Abstract
The mu-opioid system has a key role in hedonic and motivational processes critical to substance addiction. However, existing mu-opioid antagonists have had limited success as anti-addiction treatments. GSK1521498 is a selective and potent mu-opioid antagonist being developed for the treatment of overeating and substance addictions. In this study, 28 healthy participants were administered single doses of GSK1521498 20 mg, ethanol 0.5 g/kg body weight, or both in combination, in a double blind placebo controlled four-way crossover design. The primary objective was to determine the risk of significant adverse pharmacodynamic and pharmacokinetic (PK) interactions. The effects of GSK1521498 on hedonic and consummatory responses to alcohol and the attentional processing of alcohol-related stimuli, and their modulation by the OPRM1 A118G polymorphism were also explored. GSK1521498 20 mg was well tolerated alone and in combination with ethanol. There were mild transient effects of GSK1521498 on alertness and mood that were greater when it was combined with ethanol. These effects were not of clinical significance. There were no effects of GSK1521498 on reaction time, hedonic or consummatory responses. These findings provide encouraging safety and PK data to support continued development of GSK1521498 for the treatment of alcohol addiction.
Keywords: mu-opioid receptor, pharmacokinetics, pharmacodynamics, safety, OPRM1, pharmacogenetics, alcohol, addiction
The development of drug addiction is characterized by the transition from hedonic drug taking under voluntary control, to compulsive and habitual consumption despite the negative consequences, with an increased motivation to consume and an inability to control consumption,1,2 This transition is marked by critical neuroadaptations in key nodes of the dopamine reward circuitry such as the nucleus accumbens.2,3 The mu-opioid receptor (MOR) system is a key modulator of this circuitry and has important inhibitory effects on accumbens dopamine via GABAergic projections from the ventral tegmental area (VTA).4,5 Alcohol produces its effect by enhancing dopamine release in the nucleus accumbens by disinhibiting the VTA.6,7 Its effects are modulated by the OPRM1 A118G polymorphism of the MOR8,9 and carriage of the G allele has been associated with the risk of addiction in some reports.10,11 In alcohol dependence, the opioid system becomes dysregulated and MOR levels increase. This change correlates with the severity of alcohol craving and persists during abstinence.12,13 Given this critical role of MOR, opioid antagonism is an important therapeutic strategy in alcoholism. The non-selective MOR antagonist naltrexone has been shown to reduce drinking in social and dependent drinkers with some evidence for greater efficacy in G carriers.14–16 However, naltrexone has had modest clinical success17–19 and there is a clear need for more effective treatments.
GSK1521498 (Figure 1) is a MOR antagonist being developed for the treatment of overeating in obesity, and substance addiction. Its binding affinity is approximately 14–20-fold greater for the MOR (than for κ and δ subtypes), compared to 4–10-fold selectivity reported for naltrexone.20,21 In rodent models of cocaine and heroin addiction, GSK1521498 strikingly reduced drug seeking under conditions of abstinence with demonstrable superiority over naltrexone.22 In healthy human volunteers, it has been shown to be generally well tolerated up to 100 mg as a single dose23 and up to 10 mg for 10 days.24 In a 28-day proof of concept study in obese binge eaters, GSK1521498 5 mg/day was well tolerated with significant effects on hedonic and consummatory behavior25,26 and attentional processing of food stimuli.27 An exploratory post hoc pharmacogenetic (PGx) analysis suggested that weight loss may be mediated by the OPRM1 A118G polymorphism, with G-carriers demonstrating increased weight loss compared to AA homozygotes.25
Figure 1.
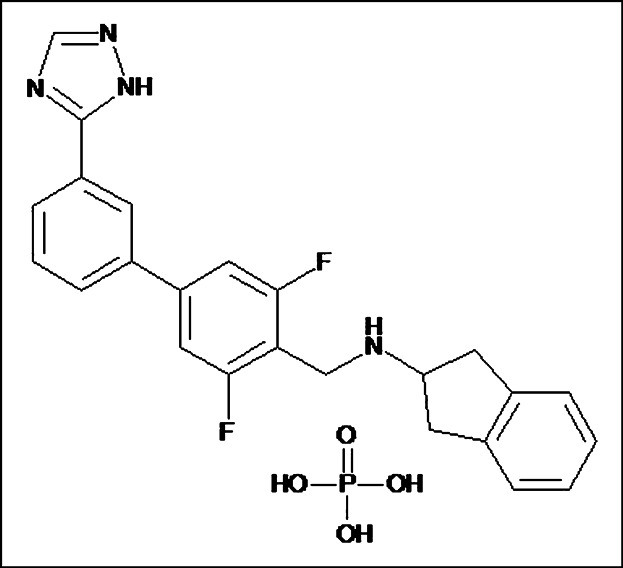
The chemical structure of GSK1521498. GSK1521498 (N-{[3,5-difluoro-3′-(1H-1,2,4-triazol-3-yl)-4-biphenylyl]methyl}-2,3-dihydro-1H-inden-2-amine phosphate (1:1)), GlaxoSmithKline, Research Triangle Park, NC.23
These findings strongly suggest that GSK1521498 may be a useful treatment for alcohol and other addictions. A first step to investigating this was to assess the possibility of any adverse interactions between alcohol and GSK1521498. To examine this we carried out a single-dose double-blind placebo controlled four-way crossover study in healthy participants with GSK1521498 20 mg, ethanol (0.5 g/kg body weight) and both agents in combination. The primary objective was to assess the risk of significant adverse pharmacokinetic (PK) and pharmacodynamic (PD) interactions between alcohol and GSK1521498 and the safety and tolerability of GSK1521498 in combination with alcohol. Additional exploratory objectives were to examine GSK1521498's effects on hedonic and consummatory aspects of alcohol consumption and any modulation of these by the OPRM1 A118G polymorphism.
Methods
The study was conducted in two parts. In part 1, a pilot assessment of the potential for interactions between alcohol and GSK1521498 was carried out and dosing and sampling times for both agents were optimized. Part 2 was a double blind crossover design to investigate the potential for PK and PD interactions, particularly sedative effects, between GSK1521498 and ethanol. In addition the PK, safety and tolerability of GSK1521498 20 mg separately and in combination with ethanol were investigated. In exploratory analyses, the effects of GSK1521498 on hedonic and consummatory aspects of alcohol consumption and on attentional and perceptual bias to alcohol-related stimuli were examined.
Participants
Twenty-eight healthy participants aged 21–55 years and within 20% of normal weight for their height and body build were recruited into the study. All subjects had to have a history of regular alcohol consumption, defined as an average weekly intake of up to 14 drinks/week for men and 7 drinks/week for women, within the previous 6 months. Participants were excluded if they had a current or chronic history of liver disease, neurological disorders, previous or current psychiatric history, a past history of DSM-IV alcohol dependence or abuse, or if they were trying to quit alcohol. A total of 28 subjects participated in parts 1 (N = 4) and 2 (N = 28). The four participants from part 1 also participated in part 2. The study was approved by the Integreview Ethical Review Board, Texas. It was sponsored by GlaxoSmithKline and conducted at the PPD Phase 1 Clinic in Texas, United States (ClinicalTrials.gov identifier: NCT01366573). All participants gave written informed consent for the study.
