Abstract
Objectives
Parasitological confirmation of malaria prior to treatment is recommended for patients of all ages, with malaria rapid diagnostic tests (mRDTs) an important tool to target artemisinin-based combination therapies (ACTs) to patients with malaria. To evaluate the impact on case management practices of routine government implementation of mRDTs, we conducted large-scale health facility surveys in three regions of Tanzania before and after mRDT roll-out.
Methods
Febrile patients at randomly selected health facilities were interviewed about care received at the facility, and blood samples were collected for reference blood smears. Health facility staff were interviewed about their qualifications and availability of malaria diagnostics and drugs.
Results
The percentage of febrile patients tested for malaria at the facility increased from 15.8% in 2010 to 54.9% in 2012. ACTs were obtained by 65.8% of patients positive by reference blood smear in 2010 and by 50.2% in 2012 (P = 0.0675); no antimalarial was obtained by 57.8% of malaria-negative patients in 2010 and by 82.3% in 2012 (P < 0.0001). Overall, ACT use decreased (39.9–21.3%, P < 0.0001) and antibiotic use increased (31.2–48.5%, P < 0.0001).
Conclusion
Roll-out of mRDTs in Tanzania dramatically improved diagnostic testing for malaria and reduced overuse of ACTs for patients without parasitemia. However, post–roll-out almost 50% of febrile patients did not receive a diagnostic test, and almost 50% of patients testing positive did not receive ACTs. Stock-outs of ACTs and mRDTs were important problems. Further investigation is needed to determine reasons for not providing ACTs to patients with malaria and potential for inappropriate antibiotic use.
Keywords: malaria, malaria rapid diagnostic test, implementation, artemisinin-based combination therapie, Tanzania, drug stock-outs, antibiotic use
Introduction
Artemisinin-based combination therapies (ACTs) are the first-line drugs for malaria in most endemic countries, but there are concerns that targeting of ACTs to those in need remains poor. Many patients with malaria do not obtain ACTs, while many others with febrile illness obtain ACTs but do not have malaria parasitaemia (Whitty et al. 2008). Because microscopic testing of malaria has been limited in availability and is often of poor quality (McMorrow et al. 2008; Drakeley & Reyburn 2009; Kahama-Maro et al. 2011), it has been standard practice to diagnose malaria presumptively based on fever, resulting in over-treatment with antimalarials and under-treatment of non-malarial infections (Reyburn et al. 2004; D'Acremont et al. 2009). Such non-malarial febrile illnesses in a minority of cases are caused by bacterial infections but are most often due to self-limiting viral illnesses (Mtove et al. 2011; Leslie et al. 2012; McMorrow et al. 2012). Malaria rapid diagnostic tests (mRDTs) are thought to be an important tool to improve malaria diagnosis and targeting of ACTs (Murray et al. 2008; Drakeley & Reyburn 2009). Parasitological confirmation of malaria prior to treatment is now recommended for patients of all ages by World Health Organization (2010), with mRDTs an important part of the ‘T3: Test, Treat, Track’ initiative to ensure that every suspected malaria case is tested and every confirmed case is treated and tracked in a surveillance system (World Health Organization 2012). Decreases in the proportion of febrile illnesses associated with malaria in many settings (D'Acremont et al. 2010) have led commentators to stress the urgency of expanding mRDT coverage (D'Acremont et al. 2009), although important challenges to effective implementation have been highlighted (McMorrow et al. 2008; English et al. 2009).
Malaria rapid diagnostic test implementation has had a varied impact on clinical decisions. While some studies have demonstrated significant reductions in the proportion of patients obtaining an antimalarial drug (Williams et al. 2008; Msellem et al. 2009; Thiam et al. 2011; Yukich et al. 2012) or reductions in over-treatment of malaria (Kyabayinze et al. 2010; Bastiaens et al. 2011; D'Acremont et al. 2011; Masanja et al. 2012), others have reported under-utilisation of diagnostic tests (Hamer et al. 2007; Nyandigisi et al. 2011) and frequent antimalarial treatment of patients with negative test results (Hamer et al. 2007; Reyburn et al. 2007; Bisoffi et al. 2009; Skarbinski et al. 2009; Nyandigisi et al. 2011). This variability has been documented across studies within the same country. For example, in Tanzania, D'Acremont et al. (2011) demonstrated a reduction in over-treatment with antimalarials, while Reyburn et al. (2007) did not, and McMorrow et al. (2008) reported significant challenges in both microscopy and mRDT use under routine conditions. Another key concern is that increased mRDT use will lead to greater and often inappropriate use of antibiotics as clinicians are faced with unclear treatment decisions for mRDT-negative patients, with potentially adverse consequences for antibiotic resistance (Baiden et al. 2012).
Artemether–lumefantrine (ALu) has been the first-line drug for treatment of malaria in Tanzania since 2004, although roll-out to the public sector did not begin until 2006. Treatment at government hospitals, health centres and dispensaries is intended to be free of charge for children below 5 years old and pregnant women, although this policy is not always adhered to (Njau et al. 2006). In 2006, the National Malaria Control Programme revised policies favouring presumptive diagnosis of malaria to treatment based on parasitological confirmation for patients aged 5 years and above, with treatment based on clinical diagnosis permitted for children under age 5 years (United Republic of Tanzania Ministry of Health & Social Welfare 2006), although implementation remained limited in practice. In 2010, the policy was amended to include parasitological confirmation of suspected malaria cases for all ages (United Republic of Tanzania Ministry of Health & Social Welfare 2011), corresponding with the phased roll-out of mRDTs to all levels of government health facilities from 2009 to 2012.
While mRDT implementation has been studied in many settings, very few studies have reported evaluations of mRDT roll-out under routine operational conditions, where implementation has been purely the responsibility of the government (Bastiaens et al. 2011; Thiam et al. 2011; Masanja et al. 2012; Yukich et al. 2012). Two studies from Senegal and Zambia used routinely collected data to measure the impact on antimalarial consumption but did not assess the appropriateness of case management (Thiam et al. 2011; Yukich et al. 2012), while two studies in Tanzania examined very early results of mRDT roll-out in limited settings [two hospitals (Bastiaens et al. 2011) and one demographic surveillance site (Masanja et al. 2012)]. More commonly, study teams in these studies had participated in implementation to some degree, potentially influencing the findings and limiting their generalisability.
In this article, we report the impact on case management practices of routine government implementation of provision and use of mRDTs in Tanzania, based on large-scale health facility surveys conducted before and after mRDT roll-out. The surveys were conducted in three of mainland Tanzania's 21 regions, reflecting the country's diversity of malaria epidemiology and commodity availability, thereby allowing us to explore the impact of policy implementation under a range of contexts.
