Abstract
Porphyromonas gingivalis is a keystone periodontal pathogen. Histologocally, the gingival tissue in periodontitis shows dense infiltration of plasma cells. However, antigens recognized by antibodies secreted from the immunocytes remain unknown. The enzyme-labeled antigen method was applied to detecting plasma cells producing P. gingivalis-specific antibodies in biopsied gingival tissue of periodontitis. N-terminally biotinylated P. gingivalis antigens, Ag53 and four gingipain domains (Arg-pro, Arg-hgp, Lys-pro and Lys-hgp) were prepared by the cell-free protein synthesis system using wheatgerm extract. With these five labeled proteins as probes, 20 lesions of periodontitis were evaluated. With the AlphaScreen method, antibodies against any one of the five P. gingivalis antigens were detected in 11 (55%) serum samples and 17 (85%) tissue extracts. Using the enzyme-labeled antigen method on paraformaldehyde-fixed frozen sections of gingival tissue, plasma cells were labeled with any one of the five antigens in 17 (94%) of 18 specimens, in which evaluable plasma cells were detected. The positivity rates in periodontitis were significantly higher than those found previously in radicular cysts (20% in sera and 33% in tissue extracts with the AlphaScreen method, and 25% with the enzyme-labeled antigen method). Our findings directly indicate that antibodies reactive to P. gingivalis are locally produced in the gingival lesions, and that inflammatory reactions against P. gingivalis are involved in periodontitis.
Keywords: antigen 53, AlphaScreen method, gingipain, wheat germ cell free protein synthesis
Introduction
Periodontitis results in erosion of alveolar bone around the teeth, and is a major cause of tooth loss in adults (Pihlstrom et al., 2005). Histologically, the gingival tissue of periodontitis is characterized by dense infiltration of plasma cells (Kim et al., 2010). However, antigens recognized by antibodies secreted from these immunocytes remain unknown. It is highly likely that the plasma cells locally secrete disease-related antibodies, simply because they are distributed within the lesion. The detection of such antibodies may give us a novel breakthrough in analyzing the pathogenesis and disease process.
Porphyromonas gingivalis is a black-pigmented, non-motile, obligatory anaerobic, gram-negative bacillus normally residing in the human oral cavity and abnormally colonizing the lesion of periodontitis or pyorrhea gingivitis (Cutler et al., 1995; Lamont & Jenkinson, 1998; Holt et al., 1999; Slots & Ting, 1999). Biofilm is formed on the gingival lesion (Lamont & Jenkinson, 1998).
It is well known that P. gingivalis is a keystone pathogen of periodontitis (Lamont & Jenkinson, 2000; Hajishengallis & Lamont, 2014), and it interferes in the host immunity through the following mechanisms. Gingipains of P. gingivalis manipulate complement activation by readily degrading complement C3. This process suppresses the deposition of C3b opsonin or the complement complex on the surface of bacteria (Hajishengallis & Lamont, 2014). Gingipains further degrade complement C5 to C5a, and C5a binds to C5a receptors on macrophages, resulting in the inhibition of inducible nitric oxide synthase-dependent intracellular bacterial killing. The innate immune response via Toll-like receptor 4 is manipulated by P. gingivalis (Hajishengallis & Lambris, 2011). Neutrophil-mediated inflammatory responses via triggering receptor expressed on myeloid cells 1 are also regulated by this bacterium (Bostanci et al., 2013).
In periodontitis, plasma cells comprise 50% of inflammatory cells and non-plasmacytic B cells 18% (Berglundh & Donati, 2005). Reportedly, the plasma cells locally produce antibodies against an extracellular matrix to cause gingival tissue destruction (Anusaksathien et al., 1992; De-Gennaro et al., 2006). Plasma cells are also known to contribute to matrix degradation by secreting matrix metalloproteinase (Berglundh et al., 2007).
Antibodies against P. gingivalis have been detected in the serum, gingival crevicular fluid and saliva of patients with periodontitis (Kurihara et al., 1991; Schenkein, 2006). It has been reported that the immunocytes isolated from the inflamed gingiva in periodontitis secrete antibodies against P. gingivalis (Ogawa et al., 1989), but these cells have not been visualized microscopically.
We have established an enzyme-labeled antigen method for identifying antigens recognized by specific antibodies produced by plasma cells within the lesion, and localizing the plasma cells histochemically (Mizutani et al., 2013). First, a biotinylated antigen protein library is constructed by the wheatgerm cell-free protein synthesis system (Sawasaki et al., 2002). In the second step, antigens recognized by antibodies in the serum or tissue extract are screened in the library using the AlphaScreen method (Matsuoka et al., 2010). Finally, the enzyme-labeled antigen method microscopically detects antigen-specific antibody-producing cells in prefixed frozen tissue sections with the use of the biotinylated antigen as a probe (Mizutani et al., 2009).
We have applied these sequences to analyzing plasma cells producing antibodies reactive to P. gingivalis in radicular cyst lesions associated with dental caries (Tsuge et al., 2011). Briefly, five proteins were evaluated as target antigens, including Ag53, a 53-kDa membrane-associated protein (Kurihara et al., 1991; Oyaizu et al., 2001), and the hemagglutinin/adhesin domain and protease domain of P. gingivalis-associated cysteine proteases, Arg-gingipain and Lys-gingipain (O'Brien-Simpson et al., 2000; Chen et al., 2001; Grenier & Tanabe, 2010). Plasma cells producing antibodies against the hemagglutinin/adhesin domains of Lys-gingipain or Arg-gingipain were found in two of eight radicular cyst lesions.
In the present study, these techniques were applied to analyzing the site of antibody production against P. gingivalis in biopsied gingiva with periodontitis, and the pathogenetic significance of P. gingivalis-specific antibody production was evaluated.
Methods
Patients and surgical specimens
When teeth were removed for the treatment of periodontitis in Fujita Health University Hospital, Toyoake, Japan, part of the gingival tissue was sampled from a total of 20 patients. Sera were also saved. The male to female ratio was 16 : 4, and the age of patients ranged from 47 to 98 years (mean 67, median 66). Clinical data of each case are shown in Table1. The case number was arranged in the order of the plasma cell density on tissue sections (see Table4). The degree of periodontitis was severe in all 20 patients, and they had a prolonged clinical course resulting in loosened teeth. The criteria of periodontitis were in accordance with the guidelines for examination, diagnosis and treatment planning for periodontal diseases 2008 (The Japanese Society of Periodontology, 2009).
Table 1.
Summary of clinical data of 20 patients with periodontitis and three infants with cleft palate
| Case | Age/Sex | Degree of periodontitis | Sites of biopsied gingiva1 | Associated disorders |
|---|---|---|---|---|
| 1 | 83/F | Severe | 25, 26 | Hypertension, aortic dissection |
| 2 | 50/M | Severe | 12 | Renal cancer |
| 3 | 74/M | Severe | 36, 37 | Hypertension, type II diabetes |
| 4 | 75/M | Severe | 13 | Hypertension, hyperlipidemia, angina pectoris |
| 5 | 84/M | Severe | 16 | Benign prostatic hyperplasia |
| 6 | 82/M | Severe | 21 | Laryngeal cancer |
| 7 | 98/M | Severe | 16, 17 | None |
| 8 | 65/M | Severe | 38 | Maxillary sinus cancer |
| 9 | 73/M | Severe | 36 | Type II diabetes |
| 10 | 65/M | Severe | 46 | Esophageal cancer |
| 11 | 56/M | Severe | 25, 26 | Hypertension, laryngeal cancer |
| 12 | 56/M | Severe | 32 | Hypertension, duodenal ulcer |
| 13 | 59/M | Severe | 12, 13 | Hypertension |
| 14 | 47/M | Severe | 16, 17 | None |
| 15 | 52/F | Severe | 16, 17 | Tongue cancer |
| 16 | 67/F | Severe | 45, 46 | Gastric ulcer, hydronephrosis |
| 17 | 61/M | Severe | 36 | None |
| 18 | 77/M | Severe | 46 | Hypertension, gastric ulcer, angina pectoris |
| 19 | 47/M | Severe | 36 | Mitral regurgitation |
| 20 | 67/F | Severe | 26 | Hypertension, chronic renal failure |
| C1 | 1/F | None | Uvula | Cleft palate |
| C2 | 1/M | None | Uvula | Cleft palate |
| C3 | 1/M | None | Uvula | Cleft palate |
Cases 1–20, periodontitis; Cases C1–C3, cleft palate. M, male; F, female.
The number represents the site of a tooth numbered using the World Dental Federation (FDI) Two-Digit Notation.
Table 4.