Design
In Part 1 all subjects received GSK1521498 20 mg followed 1 hour later by 0.5 g/kg ethanol mixed with orange juice to total volume of 200 mL, under single blind conditions. Part 2 used a randomized, double blind, four-period, crossover design (see Figure 2). The study was double blind only with respect to GSK1521498 as it was not possible to completely blind subjects to the taste and mouthfeel of alcohol. Alcohol was administered in a beverage containing 0.5 g/kg ethanol mixed with orange juice to a total volume of 200 mL. In the non-alcohol condition, a plain orange juice beverage was provided, matched for color and volume with the alcoholic beverage. Subjects were randomized to receive each of the following treatments: (A) oral placebo matching GSK1521498 followed 4 hours later by orange juice; (B) oral placebo followed 4 hours later by alcoholic beverage; (C) oral GSK1521498 20 mg followed 4 hours later by orange juice; (D) oral GSK1521498 20 mg followed 4 hours later by alcoholic beverage. All subjects were scheduled to receive all four treatments with a wash out period of at least 10 days between treatment periods.
Figure 2.
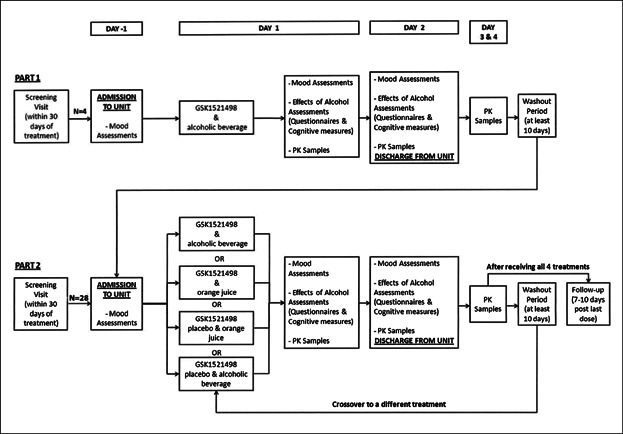
Study design schematic. The upper panel illustrates the Part 1 pilot assessment for optimization of dose administration and sampling for pharmacokinetic assessments. The bottom panel illustrates the double-blind crossover Part 2 study.
Procedures
All subjects attended a medical screening visit within a 30-day period prior to the first dosing session. In part 1, serial PK samples were taken following the administration of GSK1521498 20 mg and ethanol, to determine the time to maximum concentration (Tmax) for both agents. From these data, it was determined that to achieve near simultaneous peak levels of GSK1521498 and alcohol, alcohol had to be dosed 4 hours after GSK1521498 (see Figure S1).
In part 2, for each study visit, subjects were admitted to the clinical research unit on the evening of day −1. They were fasted from midnight until approximately 6:00 a.m. on day 1 when they received breakfast. At approximately 9:00 a.m. they received GSK1521498 or placebo and 4 hours later received the alcoholic beverage or orange juice. A set of assessments was performed over the subsequent 24 hours (described below, see Supplementary Methods for details of mood, cognitive and PD measures). Participants remained in the unit throughout and were discharged following satisfactory medical review on day 2. All subjects returned to the unit for outpatient visits at 48 and 72 hours post dosing and for a final follow-up visit 7–10 days after the last treatment session. Participants were required to refrain from caffeine and alcohol from 24 hours prior to dosing until the completion of the 72-hour visit. They were not permitted to use tobacco during the inpatient stay and were advised to limit alcohol consumption between visits to no more than 2 units/day.
Safety and Tolerability
Adverse events and serious adverse events
Adverse event (AE) and serious adverse event (SAE) data were recorded from the point of consent and until the final follow-up visit.
Vital signs and ECG
Systolic and diastolic blood pressure, pulse rate and single 12-lead ECGs were recorded prior to dosing on each study visit and then repeated at pre-specified time points over the 24 hours following dosing.
Clinical laboratory assessments
Clinical chemistry, hematology, and urinalysis were performed at screening and on days −1, day 2, and the 72-hour follow up visit of each treatment period.
Assessment of mood and alertness
Subjective changes in mood and alertness were measured using the Bond and Lader Visual Analogue Mood Scale (VAS),28 the Profile of Mood States-Brief questionnaire (POMS-B),29 the Hospital Anxiety and Depression Scale (HADS),30 Beck Depression Inventory (BDI-II),31 Beck Anxiety Inventory (BAI),32 and the Columbia Suicide Severity Rating Scale.33 The VAS consists of 16 bipolar scales, anchored at each end of a 100-mm line. Participants placed a mark on each line that best described their current state. The VAS measures three-dimensions: alertness (nine items), contentedness (five items), and calmness (two items). The POMS-B has six mood dimensions: anger, vigor, anxiety, fatigue, depression, and confusion. VAS and POMS were assessed on day −1 and on day 1 pre-dose then 4, 7, and 24 hours post GSK1521498/placebo dosing, and at the final follow-up visit. The HADS and BAI were assessed on day −1 and at 24 hours post dosing. BDI-II and CSRRS were assessed at the screening visit, 24 hours post dose on day 1 and at the final follow-up visit.
Neurological assessments
Assessments of balance, gait, coordination, eye movements, and speech were performed. The Purdue Pegboard Test, a test of manual dexterity, was included as an assessment of coordination. These assessments were performed pre-dose and 0.5, 4.5, 6, 8, and 24 hours after dosing.
Cognitive measures
The Cognitive Drug Research cognitive test battery (United BioSource Corporation, Wayne, PA) was used to measure possible effects on psychomotor processing speed, sustained attentional control and alertness as previously described.24 The Digit Vigilance (DV), Simple Reaction Time (SRT), and Choice Reaction Time (CRT) tests were included from the battery. Scores from the three tests were combined to produce a measure of attention called “Power of Attention (PoA)”, which has been shown to be sensitive to drug-induced sedation.34 Subjects completed two training sessions prior to their first treatment period. On each visit, the training was repeated on day −1 and PoA was assessed on day 1 pre-dose and 5, 7, 9, and 24 hours post GSK1521498/placebo dosing.
Secondary Pharmacodynamic Assessments
The following PD assessments were performed at each visit
Alcohol Urge Questionnaire (AUQ)35
This self-report scale provides an index of acute craving and was administered 30 minutes prior to the administration of ethanol.
Hedonic Preference Scale (HPS)
Subjects rated how much they liked the beverage at 5 and 10 minutes after the start of the drinking period.
Rate of Consumption
The rate of drinking was measured by weighing the glass and test beverage at baseline and 5 and 10 minutes after start of drinking period.
