Abstract
Background
In patients with known atrial fibrillation (AF) different scores are utilized to estimate the risk of thromboembolic events and guide oral anticoagulation. Diagnosis of AF strongly depends on the duration of electrocardiogram monitoring. The aim of this study was to use established scores to predict the prevalence of AF.
Methods
The CHADS2- (Congestive Heart failure, hypertension, Age >75 years, Diabetes, Stroke [doubled]) and CHA2DS2VASc-score (Congestive Heart failure, hypertension, Age ≥75 years [doubled], Diabetes, Stroke [doubled], Vascular disease, Age 65–74 years, Sex category [female sex]) was calculated in 150,408 consecutive patients, referred to the University Hospital of Rostock between 2007 and 2012. All factors constituting these scores and a history of AF were prospectively documented with the ICD-10 admission codes.
Results
Mean age of our study population was 67.6 ± 13.6 years with a mean CHADS2-score of 1.65 ± 0.92 and CHA2DS2VASc-score of 3.04 ± 1.42. AF was prevalent in 15.9% of the participants. The prevalence of AF increased significantly with every CHADS2- and CHA2DS2VASc-score point up to 54.2% in CHADS2-score of 6 and 71.4% in CHA2DS2VASc-score of 9 (P < 0.001).
Conclusion
The prevalence of AF increases with increasing CHADS2- and CHA2DS2VASc-score. In intermediate scores intensified monitoring may be recommended. In high scores, thromboembolic complications occurred irrespective of the presence of AF and anticoagulant therapy may be initiated irrespective of documented AF.
Keywords: atrial fibrillation, prevalence, risk factors, stroke prevention
Introduction
Atrial fibrillation (AF) is a frequent arrhythmia with an estimated prevalence of 1.5–2% in developed countries.1,2 The occurrence is suspected to rise due to an ageing population and the progressive nature of the arrhythmia.2–6 Besides, AF is associated with increased morbidity, mortality, and risk of thromboembolism, which can be significantly reduced with oral anticoagulation.2,7–11 The CHADS2- (Congestive Heart failure, hypertension, Age >75 years, Diabetes, Stroke [doubled]) and CHA2DS2VASc- (Congestive Heart failure, hypertension, Age ≥ 75 years [doubled], Diabetes, Stroke [doubled], Vascular disease, Age 65-74 years, Sex category [female sex]) score have been established to guide antithrombotic therapy in individuals with known AF.8,12,13 However, AF is often not diagnosed until patients present with thromboembolic complications.1,2,11,14 In up to 25% of patients, AF is suspected to be the cause of a cryptogenic stroke.11,14 Therefore, early identification of individuals with AF seems to be warranted in order to prevent associated complications.1,8
Many efforts have been undertaken to create models for the identification of patients at risk of AF before complications become apparent. So far, risk stratification has been limited to small cohorts or restricted age groups. Finally, risk stratification was not easily adopted in daily practice.3,15 The CHADS2- and CHA2DS2VASc-score are well-established tools to estimate the risk of thromboembolic events in individuals with known AF.1,8,12,13 Some features of these scores are not only used to predict the risk of thromboembolic complications, but also to predict the occurrence of AF.3,8,15
In this study, we hypothesized that the CHADS2- and CHA2DS2VASc-score may predict the prevalence of AF and may be used to guide cardiac monitoring.
Methods
A total of 150,408 patients who were referred to different medical departments in the University Hospital of Rostock between January 1, 2007 and December 31, 2012 were included in this study. The CHADS2- and CHAD2DS2VASc-scores were prospectively and electronically documented using the International Statistical Classification of Diseases, 10th Revision (ICD-10) codes: Hypertension (ICD-10 codes I10–I15), previous transient ischemic attack (TIA), stroke or arterial thromboembolism (ICD-10 codes G45.9, I63.0–I63.9, and I74–I74.9), congestive heart failure (ICD-10 codes I50.00–I50.01, I50.9, I50.11–I50.14, and I50.19 ), and diabetes (ICD-10 codes E10.0–E14.91). In addition, vascular diseases such as myocardial infarction (ICD-10 codes I21.0–I21.9, I22.0–I22.9, and I25.20–I25.29), coronary artery disease (ICD-10 codes I25.0–I25.19), peripheral arterial occlusive disease (ICD-10 codes I70.2–I70.25), or atherosclerosis of the aorta (ICD-10 code I70.0) were documented. Age at the time of admission and gender were also recorded in all patients. Based on this data, the CHADS2- and CHA2DS2VASc-score was calculated for each patient. Finally, patients were identified with an admission code of AF (ICD-10 codes I48.0–I48.2, and I48.9) based on anamnestic data, electrocardiogram (ECG)-recording, Holter monitoring, and data from cardiac devices. Precisely, in patients with anamnestic AF there had to be at least one ECG with documented AF before hospitalization, based on a physician recall. In patients with first detected AF during the hospital stay, we used ECG recording, Holter monitoring, and also data from loop recorders and cardiac devices for the diagnosis of AF. An episode of AF was defined as an event lasting greater than 30 seconds in duration. Patients with paroxysmal as well as persistent AF were included in our study.
