Abstract
Haemophilia and its treatment interfere with patients' life, so health-related quality of life (HRQoL) should be assessed when evaluating treatments. This study investigated the HRQoL of patients with haemophilia A treated prophylactically with a new recombinant factor VIII. Two phase 3 trials investigated turoctocog alfa in patients with severe haemophilia A: one in children, one in adults and adolescents. HRQoL was a secondary endpoint assessed by the HAEMO-QOL age-specific, self-administered questionnaires. Parent-completed versions were also included for parents of children and adolescents. All HAEMO-QOL questionnaires allow the calculation of domain-specific and total scores ranging from 0 to 100, lower scores indicating better HRQoL. Mean change in all scores was described for 25 children aged 4–7 years, 21 children aged 8–12 years, 18 adolescents aged 13–18 years and 129 adults, overall, and according to the treatment regimen received prior to the study (on-demand; prophylaxis; mixed). Mean changes in HAEMO-QOL total score were 1.4 for children aged 4–7 years, −2.6 for children aged 8–12 years, −5.8 for adolescents and −1.6 for adults. In parent-completed versions, mean changes in total score were −6.0 for children aged 4–7 years, −4.7 for children aged 8–12 years, and −10.0 for adolescents. Patients receiving on-demand treatment before the trial showed greater improvement in HRQoL scores than patients already on prophylaxis. HRQoL of patients remained fairly stable over the course of the trials. However, improvements were observed for adolescents. Switching to prophylaxis was identified as a potential driver of improvement of HRQoL in patients with haemophilia A.
Keywords: haemophilia, health-related quality of life, prophylaxis, questionnaires, turoctocog alfa
Introduction
Haemophilia A is an X-linked recessive hereditary bleeding disorder resulting from a coagulation factor VIII (FVIII) deficiency. Haemophilia A is the most common type of haemophilia with a prevalence ranging from 8 to 20 per 100 000 male patients depending on the geographical location 1. Symptoms are mostly represented by bleeding episodes that can occur spontaneously or following an injury, trauma or surgical procedure. Repeated bleeding episodes can lead to long-term musculoskeletal complications including synovitis, degenerative arthropathy and articular deformities 2. Bleeding episodes in patients with haemophilia A are primarily treated by replacing the missing coagulation factor with recombinant or plasma-derived FVIII concentrate 3. Treatment of bleeding as they manifest (on-demand therapy) may delay the appearance of long-term complications, but does not prevent them. Therefore, prophylaxis with regular preventative FVIII infusions unrelated to the occurrence of bleeding is recommended, particularly in patients with severe haemophilia, from early childhood to at least 18 years of age 4–6. Several studies have shown that patients receiving prophylaxis have significantly fewer bleeding per year compared to those receiving on-demand treatment 7–9. After the introduction of factor replacement therapy, life expectancy and quality of life of haemophilia patients have greatly improved 10,11. However, treatment-related complications including development of inhibitors to FVIII, venous access problems, infusion-related pain and interference with lifestyle associated with the time-consuming nature of the treatment affect patients' quality of life in different ways and may affect adherence to treatment 12.
The human, recombinant, B-domain truncated factor VIII, turoctocog alfa, is a potential new treatment option for patients with haemophilia A 13. Two phase 3, multinational, open-label, non-randomized, non-comparative trials, guardian™ 1 in 24 adolescents and 126 adults and guardian™ 3 in 63 children have shown that turoctocog alfa is a well-tolerated, safe and effective option for the treatment of bleeding episodes 14,15. The observed success rate in the intention to treat analysis of haemostatic response was 81% and 92%, in the two trials respectively (85% and 94% when excluding missing data in the per protocol analysis). None of the patients developed FVIII inhibitors during the trials. Both trials demonstrated the efficacy of prophylactic treatment with turoctocog alfa in decreasing the number of bleeding per patient per year; the estimated mean annualized bleeding rates were 5.6, 6.7 and 5.3 bleeding/patient/year in adolescents, adults and children, respectively, which were lower than rates reported for previously treated patients on on-demand treatment 8,16.
In view of the increased awareness of the impact of haemophilia and its treatment on patients' lives (social, work or school, physical and psychological well-being), assessment of health-related quality of life (HRQoL) was a secondary endpoint assessed in the turoctocog alfa clinical trials. The specific objective of this article is to report the results of the assessment of HRQoL of patients with severe haemophilia in the guardian™ 1 and 3 trials and additionally to explore the effect of the switch from on-demand therapy to prophylaxis.
Materials and methods
Study design and patient population
This study reports outcomes recorded during the phase 3 multinational, open-label, non-controlled trials guardian™ 1 and 3. Further details on the study design of guardian™ 1 and 3 are reported elsewhere 14,15.
