Abstract
Kidney transplant recipients receiving calcineurin inhibitor-based immunosuppression incur increased long-term risks of cancer and kidney fibrosis. Switch to mammalian target of rapamycin (mTOR) inhibitors may reduce these risks. Steroid or Cyclosporin Removal After Transplant using Everolimus (SOCRATES), a 36-month, prospective, multinational, open-label, randomized controlled trial for de novo kidney transplant recipients, assessed whether everolimus switch could enable elimination of mycophenolate plus either steroids or CNI without compromising efficacy. Patients received cyclosporin, mycophenolate and steroids for the first 14 days then everolimus with mycophenolate and CNIwithdrawal (CNI-WD); everolimus with mycophenolate and steroid withdrawal (steroid-WD); or cyclosporin, mycophenolate and steroids (control). 126 patients were randomized. The steroid WD arm was terminated prematurely because of excess discontinuations. Mean eGFR at month 12 for CNI-WD versus control was 65.1 ml/min/1.73 m2 vs. 67.1 ml/min/1.73 m2 by ITT, which met predefined noninferiority criteria (P = 0.026). The CNI-WD group experienced a higher rate of BPAR(31% vs. control 13%, P = 0.048) and showed a trend towards higher composite treatment failure (BPAR, graft loss, death, loss to follow-up). The 12 month results from SOCRATES show noninferiority in eGFR, but a significant excess of acute rejection when everolimus was commenced at week 2 to enable a progressive withdrawal of mycophenolate and cyclosporin in kidney transplant recipients.
Keywords: cyclosporin, everolimus, kidney transplantation, mammalian target of rapamycin, steroids
Introduction
A regimen containing calcineurin inhibitors, mycophenolic acid and steroids is the cornerstone of modern immunosuppressive therapy for kidney transplant recipients, yielding low rates of acute rejection and excellent short- to medium-term graft survival 1–3. However, longer-term adverse effects on graft and patient contribute to premature graft loss because of interstitial fibrosis and tubular atrophy 4 and premature death due to cardiovascular disease 5 or cancer 6.
Calcineurin inhibitors (CNI) are associated with short- and long-term toxicities including acute and chronic nephrotoxicity 7 and development or exacerbation of cardiovascular risk factors including diabetes in particular 1,8. Mycophenolic acid (MPA) is associated with bone marrow toxicity and gastrointestinal intolerance 9. Oral corticosteroids incur well-documented complications including osteoporosis, diabetes and cardiovascular disease 10.
Everolimus is a mammalian target of rapamycin inhibitor (mTORi) that is a macrocyclic lactone with immunosuppressive as well as antimalignant properties 11. mTORis, when used in combination with full-dose CNI, have been shown to exacerbate CNI nephrotoxicity 12. Trials have demonstrated the beneficial effects of CNI withdrawal for selected patients receiving mTORis in terms of improved renal function 13,14, although data to demonstrate that such improvement leads to improved graft survival are awaited. Conversion from CNI- to mTORi-based therapy for kidney transplant recipients with a past history of nonmelanoma skin cancer has recently been shown to reduce recurrence and development of new skin cancers 15,16. Definitive data on the impact of mTORi on other post-transplant malignancies are awaited.
The Steroid or Cyclosporin Removal After Transplant using Everolimus (SOCRATES) study was designed to assess whether the use of everolimus could enable the elimination of mycophenolate plus either steroids or CNI without compromising efficacy, in a bid to reduce the morbidity associated with long-term usage of the other molecules.
We present the 12 month results which assessed whether everolimus and steroids or everolimus and reduced dose CsA are able to provide noninferior efficacy and safety compared with cyclosporin (CsA), MPA and steroids, but are able to minimize long-term kidney, cardiovascular and metabolic risks.
Materials and methods
The SOCRATES study is a 36-month, prospective, multinational, open-label, randomized, controlled trial which was designed by the authors and sponsored by Novartis. The study was conducted across 11 centres, in Australia (five centres), Korea (two centres), Malaysia (one centre), New Zealand (one centre) and Taiwan (two centres).
De novo kidney transplant recipients aged 18–65 were eligible. Key exclusions were recipients of multi-organ, ABO-incompatible or T cell cross-match positive grafts, peak PRA >50% or loss of a previous allograft within 6 months of transplantation due to acute rejection.
After provision of written informed consent, patients were enrolled and randomized to a treatment on the day of transplantation. Basiliximab 20 mg (Simulect®, Novartis) was initially only given for delayed graft function, but after a protocol amendment in July 2008, it was given according to local centre practice. For the first 14 days, all patients received cyclosporin microemulsion (CsA, Neoral®, Novartis) adjusted to achieve a C2 target of 1500 ng/ml, mycophenolate sodium (MPA, Myfortic®, Novartis) 720 mg bd and corticosteroids.
From day 15 to 60, different treatment allocations were started. Subjects in the CNI withdrawal (CNI-WD) and corticosteroid withdrawal (steroid-WD) groups were commenced on everolimus (to achieve a trough concentration of 6–10 ng/ml), CsA was reduced by 50%, steroids were continued, and MPA was discontinued once the everolimus trough concentration exceeded 6 ng/ml. The control group was continued on CsA, MPA and steroids for the duration of the trial.
