Abstract
The host inflammatory response to the Onchocerca volvulus endosymbiont, Wolbachia, is a major contributing factor in the development of chronic pathology in humans (onchocerciasis/river blindness). Recently, the toll-like pattern recognition receptor motif of the major inflammatory ligands of filarial Wolbachia, membrane-associated diacylated lipoproteins, was functionally defined in murine models of pathology, including mediation of neutrophil recruitment to the cornea. However, the extent to which human neutrophils can be activated in response to this Wolbachia pattern recognition motif is not known. Therefore, the responses of purified peripheral blood human neutrophils to a synthetic N-terminal diacylated lipopeptide (WoLP) of filarial Wolbachia peptidoglycan-associated lipoprotein (PAL) were characterized. WoLP exposure led to a dose-dependent activation of healthy, human neutrophils that included gross morphological alterations and modulation of surface expressed integrins involved in tethering, rolling and extravasation. WoLP exposure induced chemotaxis but not chemokinesis of neutrophils, and secretion of the major neutrophil chemokine, interleukin 8. WoLP also induced and primed the respiratory burst, and enhanced neutrophil survival by delay of apoptosis. These results indicate that the major inflammatory motif of filarial Wolbachia lipoproteins directly activates human neutrophils in vitro and promotes a molecular pathway by which human neutrophils are recruited to sites of Onchocerca parasitism.
Keywords: filariasis, human neutrophils activation, Onchocerca volvulus, river blindness, Wolbachia, Wolbachia lipoproteins
Introduction
Onchocerciasis (river blindness) is a parasitic disease affecting 37 million people worldwide. An estimated 270 000 people have been blinded by infection with an additional 500 000 suffering visual impairment 1,2. The causative agent, the filarial worm Onchocerca volvulus, resides in subcutaneous nodules, also known as onchocercomas. Disease is caused by the migration into and subsequent death of numerous microscopic larval progeny (microfilariae; mf) in dermal and ocular tissues. Inflammatory responses invoked by the release of somatic antigens from dead mf are important in the initiation of disease pathogenesis. In particular, liberated Wolbachia, endosymbiotic bacteria found in many filarial species, are major innate inflammatory stimuli 3.
Granulocytes are the principal component of the early inflammatory infiltrate around damaged and dying mf in the cornea. Neutrophils contribute to the Wolbachia-mediated pathogenesis of onchocerciasis 4–7 and their infiltration into ocular tissues following mf exposure is dependent on the presence of Wolbachia. In mouse models of ocular Onchocerca keratitis, neutrophils surround mf in the cornea and are recruited early after injection of isolated Wolbachia bacteria and Wolbachia-containing filarial extracts but not of extracts from worms Wolbachia-depleted or naturally devoid of the symbiont, resulting in corneal haze and opacity 4,8,9. After injection of mf into the cornea, Wolbachia are found in neutrophil phagosomes fusing with granules 4.
In dermal and subcutaneous tissues, recruitment and maintenance of neutrophils at the site of Onchocerca infection and inflammation appears to mirror that occurring in the cornea 5,10. In both human and bovine onchocerciasis, neutrophil recruitment and maintenance within adult worm nodules is sensitive to anti-Wolbachia tetracycline-based chemotherapy 11–14. In the latter infection system, neutrophil depletion occurs at a point following sterilisation of the adult Onchocerca tissues of endosymbionts but prior to significant decline in adult worm viability 14. Further evidence to support a central role of nematode Wolbachia in promoting neutrophilic responses to filariae comes from observations that neutrophils accumulate around Wolbachia-containing Onchocerca spp but not around Onchocerca spp. naturally devoid of the endosymbiont 11,15.
Systemically, neutrophil activation is also observed during the adverse reactions following microfilaricidal treatment of filariasis patients, which correlate with pretreatment mf loads and the presence of liberated Wolbachia DNA or whole bacterial cells in the circulation 16–18. Systemic adverse events and levels of liberated circulating Wolbachia also positively correlate with neutrophilia, circulating levels of pro-inflammatory cytokines, including the neutrophil CXC chemokine IL-8 (CXCL8) and neutrophil-derived molecules, such as calprotectin and calgranulin B 16–19.
Despite being pivotal effector cells in the pathogenesis of onchocercal disease, few studies have addressed the mechanisms of neutrophil activation and recruitment by filarial Wolbachia. In mice, it has been established that production of neutrophil-specific chemokines, neutrophil recruitment and subsequent development of corneal opacity is Toll-like Receptor (TLR)2 and MyD88-dependent, implicating Wolbachia TLR ligands in the initiation of neutrophil responses 8,9. Recently, the diacylated membrane-associated lipoproteins of filarial Wolbachia have been promoted as the major pro-inflammatory TLR ligands expressed by filarial endosymbionts and their biosynthetic pathway have been suggested as a potential target for anti-Wolbachia filarial therapy 20,21. Bioinformatic and database searches consistently predict the presence of two lipoproteins in Wolbachia: peptidoglycan-associated lipoprotein (PAL), and a type IV secretion system protein (VirB6), which share an N-terminal motif 20. Wolbachia PAL of Brugia malayi (wBmPAL) had a predicted outer membrane location 20 and has been identified as an abundant Wolbachia protein in the secretome and proteome of B. malayi 22,23. Depletion of lipid or protein from total B. malayi female worm extracts nullifies innate immune activation, supporting a role of native Wolbachia lipoproteins as the ligands of TLR2 innate immune reactivity 20. A synthetic, lipolated version of the N-terminus of Wolbachia PAL, WoLP, has been identified as important in the mediation of filarial inflammation and modulation of antifilarial adaptive immune responses via the ligation of (TLR)2/6 and to a minor extent of TLR2/1 on myeloid immune cells and stromal cells 20. Most importantly, WoLP can replicate the effects of Wolbachia bacteria in mediating experimental Onchocerca keratitis in a TLR2/6 dependent manner 20.
