Abstract
For two decades the search for genes involved in Alzheimer's disease brought little reward; it was not until the advent of genome-wide association studies (GWAS) that genetic associations started to be revealed. Since 2009 increasingly large GWAS have revealed 20 loci, which in itself is a substantial increase in our understanding, but perhaps the more important feature is that these studies have highlighted novel pathways that are potentially involved in the disease process. This commentary assembles our latest knowledge while acknowledging that the casual functional variants, and undoubtedly, other genes are still yet to be discovered. This is the challenge that remains and the promise of next-generation sequencing is anticipated as there are a number of large initiatives which themselves should start to yield information before long.
Keywords: Alzheimer's, GWAS, NGS
Fifteen years separated the discovery of APOE, the first genetic risk factor for sporadic, late-onset Alzheimer's disease (LOAD, also referred to as sporadic Alzheimer's disease – sAD), and the first replicable genome-wide associations in 2009. However with patience comes reward; a recent meta-analysis by the International Genomics of Alzheimer's Project (IGAP) reported 11 new Alzheimer's susceptibility loci (CASS4, CELF1, FERMT2, HLA-DRB5/HLA-DRB1, INPP5D, MEF2C, NME8, PTK2B, SLC24A4/RIN3, SORL1 and ZCWPW1), and confirmed eight (CR1, BIN1, CD2AP, EPHA1, CLU, MS4A6A, PICALM and ABCA7) of the nine previously reported genome-wide associations in addition to APOE [1]; the exception being CD33 which failed to replicate. Consequently genetic discoveries within the last 5 years account for ∼47% of the population attributable risk (PAR) of LOAD. This rises to ∼61% with the established APOE haplotype (Table 1). However there is still a substantial component of ‘missing heritability’ waiting to be detected, which may be accounted for by multiple variants imparting modest effect (polygenic model) and/or fewer rare mutations of larger effect [4].
Table 1.
Population attributable fraction (PAF) calculations for alleles associated with Alzheimer's disease
| SNP | Gene | MAF | Location | Major/minor alleles | OR | PAF |
|---|---|---|---|---|---|---|
| (a) | ||||||
| rs4147929 | ABCA7 | 0.16 | Intronic | G/A | 1.15 (1.11–1.19) | 2.8 |
| rs6733839 | BIN1 | 0.37 | Intergenic | C/T | 1.22 (1.18–1.25) | 8.1 |
| rs10948363 | CD2AP | 0.26 | Intronic | A/G | 1.10 (1.07–1.13) | 2.3 |
| rs9331896 | CLU | 0.40 | Intronic | T/C | 0.86 (0.84–0.89) | 5.3 |
| rs6656401 | CR1 | 0.19 | Intronic | G/A | 1.18 (1.14–1.22) | 3.7 |
| rs11771145 | EPHA1 | 0.35 | Intergenic | G/A | 0.90 (0.88–0.93) | 3.1 |
| rs983392 | MS4A6A | 0.41 | Intergenic | A/G | 0.90 (0.87–0.92) | 4.2 |
| rs10792832 | PICALM | 0.37 | Intergenic | G/A | 0.87 (0.85–0.89) | 5.3 |
| rs429358 | APOE4 | 0.12 | Nonsynonymous | T/C | 4.89 (4.45–5.39) | 27.3 |
| rs75932628 | TREM2 | 0.002 | Nonsynonymous | C/T | 4.5 (1.7–11.9) | 0.8 |
| (b) | ||||||
| rs7274581 | CASS4 | 0.08 | Intronic | T/C | 0.88 (0.84–0.92) | 1.1 |
| rs10838725 | CELF1 | 0.31 | Intronic | T/C | 1.08 (1.05–1.11) | 2.4 |
| rs17125944 | FERMT2 | 0.08 | Intronic | T/C | 1.14 (1.09–1.19) | 1.5 |
| rs9271192 | HLA | 0.28 | Intergenic | A/C | 1.11 (1.08–1.15) | 3.2 |
| rs35349669 | INPP5D | 0.46 | Intronic | C/T | 1.08 (1.05–1.11) | 4.6 |
| rs190982 | MEF2C | 0.39 | Intergenic | A/G | 0.93 (0.90–0.95) | 2.7 |
| rs2718058 | NME8 | 0.37 | Intergenic | A/G | 0.93 (0.90–0.95) | 2.9 |
| rs28834970 | PTK2B | 0.36 | Intronic | T/C | 1.10 (1.08–1.13) | 3.1 |
| rs10498633 | SLC24A4 | 0.21 | Intronic | G/T | 0.91 (0.88–0.94) | 1.5 |
| rs11218343 | SORL1 | 0.04 | Intronic | T/C | 0.77 (0.72–0.82) | 1.1 |
| rs1476679 | ZCWPW1 | 0.29 | Intronic | T/C | 0.91 (0.89–0.94) | 3.2 |
| Total PAF | 61.8 | |||||
The top table (a) documents established alleles whereas the bottom table (b) is for the newly identified genes. For each gene the documented SNP is the one achieving the greatest association in the IGAP publication [1]. The exception is TREM2 which is the first rare variant to be identified from next-generation sequencing efforts (SNP details taken from Guerreiro et al. [2]), and APOE where the odds ratio (OR) is calculated from in-house data sets (C. Medway and K. Morgan, unpublished). Combined PAF calculated according to equation reported by Naj et al. [3].