Summary of (i) IgG concentration in the serum and tissue extract, (ii) plasma cell density in the subepithelial layer of the biopsied gingival tissue, (iii) percentage of plasma cells producing antibodies reactive to Porphyromonas gingivalis, and (iv) the ΔΔCt value, representing a relatively quantitative value, for the P. gingivalis 16S ribosomal RNA gene
| Case | IgG concentration (mg ml−1) | Plasma cell density (cells mm−2) | Percentage of specific plasma cells in tissue1 | Real-time PCR for P. gingivalis genome2 | |
|---|---|---|---|---|---|
| Serum | Tissue extract | ||||
| 1 | 9.3 | 0.25 | 392 | 10.4 | 1.00 |
| 2 | 10.8 | 0.19 | 307 | 22.3 | 0.46 |
| 3 | 17.7 | 0.32 | 168 | 2.8 | 13.93 |
| 4 | 10.8 | 0.19 | 164 | 1.3 | 0.01 |
| 5 | 9.0 | 0.27 | 132 | 1.5 | 112.21 |
| 6 | 16.2 | 0.17 | 132 | 10.0 | 709.18 |
| 7 | 12.9 | 1.10 | 130 | 6.8 | – |
| 8 | 7.8 | 0.14 | 128 | 0.9 | 194.01 |
| 9 | 15.0 | 0.38 | 127 | 4.2 | 1.96 |
| 10 | 18.0 | 0.26 | 77 | 1.1 | 2.64 |
| 11 | 9.3 | 0.33 | 71 | 3.3 | 44.63 |
| 12 | 8.1 | 0.27 | 52 | 3.7 | 0.00 |
| 13 | 12.9 | 0.31 | 51 | 7.0 | 59.30 |
| 14 | 4.8 | 0.16 | 42 | 0.0 | 15.78 |
| 15 | 11.7 | 0.23 | 40 | 0.2 | 11.39 |
| 16 | 10.5 | 0.30 | 37 | 8.4 | 3.18 |
| 17 | 12.9 | 0.56 | 30 | 2.9 | 0.02 |
| 18 | 14.1 | 0.23 | 12 | 8.2 | 0.26 |
| 19 | 14.4 | 0.66 | 5 | 0.0 | 0.07 |
| 20 | 12.0 | 0.14 | 0 | 0.0 | – |
| C1 | 4.5 | 0.05 | 33 | 0.0 | – |
| C2 | 5.4 | 0.02 | 9 | 0.0 | – |
| C3 | 7.8 | 0.02 | 2 | 0.0 | – |
The percentage of specific plasma cells in the tissue was calculated as follows: anti-Ag53 + anti-Arg-hgp/Lys-hgp + anti-Arg-pro + anti-Lys-pro. The higher figures of anti-Arg-hgp or anti-Lys-hgp were chosen as the data for anti-Arg-hgp/Lys-hgp.
The number represents the ΔΔCt value in real-time PCR analysis, and – indicates that it was undetectable.
As a normal control group, sera and uvular mucosal tissues were sampled at plastic surgery from three infants (two boys, one girl) with cleft palate, all aged 1 year. The gingival tissues and sera were analyzed in the same way.
Written informed consent was obtained from each patient or from parents of the infants, and the analysis using the human specimens was authorized by the Committee of Ethics for Clinical and Epidemiological Study, Fujita Health University School of Medicine (acknowledgment no. 12-085, August 2011).
Gingival tissue preparation
Part of the biopsied gingival tissue was fixed in 100 mm phosphate-buffered 4% paraformaldehyde, pH 7.4, at 4°C for 4 h to cut 4-μm-thick frozen sections, as described previously (Tsuge et al., 2011). The other parts were kept fresh-frozen at −80°C until homogenization or DNA extraction.
Preparation of gingival tissue extract
Frozen gingival tissues (7–63 mg) were homogenized in 12 μl of 10 mm phosphate-buffered saline (PBS) per 1 mg of the tissue with a handheld rotor-stator homogenizer (TissueRuptor; Qiagen, Hilden, Germany). The homogenates were centrifuged at 20,400 g for 5 min twice, and the supernatants were stored at −80°C for the AlphaScreen assay.
DNA extraction
For detecting P. gingivalis genome in the gingival tissue, total DNA was extracted from the frozen tissue samples using a DNeasy Blood & Tissue Kit (Qiagen), according to the manufacturer's instruction.
Measurement of IgG concentration in the serum and tissue extract
Imunoglobulin G (IgG) in the serum and tissue extract was assayed by the enzyme-linked immunosorbent assay (ELISA) using Human IgG ELISA Quantitation Kit (Bethyl Laboratories, Montgomery, TX), according to the manufacturer's instruction.
Target bacterial proteins
In the present study, a total of five proteins of P. gingivalis origin were targeted: Ag53 and four gingipain components – the proteinase domain of Arg-gingipain (Arg-pro), the hemagglutinin/adhesin domain of Arg-gingipain (Arg-hgp), the proteinase domain of Lys-gingipain (Lys-pro), and the hemagglutinin/adhesin domain of Lys-gingipain (Lys-hgp). SpaP, a representative pathogenic protein derived from dental caries-related Streptococcus mutans (Lee et al., 1988; Aguilera Galaviz et al., 2002), was also evaluated. The molecular weights of the respective proteins are as follows: Ag53 = 53 kDa, Arg-pro = 44 kDa, Arg-hgp = 103 kDa, Lys-pro = 51 kDa, Lys-hgp = 103kDa, SpaP = 185 kDa.
Plasmid construction for the cell-free protein synthesis
Plasmid vectors inserted with DNA encoding target proteins were previously prepared (Tsuge et al., 2011). Briefly, six bacterial genes, Ag53, Arg-pro, Arg-hgp, Lys-pro, Lys-hgp and SpaP, were amplified from bacterial genomic DNA by polymerase chain reaction (PCR) using primers added with both biotin ligase recognition sites and restriction enzyme recognition sites. The PCR products were digested with restriction enzymes, and then cloned into the corresponding sites of the pEU-E01-His-MCS vector (CellFree Sciences, Matsuyama, Japan). After nucleotide sequences of the DNA inserts in all plasmid constructs were confirmed, plasmid vectors were amplified in Escherichia coli and then purified.
Protein synthesis with the cell-free protein synthesis system
Biotinylated target proteins were synthesized with the cell-free protein synthesis system, as described previously (Tsuge et al., 2011). Briefly, the wheatgerm extract (ENDEXT kits; CellFree Sciences) efficiently synthesized proteins encoded by genomic bacterial DNA in 96-well plastic plates. The plasmid vectors, incorporated with target DNA and the biotin ligase recognition sequence, were used as templates for the protein synthesis. Transcription by SP6 RNA polymerase (Takara Bio INC., Otsu, Japan) to yield messenger RNA and translation to obtain protein products using the wheatgerm extract followed. Finally, biotin labeling was performed through biotin ligase activity to obtain the target protein, to which a single biotin was labeled on the N-terminal site. The biotin-labeled target proteins lacking sugar residues on the molecules functioned as probes for the enzyme-labeled antigen method. The translation mixtures were directly used without purification or concentration.
For biotin labeling of proteins, the bilayer diffusion system was employed, as described previously (Sawasaki et al., 2002, 2008; Matsuoka et al., 2010). Briefly, 2 μl translation solution for biotin protein ligase (BirA, GenBank accession no. NP0312927) produced by the wheat cell-free system was added to the translation layer, and 5 μm D-biotin (Nacalai Tesque, Kyoto, Japan) was supplemented to both the translation and substrate layers. Unlabeled proteins were prepared without adding D-biotin.
Western blot analysis
Biotinylated proteins were Western-blotted with Alexa Fluor 488-conjugated-streptavidin (Life Technologies, Carlsbad, CA), as described previously (Tsuge et al., 2011).
The AlphaScreen method
As illustrated in Fig.1 and explained in the figure legend, the AlphaScreen method is a high-sensitivity technique for detecting a protein–protein interaction (Xu et al., 2009; Matsuoka et al., 2010). The assay was performed in the Cell-free Science and Technology Research Center, Ehime University, according to the manufacturer's instructions (PerkinElmer Life and Analytical Sciences, Boston, MA). Reactions were carried out in 25 μl of reaction mixture in 384-well Optiwel microtiter plates (PerkinElmer Life and Analytical Sciences). Each sample was diluted with the reaction buffer containing 100 mm Tris–HCl, pH 8.0, 0.01% Tween-20 and 0.1% bovine serum albumin.
Figure 1.
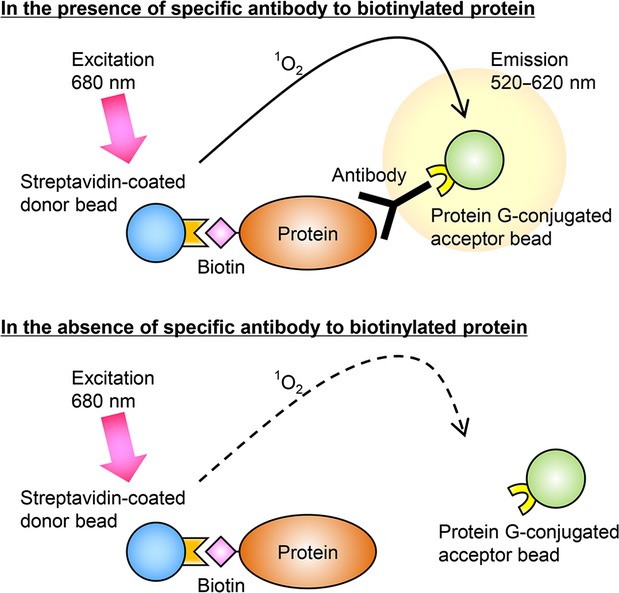
Schematic illustration of the AlphaScreen method for detecting a biotinylated protein–antibody interaction. The streptavidin-coated donor beads interact with the biotinylated target protein. The protein G-coated acceptor beads interact with the target protein via the specific antibodies. When the 680 nm excitation light is given, the donor beads generate singlet oxygens. The singlet oxygens promote the acceptor beads to emit luminescent light at 520–620 nm. This reaction occurs only in the presence of the specific antibodies in the solution, since the antigen–antibody reaction shortens the distance between both beads within 200 nm (top panel). No luminescence is seen without specific antibodies (bottom panel).