The responses of purified human neutrophils to Wolbachia lipoproteins have not been elucidated, but these likely represent responses to both PAL and VirB6, due to the shared N-terminal motif 20. In this study, we investigated the interaction between WoLP and human neutrophils in vitro and determined that this Wolbachia-specific lipoprotein motif is sufficient to activate a range of activation phenotypes. Our data therefore demonstrates a direct functional role of the major Wolbachia inflammatory ligand in promoting human neutrophil responses. We hypothesise that diacylated Wolbachia lipoproteins are critical in the induction and maintenance of neutrophil recruitment during human onchocerciasis.
Materials and Methods
Human neutrophil isolation
The use of blood neutrophils from adult healthy volunteers was approved by the Research Ethics Committee of the University of Liverpool, UK. Peripheral blood was collected by venepuncture in lithium heparin vacutainers, and neutrophils were isolated using Polymorphprep (Axis Shield Dundee, Scotland) following manufacturer's instructions. Contaminating red blood cells were lysed with 9 : 1 ammonium chloride lysis buffer (13·4 mm KHCO3, 155 mm NH4Cl, 96·7 μm EDTA) in RPMI 1640 culture media (Gibco, Life Technologies, Carlsbad, CA, USA). Cell viability was assessed by 0·2% trypan blue staining (Sigma Aldrich Gillingham, UK) and was always ≥98%. The purity of isolated neutrophils was assessed by Rapid Romanowsky stain (HD Supplies, TCS Biosciences, Buckingham, UK) of cytospins (Cytospins3, Shandon, Thermo Scientific, Loughborough, UK) followed by differential count of ≥700 cells by optical microscopy. The purity of isolated neutrophils was always ≥97% with ≤0·14% monocyte contamination. Neutrophils were incubated at 37°C in a humidified incubator in RPMI 1640 (with 25 mm HEPES and 2 mm L-glutamine) culture media (Gibco).
Neutrophil stimuli
Stimuli for neutrophil cultures were synthetic 20-mers of the N-terminal region of wBmPAL (CSKRGVNAINKMNFVVKQMK) di-palmtoylated at the N-terminal cysteine residue (WoLP) 20 (EMC Microcollections Tubingen, Germany); recombinant human TNFα (Calbiochem); recombinant, Merk Millipore, Darmstadt, Germany human GM-CSF (Roche Welwyn Garden City, UK); and N-formyl-methionine-leucine-phenylalanine (fMLP) and phorbol 12-myristate 13-acetate (PMA, both from Sigma Aldrich) at the indicated concentrations. TLR2/6 specificity of WoLP was confirmed by lack of TNFα production in response to WoLP stimulation in TLR2−/− and TLR6 −/− mouse macrophage cultures as previously performed (data not shown). Similarly, lack of LPS contamination was confirmed by determining equivalent TNFα release in WT and TLR4−/−-derived macrophages (data not shown). In all assays, vehicle Dimethyl Sulfoxide (DMSO, Sigma Aldrich) was included as control stimulus dissolved in culture media when stimuli dissolved in DMSO were used.
Cell culture
For incubations <8 h, neutrophils were cultured at 5 × 106 cells/mL in 1·5 mL screw-top tubes (Eppendorf Stevenage, UK) with gentle rotation. For incubations ≥8 h, neutrophils were cultured at 1 × 106 cells/mL in 24-well culture plates in culture media supplemented with 10% heat-inactivated human AB serum (Sigma Aldrich) in the presence of 5% CO2.
Morphological assessment of neutrophil activation
After 1·5 h culture with WoLP (1 μg/mL) and control stimuli DMSO and fMLP (0·01 μm), the morphology of neutrophils was visualized using a Zeiss Axiovert S100TV microscope (Carl Zeiss, Cambridge, UK) supporting a Hamamatsu multiformat CCD camera (Hamamatsu Corporation, Welwyn Garden City, UK) with aqm advance 6 software (Kinetic Imaging, Liverpool, UK).
Chemotactic and chemokinetic assays
Chemotactic and chemokinetic assays were performed using a transwell system (Millicell 24-wells Cell Culture Hanging Inserts, Millipore, Darmstadt, Germany) in 24-well culture plates precoated with sterile Poly-Hema (Sigma Aldrich), according to the manufacturer's instructions. For the chemotaxis assay, WoLP (0·5–5 μg/mL), and control stimuli DMSO and fMLP (0·01 μm) were added in the culture wells in RPMI 1640 culture media, while neutrophils were added at 5 × 106 cells/mL in RPMI 1640 culture media in the upper hanging insert. To differentiate between chemotaxis (migration towards a chemotactic gradient) and chemokinesis (increased random movements upon exposure to a stimulus in the absence of a gradient), the assay was then carried out with equal concentrations of stimuli WoLP (1 μg/mL) and control DMSO and fMLP (0·01 μm) in both the upper and lower chambers of the transwell system. The chambers were incubated for 1·5 h at 37°C and the cells migrating into the lower chamber were counted using a Beckman Coulter cell counter supporting multisizer 3 software (Beckman Coulter, High Wycombe, UK), counting only particles between 8 and 12 μm diameter.