Unlike the rarer, early onset familial form of Alzheimer's disease (fAD), the result of deterministic mutations in one of three amyloid processing genes (APP, PSEN1 or PSEN2), the aetiology of LOAD remains unknown. Considering LOAD shares clinical and neuropathological features with the familial disease, it is attractive to speculate that abnormalities within the amyloid pathway are similarly culpable. However, despite years of research, there is no concrete evidence that the ‘amyloid cascade’ is causally linked to late-onset disease, or to ratify its therapeutic potential.
The era of the genome-wide association study (GWAS) heralded the arrival of new LOAD alleles, each common (minor allele frequency, MAF > 5%) and conveying modest genetic effects 3,5–8. Enrichment of the first eight genome-wide associated genes within three pathways (cholesterol metabolism, immune system function and synaptic vesicle recycling/endocytosis) [9],[10] nominated novel pathways for therapeutic intervention. While these pathways may modify amyloid aggregation and/or clearance, to what extent these they are implicated in amyloid homeostasis remains to be determined [11],[12]. A summary of how these new genes link to pathways likely to be involved in sAD is presented (see Figure 1).
Figure 1.
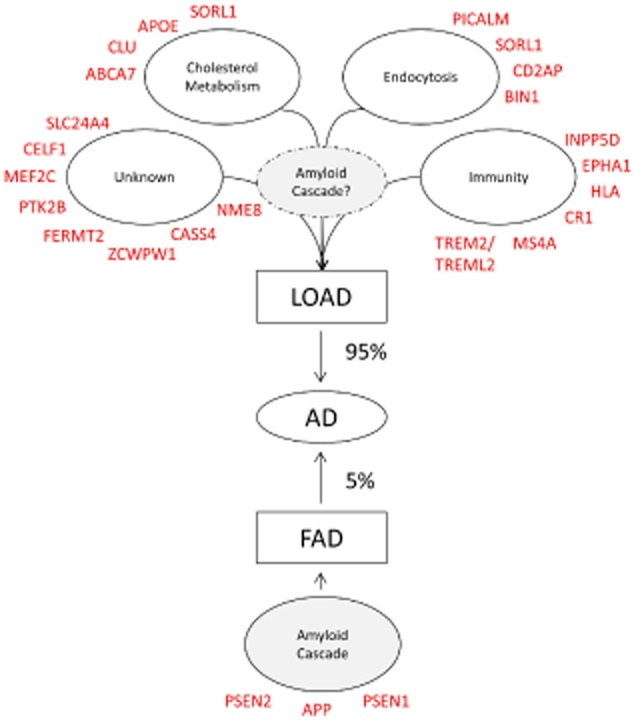
Pathways implicated in Alzheimer's disease (AD). Familial Alzheimer's disease (FAD) is caused by one of three genes (PSEN1, PSEN2 and APP) involved in the amyloid processing pathway (‘the amyloid cascade’). There is no single pathway that explains the more common nonfamilial, late-onset/sporadic Alzheimer's disease (LOAD/sAD). Candidate genes are enriched for several pathways; Cholesterol Metabolism, Immune System and Endocytosis. These pathways may be downstream dependent or independent of amyloid processing.
New sAD genes, new pathways?
The IGAP consortium recently reported 11 new genes for sAD (Table 1b) [1]. IGAP drew on a combined resource of 74 046 samples, the largest GWAS meta-analysis of LOAD to date. The ensuing power boost elevated new risk alleles previously masked due to low frequency, weak genetic effects or incomplete tagging of the causal mutation. This may explain why SORL1, which had long been suspected of harbouring genuine disease alleles using candidate gene approaches [13], attained genome-wide significance in this series but not earlier GWAS. The encoded sortilin-related receptor is a neuronal APOE receptor and is understood to direct APP trafficking to amyloidogenic endocytic pathways [14].