Methods
Context
This study was part of the IMPACT2 project, which took place in Mwanza, Mbeya and Mtwara regions of Tanzania, with populations of approximately 3 771 000, 2 822 000 and 1 375 000, respectively (National Bureau of Statistics (Tanzania) 2006). Each region includes a large municipality and smaller district towns, although the populations are predominantly rural. A greater percentage of the population is in the poorest national wealth quintile in Mtwara (35.5%), compared with Mwanza (20.8%) and Mbeya (7.7%) (National Bureau of Statistics (Tanzania) & ICF Macro 2011). According to the 2011–2012 HIV and Malaria Indicator Survey, Mwanza and Mtwara have moderately high malaria prevalence (18.6% and 17.4% among children 6–59 months of age), while Mbeya has low prevalence (0.5%) (Tanzania Commission for AIDS (TACAIDS) 2012). Malaria treatment in the public sector is provided by regional and district hospitals and a network of health centres and smaller dispensaries.
Roll-out of mRDTs took place in February 2011 in Mwanza and Mbeya, but not until May 2012 in Mtwara. A limited number of facilities in Mwanza and Mtwara had participated in small-scale mRDT pilots prior to roll-out. Roll-out involved training for 1 week of regional representatives and four representatives from each district, who then in turn trained one or two health workers from each public facility, usually the incharge clinician and laboratory technician or another health worker. The mRDT-specific training built upon earlier trainings on case management of malaria with ACTs, some of which had previously included instruction on performing mRDTs. Content included the rationale for using mRDTs, the mRDT algorithm for case management, reporting procedures for monitoring of mRDT use and the importance of compliance to test results. The trainings included demonstrations and practical exercises on performing mRDTs and reading results. Health workers receiving formal training were expected to pass on the information to colleagues who had not attended in a cascade-training approach. An initial stock of mRDTs was supplied to each facility, after which additional mRDTs could be ordered based on reported consumption.
Evaluation design and sample size
Baseline health facility surveys took place in 2010, during May–June in Mwanza, July–August in Mbeya and September–October in Mtwara. Post-implementation surveys followed in 2012 in Mwanza in April–May, Mbeya in May–June and Mtwara in June–July (Figure1).
Figure 1.
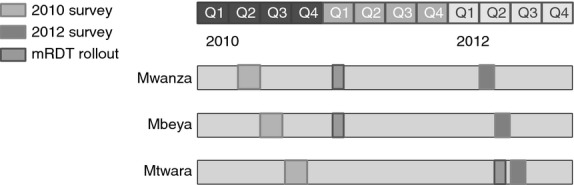
Timing of surveys and malaria rapid diagnostic test rollout.
The sample size for the study was calculated to detect a 15% absolute change in a composite indicator of appropriate treatment given true malaria parasitaemia status, as identified by reference blood smears collected by study staff. The composite indicator was defined as the proportion of patients with fever or history of fever who had a malaria-positive reference blood smear and received ACT at the health facility, or had a malaria-negative reference blood smear and did not receive any antimalarial. Assuming 5% significance, 80% power, a baseline proportion receiving appropriate treatment of 65%, 5% refusal and a design effect of 2.5, 338 patients with fever or history of fever per study region were required in each survey round.
Data collection
In each region, 35 health facilities in 2010 and 60 in 2012 were randomly selected with probability proportional to malaria outpatient utilisation, based on the most recent data available for all government health facilities. Due to slower than expected recruitment in 2010, additional facilities were added from a list of randomly selected alternates for each region, and the total number of facilities selected in 2012 was increased. A similar number of patients were sampled at each facility, resulting in an approximately self-weighting survey sample. Each facility was visited by the study team for 1 day. Patients presenting for outpatient care on the day of the study team's visit with fever or history of fever in the previous 48 hours were enrolled upon arrival at the health facility, subject to informed consent having been obtained. To achieve sufficient sample size, facilities were replaced with randomly selected alternate facilities of similar size if no eligible patients attended the facility on the day of the study team's visit. In 2010, the first 12 eligible patients under five and the first 12 eligible patients aged five and above were enrolled. Because this target was not met at most facilities in 2010 and additional randomly selected facilities had to be added, the protocol was adjusted in 2012 to enrol all patients meeting eligibility criteria at each facility during daytime operating hours.
After completion of their consultations with facility staff, patients were interviewed by research staff about demographic information, previous treatment for fever and care received at the facility, including blood tests for malaria conducted as part of the consultation (facility tests) and drugs obtained. Health facility staff seeing enrolled patients were interviewed about their qualifications, training, knowledge, and availability of antimalarial drugs, mRDTs, microscopy and antibiotics.
Definitions
Facilities were considered to have mRDTs in stock if the study team observed at least one full box containing 25 non-expired mRDTs, and facilities were considered to have microscopy upon observation of a functional microscope, slides and Giemsa stain for at least 25 smears, and a microscopist. Facilities were considered to have ALu in stock if they had had at least one observed, non-expired blister pack for any age/weight group. Antibiotics were considered to be in stock if any non-expired oral antibiotics were observed.
Finger prick blood samples were taken from enrolled patients by study staff to test for malaria parasitaemia by study mRDTs (ICT Diagnostics, Cape Town, South Africa) and thick blood smears (reference blood smears) stained with 10% Giemsa. Reference blood smears were double-read at the Ifakara Health Institute in Bagamoyo by two microscopists blinded to the initial reading and patients' study mRDT results, with discrepant readings read by a third microscopist. Parasites were counted against 200 white blood cells, and slides were considered negative if no parasites were found after examining 100 fields.
Ethics
All questionnaires and consent forms were translated into Swahili and piloted by native speakers. The study protocol was approved by the ethical review boards of the Ifakara Health Institute and the London School of Hygiene and Tropical Medicine. CDC investigators provided technical assistance in design and analysis but were not engaged in data collection. Written informed consent was obtained from health workers and patients or their caregivers prior to enrolment. Patients who tested positive by the study mRDT and had not already received ACT were given the weight-appropriate blister pack by study staff. Pregnant women in the first trimester and children below 5 kg who tested positive were referred back to the health facility incharge for treatment decisions.
Statistical analysis
Data were collected using personal digital assistants (PDAs) and were analysed in Stata 11.0 (Stata Corporation, College Station, USA) using survey commands to account for the two-stage survey design with stratification by region. Percentages with 95% confidence intervals and p-values for the Pearson design-based F-test were reported where applicable, with significance defined as P < 0.05.
Results
We visited 140 health facilities (11 hospitals, 24 health centres and 105 dispensaries) between May and October 2010, and 176 health facilities (13 hospitals, 31 health centres, 132 dispensaries) between April and July 2012.
Availability of diagnostics and drugs
In 2010, only 3.3% of health facilities had mRDTs in stock, while 10.9% had microscopy and 11.8% had either mRDTs or microscopy available (Figure2). In 2012, mRDTs were in stock in 69.0% of health facilities, with microscopy available at 16.7% and either diagnostic test available at 73.9%. The percentage of facilities with any ALu in stock was similar in both years at 77.8% in 2010 and 78.4% in 2012. All four age/weight blister packs were in stock in only 41.1% of health facilities in 2010 and 27.3% in 2012. Stock-outs of all four packs at the same time in the previous 3 months had been experienced by 35.5% of facilities in 2010 and 41.6% in 2012. During both surveys, stock-outs of ALu were most severe in Mwanza, which also faced the most mRDT stock-outs in 2012. At least one oral antibiotic (mainly cotrimoxazole and amoxicillin) was in stock at 93.1% of health facilities in both 2010 and 2012, with little difference by region.