Biphasic Alcohol Effects Scale (BAES)36
This self-report scale measures positive and negative feelings commonly experienced after alcohol.
Drug (Alcohol) Visual Analogue Scale (DVAS)
The DVAS measures current drug effects in terms of whether subjects feel drunk/do not feel drunk, want more alcohol/do not want more alcohol and like/dislike how alcohol makes them feel. Both the BAES and DVAS were administered approximately an hour after the drink had been consumed.
Visual Probe Task (VPT)37
This task measures attentional bias to alcohol-related stimuli which is measured in terms of speeded reaction times to probes that replace alcohol cues compared to non-alcohol cues. The cues are presented at 500 and 2,000 milliseconds stimulus durations and the attention bias score was averaged across both durations for the statistical analysis.38
Perceptual Processing Task (PPT)
This task measures perceptual processing of alcohol-related stimuli in terms of increased increase accuracy for alcohol-related cues relative to non-alcohol-related cues. It has the advantage of having accuracy as the primary measure, not reaction time, which can be generally slowed by alcohol.
The VPT and the PPT were performed 1.5 and 1.75 hours after the ethanol administration, respectively.
Pharmacogenetic Analyses
An exploratory post hoc PGx analysis was carried out to examine the effect of the OPRM1 A118G polymorphism on the PD effects of GSK1521498. A single blood sample was collected for genetic analysis from 13 participants who consented to take part in this aspect of the study. The OPRM1 A118G polymorphism (rs1799971) was genotyped in DNA extracted from whole blood in these subjects (see Supplementary Methods).
The following endpoints were examined by genotype: DVAS, BAES (stimulant items and sedative items), PoA, VAS (alertness), and POMS-B (fatigue/inertia and vigor/activity).
Pharmacokinetics
Blood samples for the PK analyses were collected pre-dose and then 0.5, 1, 2, 4, 6, 8, 12, 24, 48, and 72 hours post dose for GSK1521498, and pre-dose, 4.5, 5, 5.5, 6, 6.5, 7, 8, 10, 12, and 14 hours post GSK1521498 dose for ethanol. Samples were collected (∼2 mL in ethylenediaminetetraacetic acid tubes for GSK1521498 analysis, ∼4 mL in sodium fluoride/potassium oxalate tubes for ethanol analysis) and immediately cooled to 2–4°C. Samples were centrifuged in a refrigerated centrifuge (∼4°C) at 1,500g or 3,000 rpm for 10 minutes. The resultant plasma was transferred to appropriately labeled 1.8 mL (GSK1521498) or 3.6 mL (ethanol) polypropylene tubes and stored at approximately −20°C (or colder) until transferred in the frozen state to Aptuit Laboratories, Verona, Italy (GSK1521498) or PPD, Richmond VA (ethanol). All study samples were received in acceptable condition.
Plasma ethanol analysis was performed under the management of Worldwide Bioanalysis, Drug Metabolism and PKs, GlaxoSmithKline. Plasma ethanol concentrations were determined by PPD, Inc. (Richmond, Virginia) using a validated method (PPD Method GC 89 Version 1.011,2,3). Ethanol and its internal standard, n-propanol, were extracted from 100 µL human plasma and analyzed via heated headspace gas chromatography with flame ionization detector. The assay was validated over the ethanol concentration range of 20.0–1000 µg/mL in human potassium oxalate/sodium fluoride plasma. Quality controls for run acceptance were prepared and analyzed with each batch of samples against separately prepared calibration standards to assess the day-to-day performance of the assay. For the analysis to be acceptable, no more than one-third of the quality control results were to deviate from the nominal concentration by more than 15%, with at least one quality control result acceptable at each concentration.
Plasma GSK1521498 concentrations were determined by Aptuit. GSK1521498 was extracted from human plasma by protein precipitation using acetonitrile containing [13C6]-GSK1521498 as an internal standard. Extracts were analyzed by HPLC-MS/MS using a TurboIonspray™ interface with positive ion multiple reaction monitoring. This method was validated according to departmental standard operating procedures (SOPs) over the range 0.1–100 ng/mL and the lower limit of quantification (LLQ) was 0.1 ng/mL using a 50-L aliquot of human plasma. Calibration data were deemed acceptable if the back-calculated concentration did not deviate from the actual by more than 15% (20% at LLQ) and if no more than 25% of the calibration standards were rejected or lost for any other reason. A calibration standard was omitted from the regression if the back-calculated concentration deviated from actual by more than 15% (20% at LLQ). Individual QC results were deemed acceptable if the calculated concentration deviated by no more than 15% from the actual concentration. The analytical run was approved if no more than one-third of the QC results exceeded the acceptable limit and at least 50% of the results at each concentration were within the acceptable limit.
PK parameters were determined from the plasma concentration-time data for GSK1521498 and ethanol. PK analysis of plasma GSK1521498 and ethanol concentration-time data were conducted using non-compartmental Model 200 (for extravascular administration) in WinNonlin version 5.2 (Pharsight Corporation, Mountain View, CA). Actual elapsed time from dosing was used to estimate all individual plasma PK parameters for evaluable subjects. The following PK parameters were estimated:
The maximum observed plasma concentration (Cmax), the first time to reach Cmax (Tmax), and absorption lag time (tlag) were the actual observed values.
The terminal plasma elimination rate-constant (λz) was estimated from log-linear regression analysis of the terminal phase of the plasma concentration-time profile. The number of points included in the terminal phase were selected by WinNonlin, and then confirmed and changed, if necessary, by visual inspection of semi-log plots of the plasma concentration-time profiles. At least three data points in the terminal elimination phase were used for each participant for the estimation of λz. The associated apparent terminal elimination half-life (t½) was calculated as t½ = ln2/λz.
The area under the plasma concentration-time curve from time 0 to the last quantifiable time point (AUC(0–last)), from time 0 to 12 h (AUC(0–12)), and from time 0 to 24 h (AUC(0–24)) were calculated by a combination of linear (for increasing concentrations) and logarithmic (for decreasing concentrations) trapezoidal methods. AUC(0–12) and AUC(0–24) were calculated only for GSK1521498. AUC from time 0 extrapolated to infinity (AUC(0–∞) was calculated as AUC(0–∞) = AUC(0–last) + Clast/λz, where Clast is the last observed quantifiable concentration in the terminal elimination phase.
Statistical Analyses
Sample size
A target sample size of 20 evaluable subjects was set based on feasibility to address the objectives of the study. For each PK endpoint, if the within-subject coefficient of variation (CVw) was no larger than 0.24 and the true ratio of test and reference means was 1 then with this sample size the study would have an 80% probability of resulting in a 90% CI entirely contained within the conventional limits for equivalence (0.8–1.25). Estimates of CVw from a previous study were 0.20 for AUC(0–∞) and 0.28 for Cmax for GSK1521498.23 For alcohol the estimates were 0.17 and 0.1839 and 0.33 and 0.12.40 For the PoA based on a within-subject-standard deviation of 41 milliseconds (95% CI = 33, 56)24 the study would then have 90% power to detect a pair-wise difference of 45 milliseconds (with 5% type-I error). Mean values were typically around 1 second, so this was a difference of the order 5%. Alternatively, assuming no difference in PoA, there was an 80% probability that the CI for the difference would lie between ±56 milliseconds (i.e., ruling out differences of a greater magnitude than about 6%).
