Abstract
The current maximum acceptable daily intake (ADI) of ethylenediaminetetraacetic acid (EDTA) of 1.9 mg day−1 per kilogram bodyweight (mg day−1 kgbw−1) limits the daily intake of iron as iron EDTA [ferric sodium EDTA; sodium iron(III) EDTA] to approximately 2–2.5 mg day−1 for children 6–24 months of age. This limit was defined by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1973 based on data from an animal‐feed study published in 1963. Other animal studies indicate that this limit can be raised to 4.4 or possibly up to 21.7 mg day−1 kgbw−1, which is 2.3–11.4 times higher than the current value. For nearly 50 years, iron EDTA has been used in France in medicinal syrup for infants 1–6 months of age. The maximum recommended dosage of this drug is 37 times higher than the maximum ADI of EDTA. No adverse health effects have been reported as a result of this medicinal consumption of iron EDTA. Raising the maximum ADI of EDTA to only 4.4 mg day−1 kgbw−1 would enable iron EDTA, an iron fortificant with proven bioavailability in phytate‐rich meals, to be added in adequate amounts to cereal‐based meals for children 6–24 months of age, who are at risk of iron deficiency.
Keywords: iron, infant iron status, iron deficiency anaemia, EDTA, acceptable daily intake, zinc
Introduction
Millions of young children predominantly in developing and emerging countries may not reach their full physical and intellectual potential because of micronutrient deficiencies in their diet (Horton 2008). Among the most prominent micronutrient deficiencies are iron and zinc, and iron especially may adversely affect their long‐term cognitive and emotional development (EFSA 2013; Lozoff et al. 2013). Yet, these problems could be overcome with relatively low investment in providing micronutrients through food fortification (Hoddinott et al. 2012).
Minerals are often not well absorbed from food because of the presence of phytate, also referred to as phytic acid. It is the plant form of phosphorus and thus an essential component of plant foods. Plant‐based diets are rich in phytate (Gibson et al. 2010). This otherwise health‐beneficial food ingredient (Kumar et al. 2010) converts mineral ions, such as iron and zinc, to insoluble complexes that obstruct absorption in the gastrointestinal tract (Hurrell 2002). Many food products contain levels of phytic acid in excess of 100 mg per 100 g (Ma et al. 2005).
To enable sufficient iron absorption in the absence of enhancing compounds such as ascorbic acid, the phytic acid to iron molar ratio needs to be decreased to less than 1:1 and preferably to lower than 0.4:1 (Hurrell 2004). The molar mass of iron is 56 mg mmol−1 and of phytic acid 660 mg mmol−1. At a phytic acid level of 100 mg per 100 g (1000 ppm; 1.5 mmol kg−1), the level of intrinsic and added iron (except iron EDTA) should be above 85 ppm (1.5 mmol kg−1, 8.5 mg per 100 g of food) and preferably above 210 ppm (3.75 mmol kg−1, 21 mg per 100 g of food). However, the latter level of added iron could result in organoleptic and possibly also stability issues.
Various studies have shown that the inhibitory effect of phytate on iron absorption in cereal foods can be counteracted safely and effectively by iron EDTA (Hurrell et al. 2010). This compound is also known as sodium iron(III) EDTA (NaFeEDTA) or ferric sodium EDTA (FeNaEDTA), and in pharmaceutical preparations, it is invariably referred to as sodium feredetate. EDTA stands for ethylenediaminetetraacetic acid (also denoted as H4EDTA or EDTA‐H4). Ferric sodium EDTA is a yellow to yellow‐brown powder that is highly stable when stored under ambient conditions. In storage‐stable form, iron EDTA carries three molecules of crystal water: EDTA‐FeNa·3H2O. In this chemical form, it is obtained via crystallisation and should preferably be used in food applications (FCC 2012). For the structural formula of iron EDTA (EDTA‐FeNa·3H2O), see Fig. 1. The function of EDTA in iron EDTA is to ensure adequate uptake of iron by intestinal cells from food rich in phytate. Thus, the EDTA molecules in iron EDTA can be considered to be a vehicle to transport iron. EDTA could also fulfil a similar role with respect to zinc ions (Hess & Brown 2009).
Figure 1.
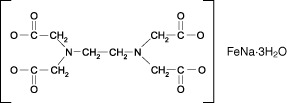
Structural formula of EDTA‐FeNa·3H2O (iron EDTA).
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) classifies iron EDTA as ‘suitable for use as a source of iron for food fortification’ (JECFA 2007). The World Health Organization (WHO) recommends iron EDTA as the only iron compound suitable for fortification of high‐extraction wheat and maize flour, and as one of only four iron compounds for fortification of low‐extraction flours (WHO 2009). Recently, the use of iron EDTA in foods and food supplements was approved in the European Union (EU) (EFSA 2010; EU 2010; EU 2011) after earlier food approvals in China (PRC 1994; PRC 1996), the Philippines (Republic of the Philippines 1995), most Latin American countries by about 2000, United States (FDA 2004; FDA 2006), Australia and New Zealand (FSANZ 2008), and India (Gazette of India 2011). In spite of wide approval, iron EDTA cannot be used in the required amounts in complementary foods for older infants and young children (6–24 months of age) because of concern about exceeding the maximum acceptable daily intake (ADI) of EDTA (Yang et al. 2011).
ADI has been defined by JECFA as an ‘estimate of the amount of a food additive [expressed in mg day−1 per kilogram bodyweight (mg day−1 kgbw−1)] that can be ingested daily over a lifetime without appreciable health risk’. The maximum ADI is derived from the highest dose level expressed in mg day−1 kgbw−1 that can be administered in long‐term animal studies without inducing adverse health effects. This level is divided by a safety margin of usually 100 in order to arrive at a maximum intake level, also expressed in mg day−1 kgbw−1, that can be deemed safe for humans (WHO 1987).
Ferric sodium EDTA consists of iron, sodium and EDTA, and for each of these three constituents, maximum intake levels are applicable (Munro 1993). In 1983, JECFA proposed a provisional maximum tolerable daily intake for iron in food of 0.8 mg day−1 kgbw−1 (JECFA 1983). In 2001, the US Institute of Medicine (IOM) published a tolerable upper limit for adults of 45 mg day−1 of iron (IOM 2001). The latest WHO recommendation on sodium is a maximum intake of 2 g day−1 (WHO 2012a). An intake of 45 mg of iron as iron EDTA is equivalent to an intake of 18 mg of sodium, which is less than 1% of the recommended maximum of 2 g day−1.
In 1973, JECFA defined a maximum ADI for calcium disodium EDTA (CaNa2EDTA) of 2.5 mg day−1 kgbw−1 (JECFA 1974). This quantity of calcium EDTA (CaNa2EDTA) contains the same number of EDTA molecules (6.7 μmol) as present in 0.375 mg iron as iron EDTA (FeNaEDTA·3H2O) or 1.95 mg of EDTA (H4EDTA). In a safety re‐evaluation, JECFA redefined the maximum ADI of EDTA to 1.9 mg EDTA (JECFA 2007). This amount is equivalent to 6.5 μmol of EDTA molecules and limits the intake of iron as iron EDTA to 0.37 mg day−1 kgbw−1.
The current maximum ADI of EDTA (JECFA 1974; JECFA 2007) allows a maximum daily dosage of 2–2.5 mg day−1 of iron as iron EDTA for infants and young children (Yang et al. 2011), whereas experts advocate that this amount should be two to four times higher (Rosenberg et al. 2011). An amount four times higher would meet the recommended iron intake for under‐fives (De Pee et al. 2011), but because of high bioavailability, a daily intake of 5 mg iron as iron EDTA may provide enough additional iron.