Statistical Analysis
All data were stored and analyzed using the SPSS statistical package 21.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were computed for continuous and categorical variables. The statistics computed included mean and standard deviation (SD) of continuous variables and are presented as mean ± SD, frequencies, and relative frequencies of categorical factors.
Comparisons between groups for categorical variables were done using the χ2 or Fisher's exact test and for continuous variables using a t-test for independent samples. The logistic regression model was used to evaluate the influence of several factors constituting the scores on risk for AF. Evaluation was done by computation of odds ratios (OR), 95% confidence intervals (95% CI), and examination the significance of the Wald statistic. All P values resulted from two-sided statistical tests and values of P < 0.05 were considered to be statistically significant.
Results
A total of 150,408 patients were included in the study; mean age was 67.6 ± 13.6 years (Table1). A total of 46,602 patients (31%) were between 65 years and 74 years and 51,720 older than 74 years of age (34.4%, Table1). The majority (56.9%) were male. AF was known in 23,905 patients (15.9%) with a mean age of 72.8 ± 10.5 years (Table1). Of 150,408 patients, 80.2% suffered from hypertension. Diabetes mellitus was present in 35.3% and vascular diseases in 30.4% of patients. Congestive heart failure was documented in 10.2%, a history of TIA or stroke in 4.0% of patients.
Table I.
Baseline Characteristics
| Without AF | With AF | ||||||
|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % | P Value |
| Patients | 150,408 | 100 | 126,503 | 84.1 | 23,905 | 15.9 | |
| Age (mean ± SD) years | 67.6 ± 13.6 | 66.6 ± 13.9 | 72.8 ± 10.5 | <0.001 | |||
| Age <65 years | 52,086 | 34.6 | 47,577 | 37.6 | 4,544 | 19.0 | |
| Age 65–74 years | 46,602 | 31.0 | 38,994 | 30.8 | 7,608 | 31.8 | |
| Age >74 years | 51,720 | 34.4 | 39,932 | 31.6 | 11,753 | 49.2 | |
| Age >75 years | 46,286 | 30.8 | 35,567 | 28.1 | 10,665 | 44.6 | |
| Female | 64,835 | 43.1 | 54,748 | 43.3 | 10,087 | 42.2 | 0.002 |
| Congestive heart failure | 15,326 | 10.2 | 9,932 | 7.9 | 5,394 | 22.6 | <0.001 |
| Hypertension | 120,631 | 80.2 | 102,830 | 81.3 | 17,801 | 74.5 | <0.001 |
| Diabetes mellitus | 53,115 | 35.3 | 44,236 | 35.0 | 8,879 | 37.1 | <0.001 |
| History of stroke | 5,984 | 4.0 | 4,302 | 3.4 | 1,682 | 7.0 | <0.001 |
| Vascular disease | 45,755 | 30.4 | 36,603 | 28.9 | 9,152 | 38.3 | <0.001 |
AF = atrial fibrillation; SD = standard deviation.
CHADS2-Score
The mean CHADS2-score was 1.65 ± 0.92. The prevalence of AF was 10.1%, 15.5%, 25.8%, 37.4%, 45.9%, and 54.2% in patients with a CHADS2-score between 1 and 6, respectively (Table2, Fig.1). This prevalence of AF increased continuously and significantly by 5.4–11.5% with every score point (P < 0.001).
Table II.