Safety and tolerability
Respiratory rate, temperature, orthostatic blood pressure measurements, systolic and diastolic blood pressure, and heart rate were summarized for each time point and treatment. A categorical summary of the ECG measures was created to determine the number and percentage of subjects per treatment who had a maximum increase from baseline QTc on day 1 (0–24 h) by pre-specified categories. Neurological examination measures were summarized as absolute and relative frequencies of assessments considered abnormal. Clinical laboratory parameters at each time point were compared to baseline measures for all participants.
Mood and alertness and PD endpoints
All statistical analyses were carried out only on the part 2 study data. The PoA score, VAS, POMS-B, Purdue Pegboard, HPS, and rate of beverage consumption were analyzed using a repeated measures analyses of variance (ANOVA) with treatment period, time, treatment and time × treatment as fixed effects, subject as a random effect and time as a repeated effect. For PoA, VAS, and POMS-B, subject and period baseline were included as continuous covariates with a period baseline × time interaction. The AUQ, BAES, DVAS, VPT, and PPT were analyzed using an ANOVA with period and treatment as fixed effects and subject as a random effect. All analyses were performed using PROC MIXED from SAS. Least squares means and 95% CI were estimated for each treatment. Pair-wise treatment differences to evaluate the effects of GSK1521498 and ethanol independently, in the presence of each other, and in combination were also estimated with 95% CI and associated P-value. Interaction of GSK1521498 and ethanol was evaluated as [μ GSK1521498 + ethanol) − μ (ethanol)] − [μ (GSK1521498) − μ (placebo)] and estimated treatment differences and 95% CI were calculated. No adjustment was made for multiple testing because the focus of the study was estimation, and it was not considered appropriate to control false-positive findings on what are primarily safety assessments.
Pharmacogenetic analyses
The influence of OPRM1 A118G on the clinical endpoints evaluated was performed using a non-parametric Wilcoxon's exact test. Box and whisker plots were generated using information from all White subjects with genotype and phenotype data available (n = 9). Analysis was undertaken by genotype within each treatment group.
Pharmacokinetic Analyses
For the assessment of the PK interactions between ethanol and GSK1521498, mixed effects ANOVA were performed on log-transformed AUC(0–∞), Cmax, AUC(0–last), and t½, with subject as a random effect and treatment and period as fixed effects. GSK1521498 plus ethanol was considered the test treatment. For the first analysis assessing the interaction of ethanol on GSK1521498 PK, GSK152498 alone was considered the reference treatment. For the second analysis assessing the interaction of GSK1521498 on ethanol PK, ethanol alone was considered the reference treatment. For both analyses point estimates and associated 90% CIs were constructed for the difference between the test and reference treatments. These were back-transformed to obtain point estimates and 90% CIs for the ratios of test to reference treatments and the magnitude of the interaction effects of ethanol and GSK1521498 on each other. Tmax and tlag of GSK1521498 and ethanol were analyzed separately. For these measures, the point estimates of the median for combined and independent administration, together with the difference of medians (test treatment vs. both reference treatments) and the corresponding 90% CIs from the Wilcoxon's test, were determined.
Results
Four patients were enrolled into and completed part 1. Twenty-eight subjects were randomized into part 2 and received at least one dose of study drug. The mean age was 32.2 years (SD = 8.93) and mean BMI 24.1 kg m2 (SD = 2.54). Eighteen (64%) were male and 23 (82%) were Caucasian (see Table S1). Twenty-two completed all study periods. Two subjects withdrew consent before completing the study, and four subjects were withdrawn (see below). All 28 subjects were included in the safety and tolerability analyses and 25 who had at least one PK sample were included in the PK analysis.
Safety and Tolerability
Adverse events
GSK1521498 20 mg was well tolerated when given alone and in combination with ethanol (see Tables1 and S3). The most frequently reported AEs that were judged to be possibly drug-related were nausea, dizziness, fatigue headache, and abnormalities of tandem walking. A lower incidence of AEs was reported with GSK151498 20 mg alone (22%) compared to GSK1521498 plus ethanol (30%) and placebo plus ethanol (44%). Nausea was the most frequent AE (4–25%) across treatment groups. Gastrointestinal AEs (nausea and vomiting) appeared slightly higher following GSK1521498 administration (either alone or with ethanol) than with placebo or ethanol alone. Gait and coordination disturbances were reported after ethanol; the incidence was similar when administered with placebo (4–8%) or GSK1521498 (4–9%). All AEs were mild or moderate in intensity. Co-administration did not appear to have a major effect on tolerability compared with each agent alone. No SAEs were reported during the study. Two subjects were withdrawn after the third treatment period owing to AEs. One developed headache and wheezing (but no other features of hypersensitivity) after GSK1521498. The other developed premature ventricular contractions (PVCs) prior to receiving any treatment.
Table 1.
Summary of On-Therapy Adverse Effects Occurring in Two or More Subjects, No. (%)
| Preferred term | Placebo | ETOH, 0.5 g/kg | GSK152149,8 20 mg | GSK1521498 + ETOH at 1 hour | GSK1521498 + ETOH at 4 hours |
|---|---|---|---|---|---|
| Total subjects | 26 | 25 | 23 | 4 | 23 |
| Any adverse event | 4 (15) | 11 (44) | 5 (22) | 4 (100) | 7 (30) |
| Nausea | 1 (4) | 1 (4) | 2 (9) | 1 (25) | 3 (13) |
| Dizziness | 0 | 4 (16) | 0 | 0 | 2 (9) |
| Fatigue | 1 (4) | 0 | 2 (9) | 0 | 2 (9) |
| Headache | 1 (4) | 0 | 1 (4) | 0 | 3 (13) |
| Vomiting | 1 (4) | 0 | 1 (4) | 1 (25) | 1 (4) |
| Tandem gait test abnormal | 0 | 2 (8) | 0 | 0 | 2 (9) |
| Gait disturbance | 0 | 1 (4) | 0 | 2 (50) | 0 |
| Coordination abnormal | 0 | 1 (4) | 0 | 1 (25) | 1 (4) |
| Positive Rombergism | 0 | 2 (8) | 0 | 0 | 1(4) |
| Feeling drunk | 0 | 1 (4) | 0 | 0 | 1 (4) |
| Abdominal distension | 0 | 0 | 1 (4) | 0 | 1 (4) |
| Somnolence | 0 | 0 | 0 | 1 (25) | 1 (4) |
| Disturbance in attention | 0 | 0 | 0 | 0 | 2 (9) |
| Skin irritation | 1 (4) | 0 | 1 (4) | 0 | 0 |
| Euphoric mood | 0 | 1 (4) | 1 (4) | 0 | 0 |
| Decreased appetite | 0 | 0 | 1 (4) | 0 | 1 (4) |
Vital signs and ECG
Apart from the subject who developed PVC, and two subjects who experienced orthostatic hypotension (one each in the placebo and ethanol periods), no abnormalities of clinical significance were seen.