Figure 2.
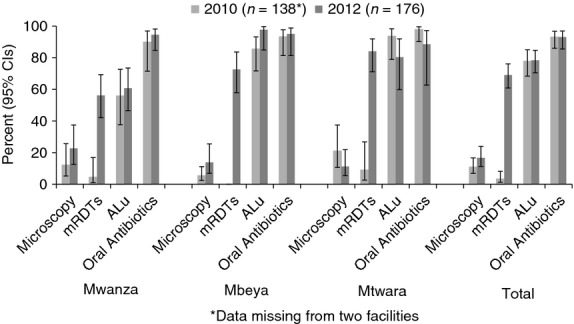
Availability of malaria diagnostics, artemether–lumefantrine and oral antibiotics in health facilities in 2010 (pre-roll-out) and 2012 (post-roll-out).
Health worker characteristics and training
We interviewed 154 health workers in 2010 and 209 in 2012. While most health worker characteristics remained similar between 2010 and 2012 (Table1), the percentage of health workers who had attended a formal in-service training on mRDTs (including training on ACTs where health workers were taught how to use mRDTs) increased from 6.5% in 2010 to 59.4% in 2012. Mtwara, the most recent region to receive mRDT roll-out, had the highest percentage of mRDT-trained health workers (79.0%), compared with 49.3% in Mwanza and 58.8% in Mbeya.
Table 1.
Health worker characteristics (Percent, 95% Confidence interval)
| Health workers with interview data (N) | Mwanza | Mbeya | Mtwara | Total | ||||
|---|---|---|---|---|---|---|---|---|
| 2010 | 2012 | 2010 | 2012 | 2010 | 2012 | 2010 | 2012 | |
| 41 | 71 | 64 | 72 | 49 | 66 | 154 | 209 | |
| Age (years) | ||||||||
| 25-34 | 15.1 (5.2, 36.9) | 34.3 (23.7, 46.8) | 29.2 (14.7, 49.7) | 31.7 (19.9, 46.3) | 10.5 (4.2, 23.7) | 21.4 (10.9, 37.7) | 20.7 (12.6, 32.0) | 30.6 (23.6, 38.7) |
| 35-44 | 32.5 (17.9, 51.6) | 15.7 (8.4, 27.4) | 19.8 (9.6, 36.5) | 9.2 (4.1, 19.5) | 23.7 (11.4, 43.0) | 19.6 (10.1, 34.6) | 24.7 (16.6, 35.2) | 14.5 (9.8, 20.9) |
| 45 and older | 52.4 (34.4, 69.8) | 50.0 (37.4, 62.5) | 51.0 (30.2, 71.3) | 59.1 (45.0, 71.9) | 65.8 (46.4, 81.1) | 59.0 (42.2, 73.9) | 54.7 (42.4, 66.4) | 55.1 (47.4, 62.6) |
| Male sex | 71.3 (51.9, 85.1) | 65.8 (54.4, 75.6) | 47.5 (27.6, 68.2) | 49.3 (34.9, 63.8) | 39.8 (23.3, 59.0) | 43.2 (26.9, 61.0) | 53.4 (40.8, 65.6) | 52.9 (45.2, 60.4) |
| Qualifications | ||||||||
| Medical or Clinical Officer | 55.8 (37.4, 72.8) | 70.1 (55.9, 81.2) | 41.9 (24.1, 62.1) | 40.9 (27.5, 55.8) | 54.9 (33.8, 74.4) | 36.3 (22.9, 52.1) | 49.1 (37.0, 61.4) | 52.7 (43.3, 61.8) |
| Nurse, midwife or assistant clinical officer | 39.3 (22.9, 58.5) | 27.2 (16.8, 40.9) | 28.7 (14.6, 48.4) | 46.8 (32.5, 61.7) | 36.5 (17.6, 60.7) | 50.5 (34.0, 66.9) | 33.7 (23.3, 46.1) | 39.0 (30.4, 48.4) |
| Assistant or attendant | 4.9 (1.0, 20.7) | 2.7 (0.7, 9.9) | 29.5 (10.6, 59.7) | 12.3 (6.1, 23.0) | 8.6 (3.0, 22.2) | 13.2 (6.1, 26.3) | 17.1 (7.1, 36.0) | 8.3 (5.2, 13.1) |
| Number of patients seen on a regular day | ||||||||
| 15 or less | 27.0 (13.7, 46.2) | 29.7 (17.9, 45.0) | 70.9 (54.0, 83.5) | 49.9 (35.5, 64.2) | 31.3 (16.8, 50.6) | 23.3 (12.8, 38.8) | 48.3 (36.4, 60.5) | 34.8 (26.6, 43.9) |
| 16-30 | 50.5 (32.7, 68.1) | 53.6 (40.2, 66.5) | 25.3 (14.1, 41.2) | 38.3 (25.6, 52.9) | 63.2 (43.6, 79.2) | 64.0 (47.8, 77.5) | 41.6 (30.7, 53.3) | 51.1 (42.7, 59.5) |
| More than 30 | 22.5 (11.5, 39.6) | 16.7 (6.6, 36.2) | 3.8 (1.6, 8.9) | 11.8 (5.8, 22.6) | 5.6 (1.5, 18.9) | 12.7 (6.0, 24.8) | 10.1 (6.0, 16.6) | 14.1 (8.3, 23.2) |
| Attended formal training on ACTs in the last 5 years | 70.3 (51.4, 84.2) | 47.3 (34.7, 60.2) | 40.2 (23.2, 60.0) | 61.1 (47.1, 73.4) | 77.1 (60.9, 88.0) | 73.6 (55.1, 86.3) | 57.8 (44.6, 70.0) | 58.0 (49.5, 66.0) |
| Attended formal training on how to use mRDTs in the last 5 years* | 8.1 (2.3, 25.9) | 49.3 (33.6, 65.2) | 3.8 (0.7, 18.4) | 58.8 (44.5, 71.7) | 9.8 (3.6, 24.4) | 79.0 (60.0, 90.5) | 6.5 (3.0, 13.5) | 59.4 (49.4, 68.7) |
| Received supervision on ACTs in the last six months | 31.8 (17.5, 50.6) | 30.8 (20.0, 44.2) | 19.1 (9.8, 33.9) | 29.1 (18.0, 43.4) | 48.2 (29.1, 67.9) | 19.2 (10.1, 33.3) | 29.5 (20.9, 39.8) | 27.5 (20.7, 35.5) |
| Reported having ACT job aid in consultation room | 78.4 (59.3, 90.1) | 62.2 (49.3, 73.6) | 64.1 (37.7, 84,1) | 66.4 (52.5, 78.0) | 88.6 (74.2, 95.4) | 77.7 (63.3, 87.6) | 74.0 (58.0, 85.4) | 67.3 (59.5, 74.3) |
| Correctly identified correct dosage regimen of ACT for a 10 kg child | 65.9 (47.4, 80.6) | 92.7 (83.6, 96.9) | 81.9 (66.4, 91.2) | 92.0 (81.4, 96.8) | 87.1 (71.2, 94.8) | 90.9 (79.3, 96.3) | 77.9 (68.3, 85.3) | 92.0 (86.9, 95.2) |
| Correctly identified correct dosage regimen of ACT for an adult | 96.0 (75.7, 99.5) | 98.5 (89.5, 99.8) | 100.0 | 98.4 (89.4, 99.8) | 100.0 | 90.0 (63.6, 97.9) | 98.7 (91.2, 99.8) | 96.4 (89.2, 98.9) |
Includes formal training on ACTs where health workers were taught how to use mRDTs.