From day 61 to 120, the CNI-WD group had the everolimus dose increased, to achieve a trough level of 8–12 ng/ml, steroids were continued, and CsA was discontinued. The steroid-WD group continued on everolimus to achieve a trough level of 6–10 ng/ml, continued on CsA at a reduced dose of 50% and had gradual withdrawal of prednisone by 1 mg/week to be discontinued by day 120 (Fig.1).
Figure 1.
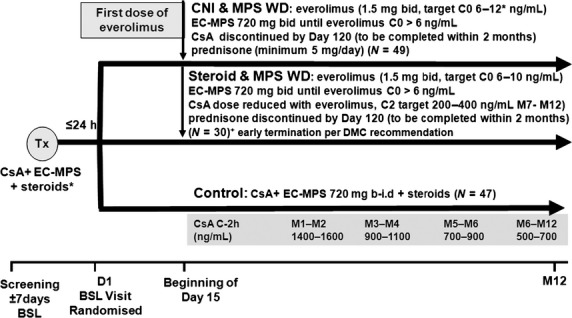
Study design. *Basiliximab induction allowed as of July 2008 by protocol amendment; EC-MPS: Myfortic; CNI + CsA: Neoral; Tx: transplant; BSL: baseline.
The study was designed and implemented in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice, with applicable local regulations and with the ethical principles laid down in the Declaration of Helsinki.
SOCRATES was registered on ClinicalTrials.gov and identified by the code NCT00371826.
Endpoints
The primary endpoint was difference in kidney function (eGFR using the Nankivell method) at 12 months after kidney transplantation. The main secondary endpoints were the incidence of biopsy-proven acute rejection (BPAR), graft survival, death and loss to follow-up and a composite of these.
Sample size
The everolimus and control groups were assumed to both have eGFR 60 ± 17 ml/min/1.73 m2 at month 12. To control for multiple comparisons, the one-sided significance level was set at 0.025. A noninferiority margin was set at 9 ml/min/1.73 m2 and measured by two independent sample t-test based on a 95% CI. A sample size of 51 patients per arm would have 75% power to show that the 12-month mean GFR value of the everolimus arm is not worse than the control arm by 9 ml/min/1.73 m2 or more. A total sample size of 177 patients (59 patients per group) was chosen to allow for a 15% dropout rate.
Randomization and blinding
Randomization was performed by Novartis drug supply management and was subjected to quality control procedures by the Novartis biostatistics quality assurance group. The investigator received a set of treatment allocation cards with sequential randomization numbers on which the treatment group information was covered by a scratch-off label. Treatment allocation cards were used to avoid bias in assignment of the patients to groups in the specified 1:1:1 ratio. Following enrolment, the investigator removed the scratch-off label from the numbered treatment allocation card to reveal the allocated treatment group.
The investigators and patients were not blinded in this open-label study.
Statistical methods
Difference in graft function (eGFR, Nankivell method) was used as the primary efficacy criterion to demonstrate that either of the CNI-WD or steroid-WD groups were not inferior to control group by 9 ml/min/1.73 m2 or more. Type I error probability was set at 0.05, one-sided. All other efficacy and safety variables are presented by descriptive statistics and were compared between both treatments using appropriate tests for unpaired or paired (laboratory data and vital signs) observations. Categorical variables were analysed with the chi-squared test or Fisher's exact test. Time to event data including rates of affected patients was assessed by Kaplan–Meier statistics and compared between the two groups with the log-rank test.
A case report form (CRF) error failed to record month 12 eGFR for patients who prematurely discontinued the study medication. This flaw was not discovered until after database lock and resulted in sites being retrospectively asked to provide the missing data. The database underwent unlock/relock to include this data. All results presented include this data and were analysed according to the prospectively planned statistical approach using intention-to-treat groups.
Results
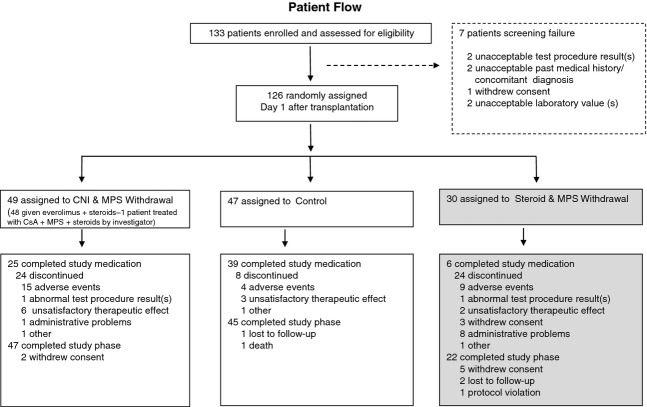
A total of 133 patients were screened for enrolment, between March 2006 and July 2010, of whom 126 were randomized and thereby provided data for this 12-month analysis (CNI-WD n = 49, steroid-WD n = 30, control n = 47). One patient was not treated as randomized, being treated with the control regimen rather than CNI-WD.