Clinical laboratory assessments
There were no laboratory findings of clinical significance and no notable differences across the treatment periods.
Mood and alertness
The results of the various measures are presented below. It should be noted that as measures at the 4-hour time point are prior to the administration of ethanol, any effects in the ethanol containing treatment periods do not reflect a PD effect of ethanol at this time point. Due to the sensitivity of some measures, in combination with the crossover study design, some very small differences between arms (e.g., 0.9 mm for VAS) reached statistical significance.
VAS: Decreases on the VAS subscales are reflected as increases in subscale scores with changes of 20 mm being clinically significant. Compared to placebo, at the 4-hour time point in treatment periods with GSK1521498 20 mg, statistically significant decreases in alertness and contentedness were seen. At 7 hours, GSK1521498 in combination with ethanol produced further reductions in alertness and contentedness, which were higher than the effects of ethanol or GSK1521498 alone. There were no significant effects at 24 hours and no impairments on the calmness subscale (see Figure 3 and Table S4).
POMS-B: Compared to placebo, at the 4-hour time point GSK1521498 20 mg significantly impaired the total mood disturbance (mean = 3.14 points, 95% CI = 0.96, 5.32). A statistically significant decrease was seen in vigor/activity (mean = −1.40, 95% CI = −2.63, −0.17) and an increase in fatigue/inertia (mean = 1.31 95% CI = 0.45, 2.18). GSK1521498 combined with ethanol produced no additional impairment (3.20, 95% CI = 1.01, 5.38). A significant mood effect persisted at the 7-hour time point, when GSK1521498 with ethanol was compared to placebo (mean 3.93, 95% CI = 0.89, 6.97) or ethanol alone (mean 3.55, 95% CI = 0.48, 6.61). In addition, GSK1521498 and ethanol in combination produced a statistically significantly increase on the confusion/bewilderment subscale than placebo, GSK1521498 or ethanol alone. There were no significant effects at 24 hours (Table S5).
Self-report scales: One subject scored 13 on the BAI following GSK1521498 with ethanol and another 16 on the BDI following GSK1521498. Both were isolated instances and no significant treatment effects were detected for BDI-II, BAI or the HADS. No suicidal ideation was reported at any point in the study (Tables S6–S8).
Figure 3.
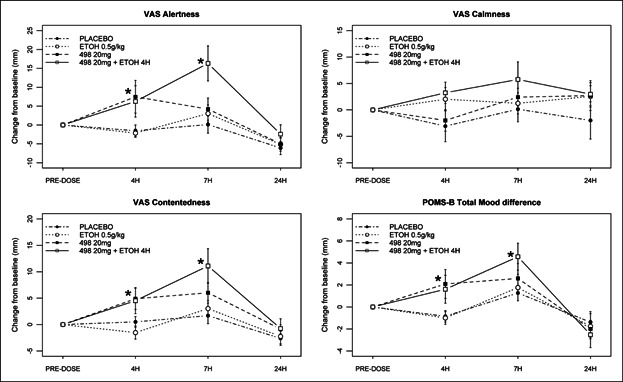
Summary of VAS and POMS-B treatment effects. Significant effects are seen in GSK1521498 treatment periods at the 4-hour time point and in the GSK1521498 and ethanol periods at 7 hours on the VAS alertness and contentedness and POMS-B total mood disturbance. No effects are seen on VAS calmness. Ethanol was administered after the 4-hour time point, so there is no PD effect of ethanol at this time point (*P < .05).
Neurological assessments
Abnormalities of gait, balance, coordination, and speech were reported following regimens that included ethanol (see adverse effects). On the Purdue pegboard there were no differences in baseline performance. Ethanol impaired performance but only 30 minutes after its administration (Table S9). Ethanol produced a mean impairment of −1.82 (95% CI = −4.27, 0.63) both alone and with GSK1521498 but this appeared to be driven by ethanol as the combination showed an impairment compared to GSK1521498 alone (−2.64, 95% CI = −5.18, −0.10).
Cognitive measures
There were no pre-dose differences among treatments on any of these measures (see Figure 4 and Tables S10–S13).
Choice Reaction Time: There were no statistically significant differences at any time point for either GSK1521498, ethanol or its combination.
Simple Reaction Time: Significant effects were only seen at the 5-hour time point. Ethanol increased SRTs by 46.26 milliseconds (95% CI = 14.41, 78.12) compared to placebo. There were no other statistically significant differences at any time point for GSK1521498, ethanol, or the combination.
Digit Vigilance: Significant effects were only seen at the 5-hour time point. Ethanol increased DV speed by 30.75 milliseconds (95% CI = 16.22, 45.28) compared to placebo. The combination of ethanol and GSK1521498 produced a smaller increase in speed of 26.61 milliseconds (95% CI = 11.20, 42.01). This effect seemed to be driven by ethanol as the combination compared to GSK1521498 alone showed an increase of 29.46 milliseconds (95% CI = 13.49, 45.53). There were no other statistically significant differences at any time point for either GSK1521498, ethanol, or its combination.
Power of Attention: Significant effects were only seen at the 5-hour time point. Ethanol reduced attention compared to placebo (mean = 142.57 milliseconds, 95% CI = 59.26, 225.87). The combination of GSK1521498 20 mg with ethanol did not cause any additional impairments in attention, although compared to placebo, the combination impaired attention less than ethanol alone (mean = 81.72 milliseconds, 95% CI = −5.7, 169.14). GSK1521498 20 mg alone did not produce any significant impairment of attention.
Figure 4.
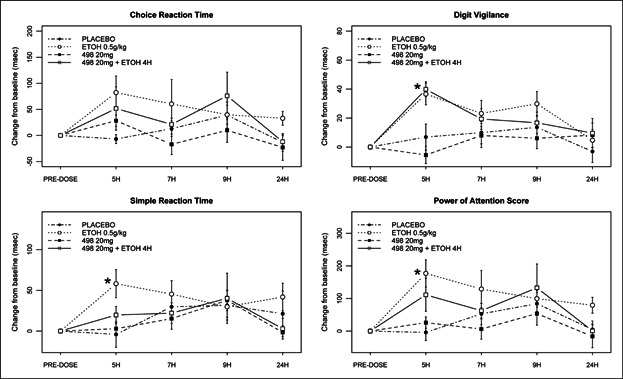
Summary of Power of Attention (PoA) scores. Increases in reaction time were seen on Digit Vigilance, Simple Reaction Time, and Power of Attention score in treatment periods with ethanol at the 5-hour time point only. There were no effects or additional effects of GSK1521498 at any time point (*P < .05).
