Abstract
Background
Oclacitinib (Apoquel®) inhibits the function of a variety of pro-inflammatory, pro-allergic and pruritogenic cytokines that are dependent on Janus kinase enzyme activity. Oclacitinib selectively inhibits Janus kinase 1.
Hypothesis/Objectives
We aimed to evaluate the safety and efficacy of oclacitinib for the control of pruritus associated with allergic dermatitis in a randomized, double-blinded, placebo-controlled trial.
Methods
Client-owned dogs (n = 436) with moderate to severe owner-assessed pruritus and a presumptive diagnosis of allergic dermatitis were enrolled. Dogs were randomized to either oclacitinib at 0.4–0.6 mg/kg orally twice daily or an excipient-matched placebo. An enhanced 10 cm visual analog scale (VAS) was used by the owners to assess the severity of pruritus from day 0 to 7 and by veterinarians to assess the severity of dermatitis on days 0 and 7. Dogs could remain on the study for 28 days.
Results
Pretreatment owner and veterinary VAS scores were similar for the two treatment groups. Oclacitinib produced a rapid onset of efficacy within 24 h. Mean oclacitinib Owner Pruritus VAS scores were significantly better than placebo scores (P < 0.0001) on each assessment day. Pruritus scores decreased from 7.58 to 2.59 cm following oclacitinib treatment. The day 7 mean oclacitinib Veterinarian Dermatitis VAS scores were also significantly better (P < 0.0001) than placebo scores. Diarrhoea and vomiting were reported with similar frequency in both groups.
Conclusions and clinical importance
In this study, oclacitinib provided rapid, effective and safe control of pruritus associated with allergic dermatitis, with owners and veterinarians noting substantial improvements in pruritus and dermatitis VAS scores.
Introduction
Dermatological problems are the second most common reason for dogs to present to veterinary practices.1,2 These frequently include pruritic conditions, such as parasitic infestations and allergic skin diseases.3–5
Veterinarians treating pruritic dogs have the following two goals: (i) to reduce or eliminate the pruritus, which breaks the itch cycle, allowing the skin to heal, preventing chronic inflammatory changes and secondary infection, and reducing patient and owner discomfort and distress; and (ii) to diagnose and manage the cause of the pruritus.6–8
Glucocorticoids are widely used to treat pruritic dogs. They are highly effective, but short-term and chronic adverse effects are common. Acute problems, such as polyuria, polydipsia, polyphagia, inappropriate urination in homes, behavioural changes and panting, can be a problem for the pet owner, interfere with the quality of life of the dog and result in decreased owner compliance. Long-term administration of glucocorticoids may result in serious health conditions, including pancreatitis, gastrointestinal ulceration, lipidaemia, diabetes mellitus, muscle wasting and iatrogenic hyperadrenocorticism.6,9 Topical glucocorticoids can be effective and well tolerated10 but are not suitable for generalized pruritus. Antihistamines have shown only minimal efficacy in the treatment of canine pruritus.11 Systemic ciclosporin and topical tacrolimus can effectively control atopic dermatitis, but the delayed onset of action makes it impractical as a stand-alone therapy for the rapid management of pruritus.11 Essential fatty acids can improve the skin barrier and help to ameliorate atopic dermatitis, but are generally not the first choice treatment to control acute pruritus.12
The pathophysiology of pruritus is complex and, until recently, poorly understood. Recent research has shown that pruritogenic cytokines are a major stimulus of pruritic behaviour in dogs.13 This knowledge has allowed researchers to investigate more targeted and effective antipruritic therapies. Oclacitinib is a novel targeted therapy that selectively inhibits key pathways involved in itch and inflammation associated with allergy.14 Oclacitinib selectively inhibits Janus kinase 1-dependent cytokines in cellular assays with minimal effects against Janus kinase 2-dependent cytokines involved in haematopoiesis. Janus kinase 1 enzyme activities play a central role in cytokine signalling and are involved in the signal transduction of many pro-inflammatory, pro-allergic and pruritogenic cytokines implicated in atopic dermatitis, including interleukin (IL)-2, IL-4, IL-6 and IL-13.15–18 Janus kinases are also involved in the signalling of IL-31, a recently identified cytokine that has been shown to play a key role in canine pruritus.13 Oclacitinib has been shown to inhibit IL-31 cytokine function strongly in dogs and thus it may significantly reduce pruritus.17
The aim of this study was to evaluate the safety and efficacy of oclacitinib compared with a placebo for the control of pruritus associated with allergic dermatitis in client-owned dogs.
Materials and methods
Study design
The study was conducted as a double-blinded, placebo-controlled clinical trial with a randomized complete block design replicated at 26 sites throughout the USA; 24 of the participating veterinarians were general practitioners with an interest in dermatology and two were veterinary dermatology specialists.
Oversight
This study complied with all applicable animal welfare regulations related to the humane care and use of animals. The protocol was approved by each study site's Institutional Animal Care and Use Committee (IACUC) prior to initiation of the study at that site. For those sites in which there was no IACUC, the protocol was reviewed and approved prior to study initiation by the Pfizer Ethical Review Board. The study was conducted in compliance with Guidance for Industry Good Clinical Practice, No. 85 (VICH GL9).19 The owners gave written informed consent for each dog to participate in the study.
Inclusion criteria
All dogs were client owned, 6 months of age or older and in overall good health based on the initial (day 0) physical examination. Dogs had to weigh between 3 and 80 kg. Dogs were assessed by their owners as having moderate to severe itching (pruritus), using a categorical scale.20 A presumptive diagnosis of pruritus associated with allergic dermatitis was established based on the dog's history, clinical signs and the owner's presenting complaint. Veterinarians attributed the dog's pruritic condition to one or more of the following presumptive diagnoses: atopic dermatitis (AD), flea allergy dermatitis, food allergy dermatitis, contact dermatitis, sarcoptic mange or an unspecified allergic dermatitis. Dogs in which sarcoptic mange was suspected were skin scraped; however, the presence of a mite was not required for enrolment.
Dogs with other conditions that required concomitant treatment could be enrolled if the treatment remained the same for at least the 6 weeks prior to the study and no change in medication was anticipated during the study. Appropriate flea or sarcoptic mange treatment was implemented where evidence was found on examination or where infestation with fleas or sarcoptic mites was suspected but not definitively confirmed. All dogs were maintained on appropriate flea prevention for the duration of the study. Dogs that were receiving a hypoallergenic diet to manage previously diagnosed adverse food reactions had to have been on that diet for at least 6 weeks prior to day 0 and must have remained on the same diet during the study. Dogs that were presumed to be food allergic on day 0 were permitted to start on a hypoallergenic diet at the time of the day 0 visit. Intradermal allergen tests had to have been conducted at least 8 weeks prior to the start of the study. Concomitant allergen-specific immunotherapy had to have been ongoing for at least 6 months prior to enrolment and the protocol must have been maintained throughout the study. If allergen-specific immunotherapy was discontinued, it had to be discontinued at least 8 weeks prior to enrolment.