Baseline demographic characteristics are summarized in Table1. The three groups were generally comparable with respect to transplant recipient demographic and background characteristics (Table1). The majority of patients were Caucasian (50.8%), were undergoing their first transplantation and had more than two human leucocyte antigen mismatches. There was a trend towards a higher proportion of deceased donors in the CNI-WD group and more living-related donors in the control group.
Table 1.
Patient demographic summary by treatment group.
| Variable | CNI withdrawal N = 49 | Control N = 47 | Steroid withdrawal N = 30 | Total N = 126 |
|---|---|---|---|---|
| Recipient age in years (SD) | ||||
| Mean | 48.4 (10.17) | 45.8 (10.83) | 43.5 (10.66) | 46.3 (10.63) |
| Range | 24–65 | 20–64 | 23–62 | 20–65 |
| Gender, n (%) | ||||
| Male | 32 (65.3) | 34 (72.3) | 24 (80.0) | 90 (71.4) |
| Female | 17 (34.7) | 13 (27.7) | 6 (20.0) | 36 (28.6) |
| Race, n (%) | ||||
| Caucasian | 26 (53.1) | 25 (53.2) | 13 (43.3) | 64 (50.8) |
| Black | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (0.8) |
| Asian | 19 (38.8) | 19 (40.4) | 14 (46.7) | 52 (41.3) |
| Pacific Islander | 0 (0.0) | 3 (6.4) | 1 (3.3) | 4 (3.2) |
| Other | 4 (8.2) | 0 (0.0) | 1 (3.3) | 5 (4.0) |
| Body Mass Index in kg/m2 (SD) | ||||
| Mean | 25.1 (4.45) | 25.0 (3.88) | 26.2 (3.86) | 25.3 (4.11) |
| Range | 16.5–34.4 | 17.4–32.0 | 20.5–35.1 | 16.5–35.1 |
| End-stage disease leading to transplantation, n (%) | ||||
| Glomerular disease | 24 (49.0) | 17 (36.2) | 10 (33.3) | 51 (40.5) |
| Pyelonephritis | 0 (0.0) | 1 (2.1) | 0 (0.0) | 1 (0.8) |
| Polycystic disease | 5 (10.2) | 9 (19.1) | 1 (3.3) | 15 (11.9) |
| Hypertension/nephrosclerosis | 1 (2.0) | 4 (8.5) | 6 (20.0) | 11 (8.7) |
| Drug-induced toxicity | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Diabetes mellitus | 3 (6.1) | 2 (4.3) | 1 (3.3) | 6 (4.8) |
| Interstitial nephritis | 0 (0.0) | 2 (4.3) | 0 (0.0) | 2 (1.6) |
| Vasculitis | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (0.8) |
| Obstructive disorder/reflux | 2 (4.1) | 2 (4.3) | 1 (3.3) | 5 (4.0) |
| Unknown origin | 10 (20.4) | 9 (19.1) | 7 (23.3) | 26 (20.6) |
| Other | 3 (6.1) | 1 (2.1) | 3 (10.0) | 7 (5.6) |
| Number of HLA mismatches, n (%) | ||||
| None | 3 (6.1) | 6 (12.8) | 2 (6.7) | 11 (8.7) |
| One | 8 (16.3) | 5 (10.6) | 0 (0.0) | 13 (10.3) |
| Two | 9 (18.4) | 6 (12.8) | 3 (10.0) | 18 (14.3) |
| >two | 27 (55.1) | 27 (57.4) | 24 (80.0) | 78 (61.9) |
| Missing | 2 (4.1) | 3 (6.4) | 1 (3.3) | 6 (4.8) |
| Number of previous renal transplantations, n (%) | ||||
| None | 47 (95.9) | 46 (97.9) | 30 (100.0) | 123 (97.6) |
| One transplantation | 1 (2.0) | 1 (2.1) | 0 (0.0) | 2 (1.6) |
| Missing | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Cold ischaemia time in hours (SD) | ||||
| Mean | 6.2 (4.90) | 5.2 (4.60) | 7.3 (6.87) | 6.1 (5.35) |
| Range | 0.1–16.0 | 0.3–20.0 | 0.1–24.5 | 0.1–24.5 |
| Donor age in years (SD) | ||||
| Mean | 48.6 (12.60) | 40.8 (13.13) | 48.1 (13.66) | 45.5 (13.47) |
| Range | 16–70 | 9–71 | 23–71 | 9–71 |
| Donor characteristics, n (%) | ||||
| Cadaveric heart beating | 20 (40.8) | 15 (31.9) | 13 (43.3) | 48 (38.1) |
| Cadaveric nonheart beating | 2 (4.1) | 1 (2.1) | 1 (3.3) | 4 (3.2) |
| Living related | 17 (34.7) | 22 (46.8) | 12 (40.0) | 51 (40.5) |
| Living unrelated | 10 (20.4) | 9 (19.1) | 4 (13.3) | 23 (18.3) |
The steroid-WD arm was terminated early by the sponsor on the recommendation of the Data Monitoring Committee in March 2008 due to a higher premature treatment discontinuation rate between this and the other two groups which rendered continuation of this arm futile. Discontinuations were mostly due to acute rejection, unsatisfactory therapeutic effect and adverse events. The most common reason for discontinuation of study medication from the remaining groups was adverse events [CNI-WD n = 15 (30.6%), control n = 4 (8.5%)] (Fig.2).