Vitamin C has also been found to enhance iron absorption from single meals, including those rich in phytate (Diaz et al. 2003; Thankachan et al. 2008; Cercamondi et al. 2013). However, long‐term studies in which vitamin C was added to diets containing foods naturally rich in iron but no fortificant iron did not show a meaningful improvement of the iron status (Cook et al. 1984; Hunt et al. 1994; Garcia et al. 2003). No studies have been found in which vitamin C has been used to improve iron status based on long‐term intake of iron‐fortified food. Furthermore, the instability of vitamin C especially at higher temperatures is considered a drawback to its use in staple food fortification (Teucher et al. 2004).
This paper reviews the basis for setting the maximum ADI of EDTA and assesses the safety of a higher level that is deemed adequate for fortifying complementary foods with iron EDTA for children 6–24 months of age. An increase in the maximum ADI of EDTA would widen the scope for iron EDTA in food fortification and also the use of suitable EDTA compounds in food fortification to improve zinc absorption.
Key messages
Test animal data support an increase of the current maximum acceptable daily intake (ADI) of EDTA by a factor 11.4 or even higher.
No negative health effects have been reported on the pharmaceutical use of iron EDTA in infants at levels exceeding the maximum ADI of EDTA by a factor 12 to 37.
A daily intake of 5 mg Fe as iron EDTA, which is twice as high as the current maximum ADI of EDTA, would provide sufficient iron for children 6 to 24 months of age.
A higher maximum ADI of EDTA could also improve zinc absorption.
EDTA
EDTA is a synthetic chemical used in a wide range of applications (Anderson & Gaunt 1960). When dissolved in water, for instance in the form of disodium EDTA (Na2H2EDTA), EDTA molecules can form highly stable complex ions with two‐ and three‐valent metal ions. An EDTA molecule is comparable with a hand holding a tennis ball, where the tennis ball is the metal ion. All metal EDTA complex ions are negatively charged and highly soluble in water.
Many applications of EDTA were introduced to the market decades ago (Anderson & Gaunt 1960; Furia 1964). For instance, solutions containing EDTA are highly effective in dissolving deposits of calcium salts and this unique property has led to a widespread use in cleaning formulations. EDTA is used in the manufacture of paper to inactivate traces of manganese, which can catalyse the rapid decomposition of the wood pulp bleaching agent, hydrogen peroxide. Additionally, metal EDTA salts of iron, manganese, zinc and copper are used in growing crops as a highly effective source for these trace metals. Various EDTA derivatives are used in cosmetics (Lanigan & Yamarik 2002), and calcium EDTA and disodium EDTA have been approved as additives for a large number of food products to prevent rancidity and discolouration (Furia 1964).
Multi‐year animal studies
From the large number of publications on EDTA, only three multi‐year studies in rats were found (Table 1). One of these studies was a PhD thesis (Yang 1952), of which only a brief summary is available in the public domain (BIBRA 1964), with no indication whether the data have been peer‐reviewed. However, these data are referred to in regulatory documents such as a recent Generally Recognized As Safe (GRAS) Notice of the US Food and Drug Administration (FDA 2011). Yang maintained intake levels of EDTA in the feed at 0.5%, 1.0% and 5% disodium EDTA (Na2H2EDTA). Based on an assumed ratio of 20:1 of dietary level vs. daily intake per kilogram bodyweight (Oser et al. 1963), these disodium EDTA concentrations in the feed correspond to oral intake levels of 250, 500 and 2500 mg day−1 kgbw−1, respectively. An intake of 2500 mg day−1 kgbw−1 of disodium EDTA is equivalent to an intake of 7440 μmol day−1 kgbw−1 of EDTA molecules. The experimental animals given the highest level of disodium EDTA suffered from continuous diarrhoea, consumed less feed than the lower dose groups and failed to produce litters. No other adverse health effects were detected in this experiment, including in the highest dose group.
Table 1.
Overview of multi‐year studies in rats with high levels of oral intake of EDTA
In an extensive study (Oser et al. 1963), the highest intake level was 250 mg day−1 kg−1 of calcium EDTA (CaNa2EDTA), which is equivalent to 668 μmol day−1 kgbw−1 of EDTA molecules. At this highest intake level, no adverse health effects were observed. This study was referred to by JECFA in 1973 in determining the maximum ADI of EDTA (JECFA 1974). Higher levels were not tested as these were known to cause diarrhoea in the experimental animals (Foreman et al. 1953). This detailed study was intended to prove the safety of calcium EDTA and disodium EDTA as food additives. By applying a safety margin of 100, JECFA arrived at the current value for the maximum ADI of EDTA of 2.5 mg day−1 kgbw−1, expressed as CaNa2EDTA (JECFA 1974). This is equivalent to 6.7 μmol day−1 kgbw−1 of EDTA molecules.
An also extensive study by the US National Cancer Institute in 1977 used trisodium EDTA trihydrate (EDTA‐Na3H·3H2O) at 7500 ppm in the highest dose level (NCI 1977). This corresponds to 375 mg day−1 kgbw−1 and is equivalent to 910 μmol day−1 kgbw−1 of EDTA molecules. This study focused on identifying potential carcinogenic properties of EDTA, but no carcinogenic or any other adverse health effects were detected.
Other toxicological data from animal studies
The data above indicate that experimental animals tolerate an oral intake of around 600–700 μmol day−1 kgbw−1 of EDTA molecules (∼250 mg day−1 kgbw−1 of calcium EDTA). At double this level (∼500 mg day−1 kgbw−1 of calcium EDTA), diarrhoea has been observed occasionally in experimental animals, but no other harmful health effects have ever been reported (Foreman et al. 1953).
At a level of 3% (30 000 ppm) disodium EDTA in the feed of gestational rats, severe malformation of offspring was observed, but at a level of 2% disodium EDTA, reproduction was impaired only slightly (Swenerton & Hurley 1971). This 3% addition level is equivalent to an oral intake of 1500 mg day−1 kgbw−1 or approximately 4500 μmol day−1 kgbw−1 of EDTA molecules. For a pregnant woman weighing 60 kg, this would mean a daily intake of 90 g of disodium EDTA. These teratogenic (embryo‐damaging) effects in the gestational rats were fully reversed with the addition of 1000 ppm zinc (∼15 mmol kg−1) to the feed containing 3% disodium EDTA (∼90 mmol kg−1). At these extremely high intake levels, EDTA may very well have removed zinc from the body of these rats and severe zinc deficiency is a teratogenic condition. The addition of a relatively small amount of zinc to the feed in a molar ratio of EDTA to zinc of 6:1 prevented developmental damage to the rat offspring.
EDTA in humans
Both calcium EDTA and disodium EDTA can be safely administered intravenously in humans. When present in the blood circulation, EDTA molecules can bind lead ions effectively (Rubin et al. 1953). Intravenous application of EDTA has been demonstrated to improve renal function and to slow down the progression of renal insufficiency in patients with a ‘high‐normal body lead burden’ (Lin et al. 2003).
When administered into the blood circulation, all EDTA molecules are bound to metal ions, such as calcium, iron and zinc. The resulting metal EDTA complex ions are not metabolised by the liver, and do not mimic any hormone in the human body (no activity as an endocrine disruptor). All metal EDTA complex ions are soluble in water and are cleared rapidly by the kidneys.
However, when disodium EDTA is injected at very high levels so that all metal ions in the blood circulation are bound by an EDTA molecule (above approximately 2500 μmol L−1), the remaining EDTA molecules that are not bound to a metal ion can be expected to extract metal ions from nearby endothelial cells. Therefore, when applied intravenously at a level exceeding around 2500 μmol L−1 in the blood circulation, disodium EDTA becomes toxic (FAO 1965a,b). This can occur, for example, when an adult dose of disodium EDTA for chelation therapy is given to a small child.