Patient characteristics
Of 1746 patients interviewed in 2010, 59.2% were under 5 years old, while in 2012, 66.2% of 1710 patients were under-fives (Table2). A lower percentage of patients had sought care at another source prior to coming to the study health facility in 2010 compared with 2012 (18.8% versus 32.4%, P < 0.0001). There was no evidence of change from 2010 to 2012 in the percentage testing positive by study mRDT in any of the three regions or overall (20.8% and 22.9%, respectively, P = 0.4), but the percentage of children over age five and adults testing positive by study mRDT increased from 17.0% in 2010 to 24.3% in 2012 (P = 0.0116). In Mwanza and Mbeya, there was no evidence of change in the percentage with a positive reference blood smear, but in Mtwara, there was an increase from 20.9% in 2010 to 31.8% in 2012 (P = 0.0048), primarily reflecting the significant increase in parasitaemia among older children and adults.
Table 2.
Patient characteristics (Percent, 95% Confidence interval)
| Mwanza | Mbeya | Mtwara | Total | |||||
|---|---|---|---|---|---|---|---|---|
| 2010 | 2012 | 2010 | 2012 | 2010 | 2012 | 2010 | 2012 | |
| Patients with interview data (N) | 689 | 750 | 559 | 388 | 498 | 572 | 1746 | 1710 |
| Percent below 5 years | 58.8 (51.4, 65.8) | 68.5 (63.0, 73.6) | 55.1 (48.1, 61.9) | 63.7 (58.0, 69.0) | 64.3 (58.5, 69.6) | 64.9 (59.7, 69.7) | 59.2 (55.1, 63.1) | 66.2 (63.0, 69.2) |
| Male sex | 43.4 (38.8, 48.1) | 43.6 (40.2,47.1) | 42.6 (38.0, 47.3) | 43.0 (38.6, 47.6) | 44.0 (40.4, 47.6) | 41.6 (37.6, 45.7) | 43.3 (40.8, 45.9) | 42.8 (40.6, 45.1) |
| Sought care at another source before coming to health facility | 30.8 (26.7, 35.2) | 38.4 (34.5, 42.5) | 10.6 (7.4, 14.9) | 30.2 (24.4, 36.6) | 11.5 (7.2, 17.7) | 25.9 (20.6, 31.9) | 18.8 (16.3, 21.5) | 32.4 (29.5, 35.4) |
| Can reach health facility within one hour | 69.1 (63.8, 74.0) | 72.4 (67.4, 76.9) | 74.8 (69.3, 79.6) | 69.7 (62.6, 76.0) | 68.5 (61.1, 75.0) | 65.1 (59.8, 70.1) | 70.8 (67.4, 74.0) | 69.3 (66.1, 72.3) |
| Slept under insecticide-treated net* last night | 81.7 (77.5, 85.2) | 88.3 (85.1, 91.0) | 75.8 (71.7, 79.5) | 79.5 (74.8, 83.5) | 81.9 (77.4, 85.7) | 84.5 (80.8, 87.6) | 80.0 (77.5, 82.1) | 85.1 (83.0, 87.0) |
| Median days since fever onset | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Percent testing positive by study mRDT† | 21.3 (16.6, 26.9) | 14.4 (9.8, 20.8) | 5.9 (3.5, 9.9) | 6.5 (3.3, 12.1) | 37.0 (29.8, 44.7) | 45.1 (38.6, 51.8) | 20.8 (17.7, 24.4) | 22.9 (19.5, 26.7) |
| Percent testing positive by study blood smear‡ | 6.6 (4.4, 9.8) | 10.7 (7.1, 15.7) | 1.6 (0.7, 3.5) | 4.8 (2.2, 10.0) | 20.9 (16.4, 26.3) | 31.8 (27.1, 36.8) | 9.2 (7.4, 11.4) | 16.5 (13.9, 19.4) |
A long-lasting insecticide-treated net (LLIN) obtained within the previous 3 years or a conventional net treated with insecticide in the previous year.
N = 1737 in 2010 and 1707 in 2012 due to 12 interviewed patients without study RDT results.
N = 1649 in 2010 and 1687 in 2012 due to 120 interviewed patients with missing blood smears.
Use of malaria diagnostics
The results show a large increase in the use of malaria diagnostics post-implementation (Figure3). In 2010, 7.2% of febrile patients in Mwanza, 12.7% in Mbeya and 31.3% in Mtwara were tested for malaria by health workers at the facility, with almost all of these patients tested by microscopy. By 2012, this had increased to 48.4% in Mwanza, 43% in Mbeya and 71.5% in Mtwara, which had most recently received mRDT roll-out. If the analysis for 2012 is restricted to facilities with diagnostics in stock, the percentage tested increases to 67.4% in Mwanza, 49.0% in Mbeya and 82.1% in Mtwara. In both surveys, children under 5 years old were no more likely to be tested than older children and adults. The majority of malaria tests in 2012 were mRDTs (80.5%). Facility tests were positive for almost half of febrile patients in Mtwara (46.5%), compared with 22.8% in Mwanza and 11.4% in Mbeya. Overall, sensitivity and specificity of facility blood smears compared with reference blood smears in 2012 were 70.6% and 63.7%, respectively, with a positive predictive value of 24.5% and a negative predictive value of 92.9% (n = 119 patients). Sensitivity and specificity of facility mRDTs compared with reference blood smears were 91.3% and 88.0%, respectively, with a positive predictive value of 69.3% and a negative predictive value of 97.3% (n = 746).
Figure 3.
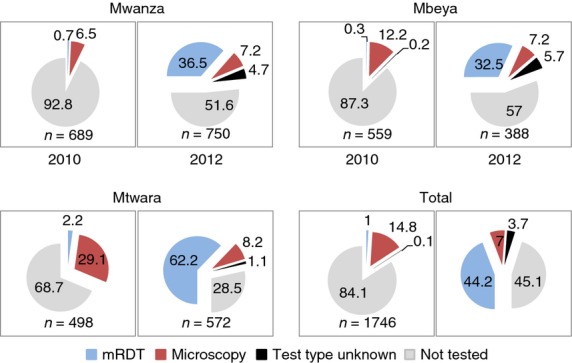
Percent of patients receiving malaria diagnostic test at health facility pre and post malaria rapid diagnostic test roll-out.