Prohibited and conditionally allowed medications and therapies
Withdrawal times for prohibited medications were as follows: long-acting injectable glucocorticoids, 6 weeks; oral glucocorticoids, ciclosporin, long-acting injectable antimicrobial agents and miscellaneous compounds with known antipruritic activity (e.g. Staphage Lysate (SPL®; Delmont Laboratories Inc., Swarthmore, PA, USA), gabapentin, monoamine oxidase inhibitors and tacrolimus), 4 weeks; topical nonsteroidal anti-inflammatory drugs and topical glucocorticoids, 3 weeks; antihistamines, 2 weeks; and oral antibacterial/antifungal agents, 1 week.
Exclusion criteria
Exclusion criteria included the following: dogs with evidence of malignant neoplasia, demodicosis or immune suppression, such as hyperadrenocorticism; dogs that were receiving or should have been receiving antimicrobial therapy for bacterial folliculitis or fungal dermatitis; and lactating bitches or dogs (male or female) intended for use as breeding animals. Dogs with clinically significant abnormalities in their pretreatment complete blood count, serum chemistry or urinalysis tests were withdrawn from the study.
Randomization and masking
Dogs were randomized to one of two treatment groups (i.e. oclacitinib or placebo) in a 1:1 ratio. Blocking was based on order of enrolment within clinic. The dog was the experimental unit.
All clinical trial personnel, the owner and the laboratory were blinded to the treatment group assignments. The placebo and oclacitinib tablets were identical in size and appearance. An interactive voice response system (IVRS; Almac Clinical Technologies, Yardley, PA, USA) was used to manage patient treatment assignment and blinded drug dispensing. Upon each dog's enrolment, the sites accessed the IVRS system, and the system then randomized the dogs to the respective treatment group.
Drug administration
Dogs in the oclacitinib treatment group were given oclacitinib maleate caplets orally at a dose of 0.4–0.6 mg/kg twice daily. The scored caplets were provided in three strengths containing 3.6, 5.4 and 16 mg of oclacitinib. Dogs in the placebo treatment group were given the same number of caplets, identical in appearance to oclacitinib maleate caplets and containing all of the same excipients except oclacitinib maleate. Owners administered the study drug at home, with or without food,21 and were instructed to maintain as close to a 12 h interval between doses as possible.
Study schedule and variables measured
Following randomization, the dogs were assigned to receive either the excipient placebo or oclacitinib at a dose of 0.4–0.6 mg/kg, orally twice daily from day 0 to day 7 (+3 days; study phase). If, in the veterinarian's clinical judgment, the pruritic condition resolved or improved to a point that no additional therapy was indicated, day 7 was regarded as the final study day. Dogs in which the underlying diagnosis (presenting complaint) was not resolved at the end of the study phase, but that had responded well to therapy, were permitted to remain on therapy (either placebo or oclacitinib) up to day 28 (±2 days; continuation phase). Certain concurrent medications not permitted for use on days 0–7 could be added on or after day 8 (e.g. systemic antimicrobial drugs). Glucocorticoids, antihistamines, ciclosporin or other immunosuppressive drugs were not permitted during either phase of the study. Dogs were withdrawn if the owner or veterinarian felt that their pruritus and/or dermatitis required treatment with a prohibited medication. Owners were free to withdraw their dog at any point.
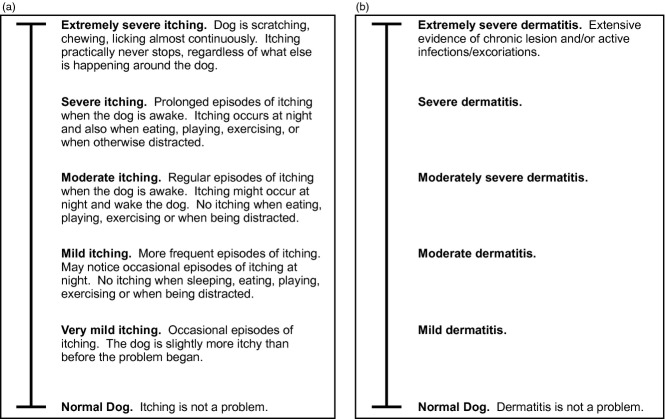
Baseline data (demographics, physical examination, assessments of pruritus and dermatitis, and whether flea control was applied on day 0) were collected on enrolment at day 0. An enhanced visual analog scale (VAS) score was used by both dog owners and veterinarians. The VAS scale consisted of a 10 cm line with word descriptors at 2 cm intervals. Owners were asked to assess the severity of the ‘itch’, and veterinarians were asked to assess the severity of ‘dermatitis’ (Figure1a,b). The enhanced Owner Pruritus VAS had six descriptors of pruritus evenly spaced at 2 cm intervals with ‘normal dog’ at 0 cm and ‘extremely severe itching’ at 10 cm. The enhanced Veterinarian Dermatitis VAS had six descriptors of dermatitis evenly spaced at 2 cm intervals with ‘normal dog’ at 0 cm and ‘extremely severe dermatitis’ at 10 cm.
Figure 1.
(a) Enhanced Owner Pruritus visual analog scale (VAS). (b) Enhanced Veterinarian Dermatitis VAS.
Owners and veterinarians were instructed to place a mark on the VAS line at the location that best represented the dog's pruritus or dermatitis, respectively. At study completion, the distance (in centimetres) from the bottom of the line (‘normal dog’) to the owner's or veterinarian's mark on the line was measured and recorded. Owners performed a VAS assessment on days 0, 1, 2, 3, 4, 5, 6 and 7. Veterinarians performed a VAS assessment on days 0 and 7 and (when applicable) at all visits occurring during the continuation phase. Assessments were required to be performed by the same owner or veterinarian at all time points.
Blood samples (complete blood count and serum chemistry) were collected on day 0 (prior to dosing), on day 7 and (when applicable) at the conclusion of the continuation phase. Samples for urinalysis were collected on day 0. All samples for haematology (complete blood count), serum chemistry and urinalysis were sent to a central laboratory (Heska Corporation, Loveland, CO, USA).
Efficacy outcome measures
To be included in the efficacy analyses, dogs had to have been on study until day 5 and had to have received a minimum of 80% of their intended doses (as recorded in a daily log) on days 0–7. The same individuals (owner and veterinarian) had to perform all assessments for the enrolled dogs. For the analysis of the owner (not veterinarian) assessments, there was an additional requirement that dogs had been properly dosed (two doses in the previous 24 h) and that there were at least five of eight evaluable Owner Pruritus VAS scores between days 0 and 7. Data were analysed using SAS version 9.2 (SAS Institute, Cary, NC, USA). The level of significance was set at P < 0.05.
Owner Pruritus VAS scores were analysed with a linear mixed model for repeated measures. The model included the fixed effects of baseline, treatment and baseline by treatment interaction, time and the interaction of treatment by time. Random effects included clinic, clinic by treatment interaction, between-animal error, clinic by treatment by time interaction and the residual variation. Baselines were centred for inclusion in the model by subtracting the mean baseline value from an individual animal's value at day 0. Given that the treatment by time interaction was significant (P < 0.05), treatment was compared at each time point.