Similarly, immune system function is represented in new candidate genes. Most notable are the association signals within the HLA region (which encompasses a number of candidate genes, the strongest associations are found at DRB1 and DRB5). These genes have been previously associated with Parkinson's disease [15] and multiple sclerosis [16]. INPP5D encodes a member of the inositol polyphosphate-5-phosphatase family of enzymes involved in second messenger signalling in myeloid cells. INPP5D affects pathways associated with cell proliferation and the regulation of inflammatory responses [17].
The remaining genes (PTK2B, MEFC2, CASS4, FERMT2, CELF1, ZCWPW1, SLC24A4, NME8) cannot be readily attributed to pathways which are biologically relevant to LOAD. Among them are cell adhesion molecules and kinases which signal in pathways attributed to neuronal function. PTK2B is a cytoplasmic protein tyrosine kinase expressed in brain, where it is understood to regulate neuronal activity via the MAP-kinase signalling pathway. Specific functions in long-term potentiation (LTP) and memory have been proposed [18]. MEF2C is also expressed in the cortex and is involved in the MAP kinase signalling pathway, responding to calcium influx to activate survival genes [19]. MEF2C encodes a transcription factor of the MEF2 family, which has a widely reported role in muscle (cardiac and skeletal) and vascular development [20]. Like PTK2B, MEFC2 has been implicated in hippocampal synaptic plasticity and LTP [21]. Haploinsufficiency of MEF2C manifests mental retardation and epilepsy [22].
PTK2B is a focal adhesion kinase (FAK2), which is known to interact with the CASS4 (also genetically implicated in the IGAP series) homologue NEDD9 and integrin to regulate apoptosis and cell adhesion [23]. While CASS4 encodes a poorly characterized scaffold protein, variants in other members of the CAS gene family (NEDD9) have been associated with dementia and Alzheimer's disease [24],[25]. Fermitin family member 2 [FERMT2 or kindling-2 (KIND2)] is a neuronally expressed adhesion molecule implicated in actin cytoskeleton organization [26]. FERMT2 also plays a role in signalling via integrin activation and the Wnt signalling pathway [27]. Abnormal Wnt signalling has previously attracted attention as a candidate pathway in Alzheimer's disease and other dementing syndromes [28].
CELF1, ZCWPW1, SLC24A4 and NME8 are particularly poorly characterized. CELF1 (CUGBP1 – CUG triplet repeat-binding protein 1) encodes an RNA-binding protein involved in alternative mRNA splicing, editing and stability. A CUG triplet repeat expansion is responsible for myotonic dystrophy type-1. Given the size of the associated locus in the IGAP publication, the causal gene may lie further afield; a MAP-kinase activating death domain 130kb downstream has attracted attention although RAPSN, a receptor-associated protein found at the synapse, is also within this region. Similarly, the association signal attributed to ZCWPW1 (rs1476679), encoding an epigenetic regulatory protein [29], is part of a larger LD block, encompassing many candidate genes. SLC24A4 (NCKX4) is a sodium/potassium/calcium exchanger expressed in human brain [30]. Variation in SLC24A4 gene has been associated with hair, eye and skin pigmentation [31]. More recently variation in SLC24A4 has been associated with lipid metabolism [32]. It is important to note that the SLC24A4 association signal (rs10498633) is towards the 3′-end of the gene, which is neighboured by RIN3. RIN3 interacts with BIN1 (amphiphysin-2), and may be part of the endocytic machinery [33]. Finally NME8, encoding a Thioredoxin Domain-containing Protein, is associated with primary ciliary dyskinesia-6 [34].
Towards identifying the causal alleles: why we find genome-wide association signals
In each instance identification of the causal allele(s) will be instrumental towards conclusively pinpointing the affected gene, accurately attributing risk to each gene/loci, and to fully appreciate the mechanism of pathogenesis. However, given that the causal mutations underpinning earlier genome-wide associations remain largely unknown this will be a considerable challenge. This has led some to postulate that we need to dig deeper, resequencing candidate loci to identify rare mutation(s) explaining GWAS associations [35]. Coined ‘synthetic associations’ [36], there is no evidence that any of the common Alzheimer's disease alleles discovered to date are precipitated through correlation (linkage disequilibrium) to rarer causal mutations. Furthermore, it would be expected that GWAS ‘hits’ conforming to the synthetic model will have allele frequencies skewed towards the lower end of the allele spectrum, which is not the rule in complex disorders [37]. The more common the associated GWAS SNPs (single nucleotide polymorphisms), the larger the effect (odds ratio) at the rare position(s) would have to be to fully explain the signal – this makes it more likely that they would have already been detected through family-based linkage approaches [37]. IGAP reports three alleles with frequencies below 10%. It will remain to be seen if, in these instances, genome resequencing unearths rare alleles evoking these signals as they represent the best potential examples to date that are possibly compatible with this concept.