Treatment according to facility test results
We present results on treatment obtained firstly according to the facility test and secondly according to the reference blood smear. Results in relation to the facility test are presented for 2012 only, as few facility tests were conducted in 2010. As explained above, we define appropriate antimalarial treatment for patients with positive test results as obtaining an ACT and for those with negative results as not obtaining an antimalarial. Only three women in 2010 and three women in 2012 reported being in the first trimester of pregnancy and had a positive reference blood smear, and of these, only one in 2010 received any treatment (quinine). Due to their negligible numbers, these women were not excluded from the analysis.
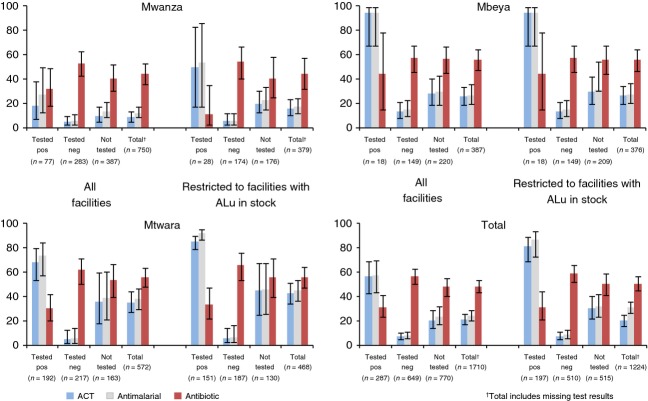
In 2012, 56.5% of patients testing positive by the facility test obtained an ACT, while 7.7% of those testing negative and 23.6% of those not tested obtained any antimalarial (Figure4). In comparison, 25.2% of those testing negative in 2010 obtained any antimalarial (denominator of n = 131), although very few patients overall were tested. While the percentage of patients in 2012 testing negative who obtained any antimalarial was low in all three regions, the percentage testing positive that obtained ACT varied from 18.2% in Mwanza to 68.2% in Mtwara and 94.6% in Mbeya. Older children and adults testing positive were more likely to obtain an ACT than children under five who tested positive (67.7% and 50.3%, respectively, P = 0.0026). Considering only facilities where ALu was in stock, 81.2% of patients testing positive by the facility test obtained an ACT, and of those who did not, 70.3% did not obtain any antimalarial, while 29.7% were treated with quinine. Of patients testing negative and of those not tested at facilities with ALu in stock, 8.6% and 32.0%, respectively, obtained any antimalarial. In both surveys, almost all antimalarials obtained were ACTs (92.2% in 2010 and 88.4% in 2012), and almost all ACTs were ALu. In 2012, antibiotics were obtained by 31.5% of those positive for malaria by the facility test, 56.9% of those testing negative and 47.9% of those not tested.
Figure 4.
Treatment obtained at health facilities according to facility malaria test (microscopy or mRDT) post-roll-out (Percent, 95% CIs).
Treatment according to reference blood smear results
We assessed treatment obtained against reference blood smear results in 2010 and 2012 (Figure5). Based on reference blood smears, the composite indicator of appropriate treatment increased from 58.5% in 2010 to 76.8% (P < 0.0001). Mwanza appeared to perform very well by this composite indicator (80.0% in 2012), although to a large degree this reflected patients testing negative being unable to obtain ACTs at facilities where they were not in stock. To address this concern, the analysis was restricted to facilities where ALu was in stock, showing an overall improvement in the percentage of patients obtaining appropriate treatment according to the reference blood smear from 47.3% in 2010 to 76.4% in 2012 (P < 0.0001).
Figure 5.
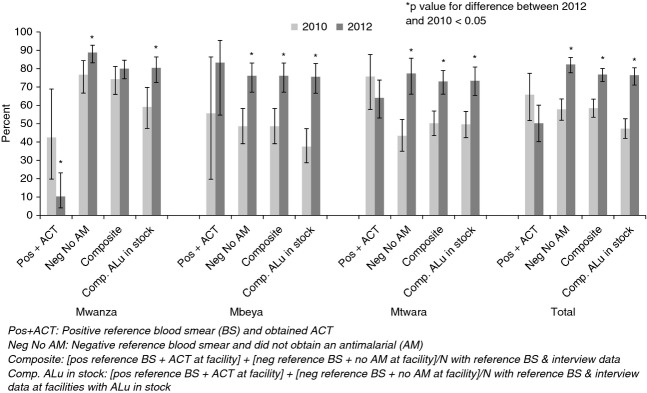
Patients receiving correct treatment according to parasitaemia status by study blood smear pre and post mRDT roll-out.
As reported above for the facility tests, we also report appropriate treatment for patients with positive reference blood smears and negative reference blood smears separately. ACTs were obtained by 65.8% of patients testing positive in 2010 (denominator of n = 152) and 50.2% in 2012 (n = 287) (P = 0.0675), while no antimalarial was obtained by 57.8% of patients testing negative in 2010 and 82.3% in 2012 (P < 0.0001). In 2012, but not 2010, older children and adults with a positive reference blood smear were more likely to obtain ACTs than children under five (45.0% and 60.0%, respectively, P = 0.0176). Considering only facilities where ALu was in stock, ACTs were obtained by 82.6% of patients of all ages testing positive in 2010 (n = 121) and 71.8% in 2012 (n = 188) (P = 0.0756), while no antimalarial was obtained by 43.2% of patients testing negative in 2010 and 77.3% in 2012 (P < 0.0001). In Mwanza, the percentages of patients positive by reference blood smear (n = 40 in 2010 and 78 in 2012) who obtained ACT were very low both years, especially in 2012 (42.5% and 10.3%, respectively), with little improvement observed when considering only facilities with ALu in stock (73.9% and 28.6%, respectively, n = 23 in 2010 and 21 in 2012) (P < 0.0161).
Overall use of ACTs and antibiotics
The consequences for overall levels of ACT and antibiotic treatment are shown in Figure6. Overall, the percentages of patients obtaining ACT decreased significantly (39.9% to 21.3%, P < 0.0001) between 2010 and 2012, while the proportion obtaining an antibiotic increased significantly (31.2% to 48.5%, P < 0.0001). The same pattern occurs in all three regions and for both patients under and over 5 years of age. The percentage obtaining both ACT and antibiotics did not change overall between 2010 and 2012 (11.7% and 8.3%, P = 0.10). Compared to all patients in 2010, antibiotic use was higher in 2012 not only in the subgroup testing negative by the facility test, but also in the subgroup not tested (Figure4).
Figure 6.
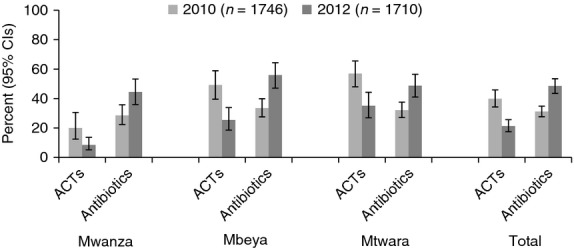
Patients receiving ACT and antibiotic by region pre and post mRDT roll-out.
