.053, Figure 2).
Figure 2.
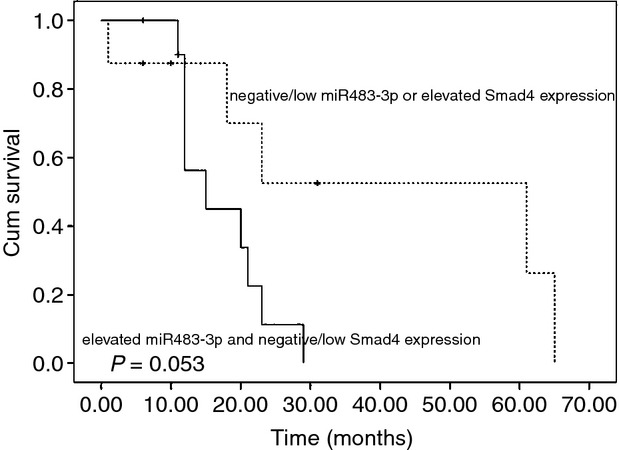
Kaplan–Meier analyses of patients with elevated miR483-3p and negative/low Smad4 expression, compared to other patients.
Discussion
Distinguishing malignant from benign adrenal cortical tumours represents a great challenge for both clinicians and pathologists. In 1974, Lewins et al.26 reported that the definitive evidence for malignancy was distant metastasis and/or local infiltration. However, with the development of imaging techniques, a growing number of unexplained adrenal incidentalomas have been found, making the diagnosis of potential malignancy more challenging in clinical practice. Therefore, the differential diagnosis of adrenal cortical carcinoma from adenoma requires comprehensive clinical characteristics, pathological morphological evaluation and a variety of auxiliary investigations.
Tumour weight and size have been important clues for malignancy. Tang and Grey27 demonstrated that tumour weights >95 g can be used to determine malignancy with 100% sensitivity. Aubert et al.7 determined that a tumour weight greater than 50 g predicted malignancy with 100% sensitivity and 90.9% specificity in a retrospective analysis of 49 adrenal masses, including 24 ACCs and 25 ACAs. Our study confirmed that malignant tumours had significantly larger sizes and greater weights than benign tumours. Furthermore, we determined that large tumours with a weight >50 g were malignant, with 100% specificity and 100% sensitivity. However, some studies have also reported tumours with a weight lower than 50 g following a malignant course.28,29 Tumour size is often underestimated by radiological investigation. Thresholds ranging from 3 to 6 are often used to predict the risk of malignancy, according to various studies.30,31 However, small thresholds are applied mainly to increase the preoperative radiological identification of non-functioning tumours. Aubert et al.7 proposed a threshold of larger than 6.5 cm for distinguishing malignancy with 100% sensitivity and 91.7% specificity. Somewhat differently, our retrospective study determined that a tumour size greater than 6.5 cm significantly predicted malignancy, with 68% sensitivity and 100% specificity. Further studies with larger sample sizes are needed to confirm the role of tumour weight and size in assessing adrenocortical malignancy. Moreover, in this retrospective study, patients presenting with primary aldosterone syndrome were found to be more likely to have a benign tumour than a malignant tumour.
Diagnosis of adrenal cortical carcinomas is not always straightforward. Lesions of intermediate size with some suspicious features are often equivocal for diagnosis. At this point, a large number of histological factors can be taken into account and several multiparametric systems have been proposed for delineating malignancy. Among various histological criteria, the Weiss system is most commonly used. To evaluate its diagnostic efficacy further, this retrospective study reviewed the Weiss criteria among all 50 samples, revealing that a total Weiss score of 3 or higher predicted malignancy significantly, with 100% specificity and 100% sensitivity. However, it has been reported previously that some tumours with three Weiss criteria maintain a clinical benign course,7–9 and individual tumours with two Weiss criteria have behaved in a malignant manner during follow-up.10,11 Thus, although the Weiss system can result in accurate diagnosis of most ACCs and ACAs, it is not applicable in some challenging cases.
To date, many approaches have been studied for the distinction of benign and malignant adrenocortical tumours, such as genotyping methods, molecular profiling studies and most commonly immunohistochemical approaches.32 Numerous molecular markers, including topoisomerase II alpha, MIB-1, p53, E-cadherin, HER-2, p27 and the IGF family members, have been validated as diagnostic and prognostic indicators.16,17,20,22–25 Among these, in most studies the MIB-1/Ki-67 labelling index was shown to possess diagnostic and prognostic value.20,22–25 However, the diagnostic threshold used was not consistent, and diagnostic accuracy still requires confirmation in a larger sample size.