The effectiveness variables assessed were as follows: (i) Owner Pruritus VAS scores at each assessment day; (ii) Veterinarian Dermatitis VAS scores on day 7; (iii) dogs achieving a 2 cm reduction compared with baseline in Owner Pruritus VAS scores at each assessment day; (iv) dogs achieving a ≥50% reduction compared with baseline in Owner Pruritus VAS scores on day 7; and (v) the proportion of dogs that were treatment successes based on the Owner Pruritus VAS assessment on days 1–7. Treatment success was defined as achieving at least a 2 cm reduction from baseline score on day X on the Owner Pruritus VAS assessment (day 0 score minus day X score ≥ 2 cm) on at least five of the seven study days assessed. If the criteria for treatment success were not met, the case was a treatment failure; this included dogs withdrawn prior to day 7 for an adverse event or for worsening skin condition.
The Veterinarian VAS scores were analysed with a linear mixed model including the fixed effects of (centred) baseline, treatment and (centred) baseline by treatment interaction, and the random effects of clinic, clinic by treatment interaction and residual variation.
A generalized linear mixed model for repeated measures using a logit link function and binomial error distribution was used to analyse a 2 cm and a 50% reduction from baseline in Owner Pruritus VAS scores. The fixed effects were treatment, time and treatment by time interaction, while the random effects were clinic, clinic by treatment interaction, animal nested within clinic and treatment, and clinic by treatment by time interaction. Treatments were compared at each time.
Treatment success was analysed using a generalized linear mixed model with a logit link and binomial error. The model included the fixed effect of treatment and the random effects of clinic and treatment by clinic interaction. The proportion of success with 95% confidence interval for each treatment and the odds ratio with 95% confidence interval comparing the treatments was reported.
To evaluate the effect of flea or sarcoptic mange treatment on the effectiveness of oclacitinib for pruritus, the primary variable, treatment success at day 7, was stratified by flea control/sarcoptic mange control/none and the proportion of treatment success calculated for each strata.
Safety outcome measures
All enrolled dogs that were administered at least one dose of test article were included in the safety analysis. For each continuous haematology and serum chemistry measure, summary statistics (mean, median, SDs, minimum and maximum) were calculated by treatment and time point. Frequencies of dogs reported to experience at least one abnormal health event were displayed by clinical sign for all unique terms. Frequency tables summarizing the number of dogs receiving each medication over the course of the study were prepared.
Results
Demographics
A total of 436 dogs were enrolled (Table1). Approximately 69% of the dogs were purebred. Retrievers and terriers were the most common dog breed groups, comprising 15.8% (Labrador retrievers 10.3% and golden retrievers 5.5%) and 13.9% of the study population, respectively.
Table 1.
Baseline characteristics of enrolled dogs
| Variable | Placebo group | Oclacitinib group |
|---|---|---|
| Breed distribution [n (%)] | ||
| Mixed breed | 153 (69.5) | 148 (68.5) |
| Purebred | 67 (30.5) | 68 (31.5) |
| Sex distribution [n (%)] | ||
| Female | 114 (51.8) | 105 (48.6) |
| Male | 106 (48.2) | 111 (51.4) |
| Mean age at study onset [years (range)] | 5.8 (1.0–16) | 6.0 (0.5–18) |
| Mean weight at study onset [kg (range)] | 20.0 (3.0–61.7) | 20.6 (3.0–56.0) |
| Owner Pruritus VAS score at study onset (arithmetic mean; cm) | 7.58 | 7.39 |
| Veterinarian Dermatitis VAS score at study onset (arithmetic mean; cm) | 6.18 | 6.20 |
Abbreviation: VAS, visual analog scale.
Presumptive diagnoses
The presumptive diagnoses for dogs enrolled in the study are shown in Table2. It was not always possible to stipulate a single presumptive diagnosis, and enrolled dogs could have had more than one presumptive cause for the reason for their pruritus associated with allergic dermatitis. The presumptive diagnoses were similar in each of the two treatment groups. Over 80% of the dogs in each group had a presumptive diagnosis of atopic dermatitis, but only 41.5% had atopic dermatitis alone. Slightly more than 30% of the dogs were presumed to have flea allergy dermatitis, slightly more than 20% had food allergy dermatitis, and approximately 10% had contact dermatitis. Approximately 5% of the dogs had a presumptive diagnosis of sarcoptic mange, although mange mites were not always observed. A variety of other reasons were noted for the remaining 5% of the dogs enrolled. All of the cases with presumptive diagnoses of ‘other’ also had atopic dermatitis, except for one oclacitinib group dog with the ‘other’ diagnosis of ‘unspecified allergic dermatitis’.
Table 2.
Presumptive diagnoses at enrolment
| Presumptive diagnosis | Oclacitinib group [n (%)] | Placebo group [n (%)] |
|---|---|---|
| Atopic dermatitis | 175 (81.0) | 179 (81.4) |
| Flea allergy dermatitis | 72 (33.3) | 70 (31.8) |
| Food allergy dermatitis | 48 (22.2) | 51 (23.2) |
| Contact dermatitis | 24 (11.1) | 23 (10.5) |
| Sarcoptic mange | 2 (0.9) | 8 (3.6) |
| Other | 12 (5.6) | 10 (4.5) |
Assessment of effectiveness
The effectiveness data set for the Owner Pruritus VAS comprised 407 (204 placebo- and 203 oclacitinib-treated) dogs. The effectiveness data set for the Veterinarian Dermatitis VAS comprised 413 (207 placebo- and 206 oclacitinib-treated) dogs. Twenty-nine dogs (13 oclacitinib treated and 16 placebo treated) were excluded from the Owner Pruritus VAS analyses, and 23 dogs (10 oclacitinib treated and 13 placebo treated) were excluded from the Veterinarian Dermatitis VAS analyses for errors in compliance with the trial and data collection protocols.
Owner Pruritus VAS scores by day of study
The mean day 0 Owner Pruritus VAS scores were very similar between the treatment groups [7.39 and 7.58 cm for the oclacitinib-treated dogs (range 4.7–10.0) and placebo-treated dogs (range 3.0–9.9), respectively; Figure2] corresponding to ‘severe itching’ on the enhanced Owner Pruritus VAS score. After 1 day of treatment, a 2.20 cm reduction of the least squares mean (mean) from the average baseline including both treatment groups in Owner Pruritus VAS scores was observed for dogs receiving oclacitinib, while the dogs receiving placebo treatment had a 0.95 cm reduction (Figure2). For oclacitinib-treated dogs, mean Owner Pruritus VAS scores continued to decrease over the remaining 6 days of the study. Owner Pruritus VAS scores in the oclacitinib-treated dogs were significantly lower than those in the placebo-treated dogs (P < 0.0001) on each day of assessment. At day 7, the Owner Pruritus VAS score had decreased for oclacitinib-treated dogs to 2.59 cm (a 4.89 cm reduction in VAS pruritus scores, which corresponds to an approximate reduction from ‘severe itching’ to ‘very mild itching’) and for placebo-treated dogs to 5.54 cm (a 1.94 cm reduction in VAS pruritus scores, which corresponds to an approximate reduction from ‘severe itching’ to ‘moderate itching’). The reduction in the Owner Pruritus VAS scores (2.20 cm) for oclacitinib-treated dogs after 1 day of treatment exceeded the reduction in pruritus scores for placebo-treated dogs after 7 full days of therapy (1.94 cm).
Figure 2.