Figure 2.
Trial profile.
Mean exposure to study medication was generally within the protocol-define target ranges. Mean ± SD everolimus concentration in the CNI-WD group was 6.6 ± 4.1 ng/ml at month 6 and 6.7 ± 2.6 ng/ml at month 12. Control group patients achieved mean ± SD cyclosporin C2 concentrations of 866 ± 307 ng/ml at month 6 and 664 ± 287 ng/ml at month 12, and received a mean ± SD daily dose of Myfortic of 1260 ± 295 mg at month 12. Use of basiliximab as induction therapy was balanced among the CNI-WD (n = 28, 57%) and control (n = 25, 53%) groups.
Primary and secondary outcomes
Renal function
The difference in mean eGFR at month 12 between CNI-WD [65.1 (SD 15.4) ml/min/1.73 m2] and control [67.1 (SD 18.2) ml/min/1.73 m2] groups was −2.4 ml/min/1.73 m2 in the ITT population (Fig.3). Difference in renal function between CNI-WD and control groups met noninferiority criteria (P = 0.026, 95% confidence interval −6.5 to 8.7). At week 2, the time at which everolimus was commenced by the CNI-WD group, eGFR was higher in the control group than CNI-WD [controls 64.7 (SD20.0) vs. CNI-WD 53.2 (SD20.1) ml/min/1.73 m2, P = 0.007] (Fig.3). When month 12 renal function was re-analysed by ancova (with week 2 eGFR results as a covariate), the statistical noninferiority was P = 0.007. Indeed, the change in mean eGFR from week 2 to month 12 was +12.8 (SD 18.8) ml/min/1.73 m2 for CNI-WD and +5.3 (SD 19.4) ml/min/1.73 m2 for control (P = 0.089) (Fig.3). Per-protocol analysis of improvement in renal function demonstrated a significant benefit in favour of the CNI-WD group of 17.5 ml/min/1.73 m2 vs. 5.7 ml/min/1.73 m2 (P = 0.03).
Figure 3.
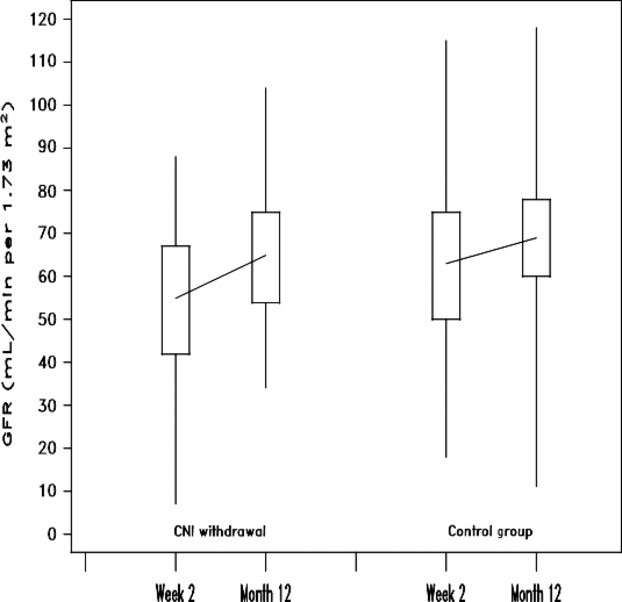
Improvement in eGFR from time of randomization (week 2) to month 12: box plot of CNI withdrawal and control groups by intention to treat, showing median (line), interquartile range (box), minimum and maximum values (whiskers). Improvement in eGFR was not different between the groups: 12.8 (SD18.8) vs. 5.3 (SD19.4) ml/min/1.72 m2 for CNI withdrawal versus control, P = 0.089.
A higher proportion of the CNI-WD group received deceased donor grafts. To exclude any confounding effect of delayed graft function on change in eGFR between week 2 and month 12, we performed a sensitivity analysis restricted to recipients of live donor grafts. Improvement from week 2 to month 12 (59.8 ± 12.8 vs. 72.6 ± 11.6, P = 0.012) was evident in the CNI-WD group, but not in controls (68.1 ± 17.9 vs. 67.3 ± 19.0, P = 0.87).
Composite treatment failure
The CNI-WD group showed a trend towards higher rates of the composite treatment failure endpoint (BPAR, graft loss, death or loss to follow-up) with 16 patients versus 12 patients in the control group (P = 0.100). The control group experienced one death which was due to sepsis on day 89 and two graft losses (renal vein thrombosis on day 10 and acute rejection on day 156). The CNI-WD and steroid-WD groups had no deaths or graft losses. The majority of the efficacy failures were due to BPAR with a significant excess seen in the CNI-WD group 15 (30.6%) versus 6 (12.8%) in the control group (P = 0.048) (Table2, Fig.4). Of those patients in the CNI-WD group who suffered BPAR, four cases occurred prior to commencement of everolimus and of the remaining 11 cases, all had ceased mycophenolate and three of these patients also had subtherapeutic everolimus levels according to protocol. Seven of CNI-WD and four of control patients who experienced BPAR had not received basiliximab which represents 47% vs. 67% of the total BPAR proportions of each group or 33% and 18% of patients in each group not exposed to basiliximab, respectively.