For the antigen–antibody reaction, 10 μl of the 1 : 10 diluted translation mixture containing the biotinylated protein was admixed with 5 μl of the 1 : 200 diluted serum or 1 : 25 diluted tissue extract, and the mixtures were incubated at 26°C for 30 min. Subsequently, 10 μl of streptavidin-coated donor beads and protein G-conjugated acceptor beads (PerkinElmer Life and Analytical Sciences) were added at a final concentration of 12 μg ml−1 per well and incubated at 26°C for 1 h in a dark box. Luminescence emission at 520–620 nm was measured with the EnVision plate reader (PerkinElmer Life and Analytical Sciences), and the data were analyzed with the AlphaScreen Detection program.
The data were expressed as a signal : noise ratio. Translation solution reacted without template RNA served as a negative control (representing a noise). In the present series, the signal : noise ratio no <5.1 was regarded as positive.
The enzyme-labeled antigen method
The enzyme-labeled antigen method was performed, as described previously (Tsuge et al., 2011). Paraformaldehyde-fixed frozen sections pretreated with 4 μg ml−1 proteinase K at room temperature for 15 min were incubated overnight at room temperature with unpurified crude solutions (translation mixtures) containing biotinylated proteins, which were synthesized in the cell-free protein synthesis system within the well. Horseradish peroxidase-labeled streptavidin was employed as the secondary reagent. After rinsing in 10 mm PBS, pH 7.2, the site of antigen–antibody reaction was then visualized in 50 mm Tris–HCl buffer, pH 7.6, containing 20 mg dl−1 diaminobenzidine (DAB) tetrahydrochloride and 0.006% hydrogen peroxide. The nuclei were lightly counterstained with Mayer's hematoxylin.
Immunohistochemical detection of CD138-positive plasma cells
Immunoperoxidase staining was performed to detect CD138-positive plasma cells in tissue sections, and compared with hematoxylin & eosin staining. After a brief dip in running water and endogenous peroxidase blockage with methanol containing 0.3% hydrogen peroxide, the prefixed frozen sections were incubated overnight with a 1 : 800 diluted mouse monoclonal antibody against CD138 (clone MI15; Dako, Carpinteria, CA). After rinsing in PBS, the sections were incubated with horseradish peroxidase-labeled amino acid polymer (Simple Stain MAX-PO multi; Nichirei, Tokyo, Japan) for 30 min, followed by diaminobenzidine coloring reaction and brief hematoxylin counterstaining. By using consecutive frozen sections, the percentage of P. gingivalis-specific antibody-producing cells among CD138-positive plasma cells was calculated.
Double immunofluorescence staining using biotinylated proteins and CD79a monoclonal antibody
To demonstrate that the cells labeled with the biotinylated proteins corresponded to plasma cells, double immunofluorescence staining using biotinylated protein probes and a monoclonal antibody against CD79a was performed. CD79a is expressed on B lymphocytes, including plasma cells (Mason et al., 1995). CD138 was not applicable in this situation because no antigenicity remained after the protease treatment. The sections were incubated with a 1 : 200 diluted mouse monoclonal antibody against human CD79a (clone JCB117; Dako), followed by incubation with Alexa Fluor 568 (colored red) -labeled goat anti-mouse IgG antibody (Life Technologies) at 1 : 500 dilution. Crude biotinylated protein solutions and then Alexa Fluor 488 (colored green) -conjugated streptavidin (Life Technologies) at 1 : 500 dilution were sequentially incubated. Fluorescence was observed under a microscope (AXIO Imager A1; Carl Zeiss, Oberkochen, Germany). The nuclei were fluoresced blue with 4′,6-diamidino-2′-phenylindole.
Absorption experiment
To confirm the specificity of the enzyme-labeled antigen method, the absorption experiment was performed in three representative lesions (cases 1, 2 and 9). Six kinds of crude unlabeled antigen solutions (Ag53, Arg-hgp, Lys-hgp, Arg-pro, Lys-pro and SpaP) were incubated overnight, and then the crude biotinylated antigen solutions, diluted at 1 : 5 with the PBS, were reacted for 1 h, and plasma cells producing antibodies against Ag53, Arg-hgp and Lys-hgp, Arg-pro and Lys-pro were visualized. As expected, an excess amount of the corresponding antigens abolished the specific staining, but indifferent antigens did not.
Real-time PCR
The 16S ribosomal RNA gene of P. gingivalis was amplified with real-time PCR. The primer pairs for P. gingivalis consisted of 5′-GGATAACCCGTTGAAAGACG-3′ (forward) and 5′-GGGACGCATGCCTATCTTAC-3′ (reverse), generating a product of 98-bp length (GenBank NR_040838).
Assays were carried out in a 25-μl final volume containing 0.5–10 μl of sample DNA, 12.5 μl of 2× reaction mixture (QuantiTect SYBR Green PCR Kits; Qiagen) and 7.5 pmol primers. The real-time PCR was performed using the Rotor-Gene Q (Qiagen), with initial holding temperature at 95°C for 15 min, followed by 50 cycles with three-step PCR at 94°C for 5 s, at 60°C for 30 s and at 72°C for 30 s, with fluorescence monitoring on SYBR Green fluorescence. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH, GenBank accession No. NG_007073) gene served as an internal control. The primer pairs for GAPDH consisted of 5′-ATCCCATCACCATCTTCCAG-3′ (forward) and 5′-TATACCCAAGGGAGCCACAC-3′ (reverse), generating a product of 98-bp length. The primers were designed using DNASYS Pro software (Hitachi Solutions, Tokyo, Japan).
Relative quantification of the P. gingivalis genome was performed, based on the ΔΔCt method, using GAPDH as an internal control.
Statistical analysis
Correlations of the AlphaScreen signals between the serum and tissue extract were evaluated by Spearman's correlation test. The percentage of plasma cells producing antibodies reactive to P. gingivalis and the relative quantity of the P. gingivalis genome were also correlated with the AlphaScreen signals of the tissue extract. For analyzing the P. gingivalis-reactive antibody-producing cells, cases 19 and 20 were excluded from study because of the absence or paucity of plasma cells in the specimen. P-values < 0.05 were regarded as statistically significant.
Results
Western blot analysis of proteins prepared with the wheatgerm cell-free protein synthesis system
Five P. gingivalis proteins, Ag53, Arg-hgp, Lys-hgp, Arg-pro and Lys-pro, as well as SpaP, were synthesized and biotinylated with the wheatgerm cell-free protein synthesis system. Crude solutions (translation mixtures) in the well were used for screening with the AlphaScreen method, the enzyme-labeled antigen method and the absorption experiment. Figure2 demonstrates the Western blot analysis of the biotinylated proteins. Protein bands showing appropriate molecular weights were visualized with streptavidin–Alexa Fluor 488.
Figure 2.
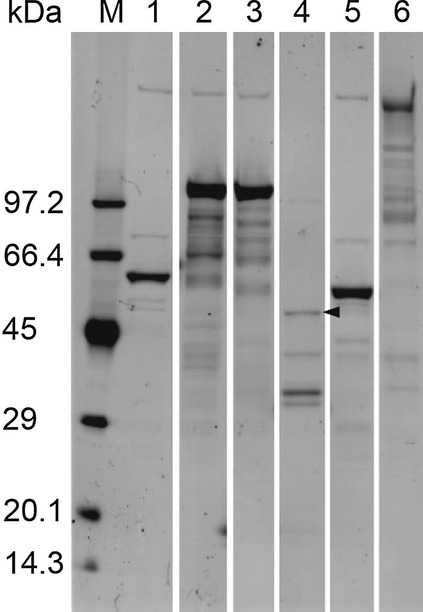
Electrophoretic analysis of biotinylated proteins without sugar moieties used in the present study. Protein bands showing appropriate molecular weights are visualized with the Western blot analysis using streptavidin-labeled Alexa Fluor 488. The estimated molecular weights of the proteins are: Ag53 = 53 kDa (lane 1), Arg-hgp = 103 kDa (lane 2), Lys-hgp = 103 kDa, (lane 3), Arg-pro = 44 kDa (lane 4), Lys-pro = 51 kDa (lane 5), and SpaP = 185 kDa (lane 6). The band of Arg-pro is relatively weak (arrowhead). Extra bands are also observed in each lane. M, molecular weight markers.
Multiple extra bands were observed on the Western blot for the synthesized proteins. Some bands were common in plural lanes. For example, the bands with the highest molecular weight were derived from the wheatgerm extract used as a protein synthesis reagent. These contaminants should be regarded as bystanders, because no positive signals were detected in either the AlphaScreen assay or with the enzyme-labeled antigen method, when the translation solution without mRNA coding target proteins was employed. The additional extra bands on the respective lanes, especially prominent in Arg-hgp and Lys-hgp, were regarded as fragments of the respective target protein due to incomplete synthesis or degradation of the protein or mRNA. The bands are expected to share the antigenicity with the complete target proteins. We believe that the extra bands did not affect the reliability of the results in the present study.
