Abstract
Children and elderly individuals are often infected easily and repeatedly with human respiratory syncytial virus (HRSV); however, the features of recurrent infection in the same individual are defined poorly. To clarify the clinical significance of repeated HRSV infections in relation to subgroup epidemiology, this study performed prospective and longitudinal analyses in children with lower respiratory tract infections over 20 consecutive epidemics between 1985 and 2005 at a pediatric outpatient clinic in Kawasaki, Japan. HRSV infections were confirmed by 2 types of reverse-transcription PCR. Samples obtained from patients with repeated infections were subjected to sequence analysis and cloning analysis. A total of 1,312 lower respiratory tract infections observed in 1,010 patients were diagnosed as HRSV infections. Repeated HRSV infections occurred in 208 of the 1,010 patients. Analysis of the patients with repeated infections revealed that children were often infected multiple times even within a single short epidemic. Some patients were re-infected with strains having the same or virtually identical N gene sequences. In patients infected more than 4 times, cloning analysis revealed more frequent dual infections with both subgroups (23.8%). The HRSV-A subgroup caused subsequent homologous infections more frequently than did HRSV-B; furthermore, HRSV-A infections provided no protection from a second homologous infection. In contrast, HRSV-B infections offered significant protection against a second homologous infection. Statistical analysis revealed alleviation of symptoms with a reduced rate of dyspnoeic attacks only in the group re-infected with homologous HRSV-A strains. Thus, this study elucidates new clinical features of recurrent HRSV infection. J. Med. Virol 86: 1629–1638, 2014.
Keywords: human respiratory syncytial virus (HRSV), repeated infections, subgroup epidemiology, clinical characteristics
INTRODUCTION
Human respiratory syncytial virus (HRSV), of the family Paramyxoviridae, subfamily Pneumovirinae, genus Pneumovirus, is the leading cause of lower respiratory tract infections in infants and children [Parrott et al., 1973]. All infants experience at least 1 HRSV infection by 2 years of age [Glezen et al., 1986]. Despite the presence of circulatory antibodies against HRSV, recurrent infections in older children and adults occur throughout life, and protective immunity against re-infections is incomplete and brief [Hall et al., 1991]. There are 2 major antigenic subgroups, A (HRSV-A) and B (HRSV-B), and viruses from these subgroups are considered genetically distinct on the basis of sequencing data [Matheson et al., 2006]. Several findings have raised the possibility that antigenic differences between HRSV subgroups may contribute to re-infection, although the results were not conclusive [Mufson et al., 1987]. Both subgroups are subdivided into several strains or genotypes according to the attachment glycoprotein (G) gene. More than 3 genotypes in each dominant subgroup usually co-circulate in epidemics, with some strains being replaced each year [Hall et al., 1990; Sullender, 2000; Galiano et al., 2005; Matheson et al., 2006]. The difference between genotypes might be considered to play a role in the establishment of re-infections; however, this requires further investigation [Sullender et al., 1998]. A birth cohort study in Kenya observed that 4 of 12 repeated infections recurred with an identical virus within the same short epidemic [Scott et al., 2005]. Furthermore, in a study with adult volunteers, clinical re-infections occurred repeatedly, even when the subjects were infected with the same strain of HRSV-A within a few months after a natural HRSV-A infection [Hall et al., 1991]. From a study of hospitalized children in Finland, no direct evidence of protection against re-infection with a homologous subgroup was found even within a single season [Waris, 1991]. To clarify the clinical characteristics and significance of HRSV re-infection, a prospective and longitudinal analysis of HRSV infections during 20 consecutive epidemics at the same outpatient clinic was conducted.
MATERIALS AND METHODS
Patients and Clinical Specimens
This study was performed at a private pediatric outpatient clinic in Kawasaki, Japan. A pediatrician in the clinic monitored children with symptoms of lower respiratory tract infection prospectively during the period from December 1985 to August 2005. The diagnosis of lower respiratory tract infection was based on major clinical manifestations such as expiratory wheezing, shortness of breath, hoarseness, barking cough with or without inspiratory stridor, deep or wet chest cough, rhonchi, and rales. Duration of fever ≥38°C and the existence of respiratory difficulty (retraction, expiratory wheezing, tachypnoea ≥50 breath/min, and/or orthopnoea) on the day of visit or during the illnesses were recorded as clinical features by the pediatrician. Nasopharyngeal secretions or nasal swabs were collected from all patients with lower respiratory tract infections, as described previously [Yui et al., 2003]. If an HRSV infection occurred ≥14 days after a previous infection or if the 2 infections were determined to involve different subgroups, the event was defined as a separate infection [Hall et al., 1991]. When a patient was diagnosed with HRSV infection for the first time during a visit to the outpatient clinic, this was determined as the first HRSV infection.
Informed consent for participation in this study was obtained from the parents of all the children. The study protocol was approved by the ethics committee of the Kitasato Institute for Life Sciences, Kitasato University.
Detection of HRSV Antigen
From a total of 1,735 clinical specimens, 1,690 were examined immediately for the presence of HRSV antigens with an enzyme-linked immunosorbent assay (ELISA) kit (Ortho Diagnostics, Raritan, NJ), a TestPack RSV enzyme immunoassay (EIA) kit (Abbott Laboratories, North Chicago, IL) or immunochromatography (IC) using ImmunoCard STAT RSV (Meridian Bioscience, Cincinnati, OH). From 1985 to 1986, an ELISA kit was used for direct antigen detection. Then, EIA was performed until December in 2004, when the manufacturer stopped providing the test kit. The IC test was used subsequently (Fig. 1).