Table 2.
Rejections, graft loss, death and loss to follow-up (ITT population).
| Time point Endpoint | CNI withdrawal N = 49 n (%) | Control N = 47 n (%) | Steroid withdrawal N = 30 n (%) | P-value* |
|---|---|---|---|---|
| Month 12 | ||||
| BPAR | 15 (30.6) | 6 (12.8) | 5 (16.7) | 0.0479 |
| Banff type IA | 7 (14.3) | 3 (6.4) | 5 (16.7) | 0.3179 |
| Banff type IB | 5 (10.2) | 4 (8.5) | 0 (0.0) | 1.0000 |
| Banff type IIA | 6 (12.2) | 0 (0.0) | 0 (0.0) | 0.0267 |
| Banff type IIB | 1 (2.0) | 1 (2.1) | 0 (0.0) | 1.0000 |
| Banff type III | 0 (0.0) | 1 (2.1) | 0 (0.0) | 0.4896 |
| Banff type unspecified | 1 (2.0) | 1 (2.1) | 0 (0.0) | 1.0000 |
| Graft loss | 0 (0.0) | 2 (4.3) | 0 (0.0) | 0.2371 |
| Death | 0 (0.0) | 1 (2.1) | 0 (0.0) | 0.4896 |
| Loss to follow-up | 1 (2.0) | 0 (0.0) | 6 (20.0) | 1.0000 |
| Treatment failure (BPAR, graft loss, death or loss to follow-up) | 16 (32.7) | 8 (17.0) | 11 (36.7) | 0.1001 |
| Treated BPAR | 13 (26.5) | 6 (12.8) | 5 (16.7) | 0.1248 |
| BPAR-treated antibodies | 0 (0.0) | 1 (2.1) | 0 (0.0) | 0.4896 |
* Comparison of CNI withdrawal and control (two-sided) Fisher's exact test.
Figure 4.
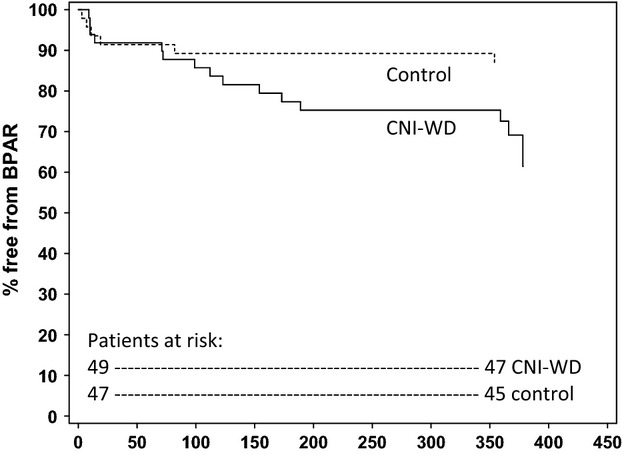
Kaplan–Meier estimate of probability of patient freedom from biopsy-proven acute rejection (BPAR). Intention to treat population, calcineurin withdrawal (CNI-WD) versus control groups.
Post hoc analysis of eGFR in patients with BPAR at month 12 did not show major differences between groups, with mean eGFR of 56.2 (SD 11.0) ml/min/1.73 m2 in CNI-WD vs. 53.0 (SD 25.9) ml/min/1.73 m2 in the control group.
Adverse events
The incidence of adverse events (AE) was similar across all groups and are summarized in Table3. Gastrointestinal disorders were the most frequently reported (CNI-WD group 45 patients (92%), control group 40 (85%), steroid-WD group 22 (73%), with diarrhoea being the most common symptom. The incidence of serious adverse events was similar between the CNI-WD (67%) and control (66%) groups. Wound healing events were similar (33% in the CNI-WD, 32% in control and 30% in the steroid-WD group). The reported incidence of skin cancer was low [two patients in CNI-WD (4%) and one (2%) control] and there were no nonskin malignancies reported for any group. More adverse events led to permanent discontinuation of study medication in the CNI-WD (n = 15, 31%) than in the control (n = 4, 9%) group (P = 0.003). Twelve of the 26 events leading to study discontinuation in the CNI-WD group and five of seven events in controls were considered as ‘not suspected’ in their relationship to the study drug.
Table 3.