Discussion
We have presented results from large-scale health facility surveys in three regions of Tanzania before and after the roll-out of mRDTs in all levels of government health facilities. We have reported on the availability and use of diagnostic testing among febrile patients and case management with ACTs based on facility test results and reference blood smears, and we have also examined the effect of this policy change on prescription of antibiotics. The findings are representative of Mwanza, Mbeya and Mtwara Regions, which encompass considerable diversity in malaria transmission, economic status and culture.
Malaria rapid diagnostic test roll-out in Tanzania led to substantial changes in the provision of malaria case management in public facilities. Post-roll-out nearly 70% of facilities had mRDTs in stock and 60% of health workers had received formal mRDT training. This was associated with a large increase in the proportion of febrile outpatients tested for malaria from 15.5% in 2010 to 54.9% in 2012, and facility mRDT testing was shown to have relatively good sensitivity and specificity. However, there were still major gaps in diagnostic coverage, with almost half of all patients not tested. In Mtwara, the proportion of patients tested (71.5%) and the proportion of health workers with formal mRDT training (79.0%) were higher than in Mwanza and Mbeya, reflecting the more recent roll-out, and therefore, lower potential for training coverage to be eroded by staff turnover or for mRDT stock-outs to arise. The latter was an important factor in suboptimal diagnostic coverage, with the per cent tested increasing to 70% if only facilities with diagnostics available were considered. Other studies have similarly reported less-than-optimal coverage of diagnostic tests (Hamer et al. 2007; Nyandigisi et al. 2011; Masanja et al. 2012). In addition to poor diagnostic availability (Ezeoke et al. 2012), reasons for poor coverage may include inadequate health worker training, negative health worker perceptions of mRDTs, provider workload or patient preferences (Chandler et al. 2008a; Williams et al. 2008; Asiimwe et al. 2012; Baiden et al. 2012). Health centre and dispensary patients paid a flat rate for treatment, so patient willingness-to-pay for tests is not expected to have been a factor in these facilities, but may have contributed in hospital settings where separate fees were sometimes charged for diagnostics.
Provider compliance with test results in terms of prescription has been a major concern in the roll-out of improved services for parasitological confirmation. Reported reasons for low compliance include providers' or patients' lack of confidence in the test result, the presence of symptoms associated with malaria, and the lack of alternative diagnoses identified by the providers or accepted by the patients (Chandler et al. 2008a,b, 2010; Asiimwe et al. 2012; Ezeoke et al. 2012). While some studies have reported poor compliance with negative test results (Hamer et al. 2007; Reyburn et al. 2007; Bisoffi et al. 2009; Nyandigisi et al. 2011). our findings correspond with other studies that have demonstrated a substantial reduction in antimalarial treatment after implementation of mRDTs (Williams et al. 2008; Msellem et al. 2009; Kyabayinze et al. 2010; Bastiaens et al. 2011; D'Acremont et al. 2011; Thiam et al. 2011; Masanja et al. 2012; Yukich et al. 2012). Our results show that treatment of patients with a negative facility test in 2012 was less than one in ten, even in facilities with ALu in stock. The proportion of patients negative by reference blood smear who did not receive any antimalarial increased significantly from 57.8% in 2010 to 82.3% in 2012.
The reduction in ACT provision for patients who were not parasitemic led to a significant increase in the composite indicator of patients appropriately treated of 18 percentage points including all facilities and of 29 percentage points considering only those facilities with ALu in stock. However, while over-prescription of ACT was substantially reduced, under-prescription remained a major problem. Only 56.5% of patients with a positive facility test obtained an ACT in 2012, with particularly poor results in Mwanza, where only 18.2% of positive patients obtained ACT. There was weak evidence of a decrease in the proportion of patients positive by reference blood smear who obtained ACT between 2010 and 2012 overall, and a significant fall in Mwanza, although the percentages of patients testing positive overall were low (9.2% in 2010 and 16.5% in 2012) resulting in small subsamples and large confidence intervals. Poor ACT availability was a major factor in the under-treatment of patients testing positive. ACT stock-outs were present in all regions but especially severe in Mwanza, with around 40% of facilities experiencing complete ACT stock-outs at the time of both surveys. Even at facilities where ALu was in stock, nearly 20% of patients overall with a positive facility test result did not obtain ACTs. Fear of future stock-outs prompting drug rationing or the absence of the appropriate weight-specific blister packs may have discouraged health workers from dispensing ACT to some patients testing positive by the facility test (Wasunna et al. 2008). Again at health centres and dispensaries, this is unlikely to be due to patients not wanting to pay for ACTs as flat fees for consultations were generally charged, but willingness-to-pay could have been a factor in hospitals which sometimes charged separately for drugs. These results are in contrast to other studies that found more than 90% compliance with positive test results (Hamer et al. 2007; McMorrow et al. 2008; Williams et al. 2008; Bisoffi et al. 2009; Msellem et al. 2009; Kyabayinze et al. 2010; D'Acremont et al. 2011), perhaps reflecting the greater risk of ACT stock-outs and more limited health worker training coverage and supervision under the routine, operational conditions we evaluated. Another study evaluating early effects of mRDT roll-out in one Tanzanian district, found that in facilities with ACT in stock, 20% of patients testing positive did not receive ACT, similar to results we present here (Masanja et al. 2012).
While the proportion of patients who obtained an ACT significantly decreased from 2010 to 2012, the proportion of patients receiving antibiotics significantly increased to just under half of all patients. An increase in antibiotic prescription after mRDT introduction was also reported by two other studies in mainland Tanzania (Bastiaens et al. 2011; D'Acremont et al. 2011) and one in Zanzibar (Msellem et al. 2009). No specific guidelines were given to health workers on when to prescribe antibiotics as part of mRDT training, but this increase might be expected, given that health workers may be more likely to consider a bacterial diagnosis following negative malaria test results. However, we also observed a significant increase from 31.3% to 47.9% in antibiotic provision among patients not tested between 2010 and 2012. The reason for this is unclear, as antibiotic availability was similarly high during both surveys, and data were not collected on symptoms warranting antibiotic prescription. While data on the aetiology of non-malarial fevers are still limited, a study of children with non-severe febrile illness in northern Tanzania found that while a bacterial pathogen was identified from blood culture in only 0.9% of children, the WHO criteria for pneumonia were met in 48% (Mtove et al. 2011). The latter is considered an indication for antibiotics although pneumonia may also be caused by a virus. In Pakistan, a trial assessed withholding of antibiotics among children with fast breathing and did not find a difference in clinical outcomes, suggesting that bacterial pneumonia may be over-diagnosed (Hazir et al. 2011). To avoid substituting overtreatment with antimalarials with overtreatment with antibiotics, there is an urgent need for better diagnostics of non-malarial fevers and improved training on case management when patients test negative or when diagnostic tests are not available.