Prevalence of Atrial Fibrillation with Increasing CHADS2-Score and Main Reason for Hospitalization
| Prevalence of AF (%) | |||||||
|---|---|---|---|---|---|---|---|
| CHADS2 | Cardiology/ | ||||||
| -Score | All | Cardiac Surgery | Dermatology | Neurology | Surgery | Otorhinolaryngology | Ophthalmology |
| 1 | 10.1 | 21.2 | 10.7 | 6.0 | 6.2 | 3.7 | 3.0 |
| 2 | 15.5 | 26.2 | 17.1 | 15.0 | 10.8 | 7.9 | 4.3 |
| 3 | 25.8 | 36.7 | 29.4 | 22.7 | 19.0 | 13.8 | 5.8 |
| 4 | 37.4 | 45.2 | 40.6 | 32.2 | 36.7 | 22.9 | 75.0 |
| 5 | 45.9 | 60.0 | 35.7 | 44.9 | 68.0 | 28.6 | 25.0 |
| 6 | 54.2 | 38.5 | 20.0 | 57.1 | 88.9 | 100.0 | 0 |
| Mean ± SD‡ | 1.65 ± 0.92 | ||||||
CHADS2-Score = Congestive Heart failure, hypertension, Age >75 years, Diabetes, Stroke (doubled); SD = standard deviation.
Figure 1.
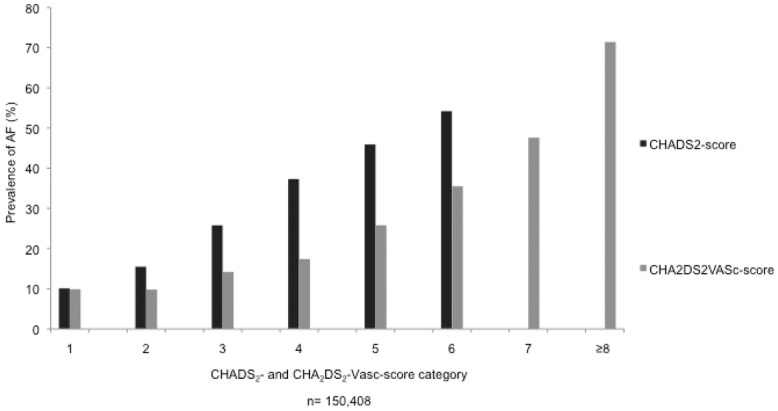
Prevalence of atrial fibrillation (AF) in different CHADS2- (Congestive Heart failure, hypertension, Age >75 years, Diabetes, Stroke [doubled]) and CHA2DS2VASc-score (Congestive Heart failure, hypertension, Age ≥75 years [doubled], Diabetes, Stroke [doubled], Vascular disease, Age 65–74 years, Sex category [female sex]) categories.
The rising prevalence of AF with an increasing CHADS2-score was present in the whole cohort irrespective of the medical department patients were admitted to and the underlying disease that caused the hospitalization (Table2). The prevalence was highest in patients who were admitted to cardiology and cardiac surgery because AF was the reason for admission in many patients. Still, the prevalence increased with an increasing score. Overall, the prevalence of AF was significantly increased in patients older than 74 years of age (OR = 2.09, 95% CI: 2.04–2.15), with congestive heart failure (OR = 3.42, 95% CI: 3.30–3.55), diabetes mellitus (OR = 1.10, 95% CI: 1.07–1.13), and a history of stroke (OR = 2.12, 95% CI: 2.00–2.24; Table3). Surprisingly, the prevalence of AF was lower in patients with hypertension (OR = 0.67, 95% CI: 0.65–0.69; Table3).
Table III.
Prevalence of Atrial Fibrillation and Factors Constituting the CHADS2-Score and the CHA2DS2VASc-Score
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| >74 years | 2.09 | 2.04–2.15 | <0.001 |
| Congestive heart failure | 3.42 | 3.30–3.55 | <0.001 |
| Hypertension | 0.67 | 0.65–0.69 | <0.001 |
| Diabetes mellitus | 1.10 | 1.07–1.13 | <0.001 |
| History of stroke | 2.12 | 2.00–2.24 | <0.001 |
| Female sex | 0.96 | 0.93–0.98 | <0.001 |
| Vascular disease | 1.52 | 1.48–1.57 | <0.001 |
CHA2DS2VASc-Score = Congestive Heart failure, hypertension, Age 75 years (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65-74 years, Sex category (female sex); CI = confidence intervals. Other abbreviations as in previous tables.
The prevalence of stroke increased from 0% to 93.8% between a CHADS2-score of 1 and 6 (Table4). Up to a score of 3, the prevalence of stroke was higher in patients with known AF. Beyond a score of 3, patients showed a high prevalence of stroke irrespective of AF (Table4).
Table IV.