Summary of adverse events (safety population).
| CNI-WD n (%) | Control n (%) | Steroid-WD n (%) | |
|---|---|---|---|
| Total adverse events | 49 (100) | 47 (100) | 30 (100) |
| Total serious adverse events | 33 (67) | 31 (66) | 16 (53) |
| Adverse events leading to study drug discontinuation | 15 (31)* | 4 (9) | 9 (30) |
| Wound complications | 8 (33) | 15 (32) | 9 (30) |
| New-onset diabetes | 8 (33) | 13 (27) | 12 (39) |
| Proteinuria | 1 (2) | 1 (2) | 0 (0) |
| Total infections | 33 (67) | 34 (72) | 18 (60) |
| CMV infection | 2 (4) | 4 (9) | 2 (7) |
| Peripheral oedema | 19 (39) | 15 (32) | 3 (10) |
| Diarrhoea | 20 (41)* | 9 (19) | 5 (17) |
| Anaemia | 18 (37) | 11 (23) | 9 (30) |
| Hypercholesterolaemia | 12 (25) | 9 (19) | 2 (7) |
| Malignancy | 2 (4)† | 1 (2)† | 0 (0) |
CNI-WD = calcineurin inhibitor withdrawal group, maintained on everolimus and prednisolone; control = control group, maintained on cyclosporin, mycophenolate and prednisolone; steroid-WD = steroid withdrawal group, maintained on everolimus and cyclosporin until group was terminated by data safety monitoring board.
P < 0.05 based on Fisher's exact test (2-sided) CNI-WD group versus control group. All other comparisons were not significant.
Skin cancer (squamous or basal cell carcinoma), with no reported cases of nonskin cancer.
Proteinuria was an uncommonly reported adverse event (one case only in CNI-WD and control groups); however, among those patients who were receiving study medication at month 12, albuminuria (ACR>3 mg/mmol) was commonly present [14 (64%) of CNI-WD group and 12 (38%) of controls] although overt albuminuria (ACR>30 mg/mmol) was present in a minority [5 (23%) of CNI-WD and 3 (9%) of controls, P = ns]. A trend towards higher rates of anaemia (37%) and erythropoietin usage (41%) was reported for the CNI-WD group as compared to controls (anaemia 23%, erythropoietin usage 19%).
Similar rates of new onset diabetes after transplant (NODAT) were reported for CNI-WD (32%) and control (27%) groups, by post hoc analysis using modified Australian National Health and Medical Research Council diabetes criteria to identify NODAT in patients who satisfied at least one of the following criteria: use of glucose lowering treatment, two fasting glucose values ≥7.0 mm or 2 random glucose values ≥11.1 mm after day 15, or diabetes reported as a treatment emergent adverse event.
Discussion
Steroid or Cyclosporin Removal After Transplant using Everolimus showed that as compared to a triple immunosuppression control regimen of cyclosporin, mycophenolate and steroids, early switch to everolimus with CNI and mycophenolate withdrawal produced noninferior eGFR at 1 year, despite incurring higher rates of BPAR and treatment discontinuation. SOCRATES also demonstrated that the use of everolimus with early steroid and mycophenolate withdrawal was associated with an extremely high rate of discontinuation, attributed to acute rejection, unsatisfactory effect or adverse reactions.
Switch from CNI to mTOR early after transplantation has, in other studies, been associated with significant improvement in kidney function as compared to ongoing CNI-based therapy 13,14. In SOCRATES, intention to treat analysis showed noninferior results for eGFR at month 12 but failed to show superiority. By chance, the control group had superior week 2 eGFR, the effective baseline eGFR as everolimus was commenced at week 2 in the CNI-WD group. The CNI-WD group experienced a greater improvement in eGFR from week 2 to month 12 as compared to controls, suggesting potential benefit; however, whether this was due to a positive effect of everolimus or simply release from CNI-induced vasospasm was not answered by this study. As with other switch studies 14–16, SOCRATES was characterized by a high rate of withdrawal from the CNI-WD group and those who withdrew were returned to CNI-based therapy which may have also mitigated against improvement in eGFR. Per-protocol analysis, undertaken as a sensitivity analysis, demonstrated a significantly higher mean improvement in the CNI-WD group compared with controls, suggesting this may have been the case.
Studies of early switch from CNI- to mTOR-based therapy have frequently shown an excess of acute rejection or a trend towards more acute rejection following switch 14–16, which was also apparent in SOCRATES. BPAR was significantly higher in the CNI-WD group; however, those experiencing BPAR included three patients who experienced BPAR prior to commencing everolimus and another 11 patients, all of whom had ceased mycophenolate and four had subtherapeutic everolimus levels according to the protocol. As has been the case in other similar studies 14, retaining, rather than ceasing, mycophenolate may have substantially reduced the incidence of acute rejection in this group. Acute rejection was more frequent among those who did not receive basiliximab induction, again suggesting insufficient overall immunosuppression in the CNI-WD group.
The overall safety profile was similar to that seen in previous mTORi studies 13–21. There were no deaths among the everolimus-treated patients and only two cases of nonmelanoma skin cancer with no other malignancies reported. Adverse events leading to drug discontinuations were more frequent in the everolimus arms. Wound healing events have been an issue with some studies of de novo mTOR usage; however, SOCRATES showed no appreciable difference between everolimus and control groups, suggesting that everolimus can be safely introduced at the 2 week point without compromising wound integrity. Anaemia and erythropoietin usage were more common in the CNI-WD group, despite early discontinuation of MPA. Hyperlipidaemia was only assessed by investigator reporting, and no difference was seen between the CNI-WD and control groups. Proteinuria was uncommonly reported by investigators, and among those remaining on therapy at month 12, the prevalence of albuminuria was not different to controls. Consistent with other everolimus trials, the incidence of cytomegalovirus (CMV) infection was low 20,21.