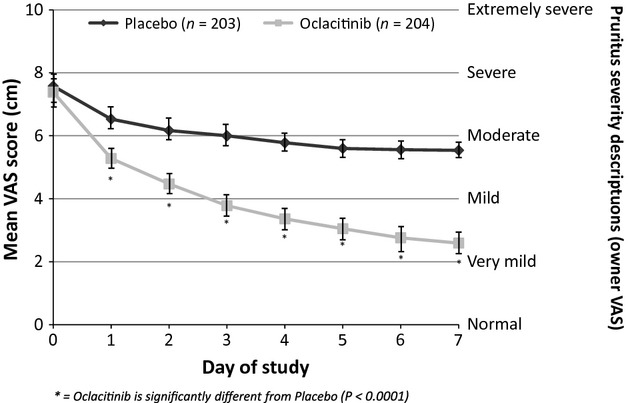
Owner Pruritus VAS score by day of study (day 0, arithmetic mean; days 1–7, least squares mean ± 95% confidence interval).
Veterinarian Dermatitis VAS scores by day of study
The mean day 0 Veterinarian Dermatitis VAS scores were similar for the two treatment groups [6.20 and 6.18 cm for the oclacitinib-treated dogs (range 4.0–10.0) and placebo-treated dogs (range 0.0–9.9), respectively]. At day 7, the mean Veterinarian Dermatitis VAS score for the oclacitinib-treated dogs had decreased to 2.22 cm (a 3.98 cm reduction in VAS dermatitis scores, which corresponds to an approximate reduction from ‘moderately severe dermatitis’ to ‘mild dermatitis’) and for the placebo-treated dogs to 4.89 cm (a 1.29 cm reduction in VAS dermatitis scores, which corresponds to an approximate reduction from ‘moderately severe dermatitis’ to ‘moderate dermatitis’; Figure3). The Veterinarian Dermatitis VAS scores in the oclacitinib-treated dogs were significantly lower than those in the placebo-treated dogs (P < 0.0001).
Figure 3.
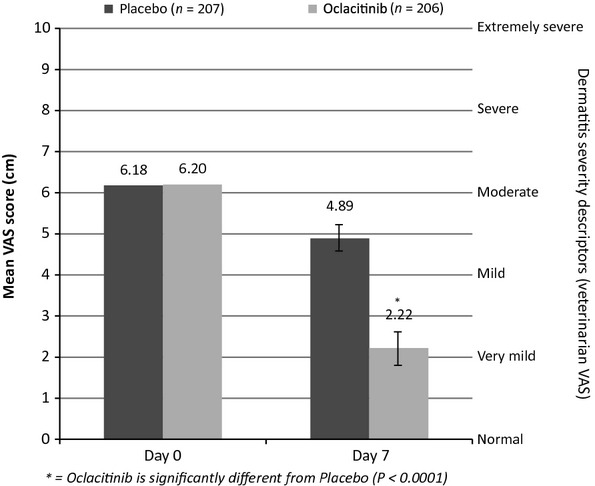
Veterinarian Dermatitis VAS score by day of study (day 0, arithmetic mean; day 7, least squares mean ± 95% confidence interval).
Veterinarian Dermatitis VAS scores were also assessed at the end of the continuation phase (days 8–28) of the study; ∼2.5 times more oclacitinib-treated dogs (n = 179) than placebo-treated dogs (n = 73) were treated during that phase. Owing to this imbalance, Veterinarian Dermatitis VAS scores were not compared during the continuation phase.
Dogs achieving a 2 cm Owner Pruritus VAS score reduction each day of the study
On day 1, a 2 cm reduction in Owner Pruritus VAS was observed in 44% of the oclacitinib-treated dogs compared with 19% of the placebo-treated dogs. By day 7, 86.4% of the oclacitinib-treated dogs compared with 42.5% of the placebo-treated dogs achieved a 2 cm reduction in Owner Pruritus VAS scores. The numbers and percentages of dogs achieving a 2 cm reduction in the Owner Pruritus VAS score for each day of the study are shown in Figure4.
Figure 4.
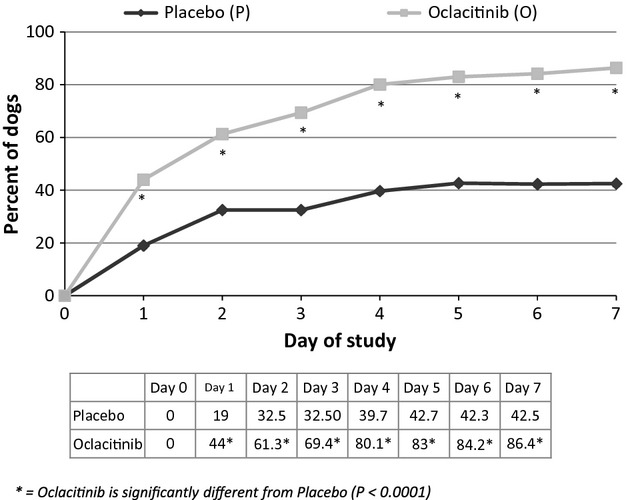
Dogs achieving a 2 cm Owner Pruritus VAS score reduction each day of the study.
Dogs achieving a ≥50% reduction from baseline in Owner Pruritus VAS and Veterinarian Dermatitis VAS scores on day 7
On day 7, 70.5% of the oclacitinib-treated dogs compared with 23.2% of the placebo-treated dogs achieved a ≥50% reduction in Owner Pruritus VAS scores (P < 0.0001). The numbers and percentages of dogs achieving a ≥50% reduction in the Owner Pruritus VAS score for each day of the study are shown in Figure5.
Figure 5.
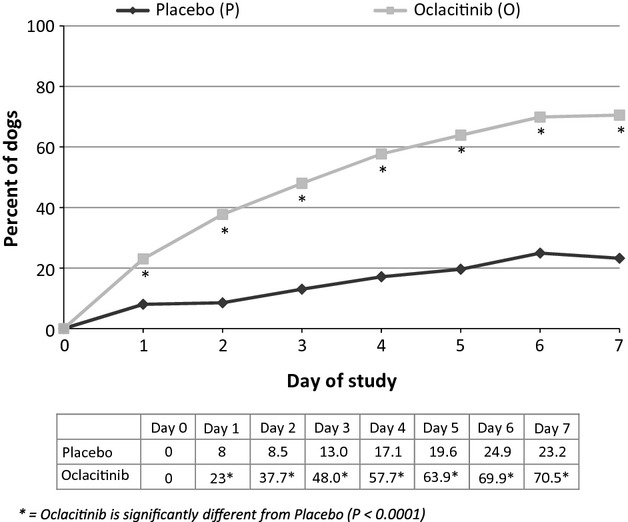
Dogs achieving a ≥50% Owner Pruritus VAS score reduction each day of the study.
Treatment success
Sixty-seven per cent of oclacitinib-treated dogs and 29% of placebo-treated dogs were considered a treatment success; the difference was significant (P < 0.0001). The study also evaluated the effect of flea treatment on treatment success. Flea treatment was initiated on day 0 for 19% (n = 41) and 13% (n = 29) of the dogs in the oclacitinib and placebo treatment groups, respectively. Within the oclacitinib group, the percentage of dogs that were treatment successes was similar regardless of whether they received or did not receive flea treatment on day 0 (63.2% success with flea treatment and 67.7% success without flea treatment). Within the placebo group, flea treatment initiated on day 0 doubled the percentage of dogs that were treatment successes, i.e. success rate increased from 25.9 to 51.9%. The small number (n = 4) of dogs receiving sarcoptic mange control treatment on day 0 made it impossible to assess the effect of treatment.