Antibodies reactive to P. gingivalis in the serum and tissue extract assayed with the AlphaScreen method
In the AlphaScreen analysis on six antigens in three infants (the control group), the highest value of the signal : noise ratio was 3.8 for Lys-hgp in the serum. The signal : noise ratio of the mean value plus two standard deviations of the Lys-hgp antibody titer in the serum in the control group was calculated as 5.1. Therefore, a signal : noise value ≥5.1 was regarded as positive in the current AlphaScreen analysis. The results of both the AlphaScreen method and the enzyme-labeled antigen method are summarized in Table2, in which both serum and tissue extracts were evaluated from 20 periodontitis patients and three infants with cleft palate.
Table 2.
Summary of the AlphaScreen method and the enzyme-labeled antigen method in 20 periodontitis cases and three cleft palate cases
| Case | Antigens | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag53 | Arg-hgp | Lys-hgp | Arg-pro | Lys-pro | SpaP | |||||||||||||
| AS | ELAM | AS | ELAM | AS | ELAM | AS | ELAM | AS | ELAM | AS | ELAM | |||||||
| S | T | S | T | S | T | S | T | S | T | S | T | |||||||
| 1 | 1.1 | 11.1 | + | 2.8 | 66.1 | + | 3.1 | 69.2 | + | 10.8 | 196.3 | + | 5.6 | 6.3 | + | 1.3 | 1.7 | − |
| 2 | 2.4 | 70.2 | + | 5.7 | 115.7 | + | 4.9 | 113.2 | + | 4.0 | 25.0 | + | 4.4 | 2.5 | + | 1.9 | 0.7 | − |
| 3 | 1.5 | 62.5 | + | 6.3 | 190.2 | + | 3.0 | 134.3 | + | 2.7 | 35.8 | + | 6.8 | 6.7 | − | 4.2 | 0.9 | − |
| 4 | 0.9 | 0.9 | − | 1.7 | 73.3 | + | 1.3 | 38.5 | + | 1.8 | 5.6 | + | 3.5 | 2.3 | − | 3.2 | 1.1 | − |
| 5 | 0.9 | 7.3 | − | 1.0 | 2.9 | + | 1.0 | 6.1 | + | 1.2 | 9.1 | + | 2.3 | 1.7 | − | 1.9 | 0.7 | − |
| 6 | 0.9 | 0.7 | − | 1.6 | 37.4 | + | 1.7 | 24.0 | + | 3.1 | 81.9 | + | 16.3 | 4.1 | + | 8.0 | 0.7 | − |
| 7 | 0.9 | 1.6 | − | 3.9 | 294.0 | + | 3.5 | 331.0 | + | 5.6 | 51.8 | + | 4.2 | 5.8 | − | 2.1 | 0.9 | − |
| 8 | 1.0 | 0.9 | − | 3.5 | 8.9 | + | 2.4 | 5.9 | + | 6.6 | 51.2 | + | 3.4 | 1.6 | + | 3.2 | 1.1 | − |
| 9 | 0.7 | 0.8 | − | 0.8 | 3.9 | + | 1.0 | 7.0 | + | 1.6 | 72.2 | + | 1.7 | 3.3 | − | 1.0 | 0.9 | − |
| 10 | 0.9 | 0.7 | − | 9.3 | 9.4 | + | 12.0 | 10.1 | + | 4.1 | 6.2 | + | 8.7 | 3.4 | − | 3.5 | 1.3 | − |
| 11 | 4.9 | 41.2 | + | 1.0 | 4.9 | + | 1.2 | 2.3 | + | 2.0 | 16.6 | + | 8.0 | 4.7 | − | 3.1 | 0.9 | − |
| 12 | 1.3 | 0.7 | − | 2.3 | 24.8 | − | 2.4 | 24.3 | + | 11.6 | 140.8 | + | 3.2 | 3.3 | − | 2.0 | 2.7 | − |
| 13 | 1.0 | 1.9 | − | 1.2 | 0.9 | + | 1.1 | 1.0 | + | 1.2 | 1.8 | + | 4.4 | 2.4 | − | 4.7 | 0.8 | − |
| 14 | 0.9 | 0.7 | − | 1.1 | 1.9 | − | 1.2 | 1.2 | − | 3.0 | 3.0 | − | 1.7 | 1.8 | − | 3.6 | 0.9 | − |
| 15 | 1.2 | 0.9 | + | 1.1 | 1.0 | − | 1.5 | 1.1 | − | 2.5 | 25.6 | − | 3.7 | 2.1 | − | 8.3 | 0.8 | − |
| 16 | 0.8 | 5.4 | − | 1.1 | 11.6 | + | 1.4 | 44.7 | + | 12.3 | 70.2 | + | 2.8 | 2.1 | − | 2.9 | 0.7 | − |
| 17 | 0.8 | 0.6 | − | 1.2 | 19.4 | + | 1.1 | 6.9 | + | 2.2 | 15.0 | + | 2.8 | 2.5 | − | 3.3 | 0.7 | − |
| 18 | 2.0 | 1.7 | − | 20.8 | 8.7 | + | 12.0 | 6.8 | + | 9.4 | 31.3 | + | 4.8 | 2.3 | − | 2.2 | 0.7 | − |
| 19 | 0.6 | 0.7 | − | 1.1 | 6.8 | − | 1.0 | 8.8 | − | 1.6 | 27.4 | − | 3.5 | 2.6 | − | 3.4 | 0.9 | − |
| 20 | 0.6 | 0.4 | − | 1.4 | 0.9 | − | 1.7 | 0.8 | − | 1.4 | 1.2 | − | 5.1 | 1.1 | − | 3.3 | 0.6 | − |
| C1 | 0.9 | 0.9 | − | 1.1 | 1.0 | − | 1.0 | 1.0 | − | 1.4 | 1.4 | − | 2.6 | 1.2 | − | 1.1 | 0.9 | − |
| C2 | 0.9 | 0.5 | − | 0.9 | 0.5 | − | 3.8 | 0.7 | − | 1.1 | 0.7 | − | 3.4 | 0.5 | − | 1.5 | 0.6 | − |
| C3 | 0.9 | 0.7 | − | 1.1 | 0.7 | − | 1.2 | 0.7 | − | 1.2 | 0.9 | − | 3.0 | 0.8 | − | 1.6 | 0.6 | − |
AS, AlphaScreen signal; S, serum sample; T, tissue extract; ELAM, enzyme-labeled antigen method; +, positive; −, negative.
Bold values indicate positive signals (≥5.1).
In the serum of 20 periodontitis cases, the AlphaScreen method detected anti-Arg-hgp antibodies in four cases, anti-Lys-hgp in two, anti-Arg-pro in six, anti-Lys-pro in five and anti-SpaP in two. No significant antibodies to Ag53 were demonstrated in the serum. The AlphaScreen method using the tissue extract demonstrated anti-Ag53 antibodies in six cases, anti-Arg-hgp in 13, anti-Lys-hgp in 15, anti-Arg-pro in 17 and anti-Lys-pro in three. The antibodies to SpaP were undetectable in the tissue extract. In most cases, the positive AlphaScreen signals against Ag53, Arg-hgp, Lys-hgp and Arg-pro were much higher in the tissue extract than in the serum. In contrast, the signal levels against Lys-pro tended to be higher in the serum than in the tissue extract.
The AlphaScreen signals against Arg-hgp and Lys-hgp were well correlated not only in the serum (r = 0.90, P < 0.01, n = 20) but also in the tissue extract (r = 0.93, P < 0.01, n = 20). This strongly suggested the presence of common antigenic epitopes on both molecules. Such strong correlation was not observed between Arg-pro and Lys-pro in the serum (r = 0.11, P > 0.05, n = 20), while mild correlation was detected in the tissue extract (r = 0.50, P < 0.05, n = 20).
The correlation of the AlphaScreen signals in the serum and tissue extract was statistically evaluated. Significant positive correlations were observed for anti-Ag53 (r = 0.65, P < 0.01, n = 20), anti-Arg-hgp (r = 0.60, P < 0.05, n = 20), anti-Lys-hgp (r = 0.45, P < 0.05, n = 20), anti-Arg-pro (r = 0.64, P < 0.01, n = 20) and anti-Lys-pro (r = 0.51, P < 0.05, n = 20). The antibody titers against SpaP showed no correlation between the serum and tissue extract (r = −0.13, P > 0.05, n = 20).
Plasma cell localization in biopsied gingival tissues
Plasma cells were observed in fixed frozen sections of the inflamed gingival tissue, and CD138 immunostaining was suitable for counting the number of plasma cells located in the subepithelial layer (Fig.3). The overlying squamous epithelial cells were also CD138-immunoreactive. The density of CD138-positive plasma cells varied from lesion to lesion, and the plasma cell density per mm2 of subepithelial stromal tissue ranged from 0 to 392 mm−2 (the data are summarized in Table4).
Figure 3.
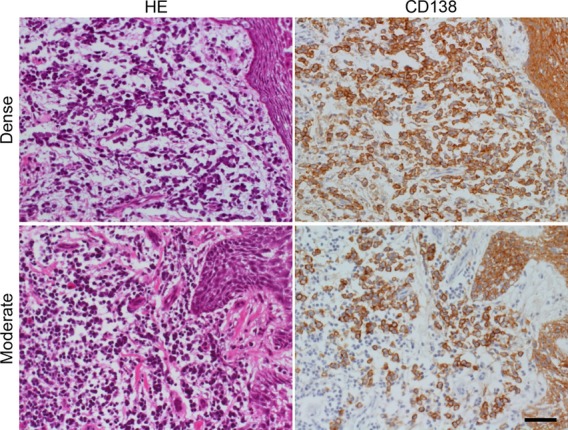
Plasma cells in consecutive frozen sections of the biopsied gingiva. Two representative lesions of gingivitis demonstrate dense (top panels: case 1) and moderate (bottom panels: case 9) infiltration of plasma cells beneath the squamous lining. Left panels: hematoxylin & eosin staining, right panels: immunostaining for CD138. Not only plasma cells located in the subepithelial layer but also covering squamous epithelial cells reveal membrane positivity for CD138. Bar indicates 50 μm.