Whether long-term improvements in patient and graft survival can be achieved by withdrawing CNI's remains to be established. The ideal way to use everolimus to eliminate or minimize the dose of CNI's in an attempt to minimize nephrotoxicity has yet to be found. The A2309 study showed that everolimus with reduced dose cyclosporin micro-emulsion had similar rates of BPAR and renal function to the CNI and MPA control group at 12 months 21, suggesting that CNI minimization exposes patients to additional mTORi side effects for equal efficacy. De novo use of everolimus with basiliximab, steroids and low or very low tacrolimus exposure has been shown to provide excellent kidney function and acceptable rates of rejection and adverse events 22,23 and is a viable alternative to switch strategies. Early CNI elimination studies have had mixed results in improving renal function with the trade-off being higher rates of BPAR, study discontinuations and other adverse events 13,17,19,24. The ORION study 24 of sirolimus with week 13 tacrolimus elimination or sirolimus with mycophenolate versus a CNI-based triple therapy control, failed to show any benefit to assessed major outcomes including BPAR, patient and graft survival. Similarly in SOCRATES, it is possible that the failure to show improvements is related to the increased rate of early BPAR in the mTORi groups, which resulted in increased discontinuations and the reintroduction of CNI and hence risk of CNI nephrotoxicity.
Perhaps the most successful mTORi regimen is that used in the ZEUS trial 14. In ZEUS, patients at increased risk of acute rejection or adverse events were excluded from switch from cyclosporin to everolimus at 4.5 months. ZEUS showed an improvement in mean eGFR of 9 ml/min/1.73 m2 from the time of switch to month 12. The control group in contrast saw a deterioration of approximately 1 ml/min/1.73 m2 over the same time period. There are a number of important differences between the SOCRATES and ZEUS studies. ZEUS enrolled lower immunological risk patients, excluded those with early rejection or other risk factors for poor outcomes after the switch, switched to everolimus-based immunosuppression at 4.5 months after transplantation and continued switch patients on mycophenolate.
Steroid or Cyclosporin Removal After Transplant using Everolimus demonstrates several issues to take forward. The trial protocol was excessively complicated for patients and investigators alike: a simplified protocol involving an abrupt switch from CNI to mTORi 25 will incur less risk of complex pharmacokinetic interactions 26 and likely better adherence and tolerance. Secondly, switch from CNI to mTOR may be achieved with a lower risk of acute rejection at a time point later than week 2, as has been used in other trials 14–17,25. Exclusion of those with prior acute rejection and those with evidence of subclinical rejection or recurrent glomerular disease on protocol biopsy performed prior to switch 25 may also be important to maximize the chances of a successful switch. Finally, use of basiliximab induction and retention of mycophenolate and steroids following switch may also be important in minimizing potential for acute rejection.
Steroid or Cyclosporin Removal After Transplant using Everolimus was ultimately limited in power as only 133 of the 177 planned patients were enrolled. This may have contributed to unequal week 2 renal function. Adverse event reporting was left to individual investigators and results may have been skewed dependent on their familiarity with everolimus, in that there were relatively low reports of hyperlipidaemia and proteinuria, known everolimus side effects. The open-label nature of the trial may have contributed to the higher discontinuation rate in the everolimus arms as investigators may have been more likely to blame and discontinue patients in the active study drug arm. Finally, use of eGFR, rather than formally measured GFR, may have detracted from the results, particularly in light of the relatively high number of Asian patients in the study for whom eGFR equations are less well validated 27.
In conclusion, the 12-month results from the SOCRATES show noninferiority in eGFR, but a significant excess of acute rejection when everolimus was commenced at week 2 to enable ultimate conversion to a double immunosuppressive regimen, by a progressive withdrawal of mycophenolate then cyclosporin in kidney transplant recipients. Whether benefits of switch become apparent at 3 years after transplantation will be examined.
Authorship
SC: study design, conduct, patient management, data analysis and manuscript preparation. JE, JK, HP, SL and GR: study design, patient management and manuscript review. PCL: patient management and manuscript review. CW: data analysis and manuscript preparation. NK: study design and manuscript review.
Funding
The study was sponsored by Novartis Pharmaceuticals.
Acknowledgments
The authors would like to acknowledge that this study would not have been possible without the collaboration and commitment of all the local investigators and their staff; Yuseun Kim (KOR), Chang Kwon Oh (KOR), Sheng Hsien Chu (TWN), Po Chang Lee (TWN), Soo Kun Lim (MAL), Helen Pilmore (NZ), Brian Hutchison (AUS), Adrian Hibberd (AUS), Steve Chadban (AUS), John Kanellis (AUS) and Graeme Russ (AUS). The authors would like to thank Wilhelm Sauermann from DATAMAP for statistical analysis. Finally, the authors would like to express their gratitude to the following Novartis employees who assisted with the trial management; Peter Bernhardt, Ana Maria Marti, Gaohong Dong and Steffen Witte.
References
- 1.Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 2.Clayton P, Campbell S, Hurst K, et al. ANZDATA 2011 Annual report. Chapter 8 Transplantation. Available via: http://www.anzdata.org.au/v1/report_2011.html.