Safety assessment
All 436 of the enrolled dogs (i.e. 220 placebo- and 216 oclacitinib-treated dogs) were included in the safety assessment, regardless of the number of doses administered during the study; these ranged from 4 to 63 doses. Safety data were also collected from 179 dogs that were treated with oclacitinib for up to 32 days after the study.
Abnormal health events
There were no fatalities and no abnormal health events that necessitated hospitalization in either the study phase [day 0–7 (+3 days)] or the continuation phase [day 8–28 (±2 days)] of the study. Given that the majority of dogs in the placebo group withdrew after the completion of the study phase, the incidence of abnormal clinical signs was similar in both groups (Table3). In most cases, the clinical signs resolved spontaneously and did not require cessation of treatment. Treatment was stopped in one oclacitinib-treated dog after 7 days because of darkening areas of skin and fur.
Table 3.
Abnormal clinical signs during study phase (days 0–7)*
| Abnormal clinical sign | Oclacitinib-treated dogs [n = 216; n (%)]† | Placebo-treated dogs [n = 220; n (%)] |
|---|---|---|
| Diarrhoea | 5 (2.3) | 2 (0.9) |
| Vomiting | 5 (2.3) | 4 (1.8) |
| Lethargy | 4 (1.8) | 3 (1.4) |
| Anorexia | 3 (1.4) | 0 (0.0) |
| Polydipsia | 3 (1.4) | 0 (0.0) |
Seen in >1% of dogs.
Number and percentage are given on a per animal basis.
The continuation phase (days 8–30) of the study was three times longer than the study phase of the study and contained approximately 2.5 times more oclacitinib maleate (179) than placebo group dogs (73). Six dogs (four oclacitinib and two placebo group) were withdrawn from the study during the continuation phase for abnormal health events. Abnormal health events were reported in 11 of 179 oclacitinib-treated dogs post-study. These were as follows: diarrhoea (four dogs; severe enough to warrant cessation of treatment in one dog); vomiting (four dogs); fever, lethargy and cystitis (one dog); an inflamed footpad and vomiting (one dog); and diarrhoea, vomiting and lethargy (one dog).
Clinical pathology
Minor changes were seen in clinical pathology parameters, but these remained within normal laboratory reference ranges. Mean lymphocyte counts for dogs in the oclacitinib group were increased at day 7, but these returned to pretreatment levels within 28 days without a break in oclacitinib administration. These dogs also had a slight decrease in mean white blood cell counts (neutrophil, eosinophil and monocyte counts), but these remained within the normal reference ranges. Serum cholesterol increased in 25% of oclacitinib-treated dogs, but levels remained within the reference range. The incidence of elevated liver enzyme activity for alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase was similar in dogs in the oclacitinib and placebo groups.
Concomitant medications
A wide variety of concomitant medications were used in conjunction with either placebo or oclacitinib treatment. The concomitant medications administered most often (in ≥2% of the oclacitinib-treated dogs) are summarized by drug class and treatment group in Table4. A variety of other products were used less frequently (in ≤2% of the oclacitinib-treated dogs) but were administered to a similar number and percentage of dogs in both treatment groups, including thyroid medications, antibacterial products, systemic and topical antifungal products, while skin emollients and skin protectives, as well as vitamins were given to slightly more dogs in the oclacitinib-treated group than in the placebo-treated group.
Table 4.
| Drug class | Oclacitinib group [n = 216; n (%)]‡ | Placebo group [n = 220; n (%)]‡ |
|---|---|---|
| Endectocides | 145 (67.1) | 150 (68.2) |
| Ectoparaciticides, insecticides and repellents | 105 (48.6) | 101 (45.9) |
| Canine vaccines | 26 (12.0) | 25 (11.4) |
| Glucosamine (with and without chondroitin) and nonsteroidal anti-inflammatory products | 6 (2.8) | 17 (7.7) |
| Systemic antibacterials§ | 17 (7.9) | 11 (5.0) |
| Omega-3 fatty acid preparations | 7 (3.2) | 7 (3.2) |
| Otologicals | 13 (6.0) | 6 (2.7) |
| Ophthalmologicals | 2 (0.9) | 5 (2.3) |
Administered to ≥2% of the oclacitinib-treated dogs.
Administered in decreasing order of frequency in the oclacitinib treatment group.
Number and percentage are given on a per animal basis.
Administered during the continuation phase.
Discussion
This study provides evidence of the effectiveness of oclacitinib in the control of pruritus associated with allergic dermatitis in dogs. There was a highly significant improvement (P ≤ 0.0001) for all of the efficacy variables in oclacitinib-treated dogs compared with placebo-treated dogs. Following 7 days of oclacitinib treatment, there was a 65% reduction in pruritus scores (from ‘severe itching’ to ‘very mild itching’) and a 64% reduction in clinical severity scores (from ‘moderately severe dermatitis’ to ‘mild dermatitis’). Within the first 24 h of treatment, pruritus scores were reduced by at least 2 cm in 44% of oclacitinib-treated dogs compared with 19% of the placebo-treated dogs. By day 7, 86.4% of the oclacitinib-treated dogs compared with <42.5% of the placebo-treated dogs achieved a 2 cm reduction in Owner Pruritus VAS scores. Additionally, by day 7, 70.5% of oclacitinib-treated dogs showed a >50% reduction in Owner Pruritus VAS scores compared with <23.2% of the placebo-treated dogs. Based on the binary treatment success analysis, the majority of oclacitinib-treated dogs (66.5%) were a treatment success compared with only 29.4% of the placebo-treated dogs, with owners and veterinarians noting substantial improvement in pruritus and dermatitis VAS scores. Oclacitinib therefore appears to improve pruritus and dermatitis substantially, affording the damaged skin an opportunity to heal, while allowing the veterinarian time correctly to diagnose and treat the underlying cause. The rapid and effective reduction in pruritus could also greatly improve the quality of life for the affected dogs and their owners.
Immediate downregulation of the action of pruritogenic cytokines, including IL-31, may in part explain the rapid reduction in pruritus following oclacitinib treatment.13 The placebo-treated group also had an immediate but lesser reduction in pruritus score on the first treatment day, which may be attributed in part to a placebo effect but could also be explained by the flea control administered at the start of the study in many of the dogs. The improvement in the clinical severity scores was probably a consequence of controlling the dogs' pruritus, but may also have reflected a direct anti-inflammatory action in the skin. These findings are consistent with the pharmacological properties of oclacitinib, which is a targeted therapy that inhibits key pathways involved in the pathophysiology of skin inflammation.14
Our study used enhanced VAS scales for the assessment of both pruritus and dermatitis. Enhanced VAS scales with severity descriptors at equally spaced intervals along the line have been shown to be an easy and repeatable method for users to assess the severity of pruritus.20,22 Unique to this study was the use of a Veterinarian Dermatitis VAS to assess changes in the severity of the dog's dermatitis at each clinic visit. In previous studies, veterinarians have used a VAS scale to assess pruritus.23 However, dogs may not reliably demonstrate pruritic behaviour in the veterinary clinic and therefore the veterinarian's pruritus VAS score may have to rely heavily on what the owner describes rather than what is observed. By comparison, the dermatitis VAS allowed the veterinarian to assess changes in the dog's skin lesions. More objective and validated assessment tools, such as Canine Atopic Dermatitis and Severity Index (CADESI), are available, but these are limited to specific dermatoses, such as atopic dermatitis, and would have been unsuitable to assess the severity of the variety of skin conditions seen in this study. The enhanced dermatitis VAS was simple and could be used successfully and reliably by general practitioners without special training in dermatology.