Genome resequencing has already contributed to our understanding of sAD genetics. Two independent groups have reported rare coding variants within TREM2 (exon 2) conferring increased risk for LOAD [2],[38]. TREM2 encodes a receptor expressed on myeloid cells, and is understood to mediate anti-inflammatory responses. Interestingly, this gene has been previously associated with other dementing phenotypes; the rare recessive Nasu-Hakola disease [39], early-onset dementia [40] and frontotemporal-like dementia [41]. Although there is evidence of allelic heterogeneity, with greater burden in cases, carriers of one nonsynonymous change (rs75932628, R47H) displayed effects sizes on par with APOE ε4 allele carriers.
International Genomics of Alzheimer's Project reports a common variant (rs9381040), 3.4 kb downstream of TREM2's neighbour TREML2, which falls just short of genome-wide significance. The fact that the alleles (R47H and rs9381040) are in complete linkage disequilibrium (D′ = 1) (there are no recombination hotspots segregating these positions), coupled with the observation that the TREM2 risk allele is in phase with the major allele at the TREML2 SNP, is consistent with a synthetic model. This would explain why the TREML2 minor allele reports a protective effect while the TREM2 allele is associated with risk. Interestingly, there is also prior linkage evidence of the TREM2 locus in Alzheimer's disease [42]. In order to fully evaluate to what extent rare mutations in the TREM2 locus account for the TREML2 signal, a greater understanding of the allelic heterogeneity will have to be sought. Interestingly this observation may represent the interface, or juxta-positioning, of GWAS and NGS technologies. As GWAS are conducted with ever increasing sample sizes they will undoubtedly start to uncover rarer disease associated variants as exemplified by the IGAP study. This bodes well for the concept that more in-depth NGS will be able to contribute to some of the remaining ‘missing heritability’.
One recent observation that disease causing, coding mutations for common disorders are relatively ‘young’ – in fact occurred within the last 10 000 years [43] adds impetus to the NGS drive. For a common variant (MAF > 5%) to become fixed, and hence readily detectable in a GWAS, the change needs to have occurred over 200 000 years ago. Previously we have been using modern technologies to investigate ancient DNA changes; we are on the verge of an era where we are now able to use these approaches to look at ‘modern’ DNA changes, that is, the contribution of rare variants with much lower MAFs.
Putting flesh on the bones; osteoclasts, microglia and Alzheimer's disease
Defects in the TREM2-DAP12 signalling pathway in osteoclasts and microglia, both of which are derived from myeloid progenitor cells, may explain the duel bone and brain manifestations in Nasu-Hakola disease. DAP12 and TREM2 are both expressed in osteoclasts and microglia (and dendritic cells and oligodendrocytes). Osteoclasts oversee the demineralization, reabsorption and recycling (via endocytosis) of bone; a similar recycling/removal mechanism for microglia within the brain is plausible.
It is interesting to note that other LOAD candidate genes have been implicated in osteoclast function downstream of TREM2-DAP12 signalling. PTK2B (Pyk2) is one of the kinases acting downstream of DAP12 [44]. INPP5D negatively regulates osteoclast formation and function by directly binding to DAP12 and preventing PI3K signalling [45],[46]. CASS4 family members (CASL and CASS1), a downstream target of PTK2B, are required for bone matrix remodelling [23] and expression is reduced in TREM2 knockouts [40]. Siglecs (CD33-related family) expressed in myeloid derived cells also signal via DAP12, suggesting a common signalling pathway [47]. A recent systems approach has championed microglia phagocytosis as a prominent functional group, where DAP12 is a key regulator [48].
Consequently, there may be a mechanistic overlap between TREM2/DAP12 and some of the GWAS derived genes. The possibility of a shared mechanism between the bone and neurological manifestations of Alzheimer's disease has been raised [49].