Assessment of expression of surface adhesion molecules and apoptosis by flow cytometry
After 1 h culture in the presence of WoLP (0·1 μg/mL) and control stimuli DMSO and GM-CSF (5 ng/mL), the surface expression of β2-integrins CD11b and CD18, and of L-Selectin by neutrophils was assessed by flow cytometry (FC). Briefly, neutrophils were stained for 30 min on ice in FC buffer (0·2% Bovine Serum Albumin (Sigma Aldrich) in PBS) with FITC-conjugated rat antihuman CD11b IgG2b (Miltenyi Biotec, Bisley, UK), mouse antihuman CD18 IgG1 (R&D Systems, Minneapolis, MN, USA), mouse antihuman L-Selectin IgG1 (R&D Systems) and mouse IgG1 isotype control (Santa Cruz Biotechnology Dallas, TX, USA). After fixation in 2% paraformaldehyde in FC buffer, cells (2 × 105/mL in FC buffer) were analysed with a Guava EasyCyte Plus (Millipore) flow cytometer and cytosoft 5.3 software.
For the assessment of apoptosis, neutrophils were cultured with WoLP (1 ng–5 μg/mL) and control stimuli DMSO and GM-CSF (5 ng/mL) for 15 and 20 h. Apoptotic cells were labelled for 15 min at room temperature with Annexin-V-FITC (Biosource Life Technologies, Carlsbad, CA, USA) 1 : 100 in HBSS (Gibco). Propidium iodide (PI, from Sigma Aldrich) was added at 1 μg/mL in HBSS to wells to label late apoptotic and necrotic cells. Unstained cells in HBSS were included as control for background fluorescence. Neutrophils (1 × 105/mL) were analysed as above.
Respiratory burst assay
Production of total intra- and extra-cellular reactive oxygen species (ROS) was measured using a luminol-enhanced chemiluminescence assay. Neutrophils were primed for 30 min with WoLP (1 ng–5 μg/mL) and control stimuli DMSO and TNFα (10 ng/mL). Cells were then added to white, low-adhesion 96-well plates and stimulated with fMLP (1 μm), PMA (100 ng/mL) or DMSO (unstimulated cells) in the presence of luminol (10 μm in HBSS, from Sigma Aldrich). Chemiluminescence was read every 30 s for 30 min in a Wallac Victor™ Light 1420 Luminescence Counter (Perkin Elmer, Waltham, MA, USA) at 37°C. Background chemiluminescence was assessed by inclusion of one well without cells per each stimulus. Total chemiluminescence was calculated using the area under the curve (AUC) method. Cell viability at the time of peak ROS production was assessed by 0·2% trypan blue staining and was always ≥93%.
Assessment of cytokine production by ELISA
Levels of IL-1β, IL12-p70, IL-8, GM-CSF and TNFα in neutrophil culture supernatants were analysed using DuoSet ELISA Development kits (R&D Systems) as per the manufacturer's instruction. Absorbance was read in a FLUOstar Omega plate reader supporting mars data analysis software 1.20 (BMG Labtech, Ortemberg, Germany). The best-fit curve method was used to calculate the cytokine concentration in each sample.
Statistical analysis
Samples from ≥3 donors were used for cell cultures and neutrophil functional assays. Means were compared using independent samples t-test. For neutrophil surface adhesion molecule expression, mean percentage changes in mean fluorescence intensity compared with control were analysed using one-sample t-test. A P ≤ 0·05 was considered significant. All analyses were carried out using spss statistics 20.0 (IBM Postsmouth, UK).
Results
Neutrophil morphology
Activation of neutrophils resulted in a change in cell morphology, with resting neutrophils having a typical round shape, but an elongated aspect after activation. After 1·5 h exposure to WoLP, neutrophils from peripheral blood of human volunteers showed an evident activated cell shape (Figure1a), similar to that obtained by exposure to fMLP (Figure1b), while cells exposed to culture media ± DMSO had a resting morphology (Figure1c, d).
Figure 1.
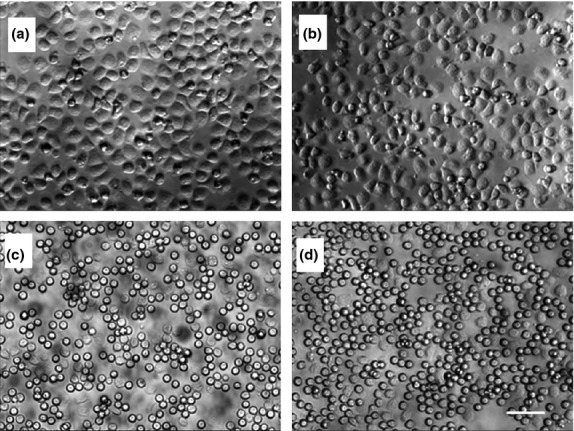
Isolated neutrophils acquire activated cell morphology upon exposure for 1·5 h to WoLP (a), similar to that induced by N-formyl-methionine-leucine-phenylalanine (fMLP) (b). Exposure to media alone (c) and Dimethyl Sulfoxide (DMSO) control (d) did not result in any evident change in cell morphology. Original magnification 32×. Scale bar, 50 μm. Images are representative of n = 3 independent experiments.
Chemotaxis and chemokinesis
Migration of neutrophils in response to WoLP was analysed in a transwell system using fMLP as a positive control. In the presence of a concentration gradient, WoLP and fMLP induced the migration of cells in higher numbers compared with control stimuli at all concentration used (P ≤ 0·004, Figure2a). It was then assessed whether this enhanced migration was due to chemotaxis or to increased random movement (chemokinesis). As shown in Figure2b, the WoLP- and fMLP-induced migration of neutrophils into the lower chamber was significantly impaired when the stimulus was present in both chambers, that is, in the absence of a concentration gradient (P ≤ 0·007), indicating that WoLP exerts a chemotactic rather than chemokinetic effect on human neutrophils in vitro.