Histochemical demonstration of plasma cells producing antibodies reactive to P. gingivalis with the enzyme-labeled antigen method
On proteinase K-pretreated prefixed frozen sections, infiltrating plasma cells were evaluable in a total of 18 lesions of periodontitis. In one lesion (case 19), only a few plasma cells were seen, and in another (case 20) no plasma cells were observed. As summarized in Table2, the enzyme-labeled antigen method using the biotinylated proteins as probes visualized plasma cells producing antibodies against Ag53, Arg-hgp, Lys-hgp, Arg-pro and Lys-pro in five, 15, 16, 16 and four lesions, respectively. In 17 (94%) of 18 lesions, antibodies specific to the P. gingivalis antigens were identified histochemically. Antibodies against SpaP were undetectable. Representative photomicrographs of plasma cells producing antibodies against the respective antigens are illustrated in Fig.4. Plasma cells with positive signals were fundamentally dispersed in the inflamed gingiva, and localized dense cluster formation was scarcely discerned.
Figure 4.
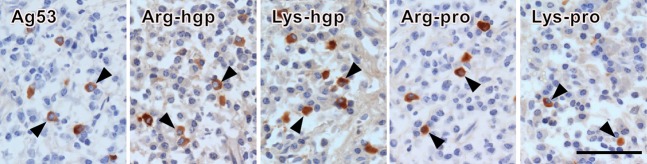
The enzyme-labeled antigen method visualizing plasma cells producing antibodies reactive to Porphyromonas gingivalis proteins in proteinase K-pretreated, prefixed frozen sections of the biopsied gingiva. Arrowheads indicate plasma cells showing cytoplasmic labeling with the biotinylated proteins. The panels demonstrate anti-Ag53 antibodies, anti-Arg-hgp antibodies, anti-Lys-hgp antibodies, and anti-Arg-pro antibodies in case 2, and anti-Lys-pro antibodies in case 1. The plasma cells producing the pathogen-reactive antibodies are dispersed in the inflammatory stroma. Bar indicates 50 μm.
It should be noted that the number and percentage of plasma cells producing antibodies against Arg-hgp and Lys-hgp were comparable. Antibodies to Ag53 were visualized only in five lesions. In case 6 active in producing antibodies reactive to P. gingivalis, no anti-Ag53 antibodies were demonstrated, whereas in case 15, a few plasma cells produced anti-Ag53 alone.
In the uvula of the control infant where plasma cells were only sparsely distributed, no positivity was noted.
Percentages of P. gingivalis-reactive antibody-producing cells among CD138-positive plasma cells
Porphyromonas gingivalis-reactive antibody-producing cells visualized with the enzyme-labeled antigen method were counted on consecutive frozen sections of the 17 gingival tissues with positive signal. The percentages of the P. gingivalis-reactive antibody-producing cells among CD138-positive total plasma cells were calculated as 0.2–2.3% in Ag53, 0.3–10.2% in Arg-hgp, 0.2–12.9% in Lys-hgp, 0.1–7.6% in Arg-pro and 0.1–0.7% in Lys-pro. The data of the individual cases are summarized in Table3.
Table 3.
Percent positivity with the enzyme-labeled antigen method among the CD138-positive plasma cells in frozen sections of the biopsied gingiva
| Case | anti-Ag53 | anti-Arg-hgp | anti-Lys-hgp | anti-Arg-pro | anti-Lys-pro |
|---|---|---|---|---|---|
| 1 | 1.3 | 4.4 | 5.3 | 3.1 | 0.7 |
| 2 | 2.3 | 10.2 | 12.9 | 7.0 | 0.1 |
| 3 | 0.4 | 1.1 | 1.8 | 0.6 | 0.0 |
| 4 | 0.0 | 1.2 | 0.5 | 0.1 | 0.0 |
| 5 | 0.0 | 0.6 | 1.2 | 0.3 | 0.0 |
| 6 | 0.0 | 4.8 | 6.4 | 3.5 | 0.1 |
| 7 | 0.0 | 4.9 | 5.5 | 1.3 | 0.0 |
| 8 | 0.0 | 0.4 | 0.7 | 0.1 | 0.1 |
| 9 | 0.0 | 0.3 | 0.2 | 3.9 | 0.0 |
| 10 | 0.0 | 0.4 | 0.4 | 0.7 | 0.0 |
| 11 | 1.2 | 1.6 | 1.0 | 0.5 | 0.0 |
| 12 | 0.0 | 0.0 | 1.2 | 2.5 | 0.0 |
| 13 | 0.0 | 3.5 | 1.7 | 3.5 | 0.0 |
| 14 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 15 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 16 | 0.0 | 2.1 | 2.1 | 6.3 | 0.0 |
| 17 | 0.0 | 1.7 | 0.4 | 1.2 | 0.0 |
| 18 | 0.0 | 1.0 | 5.1 | 3.1 | 0.0 |
| 19 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 20 | – | – | – | – | – |
| C1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| C3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
The percentage of plasma cells producing antibodies to any one of the P. gingivalis antigens in the tissue was evaluated in the following formula: anti-Ag53 + anti-Arg-hgp/Lys-hgp + anti-Arg-pro + anti-Lys-pro. The higher figures of anti-Arg-hgp or anti-Lys-hgp were chosen for the data anti-Arg-hgp/Lys-hgp. Among the 17 gingival lesions, 0.2–22.3% (mean 5.6%, median 3.7%) of plasma cells produced antibodies against P. gingivalis antigens. Some lesions (cases 1 and 2) showing dense plasma cell infiltration with high percentages of specific antibody production, while others (cases 3, 4, 5 and 8) demonstrated relatively low percentages of specific antibody production. In cases 13, 16 and 18, the density of plasma cells was low but with relatively high percentages of specific antibody production. Table4 shows the individual data.
The correlation between the percentages of P. gingivalis-reactive antibody-producing cells and the AlphaScreen signals of the tissue extract was statistically evaluated. Significant positive correlations were observed for Ag53 (r = 0.67, P < 0.01, n = 18), Arg-hgp (r = 0.55, P < 0.05, n = 18) and Lys-hgp (r = 0.60, P < 0.01, n = 18), while there were no significant correlations for Arg-pro and Lys-pro.
Absorption experiment confirming the specificity of the enzyme-labeled antigen method
In the absorption experiment, positive signals of anti-P. gingivalis proteins in plasma cells were eliminated with an excess amount of the corresponding unlabeled proteins. Representative features are demonstrated in Figs5 and 6. The signals were not affected with unlabeled indifferent proteins, except for anti-Arg-hgp and anti-Lys-hgp signals. The anti-Arg-hgp or anti-Lys-hgp signals were partly eliminated by either of the unlabeled Lys-hgp or Arg-hgp, and not by other indifferent unlabeled proteins, again indicating the presence of common epitopes on the Arg-hgp and Lys-hgp molecules. It should be noted that Arg-hgp and Lys-hgp also possessed the antigenicity unique on the respective molecules, as illustrated in Fig.6.
Figure 5.
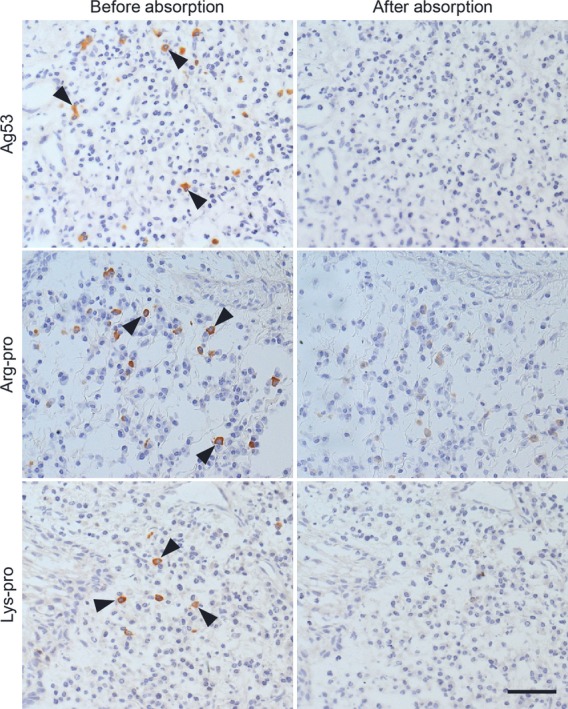
Absorption experiment using unlabeled Ag53, Arg-pro and Lys-pro. Anti-Ag53 reactivity in case 2, anti-Arg-pro reactivity in case 9, and anti-Lys-pro reactivity in case 1 are shown. Positive signals against the respective proteins (left panels) were abolished after absorption with an excess amount of the corresponding unlabeled proteins (right panels). Arrowheads indicate plasma cells producing the specific antibodies. Bar indicates 50 μm.
Figure 6.