A total of 150 and 63 patients with severe haemophilia A were enrolled in the guardian™ 1 and 3 trials respectively. As per study protocol, enrolled patients had a documented history of at least 50 exposure days (ED) to FVIII products for children (with a median of 200 ED) or at least 150 ED for adolescents and adults (with a median of 516 ED) and a negative history of FVIII inhibitors, no increased risk of thromboembolic events and, if HIV positive, a CD4 + lymphocyte count above 200 μL−1. During the trials, patients were exposed to turoctocog alfa for a mean duration of 85 ED (ranging from 11 to 172) in guardian™ 1 and 60 ED (ranging from 10 to 104) in guardian™ 3; this corresponds to approximately 6 and 4.5 months respectively.
HRQoL was a secondary endpoint in these trials. It was assessed using the Haemophilia-Quality of Life (HAEMO-QOL) age-specific questionnaires twice, before starting the treatment when entering the trial and at the end-of-treatment visit. For children and adolescents, both the patients and their parents responded to the questionnaire to capture a comprehensive picture of their quality of life.
Both trials were approved by all relevant independent ethics committees and institutional review boards. All patients or their legally authorized representatives provided written informed consent before any trial-related activities. Both trials were conducted in accordance with the declaration of Helsinki and Good Clinical Practice and are registered at http://www.clinicaltrials.gov (NCT01138501 and NCT00840086).
The HAEMO-QOL questionnaires
The HAEMO-QOL family of questionnaires comprise four age-specific patient-reported outcome (PRO) instruments designed to assess HRQoL in patients with haemophilia. Two versions of HAEMO-QOL, one completed by parents and one by children, are available for each of the following age groups: 4–7 years (21 items covering 8 dimensions), 8–12 years (64 items covering 10 dimensions) and 13–16 years (77 items covering 12 dimensions). All versions are self-administered except the version for children aged 4–7 that is interviewer-administered. The development of the HAEMO-QOL was based on literature review and expert discussions. The age-specific preliminary questionnaires were tested with children and adolescents and their parents. The resulting questionnaires were created and further psychometrically validated with children aged 4–7 years (N = 95), children aged 8–12 years (N = 122) and adolescents aged 13–16 years (N = 103), and their parents (N = 309). The HAEM-A-QOL questionnaires demonstrated satisfactory internal consistency and test-retest reliability, and satisfactory construct validity 17. The adult HAEM-A-QOL questionnaire (46 items covering 10 dimensions) was developed afterwards to assess HRQoL in adults aged 17 years and above 18. The development of the HAEM-A-QOL included focus groups with patients, physicians and nurses, the draft version of the questionnaire was first compared with the items of the HAEMO-QoL questionnaire to have a core instrument available both for children and adults, before being comprehension tested and psychometrically validated 19. For all questionnaires in the HAEMO-QOL family, scoring rules have been defined to specify how dimension scores and a total score summarizing HRQoL of patients can be calculated, in particular when some items are missing. Scores range from 0 to 100; lower scores indicate better HRQoL.
Statistical analysis
Descriptive statistics were applied to study the changes in HAEMO-QOL scores from baseline to end-of-treatment by age group and, within each age group, according to the type of treatment regimen (prophylaxis, on-demand, or mixed) received within the 12 months preceding enrolment.
The percentage of patients with a change in score between baseline and end-of-treatment greater than a threshold representing a meaningful change in score (“HRQoL responders”) was also calculated for each HAEMO-QOL score in all age groups. Defining a “responder threshold” for a PRO measure is generally done using an “anchor-based” approach, i.e. using an external variable characterizing the meaning of a given change in HRQoL for a given patient 20. However, since no external variable that could be used as a relevant anchor was available, a “distribution-based” approach was used. The threshold used to categorize patients as “responders” for each HAEMO-QOL dimension score was defined by the standard error of measurement (SEM) of this dimension score 21,22. SEM was calculated as the product of standard deviation of the score for each dimension at baseline by the square root of one minus the reliability coefficient of the dimension 23. In this context, reliability coefficients were estimated using internal consistency reliability coefficients 24. As a lay interpretation, the “response threshold” calculated as proposed can be assimilated to the minimally detectable difference, while the use of an external anchor would have configured a clinically minimal important difference.
Categorical variables are presented as absolute and relative frequencies, while continuous variables are presented as mean (standard deviation). For graphical representation of continuous variables, box-and-whiskers plots are used to give a complete image of the distribution of the variable. All data processing and analyses were performed using SAS software for Windows version 9.2 (SAS institute, Inc., Cary, NC, USA).
Results
Study population
Some patients had to be excluded from HRQoL analyses because they did not match the age-specific groups defined by the HAEMO-QOL questionnaires collected in the trial in which they were included: three patients in guardian™ 1 were 12 years old and completed the HAEMO-QOL version for 8–12 years but could not be included in the analyses because they did not follow the same treatment protocol as the other patients aged 8–12 years included in guardian™ 3 and were too few to be analyzed meaningfully; 17 patients in guardian™ 3 were younger than 4 years old and thus too young to complete a HRQoL questionnaire. The remaining 193 patients for whom HAEMO-QOL data were available and included in this study were distributed across four age groups: 25 children aged 4–7 years, 21 children aged 8–12 years, 18 adolescents aged 13–16 years, and 129 adults aged 17–60 years. Mean age within each group was 4.8, 9.4, 14.4 and 30.1 years respectively. Two-thirds of children aged 4–12 years, 22% of adolescents and 36% of adults received full prophylaxis prior to the trial (Table1).