Figure 2.
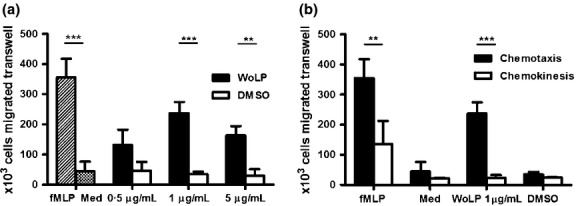
(a) In a transwell chemotaxis assay isolated neutrophils migrate towards a concentration gradient of WoLP (0·5–5 μg/mL) and control stimulus N-formyl-methionine-leucine-phenylalanine (fMLP), but not Dimethyl Sulfoxide (DMSO) and media alone. **P = 0·004; ***P = 0·001. (b) Chemokinesis assay: isolated neutrophils migrate only towards (chemotaxis, black bars) but not in the absence of (chemokinesis, white bars) concentration gradient of WoLP and fMLP. **P = 0·007; ***P < 0·001. Bar graphs mean ± SD of cells migrated transwell of n = 3 donors each tested in duplicate for chemotaxis and of n = 6 donors for chemochinesis experiment.
Surface adhesion molecule expression
L-Selectin is the major selectin expressed on neutrophils, contributing to leucocyte rolling on endothelial cells of blood capillaries, and is rapidly shed from the cell surface upon activation. CD11b/CD18 is one of the major neutrophil integrins, involved in strong binding of activated neutrophils to the endothelium, and its surface expression is upregulated upon activation. One hour incubation with WoLP led to significant downregulation of surface expression of L-Selectin (P < 0·001) and upregulation of CD11b (P = 0·001) and CD18 (P = 0·025) compared with incubation with DMSO control, mirroring the effects of stimulation with the positive control, GM-CSF (Figure3).
Figure 3.
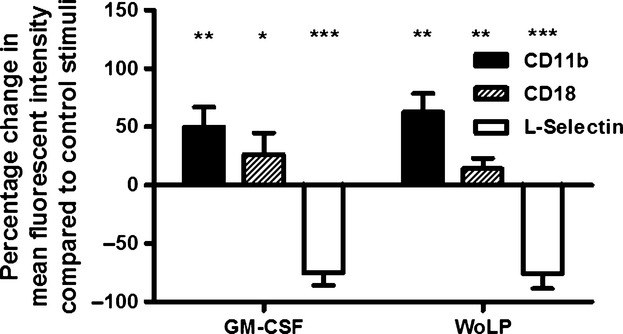
WoLP (0·1 μg/mL) induces upregulation of integrins (CD11b and CD18) and shedding of L-Selectin on isolated neutrophils, similar to the effects of GM-CSF. *P = 0·025 for WoLP and P = 0·033 for GM-CSF; **P = 0·003; ***P ≤ 0·001. Bar graph represents percentage change (mean ± SD) in mean fluorescence intensity compared with stimulation with control Dimethyl Sulfoxide (DMSO) (for WoLP) and media alone (for GM-CSF) of neutrophils from n = 5 donors.
Apoptosis
While resting neutrophils are short-lived cells undergoing apoptosis after ∼12 h, their activation leads to an increase in their lifespan. A marker of apoptosis is the re-arrangement of molecules present on the inner and outer leaflet of the cell membrane. In particular, phosphatidylserine appears on the outer surface of the cell membrane of apoptotic cells, where it can be labelled with Annexin-V-FITC. Cells in late apoptosis and necrosis show an increased cell membrane permeability which can be detected by PI staining. After 20 h culture in media alone (Figure4), 56·96% (±8·11%) neutrophils underwent apoptosis. At this time point, WoLP induced a decrease in the percentage of neutrophils undergoing apoptosis (Annexin-V+ PI−) in a concentration-dependent manner (P = 0·004 upon stimulation with WoLP 0·1 μg/mL and P = 0·047 upon stimulation with WoLP 1 μg/mL) (Figure4). Comparable findings were observed after 15-h incubation (data not shown). No differences were found in the percentage of cells in late apoptosis (Annexin-V+ PI+, range 0·06%-4·98%) or necrosis (Annexin-V− PI+, range 0·1–2·84%) (data not shown).
Figure 4.
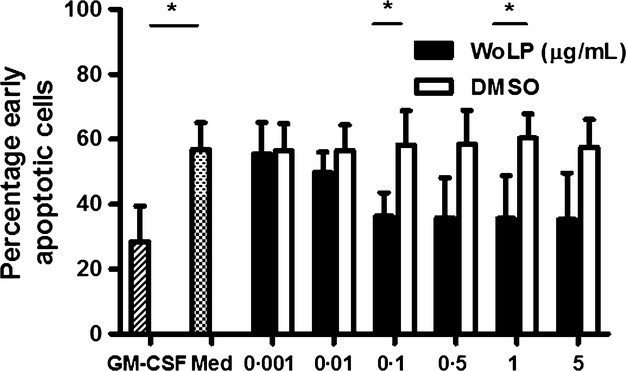
Exposure to WoLP (0·001–5 μg/mL) for 20 h delays apoptosis of isolated neutrophils similar to exposure to the positive control stimulus, GM-CSF. *P = 0·040 WoLP 0·1 μg/mL and P = 0·047 WoLP 1 μg/mL; P = 0·023 GM-CSF. Bar graphs represent mean ± SD percentage of early apoptotic neutrophils from n = 3 donors each tested in duplicate.