For the treatment success analysis, the continuous variable of Owner Pruritus VAS scores collected repeatedly over 7 days was converted to a single binary score for each case, either treatment success or treatment failure. To be classified as a treatment success, the following two criteria had to be met: first, the pruritus score had to improve by a full category (2 cm or more reduction from baseline) on the enhanced Owner Pruritus VAS; and second, the reduction in pruritus (≥2 cm) had to be achieved on ≥5 of the first 7 days of Owner Pruritus VAS assessments. Analysis of treatment success established that the pruritus score improvement was not only statistically different from the placebo group but was of a repeated magnitude (≥2 cm) anticipated to be clinically relevant to both the owner and veterinarian without extrapolating data for cases withdrawn early or interpolating data for a missing case at day 7. The downside to this analysis is that cases with efficacy satisfactory to the owner (e.g. 1.9 cm pruritus reduction or ≥2 cm on four of seven days) were counted as treatment failures. Not surprisingly, the proportion of dogs that were a treatment success (66.5%) was lower than the percentage of dogs that achieved a 2 cm pruritus VAS score reduction from baseline (85%) after 7 days of oclacitinib treatment. Treatment success analysis adds methodological rigour but may underestimate the oclacitinib effectiveness at reducing pruritus observed by the owner and/or veterinarian.
It is difficult to compare the efficacy of oclacitinib directly with that of other antipruritic and anti-inflammatory treatments. Previously reported clinical trials have been conducted in different target populations, predominantly in dogs with atopic dermatitis, without the inclusion of cases with other causes of allergic dermatitis, and have used different measures to assess efficacy (predominantly CADESI).10,24–29 However, the proportion of dogs that achieved a >50% reduction in pruritus in this study is comparable to or better than the proportion of atopic dogs that improved to this extent following treatment with topical hydrocortisone aceponate, topical triamcinolone, systemic glucocorticoids and systemic ciclosporin.4,30,31
To the authors' knowledge, there are no published studies reporting the efficacy of glucocorticoids in dogs suffering from allergic dermatitis (i.e. not only atopic dermatitis) or nonspecific pruritus despite the fact that this class of drug is the most frequently used for the short-term control of pruritus in dogs. The popularity of glucocorticoids seems to be based on the fast speed of onset and reliable results in any of a number of conditions. This study shows that oclacitinib shares these advantages in dogs with pruritus associated with a number of underlying causes of allergic dermatitis, including atopic dermatitis, flea allergy, food allergy, contact dermatitis and sarcoptic mange. In particular, oclacitinib demonstrated significantly better efficacy than placebo at 24 h, indicating a rapid speed of onset. The rapid onset of response to oclacitinib administration has also been reported in a model of IL-31-induced pruritus and a model of flea allergy dermatitis.14,32 In both studies, oclacitinib administered orally as a single dose at 0.4 mg/kg resulted in a significant (P < 0.05) reduction in pruritus within 1 h after administration compared with prednisolone administered at doses of 0.25 and 0.5 mg/kg.
Oclacitinib was well tolerated in these dogs. The frequency and type of abnormal health events were similar between the oclacitinib- and placebo-treated dogs. The most common adverse events were gastrointestinal upsets, such as decreased appetite, vomiting and diarrhoea. These were mostly mild and only rarely required cessation of treatment. The acute effects commonly observed with systemic glucocorticoids (e.g. polyuria, polydipsia, panting, polyphagia and changes in serum biochemistry) were seen in <2% of the oclacitinib-treated dogs. The mean values of all of the clinical pathology parameters analysed fell within the normal reference range for both treatment groups. The favourable safety results reported here are supported by the results of a field trial, in which oclacitinib was administered for 4 months to dogs with atopic dermatitis.33
This study was carried out to good clinical practice standards.19 Selection bias in breed, age, sex, weight and clinical severity was not apparent. Randomized treatment allocation was made according to a predetermined allocation code. Detection bias by the owners and investigators was unlikely because they were blinded to treatment allocation. Performance bias was possible, because antiparasite treatment and dietary management changes for some dogs on day 0 could have improved their clinical signs. However, the impact of this on the comparison between oclacitinib and placebo is likely to have been low because the type and frequency of concomitant treatments was comparable between the treatment groups. Attrition bias was present; the analysis excluded dogs that were considered to have had a protocol deviation that affected the collection or integrity of their efficacy data. It is possible that this biased towards a favourable response to treatment, although the numbers never exceeded 8% of the treated dogs and were comparable between the treatment groups and analyses. Inclusion and exclusion criteria established before the trial were used to establish a working diagnosis of pruritus associated with allergic dermatitis. Rigorous criteria to establish a firm diagnosis were not employed, because the aim and design of this study were to assess the efficacy of oclacitinib in short-term management of allergic dermatitis. The authors have also reported a study of longer term (112 days) efficacy in the control of a more specific condition, atopic dermatitis.33
In the conditions of this study, oclacitinib, a selective Janus kinase inhibitor, administered orally at a dose of 0.4–0.6 mg/kg twice daily, was safe and efficacious in controlling the pruritus associated with allergic dermatitis. Oclacitinib provided itch relief within 24 h that persisted through the treatment period, with >70% of the treated dogs achieving a >50% reduction in pruritus by day 7.
Acknowledgments
We would like to thank the following veterinarians who enrolled dogs in this study and performed the clinical investigations: Brett Berryhill, Glen Burkett, Jay Butan, Randall R. Carpenter, Terry Clekis, Jeffrey N. Dizik, Sam Geller, Mary Grabow, Robert Jackson, Mark Lelli, Marc Leven, David Lukof, Patrick McSweeney, Kathleen Neuhoff, Karan Oberhansley, Gregory Paplawsky, Andrew Pickering, Jeffrey Pinkston, Dean Rund, Roger Sifferman, Jason St Romain, Kathy Tater, Bradford J. Theodoroff, Philip VanVranken, Philip Waguespack and Melissa Wiest. We would also like to thank the following Zoetis Inc. colleagues for their invaluable assistance: Candace A. Sousa, Marcia J. Adams, Anne E. Daniels and Nancy L. Savicke.
Résumé
Contexte
L'oclacitinib (Apoquel®) inhibe la fonction d'une variété de cytokines pro-inflammatoires, pro-allergiques et pruritogènes qui dépendent de l'activité de l'enzyme Janus kinase. L'oclacitinib inhibe sélectivement la Janus kinase 1.
Hypothèses/Objectifs
Nous voulons évaluer l'efficacité et l'innocuité de l'oclacitinib pour le contrôle du prurit associé à la dermatite allergique dans une étude contrôlée, randomisée, en double-aveugle, contre placébo.