Secondary Pharmacodynamic Assessments
Alcohol Urge Questionnaire
There was no significant effect of GSK1521498 on the urge to consume alcohol (Table S14).
Hedonic Preference Scale and Rate of Beverage Consumption
The pleasurable response 10 minutes after consumption and rate of consumption were reduced by ethanol relative to orange juice alone, whether alone or in combination with GSK1521498. GSK1521498 had no effect on hedonic preference or rate of consumption, alone or in combination with ethanol (Tables S15 and S16).
Biphasic Alcohol Effects Scale
Ethanol produced statistically significant increases compared to placebo on the stimulant (mean = 10.8, 95% CI = 2.6, 19.0) and sedative subscales (mean = 12.3, 95% CI = 5.8, 18.8). However there was no effect of GSK1521498 alone or any additional effect in combination with ethanol on these subjective feelings (Table S17).
Drug (Alcohol) Visual Analogue Scale
Statistically significant increases on the do not feel drunk/feel drunk scale were seen in treatment periods with ethanol both alone (mean = 51.5, 95% CI = 41.8, 61.2) and in combination with GSK1521498 (mean = 55.8, 95% CI = 45.9, 65.6). There were no additional effects of the combination over ethanol alone (mean = 4.3, 95% CI = −5.8, 14.3). Ethanol significantly decreased ratings on the want more alcohol/do not want more alcohol scale (mean = -21, 95% CI = −33, −10) but the effect of the combination did not reach significance (mean = −10.2, 95% CI = −22, 1.5). No significant differences were found on the like how alcohol is making me feel/dislike how alcohol is making me feel scale (Table S18).
Visual Probe Task and Perceptual Processing Task
On the VPT there were no significant effects of GSK1521498, alcohol, or their interaction on attentional bias to alcohol-related cues. No significant differences were observed on the PPT (Tables S19 and S20).
Pharmacogenetics
Thirteen subjects (10 of white, and 3 of African ethnicity) provided consent for PGx research and were genotyped for OPRM1 A118G. Of the 10 white subjects, four carried at least one G allele (3 AG, 1 GG) but the necessary clinical phenotype data were unavailable for GG individual (see Table S2 for a comparison of the PGx sample and the full study sample). Effects of ethanol appeared more pronounced on the DVAS subscales in the white G-carriers (Figure S1). White G carriers demonstrated trends towards increased levels of sedation on the BAES when GSK1521498 was combined with ethanol (Figure S2). However, these trends were not seen across four related end-points: PoA, VAS alertness, POMS-B fatigue/inertia, and vigor/activity.
Pharmacokinetics
Summaries were not generated for PK parameters from part 1 and only data from part 2 are presented (see Table2). Dosing of ethanol 4 hours following GSK1521498 administration provided reasonable synchronization of peak exposures of both during the co-administration periods (Figures S2 and S3). The geometric least square mean ratio estimates of the interaction effects of ethanol on GSK1521498 PK were 1.02 (90% CI = 0.95, 1.09) and 0.92 (0.85, 0.99) for AUC(0–∞) and Cmax, respectively. The corresponding estimates for the effects of GSK1521498 on ethanol PK were 1.00 (0.94, 1.07) and 1.04 (0.99,1.09); 90% CIs for all measures were contained within the standard bioequivalence range (0.8–1.25) indicating no effect of either agent on the PK of the other.
Table 2.
Summary of Plasma Pharmacokinetics of GSK1521498 and Ethanol
| Parameter | Treatment | N (n) | Estimatea | 95% CIb |
|---|---|---|---|---|
| GSK1521498 | ||||
| AUC(0–∞) (ng h/mL) | GSK1521498 20 mg | 23 (23) | 2839 (29.7) | 2503, 3219 |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 2877 (31.3) | 2520, 3284 | |
| Cmax (ng/mL) | GSK1521498 20 mg | 23 (23) | 118 (26.7) | 105, 132 |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 107 (24.6) | 96, 119 | |
| t1/2 (h) | GSK1521498 20 mg | 23 (23) | 22.7 (26.9) | 20.2, 25.4 |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 22.8 (21.6) | 20.8, 25.0 | |
| tlag (h) | GSK1521498 20 mg | 23 (23) | 0.50 (0–1.10) | — |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 0.50 (0–1.00) | 0.25 (0.00, 0.25) | |
| Tmax (h) | GSK1521498 20 mg | 23 (23) | 3.92 (2.00–6.32) | |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 3.92 (2.00–8.05) | 0.95 (−0.04, 1.04) | |
| Ethanol | ||||
| AUC(0–∞) (µg h/mL) | Ethanol 0.5 g/kg | 25 (23) | 2321 (21.5) | 2117, 2545 |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 2353 (16.2) | 2195, 2522 | |
| Cmax (µg/mL) | Ethanol 0.5 g/kg | 25 (23) | 732 (18.0) | 679, 790 |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 781 (20.0) | 718, 851 | |
| t1/2 (h) | Ethanol 0.5 g/kg | 25 (23) | 1.09 (40.3) | 0.93, 1.29 |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 1.01 (35.9) | 0.87, 1.18 | |
| tlag (h) | Ethanol 0.5 g/kg | 25 (23) | 0 (0–0) | — |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 0 (0–0) | 0.0 (0.0, 0.0) | |
| Tmax (h) | Ethanol 0.5 g/kg | 25 (23) | 1.00 (0.50–2.00) | — |
| GSK1521498 20 mg + ethanol 0.5 g/kg | 23 (23) | 1.00 (0.50–1.50) | −0.24 (−0.38, 0) | |
Presented as geometric mean (between subject CV%) with the exception of Tmax and tlag which are presented as median (min, max).
Presented as upper and lower limits of 95% CI apart from Tmax and tlag which are presented as estimate of median difference (95% CI).