- 3.Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 4.Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft Nephropathy. N Engl J Med. 2003;349:2326. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 5.Pilmore H, Dent H, Chang S, et al. Reduction in cardiovascular death after kidney transplantation. Transplantation. 2010;89:851. doi: 10.1097/TP.0b013e3181caeead. [DOI] [PubMed] [Google Scholar]
- 6.Vajdic CM, McDonald SP, McCredie MRE, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 7.Burdmann E, Andoh T, Yu L, Bennett W. Cyclosporine nephrotoxicity. Semin Nephrol. 2003;23:465. doi: 10.1016/s0270-9295(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 8.Wong W, Tolkoff-Rubin N, Delmonico F, et al. Analysis of the cardiovascular risk profile instable kidney transplant recipients after 50% cyclosporine reduction. Clinical Transplant. 2004;18:341. doi: 10.1111/j.1399-0012.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 9.A blinded, randomised clinical trial of Mycophenolate Mofetil for the prevention of acute rejection in cadaveric renal transplantation (Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group) Transplantation. 1996;61:1029. [PubMed] [Google Scholar]
- 10.Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89:1. doi: 10.1097/TP.0b013e3181c518cc. [DOI] [PubMed] [Google Scholar]
- 11.Schuler W, Sedrani R, Cottens S, et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 12.Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet. 2000;356:194. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- 13.Baboolal K. A phase III prospective, randomized study to evaluate concentration-controlled sirolimus (rapamune) with cyclosporine dose minimization or elimination at six months in de novo renal allograft recipients. Transplantation. 2003;75:1404. doi: 10.1097/01.TP.0000063703.32564.3B. [DOI] [PubMed] [Google Scholar]
- 14.Budde K, Becker T, Arns W, et al. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet. 2011;377:837. doi: 10.1016/S0140-6736(10)62318-5. [DOI] [PubMed] [Google Scholar]
- 15.Campbell SB, Walker R, Tai SS, Jiang Q, Russ GR. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant. 2012;12:1146. doi: 10.1111/j.1600-6143.2012.04004.x. [DOI] [PubMed] [Google Scholar]
- 16.Euvrard S, Morelon E, Rostaing L, et al. Sirolimus and secondary skin cancer prevention in kidney transplantation. New Eng J Med. 2012;367:329. doi: 10.1056/NEJMoa1204166. [DOI] [PubMed] [Google Scholar]
- 17.Kreis H, Oberbauer R, Campistol JM, et al. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004;15:809. doi: 10.1097/01.asn.0000113248.59077.76. [DOI] [PubMed] [Google Scholar]
- 18.Vitko S, Tedesco H, Eris J, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6-month safety and efficacy results of two randomized studies. Am J Transplant. 2004;4:626. doi: 10.1111/j.1600-6143.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 19.Lebranchu Y, Thierry A, Toupance O, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant. 2009;9:1115. doi: 10.1111/j.1600-6143.2009.02615.x. [DOI] [PubMed] [Google Scholar]
- 20.Vitko S, Margreiter R, Weimar W, et al. Everolimus (Certican) 12-month safety and efficacy versus mycophenolate mofetil in de novo renal transplant recipients. Transplantation. 2004;78:1532. doi: 10.1097/01.tp.0000141094.34903.54. [DOI] [PubMed] [Google Scholar]
- 21.Tedesco H, Cribrick D, Johnston E, et al. Everolimus plus reduced-exposure CsA versus Mycophenolic acid plus standard-exposure CsA in renal- transplant recipients. Am J Transplant. 2010;10:1401. doi: 10.1111/j.1600-6143.2010.03129.x. [DOI] [PubMed] [Google Scholar]
- 22.Chan L, Greenstein S, Hardy MA, et al. Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness. Transplantation. 2008;85:821. doi: 10.1097/TP.0b013e318166927b. [DOI] [PubMed] [Google Scholar]
- 23.Langer RM, Hene R, Vitko S, et al. Everolimus plus early tacrolimus minimization: a phase III, randomized, open-label, multicentre trial in renal transplantation. Transplant Int. 2012;25:592. doi: 10.1111/j.1432-2277.2012.01465.x. [DOI] [PubMed] [Google Scholar]
- 24.Flechner SM, Glyda M, Cockfield S, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant. 2011;11:1633. doi: 10.1111/j.1600-6143.2011.03573.x. [DOI] [PubMed] [Google Scholar]
- 25.Holdaas H, Bentdal O, Pfeffer P, et al. Early, abrupt conversion of de novo renal transplant patients from cyclosporine to everolimus: results of a pilot study. Clin Transplant. 2008;22:366. doi: 10.1111/j.1399-0012.2008.00795.x. [DOI] [PubMed] [Google Scholar]
- 26.Kovarik JM, Kalbag J, Figueiredo J, et al. Differential influence of two cyclosporine formulations on everolimus pharmacokinetics: a clinically relevant pharmacokinetic interaction. J Clin Pharmacol. 2002;42:95. doi: 10.1177/0091270002042001011. [DOI] [PubMed] [Google Scholar]
- 27.Ho E, Teo BW. Assessing kidney function in Asia. Singapore Med J. 2010;51:888. [PubMed] [Google Scholar]