Méthodes
Des chiens (n = 436) présentant un prurit évalué comme sévère par leurs propriétaires et un diagnostic probable de dermatite allergique, ont été enrôlés. Les chiens ont reçu au hasard soit de l'oclacitinib à 0.4–0.6 mg/kg oralement, deux fois par jour soit un placebo. Une échelle visuelle analogue de 10 cm (VAS) a été utilisée par les propriétaires pour évaluer la sévérité du prurit des jours 0 à 7 et par les vétérinaires pour évaluer la sévérité de la dermatite aux jours 0 et 7. Les chiens pouvaient rester dans l'étude pendant 28 jours.
Résultats
Les scores de VAS de prétraitement des propriétaires et des vétérinaires étaient identiques pour les deux groupes de traitement. L'oclacitinib a produit rapidement un début d'effet dans les 24h. Les scores moyens de VAS de prurit par les propriétaires étaient significativement meilleurs que les scores du placebo (P < 0.0001) pour chaque jour d'évaluation. Les scores de prurit ont diminué de 7.58 à 2.59 cm suite au traitement à l'oclacitinib. A jour 7, les scores moyens de dermatite par les vétérinaires étaient significativement meilleurs pour l'oclacitinib (P < 0.0001) que les scores du placebo. Diarrhée et vomissement ont été rapportés avec une fréquence identique dans les deux groupes.
Conclusions et importance clinique
Dans cette étude, l'oclacitinib a permis un contrôle rapide, efficace et sûr du prurit associé à une dermatite allergique, avec les propriétaires et les vétérinaires notant des améliorations conséquentes dans les scores VAS de prurit et de dermatite.
Resumen
Introducción
Oclacitinib (Apoquel®) inhibe la función de una variedad de citoquinas proinflamatorias, proalergénicas y pruritogénicas que dependen de la actividad de la enzima Janus quinasa 1.
Hipótesis/Objetivos
nuestro propósito fue evaluar la seguridad y eficacia de oclacinitib para el control del prurito asociado con dermatitis alérgica en un estudio al azar, doble ciego y controlado con placebo.
Métodos
perros de propietarios particulares (n = 436) con prurito de moderado a severo en opinión de los propietarios y con un diagnostico presuntivo de dermatitis alérgica se incluyeron en el estudio. Los perros fueron distribuidos al azar para recibir oclacitinib a dosis de 0,4-0,6 mg/kg dos veces al día o un placebo compuesto del mismo excipiente. Se utilizó una escala visual análoga aumentada de 10 cm (VAS) para que los propietarios evaluaran la severidad del prurito desde el día 0 al 7 y por los veterinarios para evaluar la severidad de la dermatitis en los días 0 y 7. Los perros podían permanecer en el estudio hasta 28 días.
Resultados
los valores de VAS pretratamiento de los propietarios y veterinarios fueron similares en los dos grupos de tratamiento. Oclacitinib produjo un efecto eficaz y rápido a las 24 h. Los valores medios de VAS obtenidos por los propietarios en perros tratados con oclacitinib fueron significativamente mejores que los valores de placebo (P < 0,0001) en cada uno de los días evaluado. Los valores de prurito decrecieron de 7,58 a 2,59 cm tras el tratamiento con oclacitinib. La media de los valores de VAS en el día 7 obtenidos por los veterinarios también fue significativamente mejor (P < 0,0001) que los valores de animales con placebo. Vómitos y diarrea fueron descritos con igual frecuencia en ambos grupos.
Conclusiones e importancia clínica
en este estudio oclacitinib causó un control rápido, efectivo y seguro del prurito asociado con la dermatitis alérgica, y tanto propietarios como veterinarios notaron una mejora sustancial del prurito y de los valores VAS de dermatitis.
Zusammenfassung
Hintergrund
Oclacitinib (Apoquel®) inhibiert die Wirkung einer Vielzahl von proinflammatorischen, pro-allergenen und juckreizauslösenden Zytokinen, die von der Aktivität der Janus Kinase abhängen. Oclacitinib inhibiert selektiv die Janus Kinase 1.
Hypothese/Ziele
Unser Ziel war eine Evaluierung der Sicherheit und der Wirksamkeit von Oclacitinib zur Juckreizkontrolle im Zusammenhang mit allergischer Dermatitis in einer randomisierten, doppelblinden, Plazebo-kontrollierten Studie.
Methoden
Hunde im Privatbesitz (n=436) mit moderatem bis starkem von den BesitzerInnen beurteiltem Juckreiz und der Verdachtsdiagnose einer allergischen Dermatitis wurden in die Studie aufgenommen. Die Hunde wurden zufällig aufgeteilt und entweder mit Oclacitinib bei einer Dosierung von 0,4-0,6mg/kg per os zweimal täglich oder mit dem Trägermedium angepasstem Plazebo behandelt. Eine verstärkte 10cm visuelle Analogskala (VAS) wurde von den BesitzerInnen verwendet, um den Schweregrad des Juckreizes von Tag 0 bis 7 zu beurteilen und von TierärztInnen wurde sie verwendet, um den Schweregrad der Dermatitis an den Tagen 0 und 7 zu beurteilen. Die Hunde konnten 28 Tage lang an der Studie teilnehmen.
Ergebnisse
Die Beurteilungen der BesitzerInnen und der TierärztInnen waren in den beiden Behandlungsgruppen ähnlich. Oclacitinib zeigte innerhalb von 24 h eine rasche Wirksamkeit. Die durchschnittlichen Oclacitinib Pruritus VAS Bewertungen der BesitzerInnen waren an jedem Bewertungtag signifikant besser als die Plazebobewertungen (P<0,0001). Die Pruritus Bewertungen nahmen nach Oclacitinib von 7,58 auf 2,59cm ab. Die Dermatitis VAS Durchschnittswerte der TierärztInnen waren am Tag 7 signifikant besser (P<0,0001) nach Oclacitinib Behandlung im Vergleich zu den Bewertungen mit Plazebobehandlung. Von Durchfall und Erbrechen wurde in beiden Gruppen mit ähnlicher Frequenz berichtet.
Schlußfolgerungen und klinische Bedeutung
In dieser Studie bewirkte Oclacitinib eine rasche, wirksame und sichere Kontrolle des Pruritus, der im Zusammenhang mit einer allergischen Dermatitis auftrat, wobei sowohl BesitzerInnen als auch TierärztInnen bedeutende Verbesserungen des Juckreizes sowie der Dermatitis VAS Werte bemerkten.
References
- 1.VPI®. Pet Insurance website. Top 10 pet medical conditions of 2010. Available at: http://press.petinsurance.com/pressroom/02222011Pet_Conditions_2010.aspx. Accessed Dec 28, 2012.