Unlike intravenous administration, this acute toxic affect is not displayed when, after oral intake, EDTA molecules enter the blood circulation via the gastrointestinal tract and are able to combine with a metal ion. On an assumption that a small child ingests 5.6 mg iron (100 μmol) as iron EDTA and 5% of the EDTA molecules are absorbed from the gastrointestinal tract (Foreman & Trujilio 1954; Heimbach et al. 2000), 5 μmol of EDTA molecules will enter the blood circulation. Assuming a total blood volume of 0.5 L in a small child, the concentration of EDTA molecules is at most 10 μmol L−1. This is far below the danger threshold of 2500 μmol L−1 stated earlier.
In the 1970s, there was concern in the United States that the presence of calcium EDTA and disodium EDTA in food might depress iron absorption as a consequence of strong binding of the intrinsic iron ions (Cook & Monsen 1976). Meantime, iron EDTA has emerged as an effective source of this mineral in food (Hurrell et al. 2010). Since the 1980s, the safety of the widespread use of these two food additives has not been questioned in relation to the risk of mineral deficiencies. In 1995, the EU approved calcium EDTA for a number of food products (European Community 1995). Whether extremely high levels of calcium EDTA and disodium EDTA in food could pose a risk of inducing mineral deficiencies in humans is not known, although with current guidelines they are more likely to enhance absorption of iron and maybe even of zinc (Davidsson et al. 1994; Hettiarachchi et al. 2004).
Iron EDTA in food
When iron EDTA is consumed in food, the ferric ions remain bound to EDTA as ferric EDTA complex ions in the stomach and the small intestine, including the duodenum. EDTA molecules can bind to both ferric and ferrous ions. When bound to EDTA, the ferric form is strongly preferred from a thermodynamic point of view, but nevertheless reduction to the ferrous form is possible (Seibig & Van Eldik 1997). Unlike ferric EDTA complex ions, the stability of ferrous EDTA complex ions is relatively low under acidic conditions. The cell walls of duodenal cells contain the proteins DCytB (duodenal cytochrome B), which is a strong reducing agent, and DMT1 (divalent metal‐ion transporter 1), which allows specifically ferrous ions to permeate the cell wall into the cytoplasm. It is probable that the ferric EDTA complex ions first undergo reduction by DCytB to the corresponding ferrous EDTA complex ions and, subsequently, the DMT1 proteins detach the ferrous ions from their accompanying EDTA molecules and transport them to the cytoplasm. This process is driven by the low pH of the outer membrane microenvironment during this transport of ferrous ions (Gunshin et al. 1997). Animal experiments support this mechanism of a separation of the iron ions from the EDTA molecules prior to entering the blood circulation (Zhu et al. 2006).
An EDTA molecule that is left behind after having released an iron ion to an intestinal cell can pick up another iron ion in the lumen of the gastrointestinal tract and also present it to an intestinal cell. That iron ion could very well be one that had been precipitated by phytate. This mechanism is known as the shuttle effect of EDTA and is believed to enhance the effectiveness of iron absorption even further (Lynch et al. 1993).
The use of iron EDTA in food fortification has grown in the past 10 years (Bothwell & MacPhail 2004, Wreesmann 2009). Iron EDTA is used to fortify powdered beverages in Brazil and the Philippines (Mondelez International 2011), soy sauce in China (Chen et al. 2005), wheat flour in China (Sun et al. 2007) and in Kyrgyzstan (UNICEF 2009), maize and wheat flour in Tanzania and Kenya (Andang'o et al. 2007), and fish sauce in Cambodia (Kingdom of Cambodia 2012). The World Food Programme (WFP) has recently specified that iron EDTA is one of the iron compounds that should be added to special nutritious products, and depending on the type of food, another iron fortificant could be added to meet the required iron content without exceeding the maximum ADI of EDTA (WFP 2011). The US Agency for International Development (USAID) has included iron EDTA in various food specifications (USAID 2013). Iron EDTA is added to some micronutrient powders (Troesch et al. 2011; Macharia‐Mutie et al. 2012) and is also recommended for supplementary foods for the management of moderate acute malnutrition in infants and children 6–59 months of age (WHO 2012b).
Pharmaceutical use of iron EDTA
In France, iron EDTA is approved as the active pharmaceutical ingredient for a drug with the trade name Ferrostrane (Fig. 2) as a medicinal syrup for infants and young children (Hodgkinson 1961; Kahn & Larsen 1980). This syrup contains 0.68% iron, which corresponds to ∼50 mg mL−1 of iron EDTA. This medicine has been in the market in France since the 1960s (HAS 2013) and is also commercially available in a number of other countries, including the UK (trade name Sytron), Sweden, Senegal, Algeria, Egypt and Pakistan (Akzo Nobel Functional Chemicals B.V., personal communication).
Figure 2.

Ferrostrane, medicinal syrup based on sodium feredetate (iron EDTA) and sold in 250‐mL bottles in France.
According to the package insert of Ferrostrane, the recommended intake is 5–10 mL day−1 for infants aged 1–6 months with a bodyweight of 5–8 kg and at the same dose for pregnant women, and 10–15 mL day−1 for children between 6 and 30 months (8–12 kg). In all cases, treatment duration is 3–6 months (ANSM 2012). An overview of the dosage recommendations for infants and young children, and the resulting intake of EDTA as iron EDTA is presented in Table 2.
Table 2.
Recommended dosage on the package insert of Ferrostrane for young children
| Age group in months | Bodyweight in kg | Recommended intake in mL day−1 | Intake as iron EDTA | ||
|---|---|---|---|---|---|
| in mg day−1 * | in mmol day−1 † | in μmol day−1 kgbw−1 | |||
| 1–6 | 5–8 | 5–10 | 250–500 | 0.6–1.2 | 75–240 |
| 6–30 | 8–12 | 10–15 | 500–750 | 1.2–1.8 | 100–225 |
*Based on a content of 50 mg mL−1 of iron EDTA in the syrup (ANSM 2012). †The molecular mass of iron EDTA (EDTA‐FeNa·3H2O) is 421 mg mmol−1.
A dosage of 10 mL day−1 contains 68 mg of iron, which is equivalent to 1.2 mmol (1200 μmol). For an infant with a bodyweight of 5 kg, this daily dose means an intake of 240 μmol day−1 kgbw−1 of EDTA molecules. A dosage of 5 mL day−1 for an 8‐kg infant is equivalent to an intake of 75 μmol day−1 kgbw−1.
The intake of 75–240 μmol day−1 kgbw−1 of EDTA molecules is 12–37 times higher than the current maximum ADI of 6.5 μmol day−1 kgbw−1 of EDTA molecules (JECFA 2007).
The chelates plant of AkzoNobel in Herkenbosch, the Netherlands, has been the sole supplier of the active pharmaceutical ingredient for Ferrostrane (sodium feredetate; iron EDTA) for more than 20 years. During this period, the company has never been informed of any adverse health effects (Akzo Nobel Functional Chemicals B.V., personal communication). This absence of reported adverse health effects, while not strictly a proof as safety, adds weight to the scientific evidence reported previously.