Table 1.
Demographic and clinical characteristics of the study populations at baseline.
| 4–7 years (n = 25) | 8–12 years (n = 21) | Adolescents (n = 18) | Adults (n = 129) | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 4.8 (0.9) | 9.4 (1.4) | 14.4 (1.2) | 30.1 (11.2) |
| Region*, n (%) | ||||
| North America | 5 (20.0%) | 4 (19.0%) | 1 (5.6%) | 28 (21.7%) |
| South America | 6 (24.0%) | 0 (0.0%) | 6 (33.3%) | 8 (6.2%) |
| Asia | 11 (44.0% | 8 (38.1%) | 4 (22.2%) | 41 (31.8%) |
| Europe | 3 (12.0%) | 9 (42.9%) | 7 (38.9%) | 52 (40.3%) |
| BMI, kg/m2 | ||||
| Mean (SD) | 15.5 (1.3) | 18.6 (3.5) | 19.7 (4.0) | 24.9 (4.6) |
| Time since diagnosis of haemophilia A, years | ||||
| Mean (SD) | 4.5 (1.4) | 8.8 (1.5) | 12.9 (3.1) | 28.6 (10.6) |
| Min–Max | 1.0–7.0 | 6.0–12.0 | 5.0–17.0 | 8.0–59.0 |
| Type of management in the 12 months preceding the trial, n (%) | ||||
| Prophylaxis | 17 (68.0%) | 14 (66.7%) | 4 (22.2%) | 46 (35.7%) |
| On-demand | 8 (32.0%) | 5 (23.8%) | 8 (44.4%) | 49 (38.7%) |
| Mixed regimen | 0 | 2 (9.5%) | 6 (33.3%) | 33 (25.6%) |
| Missing | 0 | 0 | 0 | 1 (0.8%) |
South America (Brazil); North America (USA); Asia (Israel, Japan, Malaysia, Russian Federation, Taiwan, Turkey); Europe (Croatia, Germany, Italy, Poland, Serbia, Spain, Switzerland, UK).
Quality of completion of the HAEMO-QOL questionnaires
More than two-thirds of the adults, as well as two-thirds of children and their parents completed the HAEMO-QOL at baseline and at end-of-treatment without any missing data (Table2). In contrast, at baseline/end-of-treatment the HAEMO-QOL was completed without any missing data by only 39%/61% of adolescents and 50%/44% of their parents. The mean percentage of missing items per patient was always lower than 10% (0.3–6.9%) for all versions, except for adolescents (12.9–19.8%) (Table2).
Table 2.
Missing assessments at baseline and end-of-treatment for all HAEMO-QOL versions.
| 4–7 years | 8–12 years | Adolescents | Adults | ||||
|---|---|---|---|---|---|---|---|
| Children | Parents | Children | Parents | Children | Parents | ||
| At baseline | |||||||
| Number of missing HAEMO-QOL items | |||||||
| Mean (SD) | 0.7 (1.6) | 1.4 (3.8) | 0.2 (0.5) | 0.1 (0.5) | 9.9 (24.5) | 15.3 (28.7) | 1.1 (5.3) |
| HAEMO-QOL questionnaire with no missing item | |||||||
| n (%) | 17 (68.0%) | 19 (76.0%) | 18 (85.7%) | 18 (85.7%) | 7 (38.9%) | 9 (50.0%) | 100 (77.5%) |
| At end-of-treatment | |||||||
| Number of missing HAEMO-QOL items | |||||||
| Mean (SD) | 0.3 (0.9) | 0.8 (1.3) | 1.0 (3.7) | 1.1 (3.5) | 13.9 (29.1) | 14.7 (28.9) | 1.5 (7.1) |
| HAEMO-QOL questionnaire with no missing item | |||||||
| n (%) | 18 (75.0%) | 16 (66.7%) | 18 (85.7%) | 16 (76.2%) | 11 (61.1%) | 8 (44.4%) | 107 (84.3%) |
Description of HAEMO-QOL scores at baseline
Mean baseline HAEMO-QOL total scores were 30.0 for children aged 4–7 years, 26.1 for children aged 8–12 years, 31.4 for adolescents and 33.4 for adults (Table3). In parent-completed versions, mean baseline HEAMO-QOL total scores were 31.0 for children aged 4–7 years, 29.3 for children aged 8–12 years, 35.6 for adolescents.
Table 3.