- 2.Hill PB, Lo A, Eden CAN, et al. Survey of the prevalence, diagnosis and treatment of dermatological conditions in small animals in general practice. Vet Rec. 2006;158:533–539. doi: 10.1136/vr.158.16.533. [DOI] [PubMed] [Google Scholar]
- 3.Nuttall T, McKeever PJ, Harvey RG. Pruritic dermatitis. In: Beynon P, editor. A Colour Handbook of Skin Diseases of the Dog and Cat. London: Manson Publishing; 2009. pp. 35–37. 2nd edn. [Google Scholar]
- 4.Olivry T, Bisikova P. A systematic review of randomized controlled trials for prevention or treatment of atopic dermatitis in dogs: 2008–2011 update. Vet Dermatol. 2013;24:97–117. doi: 10.1111/j.1365-3164.2012.01088.x. , e25–e26. [DOI] [PubMed] [Google Scholar]
- 5.Bloom P. Nonsteroidal, nonimmunosuppressive therapies for pruritus. Vet Clin North Am: Small Anim Pract. 2013;43:173–187. doi: 10.1016/j.cvsm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Scott DW, Miller WH, Griffin CE. Muller and Kirk's Small Animal Dermatology. Philadelphia: W.B. Saunders Co; 2001. Dermatologic therapy; pp. 207–274. 6th edition. [Google Scholar]
- 7.Steinhoff M, Bienenstock J, Schmelz M, et al. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006;126:1705–1718. doi: 10.1038/sj.jid.5700231. [DOI] [PubMed] [Google Scholar]
- 8.Metz M, Ständer S. Chronic pruritus – pathogenesis, clinical aspects and treatment. J Eur Acad Dermatol Venereol. 2010;24:1249–1260. doi: 10.1111/j.1468-3083.2010.03850.x. [DOI] [PubMed] [Google Scholar]
- 9.Plumb DC. Veterinary drug handbook. Ames, IA: Blackwell Publishing; 2002. Glucocorticoid agents, general information; pp. 387–389. 4th edn. [Google Scholar]
- 10.Nuttall T, Mueller R, Bensignor E, et al. Efficacy of a 0.0584% hydrocortisone aceponate spray in the management of canine atopic dermatitis: a randomised, double blind, placebo-controlled trial. Vet Dermatol. 2009;20:191–198. doi: 10.1111/j.1365-3164.2009.00756.x. [DOI] [PubMed] [Google Scholar]
- 11.Olivry T, Mueller RS. Evidence-based veterinary dermatology: a systematic review of the pharmacotherapy of canine atopic dermatitis. Vet Dermatol. 2003;14:121–146. doi: 10.1046/j.1365-3164.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 12.Scott DW, Miller WH, Griffin CE. Muller and Kirk's Small Animal Dermatology. Philadelphia, PA: W.B. Saunders Co; 2001. Skin immune system and allergic skin diseases; pp. 543–666. 6th edition. [Google Scholar]
- 13.Gonzales AJ, Humphrey WR, Messamore JE, et al. Interleukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013;24:48–53. doi: 10.1111/j.1365-3164.2012.01098.x. , e11–e12. [DOI] [PubMed] [Google Scholar]
- 14.Fleck T, Humphrey W, Coscarelli E, et al. Comparison of the janus kinase (JAK) inhibitor, oclacitinib, and prednisolone in canine models of pruritus. Vet Dermatol. 2012;23(Suppl 1):38. [Google Scholar]
- 15.Carmi-Levy I, Homey B, Soumelis V. A modular view of cytokine networks in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:245–253. doi: 10.1007/s12016-010-8239-6. [DOI] [PubMed] [Google Scholar]
- 16.Ong PY, Leung DY. Immune dysregulation in atopic dermatitis. Curr Allergy Asthma Rep. 2006;6:384–389. doi: 10.1007/s11882-996-0008-5. [DOI] [PubMed] [Google Scholar]
- 17.Felsburg PJ. Overview of immune system development in the dog: comparison with humans. Hum Exp Toxicol. 2002;21:487–492. doi: 10.1191/0960327102ht286oa. [DOI] [PubMed] [Google Scholar]
- 18.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;482:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 19.International Co-operation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH), Good Clinical Practice Website. Available at: http://www.vichsec.org/en/topics.htm. Accessed Dec 28, 2012.
- 20.Hill PB, Lau P, Rybnicek J. Development of an owner-assessed scale to measure the severity of pruritus in dogs. Vet Dermatol. 2007;18:301–308. doi: 10.1111/j.1365-3164.2007.00616.x. [DOI] [PubMed] [Google Scholar]
- 21.Collard WT, Hummel BD, Malpas PB, et al. Proceedings of the American Academy of Veterinary Pharmacology and Therapeutics, Biennial Meeting. Potomac, MD, USA: 2013. Single and multiple dose pharmacokinetics of oclacitinib maleate in the dog. (Abstract #23) [Google Scholar]
- 22.Rybnicek J, Lau-Gillard PJ, Harvey R, et al. Further validation of a pruritus severity scale for use in dogs. Vet Dermatol. 2009;20:115–122. doi: 10.1111/j.1365-3164.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- 23.Hill P, Rybnicek J, Lau-Gillard P. Correlation between pruritus score and grossly visible erythema in dogs. Vet Dermatol. 2010;21:450–455. doi: 10.1111/j.1365-3164.2010.00881.x. [DOI] [PubMed] [Google Scholar]
- 24.Olivry T, Rivierre C, Jackson HA, et al. Cyclosporine decreases skin lesions and pruritus in dogs with atopic dermatitis: a blinded randomized prednisolone-controlled trial. Vet Dermatol. 2002;13:77–87. doi: 10.1046/j.1365-3164.2002.00283.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt V, McEwan N, Volk A, et al. The glucocorticoid sparing efficacy of Phytopica in the management of canine atopic dermatitis. Vet Dermatol. 2010;21:96–105. doi: 10.1111/j.1365-3164.2009.00858.x. [DOI] [PubMed] [Google Scholar]
- 26.Steffan J, Alexander D, Brovedani F, et al. Comparison of cyclosporine A with methylprednisolone for treatment of canine atopic dermatitis: a parallel, blinded, randomized controlled trial. Vet Dermatol. 2003;14:11–22. doi: 10.1046/j.1365-3164.2003.00318.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller WH, Scott DW, Wellington JR. A clinical trial on the efficacy of clemastine in the management of allergic pruritus in dogs. Can Vet J. 1993;34:25–27. [PMC free article] [PubMed] [Google Scholar]
- 28.Hill PB, Hoare J, Lau-Gillard P, et al. Pilot study of the effect of individualised homeopathy on the pruritus associated with atopic dermatitis in dogs. Vet Rec. 2009;164:364–370. doi: 10.1136/vr.164.12.364. [DOI] [PubMed] [Google Scholar]
- 29.Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol. 2010;21:233–248. doi: 10.1111/j.1365-3164.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 30.Olivry T, Mueller RS The International Task Force on Canine Atopic Dermatitis. Evidence-based veterinary dermatology: a systematic review of the pharmacotherapy of canine atopic dermatitis. Vet Dermatol. 2003;14:121–146. doi: 10.1046/j.1365-3164.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 31.Olivry T, Foster AP, Mueller RS, et al. Interventions for atopic dermatitis in dogs: a systematic review of randomized controlled trials. Vet Dermatol. 2010;21:4–22. doi: 10.1111/j.1365-3164.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- 32.Wheeler DW, Civil J, Payne-Johnson M, et al. Oclacitinib for the treatment of pruritus and lesions associated with canine flea-allergic dermatitis. Vet Dermatol. 2012;23(Suppl 1):38–39. [Google Scholar]
- 33.Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib compared to placebo for the control of atopic dermatitis in client-owned dogs. Vet Dermatol. 2013;24:295. doi: 10.1111/vde.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]