The future
The heritable component of sAD which remains elusive may conform to a polygenic model (many more alleles of weak genetic effects), and will require larger cohorts to detect vanishingly smaller genetic effects. Polygenic risk scoring in other complex diseases suggests this is indeed the case [50]. Alternatively fewer, rarer variants conferring larger genetic effects, like TREM2, may be responsible [2]. While weak genetic effects (polygenic model) and rare allele (rare variants model) are criticized for their individually small contributions to risk, the insight they offer into disease biology is invaluable.
The time when GWAS and resequencing approaches occupy their own niche is coming to an end; GWAS as a tool to explore common variants (MAF > 5%) in sufficiently large numbers to detect weak genetic effects (OR > 1.3), and NGS as a tool to detect rare variants in modest sample numbers due to cost implications. As custom array technology begins to include rarer variants, discovered through 1000 Genomes and/or other resequencing initiatives, and whole-genome sequencing becomes cost-effective these boundaries will blur. With this will come a greater appreciation of how common and rare alleles summate to influence a phenotype at any given disease loci.
References
- 1.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, Jun G, Destefano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thornton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Hollingworth P, Ramirez A, Hanon O, Fitzpatrick AL, Buxbaum JD, Campion D, Crane PK, Baldwin C, Becker T, Gudnason V, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Morón FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fiévet N, Huentelman MJ, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuinness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossù P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F European Alzheimer's Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer's Disease (GERAD); Alzheimer's Disease Genetic Consortium (ADGC); Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannfelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O'Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Jr, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltunen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nöthen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JSK, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert J-C, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St. George-Hyslop P, Singleton A, Hardy J. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naj AC, Jun G, Beecham GW, Wang L-S, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JSK, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, St George-Hyslop P, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, DeCarli C, DeKosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin L-W, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson G. Rare and common variants: twenty arguments. Nat Rev Genet. 2011;13:135–145. doi: 10.1038/nrg3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert J-C, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ERLC, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Rüther E, Schürmann B, Heun R, Kölsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Gallacher J, Hüll M, Rujescu D, Giegling I, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel K-H, Klopp N, Wichmann H-E, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, van Duijn CM, Breteler MMB, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, Berr C, Campion D, Epelbaum J, Dartigues J-F, Tzourio C, Alpérovitch A, Lathrop M, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Björnsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossù P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O'Donovan M, Amouyel P, Williams J. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel K-H, Klopp N, Wichmann H-E, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O'Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert J-C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanché H, Dartigues J-F, Tzourio C, Gut I, Van Broeckhoven C, Alpérovitch A, Lathrop M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith A, V, Carassquillo MM, Lambert JC, Harold D, Schrijvers EMC, Ramirez-Lorca R, Debette S, Longstreth WT, Janssens ACJW, Pankratz VS, Dartigues JF, Hollingworth P, Aspelund T, Hernandez I, Beiser A, Kuller LH, Koudstaal PJ, Dickson DW, Tzourio C, Abraham R, Antunez C, Du Y, Rotter JI, Aulchenko YS, Harris TB, Petersen RC, Berr C, Owen MJ, Lopez-Arrieta J, Varadarajan BN, Becker JT, Rivadeneira F, Nalls MA, Graff-Radford NR, Campion D, Auerbach S, Rice K, Hofman A, Jonsson P, V, Schmidt H, Lathrop M, Mosley TH, Au R, Psaty BM, Uitterlinden AG, Farrer LA, Lumley T, Ruiz A, Williams J, Amouyel P, Younkin SG, Wolf PA, Launer LJ, Lopez OL, van Duijn CM, Breteler MMB. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, Pocklington A, Abraham R, Hollingworth P, Sims R, Gerrish A, Pahwa JS, Jones N, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, Heun R, Kölsch H, van den Bussche H, Heuser I, Peters O, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JSK, Cruchaga C, Nowotny P, Morris JC, Mayo K, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel K-H, Klopp N, Wichmann H-E, Rüther E, Carrasquillo MM, Pankratz VS, Younkin SG, Hardy J, O'Donovan MC, Owen MJ, Williams J. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer's disease. PLoS ONE. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan K. The three new pathways leading to Alzheimer's disease. Neuropathol Appl Neurobiol. 2011;37:353–357. doi: 10.1111/j.1365-2990.2011.01181.x. [DOI] [PubMed] [Google Scholar]
- 11.Lambert J-C, Amouyel P. Genetics of Alzheimer's disease: new evidences for an old hypothesis? Curr Opin Genet Dev. 2011;21:295–301. doi: 10.1016/j.gde.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Reitz C, Cheng R, Rogaeva E, Lee JH, Tokuhiro S, Zou F, Bettens K, Sleegers K, Tan EK, Kimura R, Shibata N, Arai H, Kamboh MI, Prince JA, Maier W, Riemenschneider M, Owen M, Harold D, Hollingworth P, Cellini E, Sorbi S, Nacmias B, Takeda M, Pericak-Vance MA, Haines JL, Younkin S, Williams J, van Broeckhoven C, Farrer LA, St George-Hyslop PH, Mayeux R. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol. 2011;68:99–106. doi: 10.1001/archneurol.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song Y-Q, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed I, Tamouza R, Delord M, Krishnamoorthy R, Tzourio C, Mulot C, Nacfer M, Lambert J-C, Beaune P, Laurent-Puig P, Loriot M-A, Charron D, Elbaz A. Association between Parkinson's disease and the HLA-DRB1 locus. Mov Disord. 2012;27:1104–1110. doi: 10.1002/mds.25035. [DOI] [PubMed] [Google Scholar]
- 16.Sawcer S, Hellenthal G, Pirinen M, Spencer CCA, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, Saarela J, Bellenguez C, Fontaine B, Gillman M, Hemmer B, Gwilliam R, Zipp F, Jayakumar A, Martin R, Leslie S, Hawkins S, Giannoulatou E, D'alfonso S, Blackburn H, Martinelli Boneschi F, Liddle J, Harbo HF, Perez ML, Spurkland A, Waller MJ, Mycko MP, Ricketts M, Comabella M, Hammond N, Kockum I, McCann OT, Ban M, Whittaker P, Kemppinen A, Weston P, Hawkins C, Widaa S, Zajicek J, Dronov S, Robertson N, Bumpstead SJ, Barcellos LF, Ravindrarajah R, Abraham R, Alfredsson L, Ardlie K, Aubin C, Baker A, Baker K, Baranzini SE, Bergamaschi L, Bergamaschi R, Bernstein A, Berthele A, Boggild M, Bradfield JP, Brassat D, Broadley SA, Buck D, Butzkueven H, Capra R, Carroll WM, Cavalla P, Celius EG, Cepok S, Chiavacci R, Clerget-Darpoux F, Clysters K, Comi G, Cossburn M, Cournu-Rebeix I, Cox MB, Cozen W, Cree BAC, Cross AH, Cusi D, Daly MJ, Davis E, de Bakker PIW, Debouverie M, D'hooghe MB, Dixon K, Dobosi R, Dubois B, Ellinghaus D, Elovaara I, Esposito F, Fontenille C, Foote S, Franke A, Galimberti D, Ghezzi A, Glessner J, Gomez R, Gout O, Graham C, Grant SFA, Guerini FR, Hakonarson H, Hall P, Hamsten A, Hartung H-P, Heard RN, Heath S, Hobart J, Hoshi M, Infante-Duarte C, Ingram G, Ingram W, Islam T, Jagodic M, Kabesch M, Kermode AG, Kilpatrick TJ, Kim C, Klopp N, Koivisto K, Larsson M, Lathrop M, Lechner-Scott JS, Leone MA, Leppä V, Liljedahl U, Bomfim IL, Lincoln RR, Link J, Liu J, Lorentzen AR, Lupoli S, Macciardi F, Mack T, Marriott M, Martinelli V, Mason D, McCauley JL, Mentch F, Mero I-L, Mihalova T, Montalban X, Mottershead J, Myhr K-M, Naldi P, Ollier W, Page A, Palotie A, Pelletier J, Piccio L, Pickersgill T, Piehl F, Pobywajlo S, Quach HL, Ramsay PP, Reunanen M, Reynolds R, Rioux JD, Rodegher M, Roesner S, Rubio JP, Rückert I-M, Salvetti M, Salvi