Discussion
GSK1521498 is a novel mu-opioid antagonist and a potential new treatment for alcohol and other addictions. In previous trials in human, it has demonstrated a good safety and tolerability profile.23–25 In this study, we investigated its safety, tolerability, PD, and PK when administered with ethanol. The selected dose of 20 mg is a clinically relevant one as it associated with an exposure following a single dose that is equivalent to 10 mg at steady-state dosing and is predicted to achieve greater than 90% receptor occupancy.21,25
GSK1521498 was well tolerated at this dose when administered on its own and in combination with ethanol. Adverse effects were mild or moderate in severity with nausea being the most common (up to 13% of subjects). Fewer adverse effects were reported with GSK1521498 alone (4–9%) than with the combination (4–13%) or with ethanol alone (4–16%). Co-administration of GSK1521498 with ethanol did not appear to substantially affect its tolerability. GSK1521498 was not associated with abnormalities of laboratory tests; vital signs or ECG measures either alone or in combination with ethanol. Minor effects were seen on neurological assessments with ethanol, which were not unexpected, and these were not affected by GSK1521498. In terms of behavioral effects, GSK1521498 produced statistically significant but mild transient effects on the VAS alertness and contentedness subscales and the POMS-B total mood score. These effects were enhanced when GSK1521498 was co-administered with ethanol. However, the effects were small and not clinically significant and consistent with findings in previous studies with this agent24,25 and these effects disappear early in treatment.25 There were no changes seen on any of the self-report measures of mood—BDI-II, BAI, or HADS. As ethanol is known to slow reaction times and a similar effect has been seen with initial administration of GSK1521498,24 a particular concern was a possible additive effect of both agents on this measure. However, while we found that ethanol did indeed affect reaction time on the PoA tasks, GSK1521498 did not and any effects of the combination were driven by the effects of ethanol.
On the exploratory PD measures that were used to examine effects on hedonic and consummatory aspects of alcohol consumption we did not find any effect of GSK1521498 either alone or in combination with ethanol. The effects of ethanol were not always as expected, for example, consumption of orange juice with ethanol was slower than orange juice alone and ethanol decreased the hedonic preference for alcohol. This may relate to the formulation of ethanol used (Everclear) which was a preparation specifically chosen for standardization of dosing and very likely different from most subjects' personally preferred alcoholic beverage. On the measure of attentional bias to alcohol-related stimuli, neither ethanol nor GSK1521498, alone or in combination affected the attentional bias to alcohol-related stimuli. These results require a cautious interpretation given the ethanol formulation used, the single dosing regimen of GSK1521498 and the study population (healthy social drinkers) for whom the motivational properties of alcohol cues may have been low. In relation to the last point, it should be noted that the effects of GSK1521498 on attentional bias to food-related stimuli were seen in obese binge eaters for whom the food cues had higher motivational value.25,38
The PK parameters of GSK1521498 in this study were in keeping with those from previous studies with this drug23,25 and there was no evidence of a PK interaction with ethanol (or vice versa).
The PGx analyses were limited by the small sample size (N = 9) of Caucasian subjects with only three G-carriers with available phenotype data. Interestingly, G-carriers showed enhanced sedative response to ethanol and GSK152498. While these results are very preliminary and not seen on related measures of sedation, they suggest that PGx considerations are very important in the future development of this drug. This is particularly relevant for targeted treatment and dose optimization; especially given the data suggesting that the A118G polymorphism may mediate the effects of alcohol,9 risk of dependence,11 treatment effects of naltrexone,15 and the preliminary weight effects seen in the recent phase 2 trial of GSK1521498 in obese binge eaters.25
In summary, GSK1521498 was generally well tolerated and had a good safety profile at the dose of 20 mg. There was no evidence of a PK or unexpected PD effect of GSK1521498 administered alone or in combination with ethanol 0.5 g/kg. There were transient effects on alertness and mood that were greater when combined with ethanol. While it is not possible to definitively rule out a small PD interaction but if present, this study indicates that it is small. Overall these findings are encouraging for the further progression of the drug for the treatment of alcohol addiction.
Declaration of Conflicting Interests
The following authors are employed by and hold shares in GSK: D.W., K.S., L.H., L.W., S.M., C.D., K. Maltby, D.B.R, L.V.J., E.T.B., and P.J.N. The following authors are contractors employed by GSK: B.S., M.Z. M.B. holds shares in GSK. B.P.B. and K. Mogg have received consultancy fees from GSK.
Funding
This study was funded and conducted by GlaxoSmithKline. H.Z. is a Clinical Translational Medicine and Therapeutics (TMAT) PhD Fellow funded by the Wellcome Trust and GSK.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
Mean GSK1521498 and ethanol concentration-time profiles.
Figure S2. Effect of OPRM1 A118G genotype on DVAS.
Figure S3. Effect of OPRM1 A118G genotype treatment differences on BAES Sedative Items Sum Score.
Table S1. Summary of subject disposition and demographic characteristics
Table S2. Comparison of PGx population with all subjects
Table S3. Summary of all drug-related on-therapy adverse events
Table S4. Summary of VAS scores
Table S5. Summary of POMS-B total mood disturbances
Table S6. Summary of Beck Depression Inventory (BDI-II) data
Table S7. Summary of Beck Anxiety Inventory (BAI) change from baseline
Table S8. Summary of Hospital Anxiety and Depression Scale (HADS) change from baseline
Table S9. Change from baseline for Purdue Pegboard Sum Hand Score
Table S10. Adjusted means and treatment differences for Choice Reaction Time
Table S11. Adjusted means and treatment differences for Simple Reaction Time
Table S12. Adjusted means and treatment differences for Digit Vigilance speed
Table S13. Adjusted means and treatment differences for Power of Attention
Table S14. Treatment differences for the Alcohol Urge Questionnaire (AUQ)
Table S15. Summary of the absolute values on the Hedonic Preference Scale
Table S16. Summary of absolute values of rate of beverage consumption
Table S17. Treatment differences for the Biphasic Alcohol Effects Scale
Table S18. Treatment differences for the DVAS
Table S19. Treatment differences in attentional bias scores (milliseconds) on the Visual Probe Task (VPT)
Table S20. Treatment differences on the Perceptual Processing Task
References
- 1.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 2.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction (Review) Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Merrer J, Becker JAJ, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89(4):1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R999–R1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- 6.Lingford-Hughes A, Watson B, Kalk N, Reid A. Neuropharmacology of addiction and how it informs treatment. Br Med Bull. 2010;96:93–110. doi: 10.1093/bmb/ldq032. [DOI] [PubMed] [Google Scholar]