Respiratory burst
The production of reactive oxygen species (ROS) by stimulated neutrophils is typically low unless cells have been pretreated (primed) with other agents, which induce the upregulation of the surface expression of the NADPH oxidase complex components. Priming typically results in a greater ROS production upon stimulation with fMLP and in a more rapid response to PMA stimulation. Levels of ROS produced by neutrophils incubated with WoLP were significantly higher compared with cells incubated in media alone, and increased in a WoLP concentration-dependent manner, reaching a plateau for concentrations ≥0·5 μg/mL WoLP (Figure5a). When cells were stimulated with fMLP after priming with WoLP, the production of ROS approximately doubled compared with unprimed cells, showing a similar concentration-dependent pattern (Figure5b). Also, stimulation of WoLP-primed neutrophils with PMA induced a concentration-dependent decrease in the time needed to reach the peak of ROS production compared with unprimed cells (Figure5c).
Figure 5.
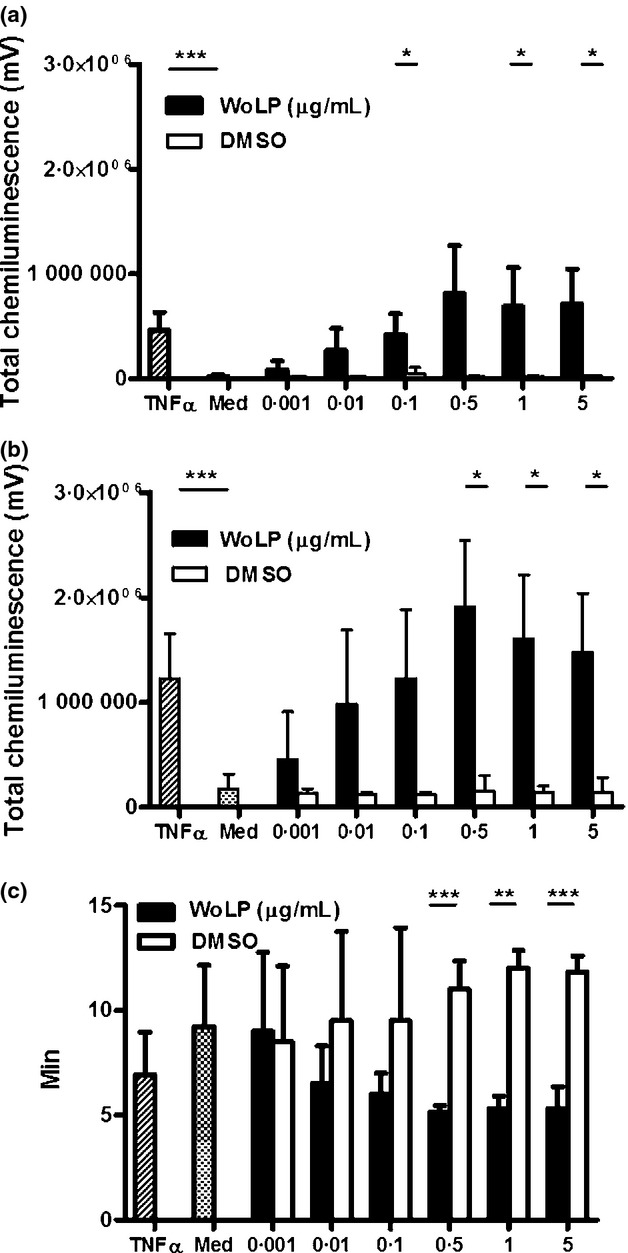
Incubation for 30 min with WoLP (0·001–5 μg/mL) alone induces reactive oxygen species (ROS) production (a) and primes ROS production upon subsequent stimulation with N-formyl-methionine-leucine-phenylalanine (fMLP) (b) in a concentration-dependent manner, as measured by total chemiluminescence over 30 min. (c) Priming with WoLP (0·001–5 μg/mL) decreases the time-to-peak ROS production upon stimulation with phorbol 12-myristate 13-acetate (PMA) in a concentration-dependent manner. (a) *P = 0·036 WoLP 0·1 and 1 μg/mL; P = 0·024 WoLP 5 μg/mL; ***P < 0·001. (b) *P = 0·040 WoLP 0·5 μg/mL; P = 0·015 WoLP 1 and 5 μg/mL; ***P < 0·001. Bar graphs represent mean AUC ± SD of n = 3 donors each tested in duplicate. (c) **P = 0·002; ***P = 0·001. Bar graph represents mean ± SD time-to-peak ROS production expressed in minutes of n = 3 donors each tested in duplicate.
IL-8 production
To investigate whether the activation of neutrophils in vitro by WoLP, resulted in cytokine/chemokine expression, levels of IL-1β, IL-8, IL-12p70, GM-CSF and TNFα were measured in the supernatant of neutrophils exposed to increasing concentrations of WoLP for 30 min, 1 h and 15 h. Only IL-8 was detected by ELISA in neutrophil culture supernatants at ≥1 h time points. Levels of IL-8 increased with time and in a WoLP concentration-dependent manner (Figure6).
Figure 6.