This study has several limitations. The sampling probability of health facilities was based on the most recent malaria outpatient data available, which may have suffered from some inaccuracies. Health workers may have been more likely to perform diagnostic tests and less likely to dispense antimalarials to negative patients as a result of the team's presence. To reduce this possible source of bias, the study team emphasised to health workers the importance of following their normal procedures. Community members may also have been drawn to the facility because of the presence of the study team in hopes of obtaining drugs for both sick household members and as a reserve for future periods of drug stock-outs. Research assistants screened all patients arriving at the facility carefully, but it is possible that some patients may not have had a true illness.
The 2012 survey took place slightly earlier in the year than the 2010 survey. The peaks in malaria incidence in Tanzania usually occur just after the short rainy season in November–December and just after the long rainy season in March–May, which may have meant that in 2010, Mtwara was visited after the peak, and in 2012, Mwanza was visited before it fully developed. However, taking into account variable weather patterns, late rains in 2010, and lack of entomological data, it is not clear that this would have affected parasitaemia prevalence.
Finally, care should be taken in interpreting facility-level data in isolation, as improvements seen at government health facilities may not necessarily translate to an increase in coverage at the community level. IMPACT2 household surveys in the same three regions in 2010 and 2012 similarly showed that among patients visiting public health facilities for fever, there was a significant increase in the proportion receiving a diagnostic test (from 28.7% to 46.6%), and a significant decrease in the proportion obtaining ACTs (from 57.4% to 46.1%) (Thomson et al., in draft). However, the household surveys showed no significant change overall in the proportion of patients reporting fever who recalled having a diagnostic test pre- and post-mRDT roll-out (Thomson et al., in draft). This appears to have reflected a reduction in the use of public facilities from 25.3% to 16.8% of reported fevers; as diagnostic coverage was much lower in the private sector, no overall increase in parasitological confirmation at the community level was observed despite the mRDT roll-out in the public sector.
Tanzania has made major strides in scaling up access to diagnostic testing for malaria within public health facilities and reducing overuse of ACTs by patients without parasitaemia. However, this study has also demonstrated the dramatically negative role that ACT and mRDT stock-outs continue to play in malaria case management. Several initiatives are underway in Tanzania to improve public sector commodity supply, including text-based stock-out reporting systems (Barrington et al. 2010), strengthening of zonal distribution capacity and direct delivery to health facilities, but this continues to be a major challenge. Maintaining buffer supplies of drugs and preventing leakage to the private sector may also potentially reduce stock-outs, as well as ensuring that mRDTs are in stock and appropriately utilised.
Priorities for further research include investigating reasons for under-prescription of ACTs for patients testing positive, and high levels of antibiotic prescription, and the potential effect on antibiotic resistance. As countries increasingly implement policies of universal testing prior to treatment for malaria, it will be important to address these concerns in order to improve case management for febrile illnesses and appropriately target ACTs.
Acknowledgments
The authors would like to thank Adam Wolkon for training and advice on PDA programming and Eugenie Poirot and Bhargavi Rao for assistance during training, piloting and start of field work. Many thanks to the field teams, regional and district health officers and study participants. This work was funded by the Bill and Melinda Gates Foundation, through the ACT Consortium.
References
- Asiimwe C, Kyabayinze DJ, Kyalisiima Z, et al. Early experiences on the feasibility, acceptability, and use of malaria rapid diagnostic tests at peripheral health centres in Uganda-insights into some barriers and facilitators. Implementation Science. 2012;7:5. doi: 10.1186/1748-5908-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiden F, Owusu-Agyei S, Okyere E, et al. Acceptability of rapid diagnostic test-based management of Malaria among caregivers of under-five children in rural Ghana. PLoS ONE. 2012;7:e45556. doi: 10.1371/journal.pone.0045556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington J, Wereko-Brobby O, Ward P, Mwafongo W. Kungulwe S. SMS for Life: a pilot project to improve anti-malarial drug supply management in rural Tanzania using standard technology. Malaria Journal. 2010;9:298. doi: 10.1186/1475-2875-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens GJ, Schaftenaar E, Ndaro A, Keuter M, Bousema T. Shekalaghe SA. Malaria diagnostic testing and treatment practices in three different Plasmodium falciparum transmission settings in Tanzania: before and after a government policy change. Malaria Journal. 2011;10:76. doi: 10.1186/1475-2875-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoffi Z, Sirima BS, Angheben A, et al. Rapid malaria diagnostic tests vs. clinical management of malaria in rural Burkina Faso: safety and effect on clinical decisions. A randomized trial. Tropical Medicine & International Health. 2009;14:491–498. doi: 10.1111/j.1365-3156.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- Chandler CI, Jones C, Boniface G, Juma K, Reyburn H. Whitty CJ. Guidelines and mindlines: why do clinical staff over-diagnose malaria in Tanzania? A qualitative study. Malaria Journal. 2008a;7:53. doi: 10.1186/1475-2875-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CI, Chonya S, Boniface G, Juma K, Reyburn H. Whitty CJ. The importance of context in malaria diagnosis and treatment decisions - a quantitative analysis of observed clinical encounters in Tanzania. Tropical Medicine & International Health. 2008b;13:1131–1142. doi: 10.1111/j.1365-3156.2008.02118.x. [DOI] [PubMed] [Google Scholar]
- Chandler CI, Whitty CJ. Ansah EK. How can malaria rapid diagnostic tests achieve their potential? A qualitative study of a trial at health facilities in Ghana. Malaria Journal. 2010;9:95. doi: 10.1186/1475-2875-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Acremont V, Lengeler C, Mshinda H, Mtasiwa D, Tanner M. Genton B. Time to move from presumptive malaria treatment to laboratory-confirmed diagnosis and treatment in African children with fever. PLoS Medicine. 2009;6:e252. doi: 10.1371/journal.pmed.0050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Acremont V, Lengeler C. Genton B. Reduction in the proportion of fevers associated with Plasmodium falciparum parasitaemia in Africa: a systematic review. Malaria Journal. 2010;9:240. doi: 10.1186/1475-2875-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Acremont V, Kahama-Maro J, Swai N, Mtasiwa D, Genton B. Lengeler C. Reduction of anti-malarial consumption after rapid diagnostic tests implementation in Dar es Salaam: a before-after and cluster randomized controlled study. Malaria Journal. 2011;10:107. doi: 10.1186/1475-2875-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakeley C. Reyburn H. Out with the old, in with the new: the utility of rapid diagnostic tests for malaria diagnosis in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:333–337. doi: 10.1016/j.trstmh.2008.10.003. [DOI] [PubMed] [Google Scholar]