Biomarkers for accurate diagnosis, prognosis or molecular therapy are needed urgently. miRNA markers are ideal for this purpose because miRNAs seem to be more stable and less prone to degradation than mRNAs. Moreover, miRNAs are short, and can therefore be easily knocked down or overexpressed for therapy. In our study, locked nucleic acid in-situ hybridization was used to determine the expression of miR483-3p in both adrenal cortical carcinomas and adenomas. We found 17 cases of overexpression in 25 subjects with primary malignancies (68%) compared to three of 25 (12%) patients with benign tumours. In line with these results, Patterson et al.33 demonstrated that miR483-3p was highly expressed in ACCs by real-time quantitative RT–PCR in 34 samples (10 primary ACCs and 24 ACAs).
Previous studies have shown that altered expression of specific miRNAs can contribute to the initiation and progression of cancer.34 Our results indicate that miR483-3p is highly expressed in primary ACCs. Therefore, it leads us to hypothesize that miR483-3p may promote the development of adrenal cortical carcinomas. In addition, some experiments have demonstrated that miR483-3p can protect cells from apoptosis in liver and colorectal cancer cell lines.13 Future functional studies are necessary to assess whether miR483-3p plays a similar role in ACCs.
MiR483-3p is located at intron 2 of IGF2, which has been reported to be one of the most commonly overexpressed genes in ACCs.16,17,25 This suggests a high likelihood of co-expression. To test this hypothesis, we measured the expression of IGF2 in all 50 samples by immunohistochemistry. We found high expression of IGF2 in 16 of 25 ACCs (64%). Furthermore, 14 patients showed simultaneous high expression of miR483-3p and IGF2. There was a positive correlation between the expression of IGF2 and miR483-3p. Thus, the IGF2 gene not only exerts mitogenic effects through interactions with the IGF-I receptor (IGF-IR)35; it may also exploit miR483-3p to regulate target gene expression post-transcriptionally. However, a small subset of patients displayed divergent expression between miR483-3p and IGF2, thus revealing a mechanism in which IGF2 and miR483-3p are not co-regulated. This is an area that requires further investigation.
A recent study reported that the expression of Smad4/DPC4 was suppressed by miR483-3p in pancreatic cancer.15 Moreover, it has been reported that approximately 55% of pancreatic adenocarcinomas show inactivation of the Smad4/DPC4 gene by one of two mechanisms: homozygous deletion and loss of heterozygosity.36–38 However, the expression status of Smad4 and its correlation with miR483-3p are still unknown in adrenal cortical tumours. Therefore, we also evaluated the expression of Smad4 in all 50 tumours. We found that ‘diffusely positive’ expression of Smad4 occurred in only two of 25 ACCs, with 23 of 25 ACCs showing ‘focally positive’ or ‘negative’ staining. According to Wilentz et al.21 cases known to have at least one wild-type Smad4 gene were expected to show diffusely positive expression of Smad4. Immunohistochemical staining for Smad4 labelled as ‘negative/low’ indicated deregulation of Smad4, regardless of the mechanism (i.e. overexpression of miR483-3p or the two types of gene inactivation). In our study, negative/low expression of Smad4 occurred in 23 of 25 ACCs, compared to 10 of 25 ACAs, a statistically significant difference. This suggests that down-regulation of Smad4 expression may play roles in the carcinogenesis of adrenocortical carcinoma. The mechanism underlying the role of miR483-3p and the down-regulation of Smad4 expression in the progression of adrenal cortical carcinomas still requires further biological investigation.
Proliferative activity measured by MIB-1 staining was reported in several previous studies to be useful for the diagnosis and prognostic assessment of ACCs.20,22–25 However, diagnostic accuracy was not verified. We tested MIB-1 expression in the same study cohort, and analysed its relationship with miR483-3p, IGF2 and Smad4 expression in ACCs. We found that 64% (16 of 25) ACCs had high expression of MIB1, compared to 4% (one of 25) of ACAs. However, the MIB1 expression level was not associated with miR483-3p, IGF2 or Smad4 expression in ACCs.
To validate further the differential diagnostic efficacy of the molecular markers and identify those that were most reliable, their expression was assessed in an independent set of 15 borderline tumours (Weiss score = 2 or 3), which were followed-up for 42–103 months. The results showed that none of the four markers alone could predict malignant potential accurately. However, the combination of elevated expression of miR483-3p and negative/low expression of Smad4 could differentiate the three tumours that followed a malignant course from others.
Based on our finding that miR483-3p and Smad4 expression together could predict clinically malignant behaviour of borderline tumours, we further analysed the prognostic value of the two markers in carcinomas. The results indicated a trend towards ACC patients with tumours having elevated miR483-3p and negative/low Smad4 expression showing a worse disease outcome than other patients, although the association was not statistically significant. Thus, a larger sample size is needed to evaluate further the diagnostic and prognostic efficacy of the two markers.