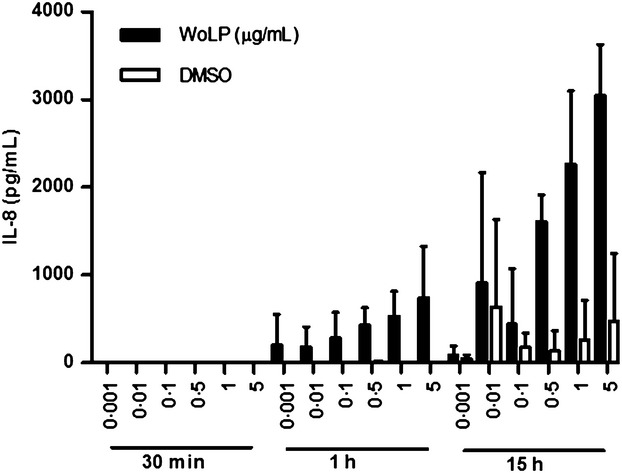
Isolated neutrophils secrete IL-8 upon stimulation with WoLP (0·001–5 μg/mL) in a concentration and time dependent manner. Bar graphs represent mean ± SD levels of IL-8 in culture supernatants of n = 3 donors each tested in duplicate.
Discussion
Neutrophils are pivotal effector cells in the pathogenesis of onchocerciasis and their presence in O. volvulus infected tissues depends upon the filarial endosymbiont Wolbachia. However, precisely how neutrophils are recruited and maintained at Onchocerca infection sites by Wolbachia has not been defined. This work investigated the role of the TLR2/6 ligand motif, WoLP, expressed within a surface filarial Wolbachia lipoprotein PAL, a major inflammatory molecule expressed by the endosymbiont, in the activation, chemotaxis and survival of isolated human neutrophils in vitro. We used a synthetically generated WoLP 20mer lipopeptide matching the N-terminus of wBmPAL in our study. The use of synthetic WoLP offers the advantage of scrutinising human neutrophil responses to dicylated Wolbachia molecules (matching the motif contained within native Wolbachia lipoproteins) rather than triacylated recombinant Wolbachia lipoproteins that are generated via expression vectors with fully intact lipolating biosynthetic pathways. This approach also nullifies the risk of inclusion of possible costimulatory effects of trace TLR ligands derived from expression systems that can contaminate recombinant preparations, as evaluated previously 20. The results obtained in this study indicate that the synthetic, diacylated analogue of the N-terminal polypeptide of Wolbachia PAL, abundantly expressed on the endosymbiont surface 20,22, exerts an activating effect on human neutrophils in vitro, as shown by changes in their cell shape and IL-8 production upon exposure to this stimulus. The analysis of cell surface adhesion molecules expression confirmed this activating effect: L-Selectin expression was downregulated while CD11b/CD18 integrins were upregulated. These results are in accordance with previous observations upon stimulation of neutrophils with ligands of TLR2/6 (MALP-2) but also TLR2/1 (Pam3CSK4) and TLR4 (LPS) 24–29. These functions appear directly mediated by TLR2 and TLR4 ligands 26,29, but are amplified in the presence of monocytes, at least where TLR4 stimulation is concerned 30. Of note, the TLR2/6 ligand MALP-1 had a 10-fold higher potency than TLR2/1 ligand Pam3CSK4 in activating neutrophils in vitro 29, comparable to that observed for activation of TLR2 dimers by WoLP 20. Shedding of surface L-Selectin was also reported in mouse neutrophils stimulated with isolated Wolbachia 8. WoLP was found to both prime and directly induce the production of ROS and to exert a chemotactic rather than a chemokinetic effect on isolated neutrophils. Moreover, WoLP was shown to induce a delay in neutrophil apoptosis. Contrasting results have been reported about the effect of TLR2 ligands on neutrophil apoptosis delay 26,29,31,32. Sabroe and coworkers suggested that apoptosis delay upon TLR2 stimulation was due to the induced production of cytokines with pro-survival activity by monocytes-contaminating neutrophil cultures 26,33. In our work, a role for WoLP-induced monocyte-derived pro-survival stimuli in the observed neutrophil apoptosis delay could not be completely excluded. However, the only measured cytokine that reached detectable levels in WoLP-stimulated neutrophil culture supernatants was IL-8, in concentrations compatible with a neutrophil, as opposed to a monocyte origin 26. IL-8 is a potent activator and chemoattractant of neutrophils, and therefore it is possible that this cytokine contributes to the WoLP-induced activation of neutrophils in a paracrine manner. Although the mechanism of chemotaxis of neutrophils towards Wolbachia is yet to be defined, the observed chemotactic response to WoLP in vitro may be reflective of a paracrine IL-8 gradient established following initial TLR2/6 ligation on the surface of neutrophils.
In conclusion, our data support an important role of the intrinsic filarial Wolbachia TLR2/6 motif expressed at the N-terminus of Wolbachia PAL, in mediating neutrophil recruitment from the blood to the skin and corneal sites of parasite infection. These are likely to be representative of neutrophil responses to both Wolbachia PAL and VirB6, due to the shared N-terminal motif 20. The interplay between parasite, host and Wolbachia in shaping neutrophil functions is intriguing. Clearly, neutrophils are recruited and activated around adult worms in a Wolbachia-dependent manner but they do not appear to be harmful to the parasite. It is possible that levels of whole Wolbachia or shed/excreted/secreted Wolbachia lipoproteins, such as PAL, slowly released by adult worms and their mf in nodular tissues would be sufficient to maintain chronic recruitment but not to fully activate macrofilaricidal responses. In comparison, the bolus release of Wolbachia and their lipoproteins after microfilaricidal drug therapy may more potently activate neutrophils, thus mediating onchocercal immunopathology. Nevertheless, defensins and calgranulins have been found on the surface of adult worms, suggesting neutrophil activation and degranulation occurs in living onchocercomas 34–36. This may indicate that living worms have a mechanism of deactivating the harmful effects of neutrophil granule-release products or are else inherently resistant to the toxic effects of neutrophil products. Indeed, several parasite molecules that neutralize host reactants, such as antioxidants and protease inhibitors, have been characterized 12. Far from being damaging to adult Onchocerca, a recent hypothesis has been proposed that the large infiltrate of neutrophils proximal to the nematode cuticle in onchocercomas may actually be beneficial to parasite survival. As such, neutrophil recruitment and release of neutrophil products adjacent to the nematode surface may form a bio-physical barrier blocking infiltration of more macrofilaricidal immune effector cells, such as eosinophils 13,14. Interestingly, it has been reported that binding of eosinophil peroxidase to human neutrophils in vitro leads to reversible inhibition of its peroxidase activity 37, and, in this regard, the recently discovered process of neutrophil death via extrusion of cellular DNA, called NETosis, deserves investigation 38,39. Finally, recent advances in the understanding of neutrophil functions illustrate that these cells are able to shape the adaptive immune response 40,41. Thus, Wolbachia and its surface lipoproteins may also influence the development of adaptive immune responses to different Onchocerca life cycle stages in humans via the activation and recruitment of neutrophils, a proposal that deserves further investigation.