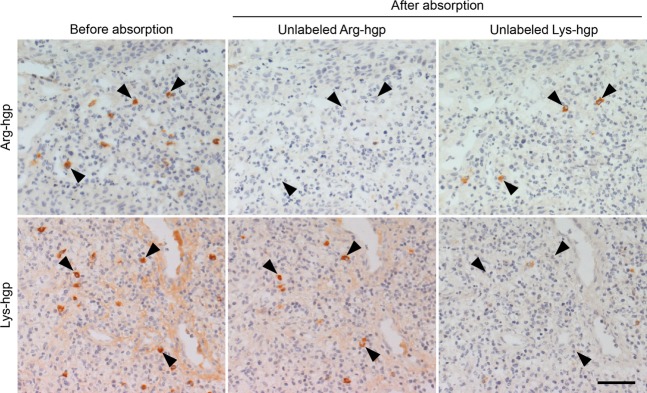
Absorption experiment using unlabeled Arg-hgp and Lys-hgp. The enzyme-labeled antigen method for Arg-hgp reactivity and Lys-hgp reactivity on consecutive frozen sections in case 1 is illustrated. The reactivities to Arg-hgp and Lys-hgp in the cytoplasm of plasma cells (left panels) are abolished with an excess amount of the corresponding unlabeled proteins (center top and right bottom panels), and partly eliminated with unlabeled Lys-hgp and Arg-hgp, respectively (center bottom and right top panels). Arrowheads indicate plasma cells producing antibodies reactive to the unique epitope on Arg-hgp or Lys-hgp. Bar indicates 50 μm.
Expression of CD79a on plasma cells producing antibodies reactive to P. gingivalis
Double immunofluorescence staining with biotinylated antigens and the mouse monoclonal antibody to CD79a was performed in the representative lesions (cases 1, 2 and 9). Cells reacted with biotinylated Ag53, Arg-hgp and Lys-hgp in case 2, biotinylated Arg-pro in case 9 and biotinylated Lys-pro in case 1 were shown to be CD79a-positive. Representative photomicrographs are demonstrated in Fig.7. The features indicated that the specific antibody-positive cells belonged to plasma cells.
Figure 7.
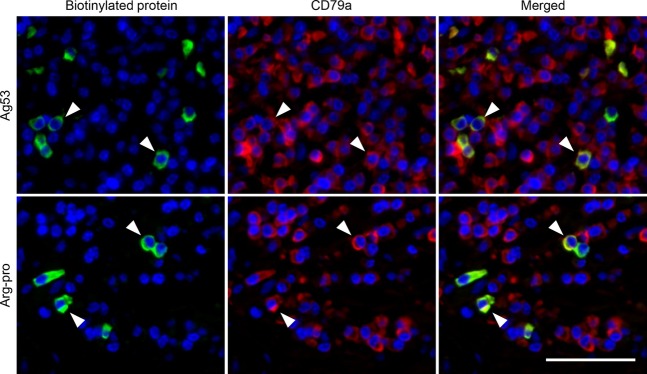
Double immunofluorescence labeling with the biotinylated protein and a mouse monoclonal antibody to CD79a. Anti-Ag53 reactivity in case 2 and anti-Arg-pro reactivity in case 9 are shown. Cytoplasmic reactivities against the Porphyromonas gingivalis proteins are labeled green with Alexa Fluor 488 (left panels). CD79a along the plasma membrane and in the cytoplasm of B lymphocytes, including plasma cells, is stained red with Alexa Fluor 568 (center panels). Arrowheads indicate plasma cells producing P. gingivalis-reactive antibodies, fluoresced in yellow (right panels, the merged figure). Bar indicates 50 μm.
IgG concentrations in the serum and tissue extract
IgG concentrations in the serum and gingival tissue extract of 20 periodontitis lesions were assayed by the ELISA. They ranged from 7.8 to 18.0 mg ml−1 (mean 11.8 mg ml−1) in the serum and from 0.14 to 1.10 mg ml−1 (mean 0.31 mg ml−1) in the tissue extract. In three control infants with cleft palate, IgG concentration ranged from 4.5 to 7.8 mg ml−1 (mean 5.9 mg ml−1) in the serum and from 0.019 to 0.050 mg ml−1 (mean 0.031 mg ml−1) in the tissue extract. The data are summarized in Table4. Although IgG concentration in the tissue extract was much lower than that in the serum, the biotinylated proteins were frequently AlphaScreen-reactive with the tissue extract.
Statistical analysis proved no correlations between the IgG concentration of the tissue extract and the plasma cell density in the tissue, between the IgG concentration and P. gingivalis-reactive plasma cell ratio in the tissue, and between the IgG concentration and the amount of P. gingivalis genome in the tissue.
Detection of P. gingivalis genome in biopsied gingival tissues with real-time PCR
With real-time PCR, the P. gingivalis genome encoding 16S ribosomal RNA was detected in 18 (90%) of the 20 gingival tissues from periodontitis patients. In uvular mucosa of the infants, the P. gingivalis genome was undetectable. Table4 summarizes (i) IgG concentration in the serum and tissue extract, (ii) plasma cell density in the tissue, (iii) the percentage of plasma cells producing antibodies reactive to P. gingivalis, and (iv) the ΔΔCt value, representing a relatively quantitative value, for the P. gingivalis genome. In case 6, ΔΔCt value and the density of specific plasma cells were both high, while in case 2, high density of specific plasma cells with low ΔΔCt value was observed. In case 7, specific plasma cells were distributed, but the bacteria were undetectable. The latter two findings suggest the production of neutralizing antibodies against P. gingivalis.
There were no significant correlations between the amount of P. gingivalis genome in the tissue and the AlphaScreen signals of the tissue extract, nor between the amount of P. gingivalis genome in the tissue and the percentage of the P. gingivalis-reactive plasma cells.
Clinicopathological correlations
Since all 20 patients showed an advanced stage of periodontitis, it was difficult to analyze the clinicopathological correlations in the present series.
Discussion
The pathogenesis of periodontitis involves polymicrobial synergy and dysbiosis, and P. gingivalis roles as a keystone pathogen of this common disease (Lamont & Jenkinson, 2000; Hajishengallis & Lamont, 2014). In the present study, we analyzed the immune response against five major antigenic proteins of the anaerobic bacteria in periodontitis. Ag53 is a major outer membrane protein of P. gingivalis (Kurihara et al., 1991; Oyaizu et al., 2001), and a positive immune response to Ag53 has been reported in patients with periodontitis (Kurihara et al., 1991; Schenkein, 2006). It is known that some strains of P. gingivalis express Ag53, but others do not (Naito et al., 2008). Porphyromonas gingivalis also secretes cysteine proteinases named gingipains (Chen et al., 2001; O'Brien-Simpson et al., 2001; Grenier & Tanabe, 2010). Two major isoforms of gingipains are known: Arg-gingipain and Lys-gingipain. Each form consists of the hemagglutinin/adhesin domain (hgp) and the proteinase domain (pro). The hgps have been implicated as adhesins that actuate colonization on the epithelium lining – the gingival sulcus. It has been reported that in cases of severe chronic periodontitis, the level of Arg-gingipain in periodontal pocket fluid was correlated with the load of P. gingivalis (Guentsch et al., 2011).
In the AlphaScreen analysis for 20 cases of periodontitis, antibodies reactive to P. gingivalis antigens were detected in 11/20 (55%) in the serum and 17/20 (85%) in the tissue extract. With the enzyme-labeled antigen method, 17/18 (94%) tissue sections, in which evaluable numbers of plasma cells were observed, showed positivity for any of the five P. gingivalis antigens. These figures were much higher than those observed in dental caries-induced radicular cyst, as we reported previously (Tsuge et al., 2011): The AlphaScreen signals were seen in 2/10 (20%) in the serum and 2/6 (33%) in the tissue extract, and the enzyme-labeled antigen method demonstrated positivity in 2/8 (25%). It should be noted that in three of 18 periodontitis lesions, the percentages of pathogen-reactive plasma cells were 10% or more (22.3, 10.4 and 10.0%). In seven lesions, more than 5% plasma cells were pathogen-reactive.
It should be noted that we analyzed the antibody response against just five bacterial proteins. The other P. gingivalis antigens may also provoke antibody reactions, as discussed later. The percentages of P. gingivalis-reactive plasma cells in the diseased gingival tissue reported here should be regarded as the minimum number.
The percentages of plasma cells producing antibodies reactive to Ag53, Arg-hgp and Lys-hgp were positively correlated with the AlphaScreen signals in the tissue extract. The results indicate that screening of the tissue extract using the AlphaScreen method is useful for detecting bacterial antigens recognized by antibodies produced within the lesions.
In case of radicular cyst, the antibodies were mainly raised against Arg-hgp and Lys-hgp, and Arg-pro and Lys-pro were much less antigenic. In only one case (1/10 = 10%), anti-Lys-pro was recorded in the serum, and no positivity was seen with the enzyme-labeled antigen method (Tsuge et al., 2011). In periodontitis, positive AlphaScreen signals were often seen for Arg-pro (6/20 in the serum and 17/20 in the tissue extract) and Lys-pro (5/20 in the serum and 3/20 in the tissue extract). The enzyme-labeled antigen method demonstrated anti-Arg-pro activity in 16/20 (80%) and anti-Lys-pro activity in 4/20 (20%) in tissue sections.