E, Santaniello A, Schaefer CA, Schreiber S, Schulze C, Scott RJ, Sellebjerg F, Selmaj KW, Sexton D, Shen L, Simms-Acuna B, Skidmore S, Sleiman PMA, Smestad C, Sørensen PS, Søndergaard HB, Stankovich J, Strange RC, Sulonen A-M, Sundqvist E, Syvänen A-C, Taddeo F, Taylor B, Blackwell JM, Tienari P, Bramon E, Tourbah A, Brown MA, Tronczynska E, Casas JP, Tubridy N, Corvin A, Vickery J, Jankowski J, Villoslada P, Markus HS, Wang K, Mathew CG, Wason J, Palmer CNA, Wichmann H-E, Plomin R, Willoughby E, Rautanen A, Winkelmann J, Wittig M, Trembath RC, Yaouanq J, Viswanathan AC, Zhang H, Wood NW, Zuvich R, Deloukas P, Langford C, Duncanson A, Oksenberg JR, Pericak-Vance MA, Haines JL, Olsson T, Hillert J, Ivinson AJ, De Jager PL, Peltonen L, Stewart GJ, Hafler DA, Hauser SL, McVean G, Donnelly P, Compston A. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware MD, Rosten P, Damen JE, Liu L, Humphries RK, Krystal G. Cloning and characterization of human SHIP, the 145-kD inositol 5-phosphatase that associates with SHC after cytokine stimulation. Blood. 1996;88:2833–2840. [PubMed] [Google Scholar]
- 18.Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- 19.Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- 20.Zweier M, Gregor A, Zweier C, Engels H, Sticht H, Wohlleber E, Bijlsma EK, Holder SE, Zenker M, Rossier E, Grasshoff U, Johnson DS, Robertson L, Firth H, V, Ekici AB, Reis A, Rauch A. Mutations in MEF2C from the 5q14.3q15 microdeletion syndrome region are a frequent cause of severe mental retardation and diminish MECP2 and CDKL5 expression. Hum Mutat. 2010;31:722–733. doi: 10.1002/humu.21253. [DOI] [PubMed] [Google Scholar]
- 21.Barbosa AC, Kim M-S, Ertunc M, Adachi M, Nelson ED, McAnally J, Richardson JA, Kavalali ET, Monteggia LM, Bassel-Duby R, Olson EN. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci U S A. 2008;105:9391–9396. doi: 10.1073/pnas.0802679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Meur N, Holder-Espinasse M, Jaillard S, Goldenberg A, Joriot S, Amati-Bonneau P, Guichet A, Barth M, Charollais A, Journel H, Auvin S, Boucher C, Kerckaert J-P, David V, Manouvrier-Hanu S, Saugier-Veber P, Frébourg T, Dubourg C, Andrieux J, Bonneau D. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J Med Genet. 2010;47:22–29. doi: 10.1136/jmg.2009.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci. 2010;67:1025–1048. doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Grupe A, Rowland C, Holmans P, Segurado R, Abraham R, Jones L, Catanese J, Ross D, Mayo K, Martinez M, Hollingworth P, Goate A, Cairns NJ, Racette BA, Perlmutter JS, O'Donovan MC, Morris JC, Brayne C, Rubinsztein DC, Lovestone S, Thal LJ, Owen MJ, Williams J. Evidence that common variation in NEDD9 is associated with susceptibility to late-onset Alzheimer's and Parkinson's disease. Hum Mol Genet. 2008;17:759–767. doi: 10.1093/hmg/ddm348. [DOI] [PubMed] [Google Scholar]
- 25.Chapuis J, Moisan F, Mellick G, Elbaz A, Silburn P, Pasquier F, Hannequin D, Lendon C, Campion D, Amouyel P, Lambert J-C. Association study of the NEDD9 gene with the risk of developing Alzheimer's and Parkinson's disease. Hum Mol Genet. 2008;17:2863–2867. doi: 10.1093/hmg/ddn183. [DOI] [PubMed] [Google Scholar]
- 26.Lai-Cheong JE, Parsons M, McGrath JA. The role of kindlins in cell biology and relevance to human disease. Int J Biochem Cell Biol. 2010;42:595–603. doi: 10.1016/j.biocel.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Yu Y, Wu J, Wang Y, Zhao T, Ma B, Liu Y, Fang W, Zhu W-G, Zhang H. Kindlin 2 forms a transcriptional complex with β-catenin and TCF4 to enhance Wnt signalling. EMBO Rep. 2012;13:750–758. doi: 10.1038/embor.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mudher A, Lovestone S. Alzheimer's disease – do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 29.He F, Umehara T, Saito K, Harada T, Watanabe S, Yabuki T, Kigawa T, Takahashi M, Kuwasako K, Tsuda K, Matsuda T, Aoki M, Seki E, Kobayashi N, Güntert P, Yokoyama S, Muto Y. Structural insight into the zinc finger CW domain as a histone modification reader. Structure. 2010;18:1127–1139. doi: 10.1016/j.str.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Li X-F, Kraev AS, Lytton J. Molecular cloning of a fourth member of the potassium-dependent sodium-calcium exchanger gene family, NCKX4. J Biol Chem. 2002;277:48410–48417. doi: 10.1074/jbc.M210011200. [DOI] [PubMed] [Google Scholar]