Overview of EDTA intake levels
An overview of maximum intakes of EDTA expressed as EDTA molecules in μmol day−1 kgbw−1 and as H4EDTA in mg day−1 kgbw−1 is given in Table 3 for observations in animal studies and in Table 4 for recommendations in humans. The highest dose level of the 1952 Yang study (7440 μmol day−1 kgbw−1 of EDTA molecules) and a safety margin of 100 would result in a value of 74.4 μmol day−1 kgbw−1 of EDTA molecules or 21.7 mg day−1 kgbw−1 as H4EDTA for maximum ADI of EDTA. This value is 11.4 times higher than the current maximum ADI of EDTA of 6.5 μmol day−1 kgbw−1 of EDTA molecules (JECFA 2007). Based on the second highest dose level of the 1952 Yang study, the maximum ADI of EDTA would be 2.3 times higher than this limit, and based on the highest dose level of the 1977 NCI study 1.4 times higher.
Table 3.
Maximum intake levels of EDTA administered in animal studies
| Reference | Expressed as EDTA molecules in μmol day−1 kgbw−1 | Expressed as H4EDTA in mg day−1 kgbw−1 | Normalised to current maximum ADI of EDTA (JECFA 2007) |
|---|---|---|---|
| BIBRA 1964 | 7440 | 2170 | 1140 |
| 1488 | 434 | 228 | |
| NCI 1977 | 912 | 265 | 140 |
| Oser et al. 1963 | 668 | 195 | 103 |
ADI, acceptable daily intake. For the 1952 Yang study (BIBRA 1964), the second highest dosage level has also been included.
Table 4.
Maximum intake levels of EDTA recommended for human consumption
| Reference | Expressed as EDTA molecules in μmol day−1 kgbw−1 | Expressed as H4EDTA in mg day−1 kgbw−1 | Normalised to current maximum ADI of EDTA (JECFA 2007) |
|---|---|---|---|
| JECFA 2007 | 6.5 | 1.9 | 1.0 |
| ANSM 2012 | 240.0 | 70.0 | 37.0 |
| Wreesmann 2007 | 17.9 | 5.2 | 2.8 |
| This publication | 74.4 | 21.7 | 11.4 |
ADI, acceptable daily intake.
In response to the announcement of a safety re‐evaluation of iron EDTA by JECFA in 2007, AkzoNobel submitted a dossier with the proposal to raise the maximum permitted level of iron as iron EDTA for 6‐ to 24‐month‐old infants and young children to 5 mg day−1 (Wreesmann 2007). This amount is equivalent to 89 μmol day−1 of iron and the same amount of μmol day−1 of EDTA molecules. For a 5‐kg infant, this intake of 5 mg day−1 iron as iron EDTA is equivalent to 17.9 μmol day−1 kgbw−1 of EDTA molecules, which is 2.8 times higher than the current maximum ADI of EDTA. The AkzoNobel dossier advocated that this maximum oral intake of 5 mg day−1 iron as iron EDTA should be considered independently of the presence of calcium EDTA and disodium EDTA in food. The intake of these two EDTA‐based food additives could remain compliant with the maximum ADI of EDTA as defined by JECFA (JECFA 1974).
The safety re‐evaluation process resulted in a monograph (Pronk & Schlatter 2008), in which JECFA outlined that they could not support a rise of the maximum ADI of EDTA. However, the previous JECFA statement on iron EDTA of 1999 (JECFA 2000) was revised from ‘could be considered safe’ to ‘is suitable’, which is clearer confirmation of its safety for human use. Furthermore, two ambiguous sentences were removed from the previous statement ‘when used in supervised food fortification programmes in response to a need for iron supplementation in a population as determined by public health officials’ and ‘such programmes would provide a daily iron intake of approximately 0.2 mg kg−1 of body weight’. This revised JECFA statement has greatly supported the acceptance of iron EDTA for nutritional purposes by decision makers and Health Authorities in the EU (EFSA 2010; EU 2010; EU 2011) and in India (Gazette of India 2011).
In their safety evaluation report, JECFA rounded the figure of 1.95 mg day−1 kgbw−1 for the maximum ADI of EDTA expressed as H4EDTA (corresponding with 2.5 mg day−1 kgbw−1 of CaNa2EDTA) downwards to 1.9 rather than upwards to 2.0 (JECFA 2007). As a result, the normalised value for the principal animal study in Table 3 is not equal to the theoretical value of 100, but to 103.
Discussion
It is now generally accepted that the EDTA molecules in iron EDTA fulfil a unique, positive role in delivering the iron ions from phytate‐rich diets to the human body (Hurrell et al. 2010). Nevertheless, there is concern that EDTA molecules may additionally have some negative impact on health. Although not justified by observations in high‐dosage animal experiments, this concern may have been aggravated by the emphasis on the maximum ADI of EDTA in regulatory documents on iron EDTA (Whittaker et al. 1993; Heimbach et al. 2000). Another view is that EDTA may represent a rare example of a synthetic chemical that is harmless to human health (Rowbury 2011). Besides a beneficial effect on iron absorption, EDTA in food could also contribute to lowering the absorption of lead (Flanagan et al. 1982; Kim et al. 2009) and to enhancing the uptake of zinc (Davidsson et al. 1994; Hettiarachchi et al. 2004).
The three long‐term animal studies summarised in Table 1 revealed no significant adverse health effects, except at the highest level (7440 μmol day−1 kgbw−1) where continuous diarrhoea, reduced appetite and no offspring were reported (BIBRA 1964). Nevertheless, extensive examination of the organs and tissues of the test animals did not reveal any change compared with the control group. The second highest dosage (1488 μmol day−1 kgbw−1) showed no adverse effects. The adverse effects at the highest dosage level could be due to the very high intake of sodium instead of the high EDTA intake. The exposure to sodium ions from the addition of 7440 μmol day−1 kgbw−1 of disodium EDTA was 14 880 μmol day−1 kgbw−1. This corresponds to 870 mg day−1 kgbw−1 NaCl, which is equivalent to 52 g daily salt intake for a 60‐kg adult. Assuming that the adverse effects were due to the high intake of sodium instead of EDTA, the highest dose would be safe and could be used as a basis for the determination of the maximum ADI of EDTA.
Two of the three long‐term studies reported positive health effects apparently induced by EDTA intake. The NCI study (NCI 1977) concluded that ‘the rats exhibited a negative dose‐related trend in survival’, indicating the rats tended to survive longer on feed containing more EDTA. In the summary of the PhD thesis, Yang stated: ‘The highest mortality occurred in group I [0 = control] and, in decreasing order, in groups II [0.5% disodium EDTA] and III [1.0% disodium EDTA]. There were no deaths in group IV [5.0% disodium EDTA]’ (BIBRA 1964).
JECFA during their 17th meeting in 1973 (JECFA 1974) considered only the study of Oser et al. 1963. Had they considered other long‐term animal studies (see Table 1), they might have proposed a higher value for the maximum ADI of EDTA. If the data from the NCI study (NCI 1977) had been available when JECFA defined the maximum ADI of EDTA in 1973, it would now likely have been 9.1 μmol day−1 kgbw−1. This is 1.4 times higher than the current level of 6.5 μmol day−1 kgbw−1. While an increase in the maximum ADI of EDTA by a factor 1.4 would be beneficial, it would still probably not meet the daily needs for iron via iron EDTA for 6‐ to 24‐month‐old children. Had JECFA in 1973 used the second highest dosage group of Yang 1952 (BIBRA 1964), which showed no adverse health effects, the maximum ADI of EDTA would now be 14.9 μmol day−1 kgbw−1, which is 2.3 times higher than the current maximum. This increase by a factor 2.3 would meet the iron requirements of 6‐ to 24‐month‐old children. Nevertheless, if JECFA in 1973 had used the data of the highest dosage group in the Yang study (BIBRA 1964), which resulted in continuous diarrhoea, reduced appetite and no offspring (but no organ changes), the maximum ADI of EDTA would have been 74.4 μmol day−1 kgbw−1. This is equivalent to 21.7 mg day−1 kgbw−1 and 11.4 times higher than the current 1.9 mg day−1 kgbw−1 (JECFA 2007), and about one‐third of the highest intake level of EDTA molecules as provided by Ferrostrane.