Prevalence of Stroke and CHADS2-Score
| Prevalence of | |||
|---|---|---|---|
| Stroke (%) | |||
| CHADS2 | |||
| -Score | All | All | With AF |
| 0 | 7,782 | 0 | 0 |
| 1 | 68,288 | 0 | 0 |
| 2 | 50,534 | 1.5 | 9.7 |
| 3 | 17,918 | 11.0 | 20.1 |
| 4 | 4,856 | 47.0 | 32.8 |
| 5 | 934 | 96.5 | 46.2 |
| 6 | 96 | 93.8 | 55.6 |
Abbreviations as in previous tables.
CHA2DS2VASc-Score
The mean CHA2DS2VASc-score was 3.04 ± 1.42 in the whole cohort. The prevalence of AF increased progressively and significantly from 9.9% in patients with a CHA2DS2VASc-score of 1–71.4% in patients with a CHA2DS2VASc-score of 9 (P < 0.001, Table5, Fig.1). The increasing prevalence of AF in patients with increasing CHA2DS2VASc-scores was apparent in all medical departments and irrespective of the underlying disease that lead to the hospitalization (Table5, Fig.2).
Table V.
Prevalence of Atrial Fibrillation with Increasing CHA2DS2VASc-Score and Main Reason for Hospitalization
| Prevalence of AF (%) | |||||||
|---|---|---|---|---|---|---|---|
| CHA2DS2 | Cardiology/ | ||||||
| VASc-Score | All | Cardiac Surgery | Dermatology | Neurology | Surgery | Oto-rhinolaryngology | Ophthalmology |
| 1 | 9.9 | 31.9 | 9.1 | 4.7 | 5.0 | 2.7 | 3.1 |
| 2 | 9.9 | 20.6 | 10.5 | 6.4 | 6.7 | 4.5 | 3.4 |
| 3 | 14.2 | 23.0 | 16.5 | 12.0 | 10.1 | 8.1 | 3.9 |
| 4 | 17.4 | 25.3 | 20.5 | 17.4 | 11.9 | 9.3 | 4.7 |
| 5 | 25.8 | 30.6 | 29.5 | 26.0 | 19.0 | 11.3 | 6.9 |
| 6 | 35.5 | 35.8 | 35.6 | 37.3 | 30.8 | 20.8 | 20.0 |
| 7 | 47.6 | 47.9 | 46.7 | 48.0 | 46.9 | 40.0 | 50.0 |
| 8 | 50.2 | 41.2 | 23.5 | 50.3 | 80.0 | 100 | 100 |
| 9 | 71.4 | 50.0 | 100 | 77.8 | 100 | 0 | 0 |
| Mean ± SD | 3.04 ± 142 | ||||||
Abbreviations as in previous tables.
Figure 2.
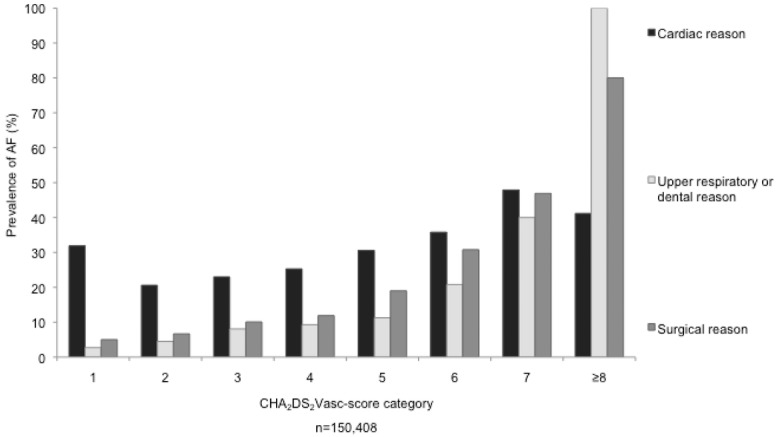
Prevalence of AF in different CHA2DS2VASc-score categories and diverse reasons for hospitalization. Abbreviations as in Figure 1.
Comparable to the CHADS2-score the prevalence of AF was also higher in cardiology and cardiac surgery patients and increased with an increasing score. The logistic regression analyses of factors constituting the CHA2DS2VASc-score revealed the same results for age, congestive heart failure, diabetes mellitus, history of stroke, and hypertension as the CHADS2-score (Table3). In addition, female patients had a slightly decreased prevalence of AF compared with males (OR = 0.96, 95% CI: 0.93–0.98). Patients with vascular disease had an increased relative risk of AF (OR = 1.52, 95% CI: 1.48–1.57) (Table3).