- 31.Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, Manolescu A, Karason A, Palsson A, Thorleifsson G, Jakobsdottir M, Steinberg S, Pálsson S, Jonasson F, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Aben KK, Kiemeney LA, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- 32.Kraja AT, Borecki IB, Tsai MY, Ordovas JM, Hopkins PN, Lai C-Q, Frazier-Wood AC, Straka RJ, Hixson JE, Province MA, Arnett DK. Genetic analysis of 16 NMR-lipoprotein fractions in humans, the GOLDN study. Lipids. 2013;48:155–165. doi: 10.1007/s11745-012-3740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kajiho H, Saito K, Tsujita K, Kontani K, Araki Y, Kurosu H, Katada T. RIN3: a novel Rab5 GEF interacting with amphiphysin II involved in the early endocytic pathway. J Cell Sci. 2003;116:4159–4168. doi: 10.1242/jcs.00718. [DOI] [PubMed] [Google Scholar]
- 34.Duriez B, Duquesnoy P, Escudier E, Bridoux A-M, Escalier D, Rayet I, Marcos E, Vojtek A-M, Bercher J-F, Amselem S. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2007;104:3336–3341. doi: 10.1073/pnas.0611405104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClellan J, King M-C. Why it is time to sequence. Cell. 2010;142:351–353. [Google Scholar]
- 36.Dickson SP, Wang K, Krantz I, Hakonarson H, Goldstein DB. Rare variants create synthetic genome-wide associations. PLoS Biol. 2010;8:e1000294. doi: 10.1371/journal.pbio.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wray NR, Purcell SM, Visscher PM. Synthetic associations created by rare variants do not explain most GWAS results. PLoS Biol. 2011;9:e1000579. doi: 10.1371/journal.pbio.1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjaerg L, Konttinen Y, Peltonen L. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chouery E, Delague V, Bergougnoux A, Koussa S, Serre J-L. Mégarbané A. Mutations in TREM2 lead to pure early-onset dementia without bone cysts. Hum Mutat. 2008;29:E194–204. doi: 10.1002/humu.20836. [DOI] [PubMed] [Google Scholar]
- 41.Guerreiro RJ, Lohmann E, Brás JM, Gibbs JR, Rohrer JD, Gurunlian N, Dursun B, Bilgic B, Hanagasi H, Gurvit H, Emre M, Singleton A, Hardy J. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol. 2013;70:78–84. doi: 10.1001/jamaneurol.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butler AW, Ng MYM, Hamshere ML, Forabosco P, Wroe R, Al-Chalabi A, Lewis CM, Powell JF. Meta-analysis of linkage studies for Alzheimer's disease-a web resource. Neurobiol Aging. 2009;30:1037–1047. doi: 10.1016/j.neurobiolaging.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Fu W, O'Connor TD, Jun G, Kang HM, Abecasis G, Leal SM, Gabriel S, Altshuler D, Shendure J, Nickerson DA, Bamshad MJ, Akey JM. Analysis of 6515 exomes reveals the recent origin of most human protein-coding variants. Nature. 2013;493:216–220. doi: 10.1038/nature11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otero K, Shinohara M, Zhao H, Cella M, Gilfillan S, Colucci A, Faccio R, Ross FP, Teitelbaum SL, Takayanagi H, Colonna M. TREM2 and β-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J Immunol. 188:2612–2621. doi: 10.4049/jimmunol.1102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeshita S, Namba N, Zhao JJ, Jiang Y, Genant HK, Silva MJ, Brodt MD, Helgason CD, Kalesnikoff J, Rauh MJ, Humphries RK, Krystal G, Teitelbaum SL, Ross FP. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat Med. 2002;8:943–949. doi: 10.1038/nm752. [DOI] [PubMed] [Google Scholar]
- 46.Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–453. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Gaiteri C, Bodea L-G, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, Xie T, Tran L, Dobrin R, Fluder E, Clurman B, Melquist S, Narayanan M, Suver C, Shah H, Mahajan M, Gillis T, Mysore J, MacDonald ME, Lamb JR, Bennett DA, Molony C, Stone DJ, Gudnason V, Myers AJ, Schadt EE, Neumann H, Zhu J, Emilsson V. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell. 2013;153:707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui S, Xiong F, Hong Y, Jung J-U, Li X-S, Liu J-Z, Yan R, Mei L, Feng X, Xiong W-C. APPswe/Aβ regulation of osteoclast activation and RAGE expression in an age-dependent manner. J Bone Miner Res. 2011;26:1084–1098. doi: 10.1002/jbmr.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]