Recently, US FDA affirmed a self‐declared determination as GRAS by Del Monte Foods for the use of disodium EDTA and calcium EDTA in packaged cooked sweet corn products (FDA 2011). In order not to exceed the current maximum ADI of EDTA, calcium EDTA and disodium EDTA were set at a maximum level of 200 and 165 ppm, respectively. To support the safety assessment, the dossier submitted also referred to the Yang animal study of 1952 (BIBRA 1964). As already stated, the ratio of the level in feed in mg kg−1 to intake level in mg day−1 kgbw−1, also known as conversion factor, is generally assumed to be 20:1 (Oser et al. 1963). However, the applicants took a conversion factor of 10 rather than 20. In referring to the Yang study of 1952 (BIBRA 1964), the dossier states: ‘Rats were fed a diet containing 0, 0.5, 1.0, or 5.0% disodium EDTA (equivalent to approximately 0, 500, 1000, or 5000 mg kg−1 day−1)’. An intake of 5000 mg day−1 kgbw−1 is twice as high as the value of 2500 mg day−1 kgbw−1 (Table 1) and thus the assumed intake is twice as high as that outlined earlier. With the same safety margin of 100, but with a conversion factor of only 10, the maximum ADI of EDTA becomes 149 μmol day−1 kgbw−1. This is equivalent to 43.5 mg day−1 kgbw−1 of EDTA and 23 times higher than the current 1.9 mg day−1 kgbw−1 (JECFA 2007). Although the use of a conversion factor of 10 rather than 20 still needs a careful defence, intakes as high as 21.7–43.5 mg day−1 kgbw−1 of EDTA as iron EDTA are similar to those achieved through the nearly 50 years' use of Ferrostrane.
An intake of 5 mg iron as iron EDTA per day, either through fortification of complementary foods or in the form of a home fortificant, such as micronutrient powders or lipid‐based nutrient supplements, is considered necessary to ensure adequate iron absorption from the complementary feeding diet of young children (6–24 months of age), especially when largely based on plant source foods rich in phytate. While encouraging results have been reported with the addition of 2.5 mg iron as iron EDTA to the diet of young children (Troesch et al. 2011; Macharia‐Mutie et al. 2012), this was under controlled circumstances. In both studies, the iron EDTA was in the form of a micronutrient powder that provided other vitamins and minerals to reduce nutritional anaemia, and the micronutrient powder in the 2011 Troesch study also contained phytase, which degraded phytic acid (Troesch et al. 2009). In a study using a diet rich in both phytate and polyphenols, iron EDTA was shown to be effective, but the dose level was 10 mg iron as iron EDTA per meal, three times a week (Abizari et al. 2012).
In summary, based on the knowledge of how EDTA functions in the human body, the results of long‐term animal studies, and the nearly 50 years' use of Ferrostrane, a rise of the maximum ADI of EDTA can be considered safe. Based on the NCI study (NCI 1977), the maximum ADI of EDTA can be raised to 9.1 μmol day−1 kgbw−1, which is 1.4 times higher than the current level. Based on the PhD thesis of Yang 1952 (BIBRA 1964), and the dosage level that did not show any adverse health effects, the maximum ADI of EDTA would be 14.9 μmol day−1 kgbw−1, which is 2.3 times higher than the current maximum. Based on the highest dosage group of Yang's study (BIBRA 1964), which resulted in continuous diarrhoea, reduced appetite and no offspring (but no organ changes), the maximum ADI of EDTA would be increased to 74.4 μmol day−1 kgbw−1, 11.4 times than the current maximum ADI of EDTA but only about one‐third of the highest exposure level to EDTA molecules as provided by Ferrostrane.
For a 5‐kg infant, this regulatory change to a maximum ADI of EDTA of 14.9 μmol day−1 kgbw−1 would allow a daily intake up to 5 mg Fe as iron EDTA rather than the currently permitted level of maximum 2.2 mg. With respect to the fortification of complementary foods for infants and young children aged 6–24 months, an addition level ensuring a daily intake of 5 mg Fe as iron EDTA is likely to be sufficient to allow adequate iron absorption and would be entirely safe.
Source of funding
Akzo Nobel Functional Chemicals B.V. provided funding for writing this article.
Conflicts of interest
The author is employed by the chemical company AkzoNobel. The Business Unit Akzo Nobel Functional Chemicals B.V. is a major global manufacturer of food‐grade EDTA products, including iron EDTA and world's largest producer of iron EDTA for agricultural purposes.
Acknowledgements
The author wishes to acknowledge Dr Saskia de Pee, World Food Programme, for guidance and support in preparing this manuscript; Dr Ian Munro, JECFA member and author of the first toxicological monograph on iron EDTA, for advice on the implications of the multi‐year animal studies before he passed away in 2011; and colleague, Mr Remi Nicolas, AkzoNobel France, for market information on Ferrostrane.
References
- Abizari A.R., Moretti D., Zimmermann M.B., Armar‐Klemesu M. & Brouwer I.D. (2012) Whole cowpea meal fortified with NaFeEDTA reduces iron deficiency among Ghanaian school children in a malaria endemic area. Journal of Nutrition 142, 1836–1842. [DOI] [PubMed] [Google Scholar]
- Andang'o P.E.A., Osendarp S.J.M., Ayah R., West C.E., Mwaniki D.L., De Wolf C.A. et al (2007) Efficacy of iron‐fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet 369, 1799–1806. [DOI] [PubMed] [Google Scholar]
- Anderson E.V. & Gaunt J.A. (1960) EDTA (ethylenediamine tetraacetic acid): a staff industry collaborative report. Industrial and Engineering Chemistry 52, 190–196. [Google Scholar]
- ANSM (French National Agency for the Safety of Medicines and Health Products) (2012) Patient Information on Ferrostrane, 0.68 Per Cent, Syrup (Publication date: 24 August 2012) . Available at: http://agence-prd.ansm.sante.fr/php/ecodex/notice/N0213394.htm (Accessed 8 October 2013).
- BIBRA (1964) Summaries of toxicological data, toxicology of EDTA. Food and Cosmetics Toxicology 2, 763–767. [Google Scholar]
- Bothwell T.H. & MacPhail A.P. (2004) The potential role of NaFeEDTA as an iron fortificant. International Journal for Vitamin and Nutrient Research 74, 421–434. [DOI] [PubMed] [Google Scholar]
- Cercamondi C.I., Egli I.M., Mitchikpe E., Tossou F., Hessou J., Zeder C. et al (2013) Iron bioavailability from a lipid‐based complementary food fortificant mixed with millet porridge can be optimized by adding phytase and ascorbic acid but not by using a mixture of ferrous sulfate and sodium iron EDTA. Journal of Nutrition 143, 1233 – 1239. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhao X., Zhang X., Yin S., Piao J., Huo J. et al (2005) Studies on the effectiveness of NaFeEDTA‐fortified soy sauce in controlling iron deficiency: a population‐based intervention trial. Food and Nutrition Bulletin 26, 177–186. [DOI] [PubMed] [Google Scholar]
- Cook J.D. & Monsen E.R. (1976) Food iron absorption in man II. The effect of EDTA on absorption of dietary non‐heme iron. American Journal of Clinical Nutrition 29, 614–620. [DOI] [PubMed] [Google Scholar]
- Cook J.D., Watson S.S., Simpson K.M., Lipschitz D.A. & Skikne B.S. (1984) The effect of high ascorbic acid supplementation on body iron stores. Blood 64, 721–726. [PubMed] [Google Scholar]
- Davidsson L., Kastenmayer P. & Hurrell R.F. (1994) Sodium iron EDTA [NaFe(IlI)EDTA] as a food fortificant: the effect on the absorption and retention of zinc and calcium in women. American Journal of Clinical Nutrition 60, 231–237. [DOI] [PubMed] [Google Scholar]
- De Pee S., Timmer A., Martini E., Neufeld L., Van Hees J., Schofield D. et al (2011) Programmatic guidance brief on use of micronutrient powders (MNP) for home fortification, a document of the Home Fortification. Technical Advisory Group (HF‐TAG): Geneva, Switzerland. Available at: http://hftag.gainhealth.org/sites/hftag.gainhealth.org/files/HF-TAG_Program%20Brief%20Dec%202011.pdf (Accessed 8 October 2013).