- English M, Reyburn H, Goodman C. Snow RW. Abandoning presumptive antimalarial treatment for febrile children aged less than five years–a case of running before we can walk? PLoS Medicine. 2009;6:e1000015. doi: 10.1371/journal.pmed.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeoke OP, Ezumah NN, Chandler CI, et al. Exploring health providers' and community perceptions and experiences with malaria tests in South-East Nigeria: a critical step towards appropriate treatment. Malaria Journal. 2012;11:368. doi: 10.1186/1475-2875-11-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer DH, Ndhlovu M, Zurovac D, et al. Improved diagnostic testing and malaria treatment practices in Zambia. The Journal of the American Medical Association. 2007;297:2227–2231. doi: 10.1001/jama.297.20.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazir T, Nisar YB, Abbasi S, et al. Comparison of oral amoxicillin with placebo for the treatment of world health organization-defined nonsevere pneumonia in children aged 2–59 months: a multicenter, double-blind, randomized, placebo-controlled trial in pakistan. Clinical Infectious Diseases. 2011;52:293–300. doi: 10.1093/cid/ciq142. [DOI] [PubMed] [Google Scholar]
- Kahama-Maro J, D'Acremont V, Mtasiwa D, Genton B. Lengeler C. Low quality of routine microscopy for malaria at different levels of the health system in Dar es Salaam. Malaria Journal. 2011;10:332. doi: 10.1186/1475-2875-10-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyabayinze DJ, Asiimwe C, Nakanjako D, Nabakooza J, Counihan H. Tibenderana JK. Use of RDTs to improve malaria diagnosis and fever case management at primary health care facilities in Uganda. Malaria Journal. 2010;9:200. doi: 10.1186/1475-2875-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie T, Mikhail A, Mayan I, et al. Overdiagnosis and mistreatment of malaria among febrile patients at primary healthcare level in Afghanistan: observational study. British Medical Journal. 2012;345:e4389. doi: 10.1136/bmj.e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masanja IM, Selemani M, Amuri B, et al. Increased use of malaria rapid diagnostic tests improves targeting of anti-malarial treatment in rural Tanzania: implications for nationwide rollout of malaria rapid diagnostic tests. Malaria Journal. 2012;11:221. doi: 10.1186/1475-2875-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorrow ML, Masanja IM, Abdulla SM, Kahigwa E. Kachur SP. Challenges in routine implementation and quality control of rapid diagnostic tests for malaria–Rufiji District, Tanzania. American Journal of Tropical Medicine and Hygiene. 2008;79:385–390. [PMC free article] [PubMed] [Google Scholar]
- McMorrow M, Ahmed S, Lyaruu P, et al. Randomized, Controlled Trial of Treatment of Febrile Children with a Negative Malaria Rapid Diagnostic Test with Artemether-Lumefantrine vs. No Antimalarial in Bagamoyo District, Tanzania in Abstract Book of 61st Meeting of the American Society for Tropical Medicine and Hygiene. Atlanta, USA: 2012. [Google Scholar]
- Msellem MI, Martensson A, Rotllant G, et al. Influence of rapid malaria diagnostic tests on treatment and health outcome in fever patients, Zanzibar: a crossover validation study. PLoS Medicine. 2009;6:e1000070. doi: 10.1371/journal.pmed.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtove G, Hendriksen IC, Amos B, et al. Treatment guided by rapid diagnostic tests for malaria in Tanzanian children: safety and alternative bacterial diagnoses. Malaria Journal. 2011;10:290. doi: 10.1186/1475-2875-10-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CK, Gasser RA, Magill AJ. Miller RS. Update on rapid diagnostic testing for malaria. Clinical Microbiology Reviews. 2008;21:97–110. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bureau of Statistics (Tanzania) Population Projections for 2012, in 2002 Population and Housing Census Analytical Report. Dar es Salaam, Tanzania: NBS; 2006. [Google Scholar]
- National Bureau of Statistics (Tanzania) and ICF Macro. Tanzania Demographic and Health Survey 2010. Dar es Salaam, Tanzania: NBS and ICF Macro; 2011. [Google Scholar]
- Njau JD, Goodman C, Kachur SP, et al. Fever treatment and household wealth: the challenge posed for rolling out combination therapy for malaria. Tropical Medicine & International Health. 2006;11:299–313. doi: 10.1111/j.1365-3156.2006.01569.x. [DOI] [PubMed] [Google Scholar]
- Nyandigisi A, Memusi D, Mbithi A, et al. Malaria case-management following change of policy to universal parasitological diagnosis and targeted artemisinin-based combination therapy in Kenya. PLoS ONE. 2011;6:e24781. doi: 10.1371/journal.pone.0024781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyburn H, Mbatia R, Drakeley C, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. British Medical Journal. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyburn H, Mbakilwa H, Mwangi R, et al. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. British Medical Journal. 2007;334:403. doi: 10.1136/bmj.39073.496829.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarbinski J, Ouma PO, Causer LM, et al. Effect of malaria rapid diagnostic tests on the management of uncomplicated malaria with artemether-lumefantrine in Kenya: a cluster randomized trial. American Journal of Tropical Medicine and Hygiene. 2009;80:919–926. [PubMed] [Google Scholar]
- Tanzania Commission for AIDS (TACAIDS); Zanzibar AIDS Commission (ZAC); National Bureau of Statistics (NBS); Office of the Chief Government Statistician (OGS); Macro International Inc. Tanzania HIV/AIDS and Malaria Indicator Survey 2011-12. Dar es Salaam, Tanzania: TACAIDS, ZAC, NBS, OGS, and Macro International Inc; 2012. [Google Scholar]
- Thiam S, Thior M, Faye B, et al. Major reduction in anti-malarial drug consumption in Senegal after nation-wide introduction of malaria rapid diagnostic tests. PLoS ONE. 2011;6:e18419. doi: 10.1371/journal.pone.0018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Republic of Tanzania Ministry of Health and Social Welfare. National Guidelines for Diagnosis and Treatment of Malaria, in Malaria control series 11. Dar es Salaam, Tanzania: National Malaria Control Program; 2006. [Google Scholar]
- United Republic of Tanzania Ministry of Health and Social Welfare. National Guidelines for Diagnosis and Treatment of Malaria, in Malaria control series 25. Dar es Salaam, Tanzania: National Malaria Control Program; 2011. [Google Scholar]
- Wasunna B, Zurovac D, Goodman CA. Snow RW. Why don't health workers prescribe ACT? A qualitative study of factors affecting the prescription of artemether-lumefantrine. Malaria Journal. 2008;7:29. doi: 10.1186/1475-2875-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitty CJ, Chandler C, Ansah E, Leslie T. Staedke SG. Deployment of ACT antimalarials for treatment of malaria: challenges and opportunities. Malaria Journal. 2008;7(Suppl. 1):S7. doi: 10.1186/1475-2875-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HA, Causer L, Metta E, et al. Dispensary level pilot implementation of rapid diagnostic tests: an evaluation of RDT acceptance and usage by providers and patients–Tanzania, 2005. Malaria Journal. 2008;7:239. doi: 10.1186/1475-2875-7-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Guidelines for the Treatment of Malaria, in 2nd Edition. Geneva, Switzerland: 2010. [Google Scholar]
- World Health Organization. World Malaria Report. 2012.
- Yukich JO, Bennett A, Albertini A, et al. Reductions in artemisinin-based combination therapy consumption after the nationwide scale up of routine malaria rapid diagnostic testing in Zambia. American Journal of Tropical Medicine and Hygiene. 2012;87:437–446. doi: 10.4269/ajtmh.2012.12-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]