Baseline scores, mean changes and percentage of responders for HAEMO-QOL dimension and total scores for all versions.
| Children-completed versions | Parents/adults-completed versions | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline mean score (SD) | Mean change (SD) | Responder threshold | Responders (%) | Baseline mean score (SD) | Mean change (SD) | Responder threshold | Responders (%) | |
| 4–7 years | ||||||||
| Physical health | 27.8 (20.7) | −9.9 (31.9) | −13.3 | 29.2 | 27.9 (19.6) | −6.8 (25.8) | −12.1 | 41.7 |
| Feeling | 17.5 (23.9) | 10.8 (28.8) | −10.1 | 16.7 | 18.4 (21.0) | −2.7 (25.9) | −10.0 | 37.5 |
| View | 14.3 (16.9) | 5.6 (20.2) | −15.6 | 12.5 | 15.8 (19.3) | −8.9 (20.2) | −12.2 | 25.0 |
| Family | 47.0 (27.1) | 4.9 (27.5) | −13.1 | 16.7 | 47.8 (29.4) | −5.4 (22.1) | −9.5 | 29.2 |
| Friend | 25.0 (33.6) | 21.1 (30.3) | –* | – | 41.7 (28.2) | −6.6 (26.1) | –* | – |
| Others | 17.0 (21.0) | 1.3 (34.8) | −16.0 | 29.2 | 16.3 (19.0) | −5.6 (19.6) | −11.7 | 37.5 |
| Sport | 37.7 (25.4) | −6.3 (26.4) | −19.4 | 12.5 | 40.4 (21.6) | 0.5 (25.1) | −18.4 | 12.5 |
| Treatment | 40.5 (34.9) | −4.2 (47.9) | −20.3 | 33.3 | 29.5 (22.3) | −0.6 (27.3) | −19.6 | 16.7 |
| Total score | 30.0 (13.6) | 1.4 (13.4) | −7.1 | 16.7 | 31.0 (14.4) | −6.0 (13.3) | −5.8 | 37.5 |
| 8–12 years | ||||||||
| Physical health | 27.0 (17.8) | −11.3 (19.3) | −8.1 | 57.1 | 28.8 (15.3) | −17.7 (14.2) | −8.1 | 61.9 |
| Feeling | 16.7 (14.7) | −5.4 (15.3) | −7.2 | 38.1 | 25.6 (17.8) | −4.0 (23.7) | −7.0 | 38.1 |
| View | 21.7 (12.5) | −1.4 (21.8) | −8.5 | 33.3 | 24.5 (14.5) | −0.9 (20.6) | −10.1 | 19.0 |
| Family | 33.1 (15.1) | 5.3 (16.6) | −12.5 | 9.5 | 33.4 (17.0) | 1.1 (13.7) | −9.3 | 28.6 |
| Friend | 38.4 (27.0) | 0.6 (33.4) | −12.7 | 23.8 | 39.1 (23.7) | 4.4 (29.4) | −10.7 | 23.8 |
| Perceived support | 44.6 (23.7) | −1.2 (25.6) | −10.9 | 33.3 | 46.1 (17.5) | −1.0 (22.2) | −10.6 | 28.6 |
| Others | 18.7 (16.6) | −3.0 (17.7) | −9.0 | 28.6 | 21.5 (17.7) | −1.4 (19.0) | −7.0 | 28.6 |
| Sport | 34.3 (15.7) | −4.2 (14.4) | −12.3 | 33.3 | 40.9 (15.6) | −10.0 (16.8) | −13.3 | 23.8 |
| Dealing | 22.8 (17.4) | 0.7 (19.3) | −7.6 | 23.8 | 24.2 (15.4) | −4.4 (17.0) | −7.3 | 38.1 |
| Treatment | 18.4 (17.3) | −3.2 (12.9) | −11.7 | 28.6 | 20.3 (11.4) | −7.0 (14.9) | −8.1 | 38.1 |
| Total score | 26.1 (8.9) | −2.6 (10.7) | −3.8 | 42.9 | 29.3 (8.5) | −4.7 (13.7) | −2.5 | 52.4 |
| Adolescents | ||||||||
| Physical health | 33.7 (23.2) | −15.0 (18.4) | −8.6 | 35.3 | 35.4 (27.2) | −11.8 (19.1) | −8.3 | 35.3 |
| Feeling | 21.7 (16.5) | −7.3 (12.3) | −9.8 | 23.5 | 34.5 (15.6) | −11.6 (19.1) | −7.7 | 29.4 |
| View | 31.8 (19.6) | −7.5 (18.4) | −16.0 | 23.5 | 30.5 (16.4) | −7.6 (19.0) | −14.0 | 29.4 |
| Family | 35.2 (20.6) | −4.4 (17.9) | −10.0 | 29.4 | 39.6 (14.7) | −3.1 (13.8) | −8.9 | 23.5 |
| Friend | 51.7 (26.9) | −1.0 (29.1) | −14.4 | 23.5 | 50.0 (27.1) | −10.9 (26.7) | −11.5 | 29.4 |
| Perceived support | 43.3 (18.2) | 7.3 (26.4) | −12.4 | 17.6 | 46.4 (18.8) | −8.3 (19.1) | −11.6 | 29.4 |
| Others | 20.8 (20.2) | −5.9 (19.3) | −9.8 | 23.5 | 28.6 (21.7) | −16.0 (20.4) | −7.7 | 47.1 |
| Sport | 44.2 (17.8) | −14.1 (17.3) | −20.3 | 17.6 | 50.7 (16.0) | −20.2 (13.3) | −20.2 | 29.4 |
| Dealing | 24.8 (15.9) | −5.5 (18.6) | −8.4 | 29.4 | 25.4 (14.1) | −4.0 (7.5) | −8.7 | 11.8 |