- Diaz M., Rosado J.L., Allen L.H., Abrams S. & Garcia O.P. (2003) The efficacy of a local ascorbic acid‐rich food in improving iron absorption from Mexican diets: a field study using stable isotopes. American Journal of Clinical Nutrition 78, 436–440. [DOI] [PubMed] [Google Scholar]
- EFSA (2010) Scientific Opinion on the use of ferric sodium EDTA as a source of iron added for nutritional purposes to foods for the general population (including food supplements) and to foods for particular nutritional uses by the European Food Safety Authority. EFSA Journal 8, 1414 [32 pp.] or doi: 10.2903/j.efsa.2010.1414. Available at: http://www.efsa.europa.eu/en/efsajournal/pub/1414.htm (Accessed 8 October 2013). [DOI] [Google Scholar]
- EFSA (2013) Scientific opinion on the substantiation of a health claim related to iron and contribution to normal cognitive development pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA Journal 11, 3335 [10 pp.] or doi: 10.2903/j.efsa.2013.3335. Available at: http://www.efsa.europa.eu/en/efsajournal/pub/3335.htm (Accessed 8 October 2013). [DOI] [Google Scholar]
- EU (2010) Commission Decision of 14 June 2010 authorising the placing on the market of ferric sodium EDTA as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Official Journal of the European Union L149(15.6.2010), 16 – 19. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:149:0016:0019:EN:PDF (Accessed 8 October 2013). [Google Scholar]
- EU (2011) Commission Regulation (EU) No 1161/2011 of 14 November 2011 amending Directive 2002/46/EC of the European Parliament and of the Council, Regulation (EC) No 1925/2006 of the European Parliament and of the Council and Commission Regulation (EC) No 953/2009 as regards the lists of mineral substances that can be added to foods. Official Journal of the European Union L296(15.11.2011), 29 – 30. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:296:0029:0030:EN:PDF (Accessed 8 October 2013). [Google Scholar]
- European Community (1995) European Parliament and Council Directive No 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners. Available at: http://ec.europa.eu/food/fs/sfp/addit_flavor/flav11_en.pdf (Accessed 8 October 2013).
- FAO (1965a) Monograph on calcium disodium ethylenediaminetetraacetate. FAO Nutrition Meetings Report Series No. 40A,B,C. Toxicological Evaluation of Some Antimicrobials, Antioxidants, Emulsifiers, Stabilizers, Flour‐Treatment Agents, Acids and Bases. Prepared by the 9th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Rome, 13–20 December, 1965 Available at: http://www.inchem.org/documents/jecfa/jecmono/40abcj09.htm (Accessed 8 October 2013).
- FAO (1965b) Monograph on disodium ethylenediaminetetraacetate. FAO Nutrition Meetings Report Series No. 40A,B,C. Toxicological Evaluation of Some Antimicrobials, Antioxidants, Emulsifiers, Stabilizers, Flour‐Treatment Agents, Acids and Bases. Prepared by the 9th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Rome, 13–20 December, 1965 Available at: http://www.inchem.org/documents/jecfa/jecmono/40abcj10.htm (Accessed 8 October 2013).
- FCC (2012) Monographs/sodium iron EDTA. Food Chemicals Codex, 8th Edition, 2012, pp. 1037–1040. US Pharmacopeia Convention, Rockville, MD, USA.
- FDA (2004) GRAS Notice No. GRN 152 by Kraft Foods Global on Sodium Iron EDTA for Use in Iron Fortification of Powdered Soft Drinks to the U.S. Food and Drug Administration . Available at: http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=grasListing&id=152 (Accessed 8 October 2013).
- FDA (2006) GRAS Notice No. GRN 178 by Akzo Nobel Chemicals on Sodium Iron EDTA for Iron Fortification of Soy, Fish, Hoisin and Teriyaki Sauces to the U.S. Food and Drug Administration . Available at: http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=grasListing&id=178 (Accessed 8 October 2013).
- FDA (2011) GRAS Notice No. GRN 363 by Del Monte Foods on Calcium Disodium EDTA and Disodium EDTA to be Used in Packaged Cooked Sweet Corn products to the U.S. Food and Drug Administration . Available at: http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=grasListing&id=363 (Accessed 8 October 2013).
- Flanagan P.R., Chamberlain M.J. & Valberg L.S. (1982) The relationship between iron and lead absorption in humans. American Journal of Clinical Nutrition 36, 823–829. [DOI] [PubMed] [Google Scholar]
- Foreman H. & Trujilio T.T. (1954) The metabolism of C(14)‐labeled ethylenediaminetetraacetic acid in human beings. Journal of Laboratory and Clinical Medicine 43, 566–571. [PubMed] [Google Scholar]
- Foreman H., Vier M. & Magee M. (1953) The metabolism of C(14)‐labeled ethylenediaminetetraacetic acid in the rat. Journal of Biological Chemistry 203, 1045–1053. [PubMed] [Google Scholar]
- FSANZ (2008) Application A570 – Ferric Sodium EDTA as a Permitted Form of Iron. Final Assessment Report of 4 June 2008 of Food Standards Australia New Zealand . Available at: http://www.foodstandards.gov.au/code/applications/Pages/applicationa570ferri3420.aspx (Accessed 8 October 2013).
- Furia T.E. (1964) EDTA in foods: a technical review. Food Technology 18, 1874–1882. [Google Scholar]
- Garcia O.P., Diaz M., Rosado J.L. & Allen L.H. (2003) Ascorbic acid from lime juice does not improve the iron status of iron‐deficient women in rural Mexico. American Journal of Clinical Nutrition 78, 267–273. [DOI] [PubMed] [Google Scholar]
- Gazette of India (2011) Notification dated 14.2.2011 (prevention of food adulteration), comments on sodium iron (III) ethylene diamine tetra acetate, trihydrate (sodium feredetate – NaFeEDTA).
- Gibson R.S., Bailey K., Gibbs M. & Ferguson E.L. (2010) A review of phytate, iron, zinc, and calcium concentrations in plant‐based complementary foods used in low‐income countries and implications for bioavailability. Food and Nutrition Bulletin 31, S134–S146. [DOI] [PubMed] [Google Scholar]
- Gunshin H., Mackenzie B., Berger U.V., Gunshin Y., Romero M.F., Boron W.F. et al (1997) Cloning and characterization of a mammalian proton‐coupled metal‐ion transporter. Nature 388, 482–488. [DOI] [PubMed] [Google Scholar]
- HAS (Haute Authorité de Santé (France), Commission de la Transparence) (2013) Product Information on Ferrostrane, 0.68 Per Cent, Syrup (Publication date: 9 January 2013) . Available at: http://www.has-sante.fr/portail/upload/docs/evamed/CT-12406_FERROSTRANE_09012013_CT12406_Avis1_RI.pdf (Accessed 8 October 2013).