Acknowledgments
This work was supported by the A•WOL Consortium through a grant of the Bill and Melinda Gates Foundation to Professor Mark J Taylor. Dr Helen L Wright was supported by Arthritis Research UK.
Author's Contribution
FT and HLW performed the experiments; FT, HLW, SWE, JDT and MJT designed the study; JDT and KLJ provided essential reagents; FT and HLW analysed the data; FT wrote the paper; all Authors revised the critically the data and the manuscript and approved the submitted final version of the manuscript.
References
- 1.Basanez MG, Pion SD, Churcher TS, Breitling LP, Little MP. Boussinesq M. River blindness: a success story under threat? PLoS Med. 2006;3:e371. doi: 10.1371/journal.pmed.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Onchocerciasis and its control. Report of a WHO Expert Committee on Onchocerciasis Control. World Health Organ Tech Rep Ser. 1995;852:1–104. [PubMed] [Google Scholar]
- 3.Tamarozzi F, Halliday A, Gentil K, Hoerauf A, Pearlman E. Taylor MJ. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin Microbiol Rev. 2011;24:459–468. doi: 10.1128/CMR.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillette-Ferguson I, Hise AG, McGarry HF, et al. Wolbachia-induced neutrophil activation in a mouse model of ocular onchocerciasis (river blindness) Infect Immun. 2004;72:5687–5692. doi: 10.1128/IAI.72.10.5687-5692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearlman E, Garhart CA, Grand DJ, Diaconu E, Strine ER. Hall LR. Temporal recruitment of neutrophils and eosinophils to the skin in a murine model for onchocercal dermatitis. Am J Trop Med Hyg. 1999;61:14–18. doi: 10.4269/ajtmh.1999.61.14. [DOI] [PubMed] [Google Scholar]
- 6.Pearlman E, Hall LR, Higgins AW, et al. The role of eosinophils and neutrophils in helminth-induced keratitis. Invest Ophthalmol Vis Sci. 1998;39:1176–1182. [PubMed] [Google Scholar]
- 7.Saint Andre A, Blackwell NM, Hall LR, et al. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002;295:1892–1895. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- 8.Gillette-Ferguson I, Daehnel K, Hise AG, et al. Toll-like receptor 2 regulates CXC chemokine production and neutrophil recruitment to the cornea in Onchocerca volvulusWolbachia-induced keratitis. Infect Immun. 2007;75:5908–5915. doi: 10.1128/IAI.00991-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillette-Ferguson I, Hise AG, Sun Y, et al. Wolbachia- and Onchocerca volvulus-induced keratitis (river blindness) is dependent on myeloid differentiation factor 88. Infect Immun. 2006;74:2442–2445. doi: 10.1128/IAI.74.4.2442-2445.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez-Pena EJ, Knab J. Buttner DW. Neutrophil granule proteins: evidence for the participation in the host reaction to skin microfilariae of Onchocerca volvulus after diethylcarbamazine administration. Parasitology. 1996;113(Pt 4):403–414. doi: 10.1017/s0031182000066543. [DOI] [PubMed] [Google Scholar]
- 11.Brattig NW, Buttner DW. Hoerauf A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 2001;3:439–446. doi: 10.1016/s1286-4579(01)01399-5. [DOI] [PubMed] [Google Scholar]
- 12.Brattig NW. Pathogenesis and host responses in human onchocerciasis: impact of Onchocerca filariae and Wolbachia endobacteria. Microbes Infect. 2004;6:113–128. doi: 10.1016/j.micinf.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Nfon CK, Makepeace BL, Njongmeta LM, Tanya VN, Bain O. Trees AJ. Eosinophils contribute to killing of adult Onchocerca ochengi within onchocercomata following elimination of Wolbachia. Microbes Infect. 2006;8:2698–2705. doi: 10.1016/j.micinf.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Hansen RD, Trees AJ, Bah GS, et al. A worm's best friend: recruitment of neutrophils by Wolbachia confounds eosinophil degranulation against the filarial nematode Onchocerca ochengi. Proc Biol Sci. 2011;278:2293–2302. doi: 10.1098/rspb.2010.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wildenburg G, Plenge-Bonig A, Renz A, Fischer P. Buttner DW. Distribution of mast cells and their correlation with inflammatory cells around Onchocerca gutturosa O. tarsicola O. ochengi, and O. flexuosa. Parasitol Res. 1997;83:109–120. doi: 10.1007/s004360050220. [DOI] [PubMed] [Google Scholar]
- 16.Cross HF, Haarbrink M, Egerton G, Yazdanbakhsh M. Taylor MJ. Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood. Lancet. 2001;358:1873–1875. doi: 10.1016/S0140-6736(01)06899-4. [DOI] [PubMed] [Google Scholar]
- 17.Keiser PB, Reynolds SM, Awadzi K, Ottesen EA, Taylor MJ. Nutman TB. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J Infect Dis. 2002;185:805–811. doi: 10.1086/339344. [DOI] [PubMed] [Google Scholar]
- 18.Turner JD, Mand S, Debrah AY, et al. A randomized, double-blind clinical trial of a 3-week course of doxycycline plus albendazole and ivermectin for the treatment of Wuchereria bancrofti infection. Clin Infect Dis. 2006;42:1081–1089. doi: 10.1086/501351. [DOI] [PubMed] [Google Scholar]