| Treatment | 24.6 (15.2) | −8.1 (9.9) | −12.1 | 23.5 | 26.2 (13.2) | −13.1 (13.6) | −11.7 | 29.4 |
| Future | 30.0 (10.1) | 9.4 (25.8) | −8.9 | 11.8 | 31.3 (20.1) | −1.0 (23.5) | −26.3 | 11.8 |
| Relationship | 9.6 (14.6) | −5.0 (19.7) | −9.4 | 23.5 | 13.6 (30.3) | −5.6 (11.0) | −7.5 | 11.8 |
| Total score | 31.4 (9.6) | −5.8 (10.0) | −4.8 | 35.3 | 35.6 (10.3) | −10.0 (7.1) | −5.0 | 52.9 |
| Adults | ||||||||
| Physical health | – | – | – | – | 41.6 (24.5) | −4.9 (20.6) | −8.9 | 39.5 |
| Feeling | – | – | – | – | 26.9 (24.0) | −1.8 (17.6) | −10.1 | 31.1 |
| View | – | – | – | – | 37.4 (21.1) | −0.8 (16.7) | −20.3 | 11.8 |
| Family planning | – | – | – | – | 17.9 (26.7) | 3.0 (16.8) | −11.9 | 10.1 |
| Work | – | – | – | – | 26.8 (26.1) | −3.8 (18.0) | −16.5 | 17.6 |
| Sport | – | – | – | – | 54.5 (22.3) | −1.0 (18.7) | −16.4 | 11.8 |
| Dealing | – | – | – | – | 19.7 (18.9) | −1.7 (20.7) | −10.0 | 20.2 |
| Treatment | – | – | – | – | 32.7 (16.9) | −0.8 (15.2) | −9.7 | 18.5 |
| Future | – | – | – | – | 39.2 (24.4) | −1.9 (15.4) | −16.0 | 16.0 |
| Partnership | – | – | – | – | 18.2 (28.8) | −0.7 (18.5) | −10.0 | 15.1 |
| Total score | – | – | – | – | 33.4 (16.0) | −1.6 (8.9) | −6.3 | 24.4 |
Single-item dimension not enabling the calculation of the internal consistency coefficient, thus threshold could not be computed.
Change in scores from baseline to end-of-treatment
Mean changes (standard deviation) in HAEMO-QOL total score from baseline to end-of-treatment were 1.4 (13.4) for children aged 4–7 years, −2.6 (10.7) for children aged 8–12 years, −5.8 (10.0) for adolescents and −1.6 (8.9) for adults (Fig.1). In parent-completed versions, mean changes in HAEMO-QOL total score from baseline to end-of-treatment were −6.0 (13.3) for children aged 4–7 years, −4.7 (13.7) for children aged 8–12 years, and −10.0 (7.1) for adolescents (Fig.1).
Fig 1.
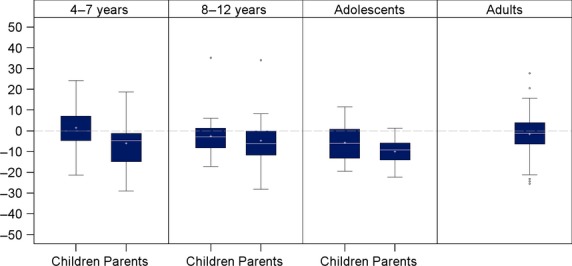
Mean change in HAEMO-QOL total score for each version from baseline to end-of-treatment. A negative change in HAEMO-QOL total score indicates improved HRQoL. Box plots present the following: interquartile range (Q1-Q3); +: mean; −: median; bottom and top bars: observed minimum and maximum observed values; ○: outliers (i.e. values that are outside the distance of 1.5 times the interquartile range from Q1 or Q3).
Mean changes in all HAEMO-QOL scores, response threshold and percentage of responders are presented in Table3. In dimensions constituting HRQoL, children aged 4–7 years reported improvement (negative mean change in score) from baseline to end-of-treatment in Physical health, Sport and Treatment and worsening in other dimensions, with the greatest worsening for Friend. Their parents reported improvement in all HAEMO-QOL dimensions, except Sport. Children aged 8–12 years reported improvement from baseline to end-of-treatment in seven dimensions and their parents in eight dimensions; the most improved was Physical health for both. Adolescents reported improvement from baseline to end-of-treatment in all dimensions except Future and Perceived support, while their parents reported improvement for their children in all dimensions. Adults reported few changes in all HAEMO-QOL scores. In summary, improvements were mainly seen on physical aspects while the few scores showing worsening were related to psychosocial aspects.