- Heimbach J., Rieth S., Mohamedshah F., Slesinski R., Samuel‐Fernando P., Sheehan T. et al (2000) Safety assessment of Iron EDTA [sodium iron (Fe3+) ethylenediaminetetraacetic acid]: summary of toxicological, fortification and exposure data. Food and Chemical Toxicology 38, 99–111. [DOI] [PubMed] [Google Scholar]
- Hess S.Y. & Brown K.H. (2009) Impact of zinc fortification on zinc nutrition. Food and Nutrition Bulletin 30, S79–S107. [DOI] [PubMed] [Google Scholar]
- Hettiarachchi M., Hilmers D.C., Liyanage C. & Abrams S.A. (2004) Na2EDTA enhances the absorption of iron and zinc from fortified rice flour in Sri Lankan children. Journal of Nutrition 134, 3031–3036. [DOI] [PubMed] [Google Scholar]
- Hoddinott J., Rosegrant M. & Torero M. (2012) Copenhagen Consensus 2012 Challenge Paper on Hunger and Malnutrition . Copenhagen Consensus Center. Available at: http://www.thousanddays.org/resource-slug/copenhagen-consensus-2012-challenge-paper (Accessed 8 October 2013).
- Hodgkinson R. (1961) A comparative study of iron absorption and utilization following ferrous sulphate and sodium ironedetate (Sytron). The Medical Journal of Australia 48, 309–311. [DOI] [PubMed] [Google Scholar]
- Horton R. (2008) Maternal and child undernutrition: an urgent opportunity. Lancet 371, 179 (Introducing The Lancet's maternal and child undernutrition series, launched 16 January 2008). Available at: http://www.thelancet.com/series/maternal-and-child-undernutrition (Accessed 8 October 2013). [DOI] [PubMed] [Google Scholar]
- Hunt J.R., Gallagher S.K. & Johnson L.K. (1994) Effect of ascorbic acid on apparent iron absorption by women with low iron stores. American Journal of Clinical Nutrition 59, 1381–1385. [DOI] [PubMed] [Google Scholar]
- Hurrell R., Ranum P., De Pee S., Biebinger R., Hulthen L., Johnson Q. et al (2010) Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food and Nutrition Bulletin 31, S7–S21. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F. (2002) Fortification: overcoming technical and practical barriers. Journal of Nutrition 132, 806S–812S. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F. (2004) Phytic acid degradation as a means of improving iron absorption. International Journal for Vitamin and Nutrition Research 74, 445–452. [DOI] [PubMed] [Google Scholar]
- IOM (2001) Iron (page 290–393) in: Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc, a report of the panel on micronutrients, subcommittees on upper reference levels of nutrients and of interpretation and use of dietary reference intakes, and the standing committee on the scientific evaluation of dietary reference intakes. Food and Nutrition Board, Institute of Medicine (IOM). ISBN 0‐309‐07279‐4.
- JECFA (1974) Toxicological evaluation of certain food additives with a review of general principles: 17th report of Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Technical Report Series 539, 18 (World Health Organization, Geneva, Switzerland). Available at: http://whqlibdoc.who.int/trs/WHO_TRS_539.pdf (Accessed 8 October 2013). [PubMed] [Google Scholar]
- JECFA (1983) Evaluation of certain food additives and contaminants: 27th report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Technical Report Series 696, 48–50. Available at: http://whqlibdoc.who.int/trs/WHO_TRS_696.pdf (Accessed 8 October 2013). [Google Scholar]
- JECFA (2000) Evaluation of certain food additives and contaminants: 53rd report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Technical Report Series 896, 27–29. Available at: http://whqlibdoc.who.int/trs/WHO_TRS_896.pdf (Accessed 8 October 2013). [Google Scholar]
- JECFA (2007) Evaluation of certain food additives and contaminants: 68th report of Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Technical Report Series 947, 48–50. (World Health Organization, Geneva, Switzerland). Available at: http://whqlibdoc.who.int/publications/2007/9789241209472_eng.pdf (Accessed 8 October 2013). [Google Scholar]
- Kahn J. & Larsen S. (1980) Irostrene (ferric sodium edetate) treatment of anaemic infants. Journal of International Medical Research 8, 258–261. [DOI] [PubMed] [Google Scholar]
- Kim H.‐S., Kim M.‐K. & Lee B.‐K. (2009) Oral supplementation with NaFeEDTA reduces blood lead in postmenopausal but not premenopausal Korean women with anemia. Nutrition 25, 66–71. [DOI] [PubMed] [Google Scholar]
- Kingdom of Cambodia (2012) For production and consumption of iron fortified fish sauce and soy sauce. Proclamation by the Ministry of Planning, National Council for Nutrition, posted on Internet. Available at: http://www.information.gov.kh/khmer/law/txt_khmer/OtherMinistry/DEC-091-2012-08-09.pdf (Accessed 8 October 2013).
- Kumar V., Sinha A.K., Makkar H.P.S. & Becker K. (2010) Dietary roles of phytate and phytase in human nutrition: a review. Food Chemistry 120, 945–959. [Google Scholar]
- Lanigan R.S. & Yamarik T.A. (2002) Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA‐EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium, EDTA, HEDTA, and trisodium HEDTA. International Journal of Toxicology 21, 95–142. [DOI] [PubMed] [Google Scholar]
- Lin J.‐L., Lin‐Tan D.‐T., Hsu K.‐H. & Yu C.‐C. (2003) Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. New England Journal of Medicine 348, 277–286. [DOI] [PubMed] [Google Scholar]
- Lozoff B., Smith J.B., Kaciroti N., Clark K.M., Guevara S. & Jimenez E. (2013) Functional significance of early‐life iron deficiency: outcomes at 25 years. Journal of Pediatrics 163, 1260–1266. doi: 10.1016/j.jpeds.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S.R., Hurrell R.F., Bothwell T.H. & MacPhail A.P. (1993) Section on: relationship of NaFeEDTA to the common nonheme iron pool (pp. 30–31) in: Iron EDTA for Food Fortification. A report of the International Nutritional Anemia Consultative Group (INACG). ISBN 0‐944398‐23‐5.
- Ma G., Jin Y., Piao J., Kok F., Bonnema G. & Jacobsen E. (2005) Phytate, calcium, iron and zinc contents and their molar ratios in foods commonly consumed in China. Journal of Agricultural and Food Chemistry 53, 10285–10290. [DOI] [PubMed] [Google Scholar]
- Macharia‐Mutie C.W., Moretti D., Van Den Briel N., Omusundi A.M., Mwangi A.M., Kok F.J. et al (2012) Maize porridge enriched with a micronutrient powder containing low‐dose iron as NaFeEDTA but not amaranth grain flour reduces anemia and iron deficiency in Kenyan preschool children. Journal of Nutrition 142, 1756–1763. [DOI] [PubMed] [Google Scholar]
- Mondelez International (2011) Developing markets' growth rockets Tang to ‘Billion‐Dollar’ status. News release posted on Internet. Available at: http://www.mondelezinternational.com/mediacenter/country-press-releases/us/2011/multi_media_06152011.aspx (Accessed 8 October 2013).
- Munro I.C. (1993) First draft on sodium iron EDTA. WHO Food Additives Series 32, JECFA Monograph No. 796 (World Health Organization, Geneva, Switzerland). Available at: http://www.inchem.org/documents/jecfa/jecmono/v32je14.htm (Accessed 8 October 2013).