- 7.Trigo JM, Martin-García E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108(3):183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Ramchandani VA, Umhau J, Pavon FJ, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray LA, Bujarski S, Mackillop J, Courtney KE, Monti PM, Miotto K. Subjective response to alcohol among alcohol-dependent individuals: effects of the mu-opioid receptor (OPRM1) gene and alcoholism severity. Alcohol Clin Exp Res. 2012;37:E116–24. doi: 10.1111/j.1530-0277.2012.01916.x. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miranda R, Ray L, Justus A, et al. Initial evidence of an association between OPRM1 and adolescent alcohol misuse. Alcohol Clin Exp Res. 2010;34(1):112–122. doi: 10.1111/j.1530-0277.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miranda R, Reynolds E, Ray L, et al. Preliminary evidence for a gene-environment interaction in predicting alcohol use disorders in adolescents. Alcohol Clin Exp Res. 2013;37(2):325–331. doi: 10.1111/j.1530-0277.2012.01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinz A, Reimold M, Wrase J, et al. Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch Gen Psychiatry. 2005;62(1):57–64. doi: 10.1001/archpsyc.62.1.57. [DOI] [PubMed] [Google Scholar]
- 13.Williams TM, Davies SJC, Taylor LG, et al. Brain opioid receptor binding in early abstinence from alcohol dependence and relationship to craving: an [11C]diprenorphine PET study. Eur Neuropsychopharmacol. 2009;19(10):9. doi: 10.1016/j.euroneuro.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Anton RF, Voronin KK, Randall PK, Myrick H, Tiffany A. Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcohol Clin Exp Res. 2012;36(11):2000–2007. doi: 10.1111/j.1530-0277.2012.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamorro A-JA, Marcos MM, Mirón-Canelo J-AJ, Pastor II, González-Sarmiento RR, Laso F-JF. Association of µ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addict Biol. 2012;17(3):505–512. doi: 10.1111/j.1369-1600.2012.00442.x. [DOI] [PubMed] [Google Scholar]
- 16.Schacht JP, Anton RF, Voronin KE, et al. Interacting effects of naltrexone and OPRM1 and DAT1 variation on the neural response to alcohol cues. Neuropsychopharmacology. 2013;38:414–422. doi: 10.1038/npp.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anton RF, O'Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 18.Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173(1–2):32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- 19.Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010;8(12):CD001867. doi: 10.1002/14651858.CD001867.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Ignar DM, Goetz AS, Noble KN, et al. Regulation of ingestive behaviors in the rat by GSK1521498, a novel-opioid receptor-selective inverse agonist. J Pharmacol Exp Therap. 2011;339(1):24–34. doi: 10.1124/jpet.111.180943. [DOI] [PubMed] [Google Scholar]
- 21.Rabiner EA, Beaver J, Makwana A, et al. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol Psychiatry. 2011;16(8):826–835. doi: 10.1038/mp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuliano C, Robbins TW, Wille DR, Bullmore ET, Everitt BJ. Attenuation of cocaine and heroin seeking by µ-opioid receptor antagonism. Psychopharmacology. 2013;227(1):137–147. doi: 10.1007/s00213-012-2949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathan PJ, O'Neill BV, Bush MA, et al. Opioid receptor modulation of hedonic taste preference and food intake: a single-dose safety, pharmacokinetic, and pharmacodynamic investigation with GSK1521498, a novel µ-opioid receptor inverse agonist. J Clin Pharmacol. 2012;52(4):464–474. doi: 10.1177/0091270011399577. [DOI] [PubMed] [Google Scholar]
- 24.Nathan PJ, Bush MA, Tao WX, et al. Multiple-dose safety, pharmacokinetics, and pharmacodynamics of the opioid receptor inverse agonist GSK1521498. J Clin Pharmacol. 2012;52(10):1456–1467. doi: 10.1177/0091270011421785. [DOI] [PubMed] [Google Scholar]
- 25.Ziauddeen H, Chamberlain SR, Nathan PJ, et al. Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry. 2012:1–7. doi: 10.1038/mp.2012.154. doi: 10.1038/mp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cambridge VC, Ziauddeen H, Nathan PJ, et al. Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biol Psychiatry. 2012;73(9):887–894. doi: 10.1016/j.biopsych.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamberlain SR, Mogg K, Bradley BP, et al. Effects of mu opioid receptor antagonism on cognition in obese binge-eating individuals. Psychopharmacology. 2012;224(4):501–509. doi: 10.1007/s00213-012-2778-x. [DOI] [PubMed] [Google Scholar]
- 28.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47(3):211–218. [Google Scholar]
- 29.McNair DM, Heuchert JP. Profile of Mood States–Technical update, 2005. New York: Multi-Health System; 2005. [Google Scholar]
- 30.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA, Brown CK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 32.Steer RA, Rissmiller DJ, Ranieri WF, Beck AT. Structure of the computer-assisted Beck Anxiety Inventory with psychiatric inpatients. J Pers Assess. 1993;60(3):532–542. doi: 10.1207/s15327752jpa6003_10. [DOI] [PubMed] [Google Scholar]
- 33.Posner K, Oquendo M, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164(7):1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah J, Wesnes KA, Kovelesky RA, Henney HR. Effects of food on the single-dose pharmacokinetics/pharmacodynamics of tizanidine capsules and tablets in healthy volunteers. Clin Therap. 2006;28(9):10. doi: 10.1016/j.clinthera.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Bohn MJM, Krahn DDD, Staehler BAB. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19(3):600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 36.Martin CSC, Earleywine MM, Musty RER, Perrine MWM, Swift RMR. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17(1):140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 37.Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology. 2004;176(1):88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- 38.Nathan PJ, O'Neill BV, Mogg K, et al. The effects of the dopamine D3 receptor antagonist GSK598809 on attentional bias to palatable food cues in overweight and obese subjects. Int. J. Neuropsychopharm. 2012;15(2):149–161. doi: 10.1017/S1461145711001052. [DOI] [PubMed] [Google Scholar]
- 39.McDowell JA, Chittick GE, Stevens CP, Edwards KD, Stein DS. Pharmacokinetic interaction of abacavir (1592U89) and ethanol in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44(6):1686–1690. doi: 10.1128/aac.44.6.1686-1690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Modi NB, Dresser M, Desai D, Edgar C, Wesnes K. Dapoxetine has no pharmacokinetic or cognitive interactions with ethanol in healthy male volunteers. J Clin Pharmacol. 2007;47(3):315–322. doi: 10.1177/0091270006297229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean GSK1521498 and ethanol concentration-time profiles.
Figure S2. Effect of OPRM1 A118G genotype on DVAS.
Figure S3. Effect of OPRM1 A118G genotype treatment differences on BAES Sedative Items Sum Score.
Table S1. Summary of subject disposition and demographic characteristics
Table S2. Comparison of PGx population with all subjects
Table S3. Summary of all drug-related on-therapy adverse events
Table S4. Summary of VAS scores
Table S5. Summary of POMS-B total mood disturbances
Table S6. Summary of Beck Depression Inventory (BDI-II) data
Table S7. Summary of Beck Anxiety Inventory (BAI) change from baseline
Table S8. Summary of Hospital Anxiety and Depression Scale (HADS) change from baseline
Table S9. Change from baseline for Purdue Pegboard Sum Hand Score
Table S10. Adjusted means and treatment differences for Choice Reaction Time
Table S11. Adjusted means and treatment differences for Simple Reaction Time
Table S12. Adjusted means and treatment differences for Digit Vigilance speed
Table S13. Adjusted means and treatment differences for Power of Attention
Table S14. Treatment differences for the Alcohol Urge Questionnaire (AUQ)
Table S15. Summary of the absolute values on the Hedonic Preference Scale
Table S16. Summary of absolute values of rate of beverage consumption
Table S17. Treatment differences for the Biphasic Alcohol Effects Scale
Table S18. Treatment differences for the DVAS
Table S19. Treatment differences in attentional bias scores (milliseconds) on the Visual Probe Task (VPT)
Table S20. Treatment differences on the Perceptual Processing Task