In conclusion, our study confirmed that tumour size, tumour weight, hormonal function and the Weiss system can differentiate malignant from benign tumours in most cases. The combined use of miR483-3p and Smad4 immunohistochemistry provides a helpful additional tool to the Weiss systems in borderline cases. However, larger sample sizes are needed to determine further the differential diagnostic and prognostic efficacy of miR483-3p and Smad4 expression. Moreover, the mechanisms through which miR483-3p modulates the development of adrenal cortical carcinoma remain to be clarified in future biological studies.
Acknowledgments
We are grateful to Dr Jing Zhang for helping to review the Weiss system. This study was supported by The National Nature Science Foundations of China (30973470 and 81172334).
Conflict of interest
The authors declare no conflicts of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
The morphological features of the 15 borderline tumours.
Table S2. Relationship between expression of miR483-3p and IGF2, and miR483-3p and Smad4 in adrenocortical carcinomas.
Table S3. Correlations between clinicopathological variables and expression of miR483-3p and IGF2 in adrenocortical carcinomas.
Table S4. Clinicopathological features of the two adrenaocortical carcinoma patients with strong Smad4 staining.
References
- 1.Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (‘incidentaloma’) Ann. Intern. Med. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 2.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J. Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 3.Favia G, Lumachi F, Carraro P, D'Amico DF. Adrenocortical carcinoma. Our experience. Minerva Endocrinol. 1995;20:95–99. [PubMed] [Google Scholar]
- 4.Wajchenberg BL, Albergaria Pereira MA, Medonca BB, et al. Adrenocortical carcinoma: clinical and laboratory observations. Cancer. 2000;88:711–736. [PubMed] [Google Scholar]
- 5.Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am. J. Surg. Pathol. 1984;8:163–169. doi: 10.1097/00000478-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Weiss LM, Medeiros LJ, Vickery AL., Jr Pathologic features of prognostic significance in adrenocortical carcinoma. Am. J. Surg. Pathol. 1989;13:202–206. doi: 10.1097/00000478-198903000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Aubert S, Wacrenier A, Leroy X, et al. Weiss system revisited: a clinicopathological and immunohistochemical study of 49 adrenocortical tumors. Am. J. Surg. Pathol. 2002;26:1612–1619. doi: 10.1097/00000478-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Gicquel C, Bertagna X, Gaston V, et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res. 2001;61:6762–6767. [PubMed] [Google Scholar]
- 9.Lucon AM, Pereira MA, Mendonca BB, et al. Adrenocortical tumors: results of treatment and study of Weiss's score as a prognostic factor. Rev. Hosp. Clin. Fac. Med. São Paulo. 2002;57:251–256. doi: 10.1590/s0041-87812002000600002. [DOI] [PubMed] [Google Scholar]
- 10.Klibanski A, Stephen AE, Greene MF, et al. Case records of the Massachusetts General Hospital. Case 36–2006. A 35-year-old pregnant woman with new hypertension. N. Engl. J. Med. 2006;355:2237–2245. doi: 10.1056/NEJMcpc069027. [DOI] [PubMed] [Google Scholar]
- 11.Pohlink C, Tannapfe A, Eichfeld U, et al. Does tumor heterogeneity limit the use of the Weiss criteria in the evaluation of adrenocortical tumors? J. Endocrinol. Invest. 2004;27:565–569. doi: 10.1007/BF03347480. [DOI] [PubMed] [Google Scholar]
- 12.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 13.Veronese A, Lupini L, Consiglio J, et al. Oncogene role of Mir-483-3p at IGF2/483 locus. Cancer Res. 2010;70:3140–3149. doi: 10.1158/0008-5472.CAN-09-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 15.Hao J, Zhang S, Zhou Y, et al. MicroRNA 483-3p suppresses the expression of DPC4/Smad4 in pancreatic cancer. FEBS Lett. 2011;585:207–213. doi: 10.1016/j.febslet.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Giordano TJ, Thomas DG, Kuick R, et al. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am. J. Pathol. 2003;162:521–531. doi: 10.1016/S0002-9440(10)63846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Fraipont F, El Atifi M, Cherradi N, et al. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J. Clin. Endocrinol. Metab. 2005;90:1819–1829. doi: 10.1210/jc.2004-1075. [DOI] [PubMed] [Google Scholar]