- NCI (1977) Bioassay of trisodium ethylenediaminetetraacetate trihydrate (EDTA) for possible carcinogenicity: CAS No. 150‐38‐9. National Cancer Institute, Bethesda (MD), USA. Technical Report Series No. 11 Available at: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr011.pdf (Accessed 8 October 2013). [PubMed]
- Oser B.L., Oser M. & Spencer H.C. (1963) Safety evaluation studies of calcium EDTA. Toxicology and Applied Pharmacology 5, 142–162. [DOI] [PubMed] [Google Scholar]
- PRC (1994) GB 14880‐94 Hygienic standard for the use of nutritional fortification substances in foods in the People's Republic of China.
- PRC (1996) GB 2760‐96 Hygienic standard for the use of food additives in foods in the People's Republic of China.
- Pronk M.E.J. & Schlatter J. (2008) First draft on sodium iron(III) ethylenediaminetetraacetic acid (sodium iron EDTA). WHO Food Additives Series 59, 125–144. (JECFA monographs, World Health Organization, Geneva, Switzerland). Available at: http://www.inchem.org/documents/jecfa/jecmono/v59je01.pdf (Accessed 8 October 2013). [Google Scholar]
- Republic of the Philippines (1995) Guidelines on Micronutrient Fortification of Foods. Administrative Order 4‐As by the Department of Health, Bureau of Food and Drugs Available at: http://old.fda.gov.ph/AO/ao%204-a%20s%201995.pdf (Accessed 8 October 2013).
- Rosenberg I., Tilahun J., Schlossman N., Bagriansky J., Johnson Q., Webb P. et al (2011) Nutritional enhancement of US Title II food aid products. Food and Nutrition Bulletin 32, 134S–151S. [DOI] [PubMed] [Google Scholar]
- Rowbury R. (2011) Miracle molecules of our age: ethylenediaminetetraacetic acid. Science Progress 94, 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M., Gignac S., Bessman S.P. & Belknap E.L. (1953) Enhancement of lead excretion in humans with EDTA. Science 117, 659–660. [DOI] [PubMed] [Google Scholar]
- Seibig S. & Van Eldik R. (1997) Kinetics of [FeII(EDTA)] oxidation by molecular oxygen revisited. New evidence for multistep mechanism. Inorganic Chemistry 36, 4115–4120. [Google Scholar]
- Sun J., Huang J., Li W., Wang L., Wang A., Huo J. et al (2007) Effects of wheat flour fortified with different iron fortificants on iron status and anemia prevalence in iron deficient anemic students in Northern China. Asia Pacific Journal of Clinical Nutrition 16, 116–121. [PubMed] [Google Scholar]
- Swenerton H. & Hurley L.S. (1971) Teratogenic effects of a chelating agent and their prevention with zinc. Science 173, 62–64. [DOI] [PubMed] [Google Scholar]
- Teucher B., Olivares M. & Cori H. (2004) Enhancers of iron absorption: ascorbic acid and other organic acids. International Journal for Vitamin and Nutrition Research 74, 403–419. [DOI] [PubMed] [Google Scholar]
- Thankachan P., Walczyk T., Muthayya S., Kurpad A.V. & Hurrell R.F. (2008) Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. American Journal of Clinical Nutrition 87, 881–886. [DOI] [PubMed] [Google Scholar]
- Troesch B., Egli I., Zeder C., Hurrell R.F., de Pee S. & Zimmermann M.B. (2009) Optimization of a phytase‐containing micronutrient powder with low amounts of highly bioavailable iron for in‐home fortification of complementary foods. American Journal of Clinical Nutrition 89, 539–544. [DOI] [PubMed] [Google Scholar]
- Troesch B., van Stuijvenberg M.E., Smuts C.M., Salomè Kruger H., Biebinger R., Hurrell R.F. et al (2011) A micronutrient powder with low doses of highly absorbable iron and zinc reduces iron and zinc deficiency and improves weight‐for‐age Z‐scores in South African children. Journal of Nutrition 141, 237–242. [DOI] [PubMed] [Google Scholar]
- UNICEF (2009) Kyrgyzstan enacts law on flour fortification to fight ‘hidden hunger’ (Publication date: 13 April 2009) . Available at: http://www.unicef.org/infobycountry/kyrgyzstan_49274.html (Accessed 8 October 2013).
- USAID (2013) Cornmeal Commodity Fact Sheet . Available at: http://www.usaid.gov/what-we-do/agriculture-and-food-security/food-assistance/resources/cornmeal-commodity-fact-sheet (Accessed 8 October 2013).
- WFP (2011) Technical Specifications for the Manufacture of: Super Cereal Plus (Corn Soya Blend) . Available at: http://home.wfp.org/stellent/groups/public/documents/manual_guide_proced/wfp251145.pdf (Accessed 8 October 2013).
- Whittaker P., Vanderveen J.E., Dinovi M.J., Kuznesof P.M. & Dunkel V.C. (1993) Toxicological profile, current use, and regulatory issues on EDTA compounds for assessing use of sodium iron EDTA for food fortification. Regulatory Toxicology and Pharmacology 18, 419–427. [DOI] [PubMed] [Google Scholar]
- WHO (1987) Environmental Health Criteria 70: Principles for the safety Assessment of Food Additives and Contaminants in Food . World Health Organization: Geneva, Switzerland. Available at: http://www.inchem.org/documents/ehc/ehc/ehc70.htm (Accessed 8 October 2013).
- WHO (2009) Nutrition: Recommendations on Wheat and Maize Flour Fortification Meeting Report: Interim Consensus Statement . World Health Organization: Geneva, Switzerland. Available at: http://www.who.int/nutrition/publications/micronutrients/wheat_maize_fortification/en/index.html (Accessed 8 October 2013). [PubMed]
- WHO (2012a) Guideline: Sodium Intake for Adults and Children . World Health Organization: Geneva, Switzerland. Available at: http://www.who.int/nutrition/publications/guidelines/sodium_intake (Accessed 8 October 2013).
- WHO (2012b) Technical Note: Supplementary Foods for the Management of Moderate Acute Malnutrition in Infants and Children 6–59 Months of Age . World Health Organization: Geneva. Available at: http://www.who.int/nutrition/publications/moderate_malnutrition/9789241504423/en/index.html (Accessed 8 October 2013).
- Wreesmann C. (2007) Ferric Sodium EDTA (Ferrazone®): A Safe and Effective Iron Source for Children from 6 Months of Age Onwards . Poster presented during the Micronutrient Forum Conference in Istanbul (Turkey), April 2007. Available at: http://www.micronutrientforum.org/meeting2007/posters/Food%20Fortification/EDTA%20Iron%20Source%20for%20Infants%20Wreesmann.pdf (Accessed 8 October 2013).
- Wreesmann C. (2009) Ferric Sodium EDTA (FeNa‐EDTA) and Its Long and Winding Road Towards Implementation as a Food Fortificant . Poster presented during the Micronutrient Forum conference in Beijing (China), May 2009. Available at: http://www.micronutrientforum.org/meeting2009/PDFs/Poster%20Presentations/4_Friday/MNPMMS/F42_Wreesmann.pdf (Accessed 8 October 2013).
- Yang S.S. (1952) Toxicological Investigation of Ethylenediaminetetraacetic Acid in the Rat . Unpublished PhD Thesis. University of Massachusetts, Amherst, MA.
- Yang Z., Siekmann J. & Schofield D. (2011) Fortifying complementary foods with NaFeEDTA – considerations for developing countries. Maternal and Child Nutrition 7, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Yeung C.K., Glahn R.P. & Miller D.D. (2006) Iron dissociates from the NaFeEDTA complex prior to or during intestinal absorption in rats. Journal of Agricultural and Food Chemistry 54, 7929–7934. [DOI] [PubMed] [Google Scholar]