A notable improvement in HAEMO-QOL total score (i.e. improvement greater than the response threshold) was more frequently observed in adolescents and children aged 8–12 years; 42.9% of children aged 8–12 years and 35.3% of adolescents were classified as “HRQoL responders,” as opposed to 24.4% of adults and only 16.7% of children aged 4–7 years.
Change in scores from baseline to end-of-treatment according to the previous treatment regimen
As shown in Fig.2, greater improvement in HAEMO-QOL total score (i.e. greater negative mean change) were consistently shown in patients receiving on-demand therapy within the 12 months preceding the trial than patients who had been receiving prophylaxis. Patients who had been receiving mixed on-demand and prophylactic therapy prior to the trial also showed generally greater improvement than patients who had been on prophylaxis; only the parent-completed version for adolescents showed a larger improvement in patients already on prophylaxis.
Fig 2.
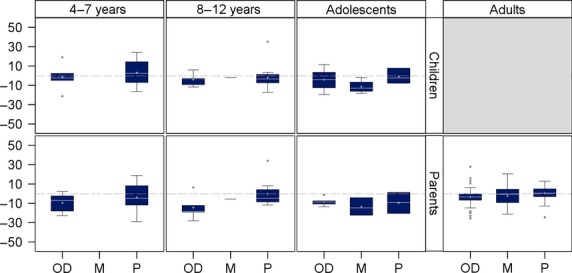
Mean change in HAEMO-QOL total score for each version from baseline to end-of-treatment, according to the type of regimen patients received within the 12 months preceding the trial. A negative change in HAEMO-QOL total score indicates improved HRQoL. (OD: on-demand/M: mixed regimen/P: prophylaxis). Box plots present the following: interquartile range (Q1–Q3); +: mean; −: median; bottom and top bars: observed minimum and maximum observed values; ○: outliers (i.e. values that are outside the distance of 1.5 times the interquartile range from Q1 or Q3).
Discussion
The objective of this study was to investigate the HRQoL of 193 patients with severe haemophilia A aged 4–60 years treated with turoctocog alfa, a new recombinant factor VIII, and additionally to explore the effect of the switch from on-demand therapy to prophylaxis. HRQoL, assessed using the HAEMO-QOL set of questionnaires, was a secondary endpoint of two phase 3 multinational clinical trials, guardian™ 1 and 3 14,15. Regardless of the type of regimen they had received before being recruited, all patients received bleeding preventative replacement therapy throughout the trials. This study showed that HRQoL during the trials was fairly stable for patients already treated with prophylaxis, while patients switching from on-demand therapy to prophylaxis seemed to benefit most in terms of HRQoL. This reinforces the importance of prophylactic treatment of haemophilia 25 and suggests that switching from on-demand to prophylactic treatment is potentially a driver for improvement of HRQoL in patients with haemophilia 8,26.
We chose to analyze the HAEMO-QOL data using two complementary approaches: the description of the mean change in score over the trials and the description of “HRQoL” responders, i.e. patients who experienced a meaningful improvement in HRQoL. The former approach gives an overall trend of the change in HRQoL scores at the group level while the latter provides an estimate of individual changes. The change in score over the trial was considered as absolute rather than relative values (i.e. percentage of change from baseline) controlling for the variability in baseline scores. The use of absolute change was enforced by the features of HAEMO-QOL scales. This can be illustrated as follows: the direction of the HAEMO-QOL scales (higher score for poorer HRQoL) is arbitrary and might as well have been reversed so that a higher score would correspond to a better HRQoL (which is the case for most other HRQoL instruments). In that case, a given change in a patient's HRQoL will correspond to the same absolute change but not the same relative change (a 10-point change from 20 and from 80 does not correspond to the same percentage). Hence, analyses of absolute change were preferred over analyses of relative change.
This study offers a set of results that could underpin hypotheses for future research related to the benefit of haemophilia treatment in terms of HRQOL. First, some domains of HRQoL may be more prone to improvement than others: improvements were shown in physical aspects of children's and adolescents' HRQoL (but not of adults') over the course of the trial, while psychosocial aspects were at best unchanged. The frequent differences observed between child and parent ratings, regardless of the age of the child, are another interesting finding. For example, the worsening of adolescents' view of future and perceived support from others was not reflected in their parents' scores. Likewise, for the younger patients' (aged 4–7 years): their perceived worsening of relationships to friends and family and in emotional aspects was not reported by their parents. These discordant results between parents' and children's reports could reflect the difference in values placed on certain aspects of HRQoL by children and adolescents compared to their parents. However, it may also pose the question of the reliability of reports of children, in particular for young children (below 8 years). In summary, our results could be useful to inform decisions on HRQoL domains of specific interest for future studies in haemophilia or to emphasize the need to carefully determine the respondent to HRQoL questionnaires (self-report vs. proxy-report).