Except for anti-Lys-pro activity, the AlphaScreen signals against P. gingivalis antigens were higher in the tissue extract than in the serum. In the present study, IgG concentration in the tissue extract was roughly 1/40 of the serum level. When the dilution rates in the AlphaScreen method (1 : 1000 in the serum, 1 : 125 in the tissue extract) are considered, IgG concentrations in the tissue extract were regarded as one-fifth of the serum. The antibody titers were positively correlated between the serum and tissue extract. It is highly likely that serum antibodies against P. gingivalis antigens derive from inflammatory gingival tissues. It should be noted that the IgG concentration in the tissue extract showed no correlation with the plasma cell density, the percentage of P. gingivalis-reactive antibody-producing plasma cells, and the amount of P. gingivalis genome in the tissue.
The AlphaScreen signals for anti-Lys-pro activity were relatively high in the sera, including those in the control cases. This may indicate antibody production in extra-gingival sites, or the antigenic cross-reactivity of Lys-pro to unrelated proteins is a possibility. The tissue samples used in the present study were primarily obtained from the severely affected gingiva, which led to dental extraction, but in two samples tissue inflammatory reactions were minimal, probably representing a sampling error.
Antibody response to Ag53 was not observed in radicular cyst (Tsuge et al., 2011), but was occasionally observed in periodontitis. AlphaScreen signals were positive in the gingival tissue extract in six cases, and the enzyme-labeled antigen method gave positive signals in five. In four cases, both the positive histochemical signals and high AlphaScreen signals were observed. The findings may be correlated with infection of Ag53-positive P. gingivalis strains. In the present series, however, no specific clinicopathological features characterizing Ag53-specific antibody response were recognized.
Kurihara et al. (1991) and Guo et al. (2000) documented the Western blot detection of antibodies against Ag53 in the serum of 70% of periodontitis patients. In the present study, the antibodies to Ag53 were not detected in the serum with the AlphaScreen method. In the reports cited above, Ag53 was extracted from P. gingivalis, and the antibody was assayed with the Western blot, a solid-phase reaction. The sera were used at a final dilution of 1 : 100–1 : 200. In contrast, we used recombinant Ag53 without sugar moieties on the molecules, which retained the three-dimensional conformation of the protein, and the antibody was assayed with the AlphaScreen method, a liquid-phase reaction. The sera were used as a 1 : 1000 dilution. These differences may explain the discrepancy.
Theoretically, antibodies secreted from plasma cells in the diseased tissue are assumed to diffuse into the serum, and so the serum concentration of the antibodies should be much lower than in the tissue extract. It is intriguing to suppose that some antibodies are produced locally in the inflamed tissue, but the efficiency of secretion into the serum is low. In our previous study of rheumatoid arthritis, antibodies to FBXO2 were locally produced in the diseased synovium, but were undetectable in the serum. In contrast, autoantibodies to TRIM21 (SS-A) were demonstrated both in plasma cells in tissue sections and in the serum (Mizutani et al., 2013). The advantage of the enzyme-labeled antigen method and the AlphaScreen method includes the detection of antibodies locally produced in the tissue but inefficiently secreted into the serum.
With the real-time PCR analysis, a variable amount of P. gingivalis genome was detected in the biopsied samples of periodontitis in 18/20 (90%). The findings re-confirmed the strong causal relationship between periodontitis and P. gingivalis infection. However, there were no statistical correlations between the detection of P. gingivalis 16S ribosomal DNA and the production of P. gingivalis-reactive antibodies in the same biopsied tissue samples. In some lesions, the bacterial DNA level and the density of pathogen-specific plasma cells were both high. It is supposed that the locally produced antibodies are devoid of neutralizing (bacteria-killing) activity; just as occurs in the case of Helicobacter pylori infection in the gastric mucosa (Bhat et al., 2005). In other lesions, a high density of pathogen-reactive plasma cells and a low level of bacterial DNA were noted, and in case 7, P. gingivalis-specific plasma cells were densely distributed and the bacteria were undetectable, suggesting the production of neutralizing antibodies. In fact, the presence of neutralizing antibodies against P. gingivalis has been reported repeatedly: the target antigens reported include Lys-hgp (DeCarlo et al., 2004), 130-kDa hemagglutinin (hgp; Shibata et al., 2005), lipopolysaccharide (Bainbridge et al., 1997), and 40-kDa outer membrane protein (Momoi et al., 2008).
In case 20, P. gingivalis DNA and anti-P. gingivalis antibody signals were negative in all assays. The negative findings are most likely caused by sampling error, because no plasma cells were identified in the tissue sample. Isolation of the pathogen from the lesion is not consistent. Condorelli et al. (1998) reported that P. gingivalis was isolated from 78.3% of subgingival plaque samples from periodontitis patients.
In case 14, no antibodies reactive to P. gingivalis were detected, in spite of the presence of plasma cells and positive signals of P. gingivalis DNA in the lesion. There are multiple antigenic molecules on P. gingivalis other than the antigens analyzed in the present study. These include lipopolysaccharide (Bainbridge et al., 1997) and a 40-kDa outer membrane antigen (Momoi et al., 2008). Antibody responses to 43-kDa fimbrial protein, Pga30 (30-kDa antigenic protein), PrtC (38-kDa collagenase), and HtpG (heat-shock protein 90 homologue) have also been reported (Condorelli et al., 1998; Hendtlass et al., 2000; Beikler et al., 2003; Shelburne et al., 2008). It is therefore plausible that antibodies to these proteins are involved in case 14.
The absorption experiment using an excess amount of unlabeled antigens confirmed the specificity of our histochemical technique, as we described previously (Tsuge et al., 2011). It should be noted that the AlphaScreen signals for Arg-hgp and Lys-hgp were correlated in both the serum and tissue extracts. In the absorption experiment of the enzyme-labeled antigen method, the anti-Arg-hgp signals were partly eliminated by unlabeled Lys-hgp, and the anti-Lys-hgp signals were partly absorbed with unlabeled Arg-hgp. These findings indicate the presence of epitopes common between Arg-hgp and Lys-hgp. Antibodies against these common epitopes were visualized in a good number of plasma cells, while the other plasma cells contained antibodies specific to epitopes unique in either Arg-hgp or Lys-hgp, as illustrated in Fig.6. We already described the same findings in radicular cyst (Tsuge et al., 2011). In fact, the homology of the amino acid sequence of both hgp domains is estimated 76% (O'Brien-Simpson et al., 2001). In contrast, the homology between Arg-pro and Lys-pro was 30% (Tsuge et al., 2011). It is intriguing that the region (or epitope) -specificity could be shown with our histochemical technique. In our previous report (Tsuge et al., 2011), plasma cells reactive to the epitopes common between Arg-hgp and Lys-hgp were clustered in some areas, while other areas contained plasma cells reactive to Arg-hgp-specific epitope. Cluster formation of plasma cells producing antibodies showing the same epitope specificity was not observed in the present study.
In conclusion, we evaluated local immune reactions against the pathogen in the diseased gingiva by using the AlphaScreen method and the enzyme-labeled antigen method. The target antigens (or even epitopes) of the antibodies produced within the lesion were demonstrated, so that the pathogenesis of the lesion can be analyzed in view of the site of antibody production. Analysis of the serum and tissue extract with the AlphaScreen method provides the specificity confirmation of our novel histochemical technique. The present approach is applicable to a variety of tissue sections showing dense plasma cell infiltration, and provides a potential histochemical tool for analyzing disease process.
Acknowledgments
Skillful technical assistance from Ms Mika Maeshima and Ms Sayaka Takeuchi, and effective office work by Ms. Chikayo Yashiro, Department of Pathology, Fujita Health University School of Medicine, Toyoake, are cordially acknowledged. Prof. Emeritus Yaeta Endo, MD, Ehime University, Matsuyama, Japan, gave us valuable advice and suggestions. This work was supported by the Research Grant from the Ministry of Education, Science, Culture, Sports and Technology, Japan (#30469035 to ST) and also in part by the Research Grant from Fujita Health University (2013 to YM). We do not have a conflict of interest to be declared in the present study.
