Figure 4.
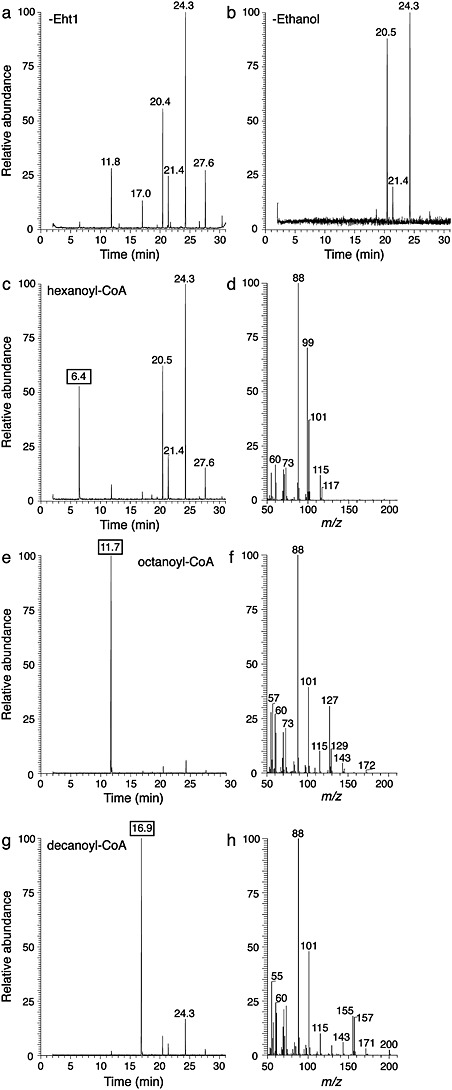
GC–MS of medium-chain FAEEs synthesized by Eht1 from acyl-CoA and ethanol. (a) Controls without Eht1 and (b) without ethanol show buffer background peaks. (c) In the full-treatment experiment combining Eht1, ethanol and hexanoyl-CoA, a modest GC peak was observed at 6.4 min. (d) MS of the 6.4 min peak gave the expected fragments for ethyl hexanoate (M+•, expected m/z = 144, weak signal; McLafferty rearrangement, m/z = 88; loss of ethoxide ion, m/z = 99; loss of ethyl group, m/z = 115; C3H5O2, m/z = 73; C5H9O2, m/z = 101). (e) GC and (f) MS of ethyl octanoate synthesized enzymatically from octanoyl-CoA and ethanol. The intense 11.7 min peak gave the expected fragments for ethyl octanoate (M+•, m/z = 172; McLafferty rearrangement, m/z = 88; loss of ethoxide ion, m/z = 127; loss of ethyl group, m/z = 143; C3H5O2, m/z = 73; C5H9O2, m/z = 101; C6H11O2, m/z = 115; C7H13O2, m/z = 129). (g) GC and (h) MS of ethyl decanoate synthesized enzymatically from decanoyl-CoA and ethanol. MS of the 16.9 min peak is characteristic of ethyl decanoate (M+•, m/z = 200; McLafferty rearrangement, m/z = 88; loss of ethoxide ion, m/z = 155; loss of ethyl group, m/z = 171; C5H9O2, m/z = 101; C6H11O2, m/z = 115)