The analysis of the limitations of of HRQoL analysis in the guardian™ 1 and 3 trials also gave rise to identification of some potential challenges for future studies investigating the impact of haemophilia treatment on HRQoL. These limitations are largely dependent on the constraints of running clinical trials in rare diseases. First, both trials were non-comparative open-label trials. It is particularly challenging to demonstrate an effect on HRQoL with this kind of design because HRQoL-specific constraints are seen in addition to limitations caused by the absence of randomization and blinding: (i) when patients are aware that they are taking a new product, it may, consciously or not, impact their perceptions and responses to HRQoL questionnaires; (ii) the interpretation of change in HRQoL scores is particularly complicated in the absence of a comparator arm because HRQoL scores generally cannot be interpreted directly in a meaningful manner. Specifically, cumulative distribution functions are often recommended to support the interpretation of PRO results 27; the absence of a comparator arm makes the use of this method less appealing since the interpretation of these functions is uneasy in this setting. Moreover, due to haemophilia being a rare disease, trials are of limited size. In addition, trial samples have to be split into subgroups for HRQoL assessment to be adapted to patient age. Therefore, HRQoL results can only be obtained for even smaller samples. This is not only an issue for the interpretation of the HRQoL findings, obtained on small samples, but it also makes some statistical techniques that are generally used with these outcomes more difficult to apply: for instance, anchor-based approach to the definition of responder, which is generally preferred over distribution-based approach 27, is difficult to apply in this context since it would require running analyses on subgroups of patients within each age category and therefore would mean performing statistical analyses on an handful of patients only. Finally, the duration of follow-up of the guardian™ trials was relatively short (about 6 months). Unfortunately the impact of a new treatment in terms of HRQoL may not be fully captured in this timeframe so HRQoL should be considered with longer follow-up (e.g. in long-term extension of trials). In conclusion, the experience of the guardian™ 1 and 3 trials could be useful for considerations on how to measure HRQoL in future haemophilia trials as they show the methodological challenges to overcome.
In this challenging context, it is also important to keep in mind that a key factor in assessing HRQoL is the choice of the measurement instrument. It is extremely important to use a very specific instrument, acceptable to the subjects (limiting the amount of missing data) and focused on very precise concepts, thus producing reliable and accurate results. These two aspects (limitation of missing data and reliability of the assessment) are critical in the case of small samples. In this context, the HAEMO-QOL questionnaires were good candidates for assessment of HRQoL in haemophilia clinical trials as they are specifically designed with and for patients with haemophilia. They were well-accepted in both guardian™ 1 and 3 as shown by the good quality of completion for all age groups and for both children and parent-completed versions supporting its use in future studies on HRQoL in patients with haemophilia.
Finally, we have identified some topics that may be of interest for future studies on HRQoL in patients with haemophilia. Exploring the link between HRQoL and frequency of treatment administration may be worthwhile, especially as it has been reported that patients would prefer a coagulation factor with a longer half-life to reduce the number of injections per week 28. Future studies could also investigate whether the time elapsed from the patient's last bleeding episode and questionnaire answering has an impact on the reported perception of HRQoL.
In conclusion, this study shows that patients switched to turoctocog alfa did not have changes in HRQoL as expected in a chronic disease with replacement therapy. The results support that treatment with turoctocog alfa did not improve nor worsen HRQoL of patients who had been on prophylaxis regimens prior to enrolment, in line with the clinically reliable performance of turoctocog alfa 14,15. Furthermore, the results support that the switch to prophylactic treatment is a potential key driver for improvement of HRQoL in patients with haemophilia who have earlier been treated on-demand.
Acknowledgments
This study was funded by Novo Nordisk A/S, Denmark. The authors thank all participating investigators, patients and trial staff. The authors also thank Juliette Meunier (Mapi) and Fatoumata Fofana (Mapi) for support with the statistical analysis, and Jérémy Lambert, PhD (Mapi) for medical writing assistance and editorial support in manuscript preparation.
Dr. Sylvia von Mackensen is the developer of all HAEMO-QOL questionnaires. The HAEMO-QOL questionnaires are a joint property of the Haemo-QoL Study Centre at the University of Hamburg, Germany and Bayer Biological Products, Research Triangle Park, North Carolina, USA.
Author contributions
ES, SL, AI and AKB made substantial contributions to analysis and interpretation of data. AR designed and performed data analyses and participated in data interpretation. All authors assisted with the manuscript outline, gave input, reviewed and approved the manuscript.
Disclosures
ES received research support from Novo Nordisk and Pfizer and has been a consultant to Bayer, Baxter, Pfizer, CSL Behring, Novo Nordisk, Kedrion and Grifols. SL received research support from Novo Nordisk and has been a consultant to Novo Nordisk. AI received research support from Bayer, Biogen, Baxter and Novo Nordisk and has been a consultant to Bayer, Baxter, Novo Nordisk and Pfizer. AKB is employee of Novo Nordisk A/S. AR, employee of Mapi, was paid consultant to Novo Nordisk A/S.