The prevalence of stroke rose from zero to a maximum of 96.4% between CHA2DS2VASc-score 1 and 9 (Table6). Up to a score of 6, the prevalence of stroke was higher in patients with AF. Beyond a score of 6, the prevalence of stroke was high irrespective of AF.
Table VI.
Prevalence of Stroke and CHA2DS2VASc-Score
| Prevalence of Stroke | |||
|---|---|---|---|
| CHA2DS2 | |||
| VASc-Score | All | All (%) | With AF (%) |
| 0 | 1,425 | 0 | 0 |
| 1 | 20,047 | 0 | 0 |
| 2 | 36,246 | 0.8 | 5.2 |
| 3 | 38,171 | 2.0 | 8.7 |
| 4 | 32,064 | 3.4 | 17.1 |
| 5 | 15,308 | 9.1 | 25.9 |
| 6 | 5,475 | 25.6 | 37.3 |
| 7 | 1,394 | 57.2 | 49.1 |
| 8 | 249 | 96.8 | 51.0 |
| 9 | 28 | 96.4 | 70.4 |
Abbreviations as in previous tables.
Discussion
Prevalence of AF
The prevalence of AF was 15.9% in our study population (mean age of 67.6 ± 13.6 years). In the general population aged over 65 years, the prevalence of AF is estimated to be between 6% and 8%.5,16,17 One reason for the higher prevalence in our study may be that many patients have been presented to our hospital for AF as reflected in the high prevalence of AF in patients who were admitted to “the cardiology-” or cardiac surgery department. Besides, patients revealed considerable comorbidities as depicted by a mean CHADS2-score of 1.65 ± 0.92 and the mean CHA2DS2VASc-score of 3.04 ± 1.42. However, the increasing prevalence of AF with higher scores was apparent in all departments and independent of the reason for hospitalization. In addition, a prevalence of AF with up to 30–34% has been described in patients with intensified monitoring with implanted devices during a follow up of 1.1–2.5 years.18,19 In line with our data Engdahl et al. reported a prevalence of 14% in a population of 75 years and 76 years of age using a special screening program.20
The Prevalence of AF and Thromboembolic Complications
In patients with known AF risk factors for stroke have been investigated in detail and “resulted in the widely accepted” CHADS2- and CHA2DS2VASc-score to identify patients who may benefit from oral anticoagulation.8 But little is known about the occurrence of AF in patients with stroke risk factors.19–22
We could demonstrate that the prevalence of AF rises significantly with every CHADS2- and CHA2DS2VASc-score point, independent of the attending medical department and the underlying disease that lead to hospitalization. Patients with a CHADS2-score of 5 and 6 had AF in 45.9% and 54.2%, respectively. Patients with a CHA2DS2VASc-score between 6 and 9 had AF in 35.3% to 71.4%. In contrast to our study, Ziegler et al. detected AF in 30% of patients during a mean follow-up of 1.1 year with implantable loop recorders.18 The occurrence of AF was not related to underlying CHADS2-score and the diagnosis was based on any episode of AF irrespective of symptoms. However, AF lasting longer than 6 hours/day was associated with a higher CHADS2-score.18 This may indicate that the scores predict persistent and longer lasting episode of AF rather than short episodes of paroxysmal AF. In addition, Zuo et al. demonstrated that in patients without documented AF but arrhythmic symptoms, a high CHADS2- and CHA2DS2VASc-score was associated with a high risk of a new onset of AF.23
In patients with CHADS2-score of more than 4 and a CHA2DS2VASc-score of more than 7 the prevalence of stroke was high and independent of AF.
In contrast, the prevalence of thromboembolic complications was two to five times higher with CHADS2-score below 4 and CHA2DS2VASc-score below 7, if AF was present.
We, therefore, conclude that using the CHADS2- and CHA2DS2VASc-score it may be possible to detect patients with a high risk of AF and thromboembolic complications if AF is present. With very high scores the risk of thromboembolic complications may no longer be dependent on AF. While intensified monitoring may be warranted in the former patients, anticoagulation may be warranted in the latter. This hypothesis should be tested in a prospective trial, which is underway in collaboration with Biotronik (Cleopatra Trial; Biotronik GmbH, Berlin, Germany). In this randomized trial, patients postmyocardial infarction with a CHADS2-score of more than 2 undergo intensified monitoring with an implantable loop recorder.
