To the Editor
We read with interest the article by Novak et al. recently published in the Scandinavian Journal of Immunology and write in support of their data.
The authors describe the profound changes with age of peripheral circulating mucosal associated invariant T (MAIT) cells in a cohort of patients whose samples were obtained post-clinical analysis 1. We conducted a similar study of a cohort of 160 patients aged <1–90 years (mean 41 years) a number of years ago of patients attending the John Radcliffe Hospital in Oxford, UK where we performed whole blood antibody staining of samples post-clinical analysis. Unlike Novak et al., we did not know the patient background and were unable to exclude patients with infective or inflammatory conditions, however, similarities between the data sets and also this recently published paper 2 are striking.
Novak et al. describe the MAIT cell population as CD3+CD161++Vα7.2+ cells; however, the naïve CD3+CD161++ population in cord blood has polyclonal T cell receptor usage and mature Vα7.2+ and Vα7.2- CD161++ T cell subsets share the same distinctive phenotype and function of the MAIT population 3,4. Our study was conducted before wider availability of the Vα7.2 antibody and describes the CD3+CD161++CD8α+ population that includes both Vα7.2+ and Vα7.2- cells, representing about 90% of the MAIT cell population, with the remainder predominantly double negative CD4-CD8- cells (DN) 3. We found a significant positive correlation between peripheral blood CD3+CD161++CD8α+ cell frequency and age up 30 years (r = 0.4651, P < 0.0001) and subsequently a significant negative correlation between MAIT cell frequency and age in those patients ≥30 years (r = −0.5171, P < 0.0001) (Fig.1A).
Figure 1.
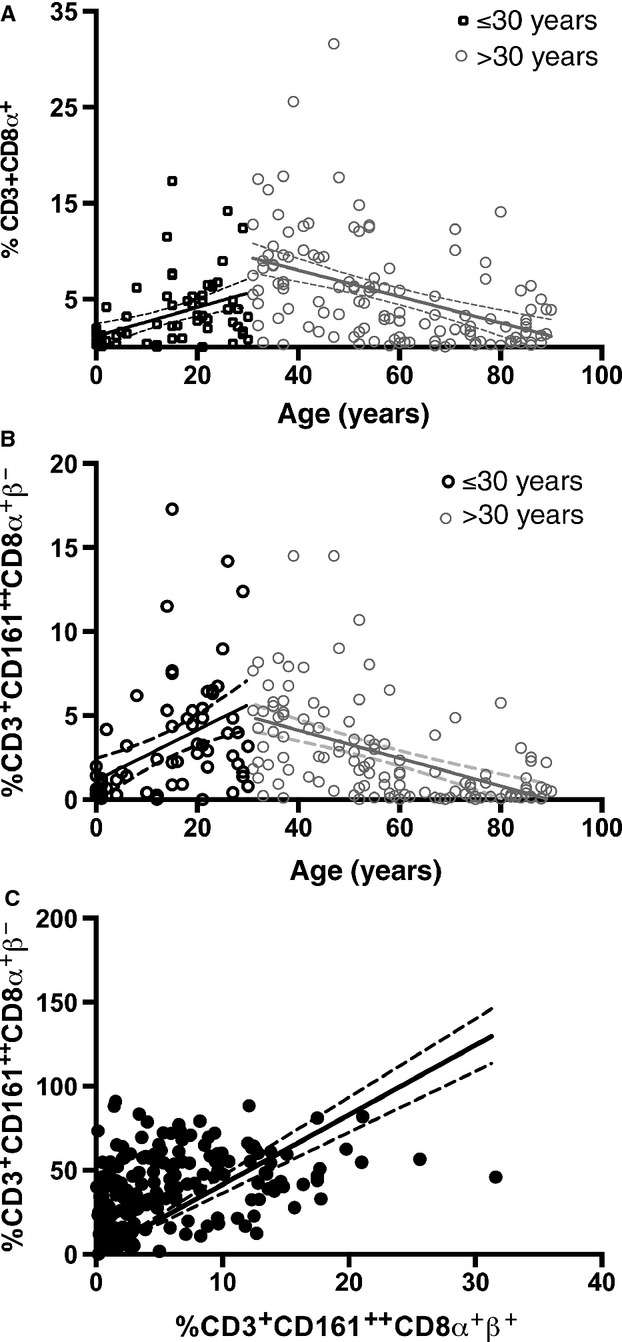
Variation with age of peripheral CD3+CD161++CD8α+ T cells. (A) Correlation between age and the size of the CD3+CD161++CD8α+β+ subset as a proportion of CD3+CD8α+β+ T cells in patients < or ≥30 years old. (<30 years r = 0.4651, P < 0.0001; ≥30 years r = −0.5171, P < 0.0001 Spearman's rank). (B) Correlation between age (years) and the size of the CD3+CD161++CD8α+β- subset as a proportion of CD3+CD161++CD8α+ T cells < or ≥30 years old (<30 years r = 0.5358 P < 0.0001; ≥30 years r = −0.5878 P < 0.0001 Spearman's rank). (C) Relationship between CD161++CD8α+β+ and CD161++CD8α+β- subsets as a proportion of CD3+ cells (r2 = 0.1151, P<0.0001 Linear regression).
CD8 can be expressed as both a CD8αβ heterodimer or a CD8αα homodimer and the CD3+CD161++CD8α+/MAIT population further subdivides into CD8α+β -/low (CD8αβ and CD8αα expressing) and CD8α+β- (CD8αα single positive) subsets 3,5,6. On further analysis, here, we describe the CD161++CD8α+CD8β- subset to vary in a similar pattern with age to the overall CD3+CD161++CD8α+ subset (<30 years r = 0.45358 P < 0.0001; ≥ 30 years r = −0.5878 P < 0.0001) (Fig.1B) and a significant positive correlation between the size of the CD161++CD8α+β+ and the CD161++CD8α+β- as a proportion of CD3+ cells (Fig.1C). Novak et al. describe a fall in CD8α+ MAIT cells and increase in proportion of DN MAITs with increasing age. Our own previously published data would indicate CD161++CD8αα MAIT cells to be derived from CD161++CD8αβ cells; however, the origin of the DN subset is not known 3. Our data would indicate that the proportion of CD161++CD8αα subset remains in a steady state as a proportion of CD161++CD8α+ MAIT population overall, regardless of possible progression to DN status.
Our data strongly support the conclusions of the authors that further work involving MAIT cells should ensure careful age and sex-matched controls are used to allow for appropriate interpretation of data suggesting changes in MAIT cell frequency within particular disease states; uncontrolled studies of MAIT cells require cautious interpretation.
Yours Sincerely
Dr Lucy Walker
Dr Hannah Tharmalingham
Professor Paul Klenerman
References
- 1.Novak J, Dobrovolny J, Novakova L, Kozak T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in males and females of reproductive age. Scand J Immunol. 2014;21 doi: 10.1111/sji.12193. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Lee O-J, Cho Y-N, Kee S-J, et al. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp Gerontol. 2014;49:47–54. doi: 10.1016/j.exger.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Walker LJ, Kang Y-H, Smith MO, et al. Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood. 2012;119:422–33. doi: 10.1182/blood-2011-05-353789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ussher JE, Bilton M, Attwod E, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reantragoon R, Corbett AJ, Sakala IG, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210:2305–20. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker LJ, Marrinan E, Muenchhoff M, et al. CD8αα expression marks terminally differentiated human CD8+ T cells expanded in chronic viral infection. Front Immunol. 2013;4:223. doi: 10.3389/fimmu.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]