- 19.Njoo FL, Hack CE, Oosting J, Stilma JS. Kijlstra A. Neutrophil activation in ivermectin-treated onchocerciasis patients. Clin Exp Immunol. 1993;94:330–333. doi: 10.1111/j.1365-2249.1993.tb03452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner JD, Langley RS, Johnston KL, et al. Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J Biol Chem. 2009;284:22364–22378. doi: 10.1074/jbc.M901528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston KL, Wu B, Guimaraes A, Ford L, Slatko BE. Taylor MJ. Lipoprotein biosynthesis as a target for anti-Wolbachia treatment of filarial nematodes. Parasit Vectors. 2010;3:99. doi: 10.1186/1756-3305-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennuru S, Meng Z, Ribeiro JM, et al. Stage-specific proteomic expression patterns of the human filarial parasite Brugia malayi and its endosymbiont Wolbachia. Proc Natl Acad Sci USA. 2011;108:9649–9654. doi: 10.1073/pnas.1011481108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennuru S, Semnani R, Meng Z, Ribeiro JM, Veenstra TD. Nutman TB. Brugia malayi excreted/secreted proteins at the host/parasite interface: stage- and gender-specific proteomic profiling. PLoS Negl Trop Dis. 2009;3:e410. doi: 10.1371/journal.pntd.0000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi F, Means TK. Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 25.Neufert C, Pai RK, Noss EH, Berger M, Boom WH. Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J Immunol. 2001;167:1542–1549. doi: 10.4049/jimmunol.167.3.1542. [DOI] [PubMed] [Google Scholar]
- 26.Sabroe I, Prince LR, Jones EC, et al. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–5275. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 27.Seifert R, Schultz G, Richter-Freund M, et al. Activation of superoxide formation and lysozyme release in human neutrophils by the synthetic lipopeptide Pam3Cys-Ser-(Lys)4. Involvement of guanine-nucleotide-binding proteins and synergism with chemotactic peptides. Biochem J. 1990;267:795–802. doi: 10.1042/bj2670795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soler-Rodriguez AM, Zhang H, Lichenstein HS, et al. Neutrophil activation by bacterial lipoprotein versus lipopolysaccharide: differential requirements for serum and CD14. J Immunol. 2000;164:2674–2683. doi: 10.4049/jimmunol.164.5.2674. [DOI] [PubMed] [Google Scholar]
- 29.Wilde I, Lotz S, Engelmann D, et al. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med Microbiol Immunol. 2007;196:61–71. doi: 10.1007/s00430-006-0027-9. [DOI] [PubMed] [Google Scholar]
- 30.Sabroe I, Jones EC, Usher LR, Whyte MK. Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 31.Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA. Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005;174:3633–3642. doi: 10.4049/jimmunol.174.6.3633. [DOI] [PubMed] [Google Scholar]
- 32.Power CP, Wang JH, Manning B, et al. Bacterial lipoprotein delays apoptosis in human neutrophils through inhibition of caspase-3 activity: regulatory roles for CD14 and TLR-2. J Immunol. 2004;173:5229–5237. doi: 10.4049/jimmunol.173.8.5229. [DOI] [PubMed] [Google Scholar]
- 33.Prince LR, Allen L, Jones EC, et al. The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival. Am J Pathol. 2004;165:1819–1826. doi: 10.1016/s0002-9440(10)63437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallin MY, Jacobi AB, Buttner DW, Schonberger O, Marti T. Erttmann KD. Human autoantibody to defensin: disease association with hyperreactive onchocerciasis (sowda) J Exp Med. 1995;182:41–47. doi: 10.1084/jem.182.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgeworth JD, Abiose A. Jones BR. An immunohistochemical analysis of onchocercal nodules: evidence for an interaction between macrophage MRP8/MRP14 and adult Onchocerca volvulus. Clin Exp Immunol. 1993;92:84–92. doi: 10.1111/j.1365-2249.1993.tb05952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottsch JD, Eisinger SW, Liu SH. Scott AL. Calgranulin C has filariacidal and filariastatic activity. Infect Immun. 1999;67:6631–6636. doi: 10.1128/iai.67.12.6631-6636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zabucchi G, Menegazzi R, Cramer R, Nardon E. Patriarca P. Mutual influence between eosinophil peroxidase (EPO) and neutrophils: neutrophils reversibly inhibit EPO enzymatic activity and EPO increases neutrophil adhesiveness. Immunology. 1990;69:580–587. [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guimaraes-Costa AB, Nascimento MT, Wardini AB, Pinto-da-Silva LH. Saraiva EM. ETosis: a microbicidal mechanism beyond cell death. J Parasitol Res. 2012;2012:929743. doi: 10.1155/2012/929743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A, Cassatella MA, Costantini C. Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 41.Abi Abdallah DS, Egan CE, Butcher BA. Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol. 2011;23:317–326. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]