- 18.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J. Gastrointest. Surg. 2008;12:2171–2176. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer-Rochow GY, Jackson NE, Conaglen JV, et al. MicroRNA profiling of benign and malignant pheochromocytomas identifies novel diagnostic and therapeutic targets. Endocr. Relat. Cancer. 2010;17:835–846. doi: 10.1677/ERC-10-0142. [DOI] [PubMed] [Google Scholar]
- 20.Soon PS, Gill AJ, Benn DE, et al. Microarray gene expression and immunohistochemistry analyses of adrenocortical tumors identify IGF2 and Ki-67 as useful in differentiating carcinomas from adenomas. Endocr. Relat. Cancer. 2009;16:573–583. doi: 10.1677/ERC-08-0237. [DOI] [PubMed] [Google Scholar]
- 21.Wilentz RE, Su GH, Dai JL, et al. Immunohistochemical labeling for dpc4 mirrors genetic status in pancreatic adenocarcinomas: a new marker of DPC4 inactivation. Am. J. Pathol. 2000;156:37–43. doi: 10.1016/S0002-9440(10)64703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta D, Shidham V, Holden J, et al. Value of topoisomerase II alpha, MIB-1, p53, E-cadherin, retinoblastoma gene protein product, and HER-2/neu immunohistochemical expression for the prediction of biologic behavior in adrenocortical neoplasms. Appl. Immunohistochem. Mol. Morph. 2001;9:215–221. doi: 10.1097/00129039-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Nakazumi H, Sasano H, Iino K, et al. Expression of cell cycle inhibitor p27 and Ki-67 in human adrenocortical neoplasms. Mod. Pathol. 1998;11:1165–1170. [PubMed] [Google Scholar]
- 24.Wachenfeld C, Beuschlein F, Zwermann O, et al. Discerning malignancy in adrenocortical tumors: are molecular markers useful? Eur. J. Endocrinol. 2001;145:335–341. doi: 10.1530/eje.0.1450335. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt A, Saremaslani P, Schmid S, et al. IGFII and MIB1 immunohistochemistry is helpful for the differentiation of benign from malignant adrenocortical tumours. Histopathology. 2006;49:298–307. doi: 10.1111/j.1365-2559.2006.02505.x. [DOI] [PubMed] [Google Scholar]
- 26.Lewinsky BS, Grigor KM, Symington T, Neville AM. The clinical and pathologic features of ‘non-hormonal’ adrenocortical tumors: report of twenty new cases and review of the literature. Cancer. 1974;33:778–790. doi: 10.1002/1097-0142(197403)33:3<778::aid-cncr2820330325>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Tang CK, Gray GF. Adrenocortical neoplasms. Prognosis and morphology. Urology. 1975;5:691–695. doi: 10.1016/0090-4295(75)90135-1. [DOI] [PubMed] [Google Scholar]
- 28.Icard P, Chapuis Y, Andreassian B, Bernard A, Proye C. Adrenocortical carcinoma in surgically treated patients: a retrospective study on 156 cases by the French Association of Endocrine Surgery. Surgery. 1992;6:972–980. [PubMed] [Google Scholar]
- 29.Sasano H, Suzuki T, Moriya T. Recent advances in surgical pathology of adrenal incidentaloma. Biomed. Pharmacother. 2000;54:169–174. doi: 10.1016/s0753-3322(00)80037-2. [DOI] [PubMed] [Google Scholar]
- 30.Dackiw AP, Lee JE, Gagel RF, Evans DB. Adrenal cortical carcinoma. World J. Surg. 2001;25:914–926. doi: 10.1007/s00268-001-0030-7. [DOI] [PubMed] [Google Scholar]
- 31.Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Incidentally discovered adrenal tumors: an institutional perspective. Surgery. 1991;110:1014–1021. [PubMed] [Google Scholar]
- 32.Giordano TJ. Molecular pathology of adrenal cortical tumors: separating adenomas from carcinomas. Endocr. Pathol. 2006;17:355–363. doi: 10.1007/s12022-006-0007-z. [DOI] [PubMed] [Google Scholar]
- 33.Patterson EE, Holloway AK, Weng J, Fojo T, Kebebew E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117:1630–1639. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu. Rev. Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 35.Logié A, Boulle N, Gaston V, et al. Autocrine role of IGF-II in proliferation of human adrenocortical carcinoma NCI H295R cell line. J. Mol. Endocrinol. 1999;23:23–32. doi: 10.1677/jme.0.0230023. [DOI] [PubMed] [Google Scholar]
- 36.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 37.Hahn SA, Hoque AT, Moskaluk CA, et al. Homozygous deletion map at 18q21.1 in pancreatic cancer. Cancer Res. 1996;56:490–494. [PubMed] [Google Scholar]
- 38.Schutte M, Hruban RH, Hedrick L, et al. DPC4 gene in various tumor types. Cancer Res. 1996;56:2527–2530. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The morphological features of the 15 borderline tumours.
Table S2. Relationship between expression of miR483-3p and IGF2, and miR483-3p and Smad4 in adrenocortical carcinomas.
Table S3. Correlations between clinicopathological variables and expression of miR483-3p and IGF2 in adrenocortical carcinomas.
Table S4. Clinicopathological features of the two adrenaocortical carcinoma patients with strong Smad4 staining.