References
- Aguilera Galaviz LA, Aceves Medina Mdel C, Estrada Garcia IC. Detection of potentially cariogenic strains of Streptococcus mutans using the polymerase chain reaction. J Clin Pediatr Dent. 2002;27:47–51. [PubMed] [Google Scholar]
- Anusaksathien O, Singh G, Matthews N, Dolby AE. Autoimmunity to collagen in adult periodontal disease: immunoglobulin classes in sera and tissue. J Periodontal Res. 1992;27:55–61. doi: 10.1111/j.1600-0765.1992.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Bainbridge BW, Page RC, Darveau RP. Serum antibodies to Porphyromonas gingivalis block the prostaglandin E2 response to lipopolysaccharide by mononuclear cells. Infect Immun. 1997;65:4801–4805. doi: 10.1128/iai.65.11.4801-4805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beikler T, Ehmke B, Wittstock M, Schmidt H, Karch H, Flemmig TF. Serum antibody reactivity against recombinant PrtC of Porphyromonas gingivalis following periodontal therapy. J Periodontal Res. 2003;38:276–281. doi: 10.1034/j.1600-0765.2003.01405.x. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;32(Suppl 6):87–107. doi: 10.1111/j.1600-051X.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Donati M, Zitzmann N. B cells in periodontitis: friends or enemies? Periodontol 2000. 2007;45:51–66. doi: 10.1111/j.1600-0757.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- Bhat N, Gaensbauer J, Peek RM, et al. Local and systemic immune and inflammatory responses to Helicobacter pylori strains. Clin Diagn Lab Immunol. 2005;12:1393–1400. doi: 10.1128/CDLI.12.12.1393-1400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, Thurnheer T, Aduse-Opoku J, Curtis MA, Zinkernagel AS, Belibasakis GN. Porphyromonas gingivalis regulates TREM-1 in human polymorphonuclear neutrophils via its gingipains. PLoS ONE. 2013;8:e75784. doi: 10.1371/journal.pone.0075784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Nakayama K, Belliveau L, Duncan MJ. Porphyromonas gingivalis gingipains and adhesion to epithelial cells. Infect Immun. 2001;69:3048–3056. doi: 10.1128/IAI.69.5.3048-3056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli F, Scalia G, Cali G, Rossetti B, Nicoletti G, Lo Bue AM. Isolation of Porphyromonas gingivalis and detection of immunoglobulin A specific to fimbrial antigen in gingival crevicular fluid. J Clin Microbiol. 1998;36:2322–2325. doi: 10.1128/jcm.36.8.2322-2325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler CW, Kalmar JR, Genco CA. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 1995;3:45–51. doi: 10.1016/s0966-842x(00)88874-5. [DOI] [PubMed] [Google Scholar]
- DeCarlo AA, Nadkarni M, Paramaesvaran M, Yun PW, Collyer CA, Hunter N. Serum antibodies against the hemoglobin-binding domain (HA2) of Porphyromonas gingivalis. J Periodontal Res. 2004;39:228–235. doi: 10.1111/j.1600-0765.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- De-Gennaro LA, Lopes JD, Mariano M. Autoantibodies directed to extracellular matrix components in patients with different clinical forms of periodontitis. J Periodontol. 2006;77:2025–2030. doi: 10.1902/jop.2006.060104. [DOI] [PubMed] [Google Scholar]
- Grenier D, Tanabe S. Porphyromonas gingivalis gingipains trigger a proinflammatory response in human monocyte-derived macrophages through the p38alpha mitogen-activated protein kinase signal transduction pathway. Toxins (Basel) 2010;2:341–352. doi: 10.3390/toxins2030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentsch A, Kramesberger M, Sroka A, Pfister W, Potempa J, Eick S. Comparison of gingival crevicular fluid sampling methods in patients with severe chronic periodontitis. J Periodontol. 2011;82:1051–1060. doi: 10.1902/jop.2011.100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Takahashi K, Kokeguchi S, Takashiba S, Kinane DF, Murayama Y. Antibody responses against Porphyromonas gingivalis infection in patients with early-onset periodontitis. J Clin Periodontol. 2000;27:769–777. doi: 10.1034/j.1600-051x.2000.027010769.x. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol. 2014;44:328–338. doi: 10.1002/eji.201344202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendtlass A, Dashper SG, Reynolds EC. Identification of an antigenic protein Pga30 from Porphyromonas gingivalis W50. Oral Microbiol Immunol. 2000;15:383–387. doi: 10.1034/j.1399-302x.2000.150608.x. [DOI] [PubMed] [Google Scholar]
- Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonas gingivalis. Periodontol 2000. 1999;20:168–238. doi: 10.1111/j.1600-0757.1999.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Kim YC, Ko Y, Hong SD, et al. Presence of Porphyromonas gingivalis and plasma cell dominance in gingival tissues with periodontitis. Oral Dis. 2010;16:375–381. doi: 10.1111/j.1601-0825.2009.01649.x. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Nishimura F, Nakamura T, et al. Humoral immune response to an antigen from Porphyromonas gingivalis 381 in periodontal disease. Infect Immun. 1991;59:2758–2762. doi: 10.1128/iai.59.8.2758-2762.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol Immunol. 2000;15:341–349. doi: 10.1034/j.1399-302x.2000.150601.x. [DOI] [PubMed] [Google Scholar]
- Lee SF, Progulske-Fox A, Bleiweis AS. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988;56:2114–2119. doi: 10.1128/iai.56.8.2114-2119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason DY, Cordell JL, Brown MH, et al. CD79a: a novel marker for B-cell neoplasms in routinely processed tissue samples. Blood. 1995;86:1453–1459. [PubMed] [Google Scholar]
- Matsuoka K, Komori H, Nose M, Endo Y, Sawasaki T. Simple screening method for autoantigen proteins using the N-terminal biotinylated protein library produced by wheat cell-free synthesis. J Proteome Res. 2010;9:4264–4273. doi: 10.1021/pr9010553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani Y, Tsuge S, Shiogama K, et al. Enzyme-labeled antigen method: histochemical detection of antigen-specific antibody-producing cells in tissue sections of rats immunized with horseradish peroxidase, ovalbumin, or keyhole limpet hemocyanin. J Histochem Cytochem. 2009;57:101–111. doi: 10.1369/jhc.2008.952259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani Y, Matsuoka K, Takeda H, et al. Novel approach to identifying autoantibodies in rheumatoid synovitis with a biotinylated human autoantigen library and the enzyme-labeled antigen method. J Immunol Methods. 2013;387:57–70. doi: 10.1016/j.jim.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Momoi F, Hashizume T, Kurita-Ochiai T, Yuki Y, Kiyono H, Yamamoto M. Nasal vaccination with the 40-kilodalton outer membrane protein of Porphyromonas gingivalis and a nontoxic chimeric enterotoxin adjuvant induces long-term protective immunity with reduced levels of immunoglobulin E antibodies. Infect Immun. 2008;76:2777–2784. doi: 10.1128/IAI.01502-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M, Hirakawa H, Yamashita A, et al. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 2008;15:215–225. doi: 10.1093/dnares/dsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien-Simpson NM, Black CL, Bhogal PS, et al. Serum immunoglobulin G (IgG) and IgG subclass responses to the RgpA-Kgp proteinase-adhesin complex of Porphyromonas gingivalis in adult periodontitis. Infect Immun. 2000;68:2704–2712. doi: 10.1128/iai.68.5.2704-2712.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien-Simpson NM, Paolini RA, Hoffmann B, Slakeski N, Dashper SG, Reynolds EC. Role of RgpA, RgpB, and Kgp proteinases in virulence of Porphyromonas gingivalis W50 in a murine lesion model. Infect Immun. 2001;69:7527–7534. doi: 10.1128/IAI.69.12.7527-7534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, McGhee ML, Moldoveanu Z, et al. Bacteroides-specific IgG and IgA subclass antibody-secreting cells isolated from chronically inflamed gingival tissues. Clin Exp Immunol. 1989;76:103–110. [PMC free article] [PubMed] [Google Scholar]
- Oyaizu K, Ohyama H, Nishimura F, et al. Identification and characterization of B-cell epitopes of a 53-kDa outer membrane protein from Porphyromonas gingivalis. Oral Microbiol Immunol. 2001;16:73–78. doi: 10.1034/j.1399-302x.2001.016002073.x. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Sawasaki T, Hasegawa Y, Tsuchimochi M, et al. A bilayer cell-free protein synthesis system for high-throughput screening of gene products. FEBS Lett. 2002;514:102–105. doi: 10.1016/s0014-5793(02)02329-3. [DOI] [PubMed] [Google Scholar]
- Sawasaki T, Kamura N, Matsunaga S, et al. Arabidopsis HY5 protein functions as a DNA-binding tag for purification and functional immobilization of proteins on agarose/DNA microplate. FEBS Lett. 2008;582:221–228. doi: 10.1016/j.febslet.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein HA. Host responses in maintaining periodontal health and determining periodontal disease. Periodontol 2000. 2006;40:77–93. doi: 10.1111/j.1600-0757.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- Shelburne CE, Shelburne PS, Dhople VM, et al. Serum antibodies to Porphyromonas gingivalis chaperone HtpG predict health in periodontitis susceptible patients. PLoS ONE. 2008;3:e1984. doi: 10.1371/journal.pone.0001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Hosogi Y, Hayakawa M, Hori N, Kamada M, Abiko Y. Construction of novel human monoclonal antibodies neutralizing Porphyromonas gingivalis hemagglutination activity using transgenic mice expressing human Ig loci. Vaccine. 2005;23:3850–3856. doi: 10.1016/j.vaccine.2005.01.159. [DOI] [PubMed] [Google Scholar]
- Slots J, Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999;20:82–121. doi: 10.1111/j.1600-0757.1999.tb00159.x. [DOI] [PubMed] [Google Scholar]
- The Japanese Society of Periodontology. The Guideline for Examination, Diagnosis and Treatment Planning for Periodontal Diseases 2008. Tokyo: Ishiyaku Publishers, Inc; 2009. [in Japanese]. [Google Scholar]
- Tsuge S, Mizutani Y, Matsuoka K, et al. Specific in situ visualization of plasma cells producing antibodies against Porphyromonas gingivalis in gingival radicular cyst: application of the enzyme-labeled antigen method. J Histochem Cytochem. 2011;59:673–689. doi: 10.1369/0022155411408906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Nagashima K, Sun D, et al. Development of high-throughput TR-FRET and AlphaScreen assays for identification of potent inhibitors of PDK1. J Biomol Screen. 2009;14:1257–1262. doi: 10.1177/1087057109349356. [DOI] [PubMed] [Google Scholar]