References
- 1.Stonebraker JS, Bolton-Maggs PH, Soucie JM, Walker I, Brooker M. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia. 2010;16:20–32. doi: 10.1111/j.1365-2516.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Merchan EC. Prevention of the musculoskeletal complications of hemophilia. Adv Prev Med. 2012;2012:201271. doi: 10.1155/2012/201271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–e47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 4.Berntorp E, Boulyjenkov V, Brettler D, et al. Modern treatment of haemophilia. Bull World Health Organ. 1995;73:691–701. [PMC free article] [PubMed] [Google Scholar]
- 5.Coppola A, Di CM, De SC. Primary prophylaxis in children with haemophilia. Blood Transfus. 2008;6(Suppl 2):s4–11. doi: 10.2450/2008.0030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Hemophilia foundation. MASAC (Medical and Scientific Advisory Council) Recommendation 179. MASAC Recommendation Concerning Prophylaxis (Regular Administration of Clotting Factor Concentrate to Prevent Bleeding) 2013. Available at http://www.hemophilia.org/NHFWeb/MainPgs/MainNHF.aspx?menuid=57&contentid=1007. Accessed June 24.
- 7.Khoriaty R, Taher A, Inati A, Lee C. A comparison between prophylaxis and on demand treatment for severe haemophilia. Clin Lab Haematol. 2005;27:320–3. doi: 10.1111/j.1365-2257.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- 8.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosendaal FR, Smit C, Varekamp I, et al. Modern haemophilia treatment: medical improvements and quality of life. J Intern Med. 1990;228:633–40. doi: 10.1111/j.1365-2796.1990.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 11.Franchini M, Mannucci PM. Past, present and future of hemophilia: a narrative review. Orphanet J Rare Dis. 2012;7:24. doi: 10.1186/1750-1172-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khair K, Lawrence K, Butler R, O'Shea E, Christie BA. Assessment of treatment practice patterns for severe hemophilia A: a global nurse perspective. Acta Haematol. 2008;119:115–23. doi: 10.1159/000121828. [DOI] [PubMed] [Google Scholar]
- 13.Thim L, Vandahl B, Karlsson J, et al. Purification and characterization of a new recombinant factor VIII (N8) Haemophilia. 2010;16:349–59. doi: 10.1111/j.1365-2516.2009.02135.x. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni R, Karim FA, Glamocanin S, et al. Results from a large multinational clinical trial (guardian3) using prophylactic treatment with turoctocog alfa in paediatric patients with severe haemophilia A: safety, efficacy and pharmacokinetics. Haemophilia. 2013;19:698–705. doi: 10.1111/hae.12165. [DOI] [PubMed] [Google Scholar]
- 15.Lentz SR, Misgav M, Ozelo M, et al. Results from a large multinational clinical trial (guardian1) using prophylactic treatment with turoctocog alfa in adolescent and adult patients with severe haemophilia A: safety and efficacy. Haemophilia. 2013;19:691–7. doi: 10.1111/hae.12159. [DOI] [PubMed] [Google Scholar]
- 16.Valentino LA, Mamonov V, Hellmann A, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10:359–67. doi: 10.1111/j.1538-7836.2011.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Mackensen S, Bullinger M. Development and testing of an instrument to assess the Quality of Life of Children with Haemophilia in Europe (Haemo-QoL) Haemophilia. 2004;10(Suppl 1):17–25. doi: 10.1111/j.1355-0691.2004.00875.x. [DOI] [PubMed] [Google Scholar]
- 18.Riva S, Bullinger M, Amann E, von MS. Content comparison of haemophilia specific patient-rated outcome measures with the international classification of functioning, disability and health (ICF, ICF-CY) Health Qual Life Outcomes. 2010;8:139. doi: 10.1186/1477-7525-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Mackensen S, Gringeri A, Ravera S, et al. Validation of the haemophilia-specific quality of life questionnaire for adult patients with haemophilia (Haem-A-QoL) Haematologica. 2005;90(Suppl. 2):115–6. [Google Scholar]
- 20.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–9. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–73. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 22.Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. 1999;37:469–78. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Nunnally JC, Bernstein IH. Psychometric theory. New York: McGraw-Hill Inc; 1994. 3rd ed. [Google Scholar]
- 24.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 25.Walsh CE, Valentino LA. Factor VIII prophylaxis for adult patients with severe haemophilia A: results of a US survey of attitudes and practices. Haemophilia. 2009;15:1014–21. doi: 10.1111/j.1365-2516.2009.02036.x. [DOI] [PubMed] [Google Scholar]
- 26.Manco-Johnson MJ, Nuss R, Geraghty S, Funk S, Kilcoyne R. Results of secondary prophylaxis in children with severe hemophilia. Am J Hematol. 1994;47:113–7. doi: 10.1002/ajh.2830470209. [DOI] [PubMed] [Google Scholar]
- 27.U.S.Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, and Center for Devices and Radiological Health. Guidance for industry. Patient-reported outcome measures. Use in medical product development to support labeling claims. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed December 2013.
- 28.Mantovani LG, Monzini MS, Mannucci PM, Scalone L, Villa M, Gringeri A. Differences between patients', physicians' and pharmacists' preferences for treatment products in haemophilia: a discrete choice experiment. Haemophilia. 2005;11:589–97. doi: 10.1111/j.1365-2516.2005.01159.x. [DOI] [PubMed] [Google Scholar]