Limitations
The study was carried out in a single community and there may be a selection bias, because all patients were referred to our hospital for various clinical reasons. However, the relationship between the prevalence of AF and the CHADS2- or CHA2DS2VASc-score was present in all departments irrespective of the reason for referral. In addition, these data were primarily collected to generate reimbursement from health insurances and not primarily for medical reasons. Clinical details such as the severity of AF were not documented. Furthermore, information concerning medication was not available. Nevertheless, in Germany under- and overcoding of diseases is under penalty, so documented data are expected to be very reliable.
Apart from that, in our study population the prevalence of AF was lower among hypertensive patients, although hypertension is considered to be a risk factor for the development of AF. We realized that the population of the eastern part of Germany has many chronic illnesses. The very fact that hypertension was prevalent in 80% of the study patients shows that concerning this disease the study patients are not comparable to general population.
Conclusion
The prevalence of AF increased considerably with increasing CHADS2- and CHA2DS2VASc-scores. The prevalence of thromboembolic complications was dependent on the presence of AF up to a CHADS2-score of 3 and CHA2DS2VASc-score of 6. This should warrant intensified monitoring in these patients. The prevalence of thromboembolic complications was high and independent of the presence of AF in patients with a CHADS2-score of 4 or more and CHA2DS2VASc-score of 7 or more. This may indicate that the need for anticoagulation may be independent of the documentation of AF. Both hypotheses should be prospectively verified.
References
- 1.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–1413. doi: 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–661. doi: 10.1016/S0140-6736(11)61514-6. [DOI] [PubMed] [Google Scholar]
- 3.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr, Newton-Cheh C, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Rho RW, Page RL. Asymptomatic atrial fibrillation. Prog Cardiovasc Dis. 2005;48:79–87. doi: 10.1016/j.pcad.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Breithardt G, Aliot E, Khatib SA, Apostolakis S, Auricchio A, Bailleul C, et al. Personalized management of atrial fibrillation: Proceedings from the fourth Atrial Fibrillation competence NETwork/European heart rhythm association consensus conference. Europace. 2013;15:1540–1556. doi: 10.1093/europace/eut232. [DOI] [PubMed] [Google Scholar]
- 8.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, et al. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin EJ, PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 10.Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA, 3rd, Page RL, et al. ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104–123. doi: 10.1161/CIR.0b013e3181fa3cf4. [DOI] [PubMed] [Google Scholar]
- 11.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 12.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 13.Lip GYR, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 14.Liao JZ, Scallan C, Morillo C, O'Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: A systematic review. Stroke. 2007;38:2935–2940. doi: 10.1161/STROKEAHA.106.478685. [DOI] [PubMed] [Google Scholar]
- 15.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: The CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullenix PS, Martin MJ, Steele SR, Lavenson GS, Jr, Starnes BW, Hadro NC, Peterson RP, et al. Rapid high-volume population screening for three major risk factors of future stroke: Phase I results. Vasc Endovasc Surg. 2006;40:177–187. doi: 10.1177/153857440604000302. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler PD, Glotzer TV, Daoud EG, Singer DE, Ezekowitz MD, Hoyt RH, Koehler JL, et al. Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. Am J Cardiol. 2012;110:1309–1314. doi: 10.1016/j.amjcard.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 20.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: Implications for stroke prevention. Circulation. 2013;127:930–937. doi: 10.1161/CIRCULATIONAHA.112.126656. [DOI] [PubMed] [Google Scholar]
- 21.Friberg L, Engdahl J, Frykman V, Svennberg E, Levin LA, Rosenqvist M. Population screening of 75- and 76-year-old men and women for silent atrial fibrillation (STROKESTOP) Europace. 2013;15:135–140. doi: 10.1093/europace/eus217. [DOI] [PubMed] [Google Scholar]
- 22.Samol A, Masin M, Gellner R, Otte B, Pavenstadt HJ, Ringelstein EB, Reinecke H, et al. Prevalence of unknown atrial fibrillation in patients with risk factors. Europace. 2013;15:657–662. doi: 10.1093/europace/eus366. [DOI] [PubMed] [Google Scholar]
- 23.Zuo ML, Liu S, Chan KH, Lau KK, Chong BH, Lam KF, Chan YH, et al. The CHADS2 and CHA 2DS 2-VASc scores predict new occurrence of atrial fibrillation and ischemic stroke. J Interv Card Electrophysiol. 2013;37:47–54. doi: 10.1007/s10840-012-9776-0. [DOI] [PubMed] [Google Scholar]