Fig 1.
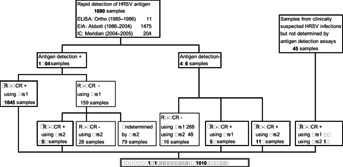
Study flow diagram. Among the 1,735 clinical specimens, 1,690 were subjected to rapid antigen detection assay and 45 samples from clinically suspected HRSV infections were subjected to RT-PCR. A total of 1,312 specimens obtained from 1,010 children with symptoms of lower respiratory tract infections were confirmed to be infected with HRSV using 2 types of RT-PCR using the primers Prs1 and Prs2. The asterisk represents confirmed HRSV infections. ELISA, enzyme-linked immunosorbent assay; EIA, enzyme immunoassay; IC, immunochromatography; Prs1, RT-PCR primer set 1 shown in Table1; Prs2, RT-PCR primer set 2 shown in Table1.
RNA Extraction and RT-PCR
Total RNA was extracted directly from respiratory specimens (nasopharyngeal secretions or nasal swabs) using acid guanidinium thiocyanate phenol–chloroform with minor modifications [Yui et al., 2003], as described previously or using the High Pure Viral RNA Kit (Roche Applied Science, Manheim, Germany) according to the manufacturer's instructions. To amplify HRSV-A and HRSV-B simultaneously, an N gene region conserved between the 2 subgroups was selected (Table1). Early in the study, reverse-transcription PCR (RT-PCR) was performed using primer set 1 (Prs1), as described previously [Yui et al., 2003]. Later in the study, an increased number of samples tested negative by PCR using Prs1, although they were positive by EIA and IC. Because HRSV sequence mutation(s) at the position of Prs1 may cause a failure of PCR amplification, another primer set (Prs2) was synthesized (Table1). First-strand cDNA synthesis was carried out using the CN3 primer (for viral RNA) and CCN6 primer (for mRNA). cDNA was amplified by the first PCR using n-F1(+) and n-B1(−), followed by nested PCR using EcoF3′ (+) and NotB3 (−). When an HRSV infection was suspected clinically or epidemiologically, RT-PCR was conducted irrespective of the rapid antigen detection assay (Fig. 1). All RT-PCR procedures were performed according to the protocol described by Kwok and Higuchi [1989]. Every assay was performed with a negative control.
TABLE I.
Sequences and Positions of the PCR Primers Used
| Primer | Sequence (5′–3′) | Positions |
|---|---|---|
| RT-PCR primer set 1 (Prs1) | ||
| C-RSN (+) | GGGTCGACAATTCACTGGGTTAATACCTAT | 1274–1295* |
| RSN-F1 (+) | GCCCCGGGGAGATAGAATCTAGAAAATCCT | 1477–1498 |
| RSN-B1 (−) | GCGGAGCTCTTTGGGTTGTTCAATATATGG | 1998–2018 |
| RSN-F2 (+) | CCGGTACCGAAATGGGAGAGGTAGCTCC | 1516–1535 |
| RSN-B2 (−) | CCGCATGCATAAACCTCAACAACTTGTTCC | 1938–1959 |
| RT-PCR primer set 2 (Prs2) | ||
| CN3 (+) | GCTCTTAGCAAAGTCAAGTTGAA | 1099–1121* |
| CCN6 (−) | TCTGTACTCTCCCATTATGCCTA | 2087–2109 |
| n-F1 (+) | GAGATAGAATCTAGAAAATCCTACAAAA | 1477–1504 |
| n-B1 (−) | TGGGTTGTTCAATATATGGTAGA | 1994–2016 |
| EcoF3′ (+) | TGGTGAATTCGCTCCAGAATACAGGCA | 1531–1547 |
| NotB3 (−) | AGTTGCGGCCGCATAAACCTCAACAACTTGTTCC | 1938–1959 |
| Colony direct PCR primers | ||
| M13m4 (+) | GTTTTCCCAGTCACGAC | 580–596** |
| M13RV (−) | CAGGAAACAGCTATGAC | 812–828 |
Restriction Fragment-Length Polymorphism
HRSV subgroups were distinguished using restriction fragment-length polymorphisms (RFLP) of the RT-PCR products, as reported previously [Yui et al., 2003]. In brief, the RT-PCR product of HRSV-A was digested with BglII and that of HRSV-B was digested with HaeIII. All PCR products were subjected to RFLP, except the clones obtained from the samples selected for cloning.
Nucleotide Sequencing
All the samples obtained from patients with repeated infections were subjected to sequence analysis. When a subgroup could not be determined by RFLP due to an atypical cutting pattern, the samples were also subjected to direct sequencing. RT-PCR products were extracted from low-melting-temperature 1% agarose gel and used for sequencing. The nucleotide sequence was determined with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Tokyo, Japan) using an automated 3130/3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA). RT-PCR products from samples that were suspected of dual infections of HRSV-A and HRSV-B, based on the RFLP pattern and samples from children infected with HRSV more than 4 times, were inserted into pBluescript II SK (−) (Stratagene, La Jolla, CA); then, nucleotide sequences of more than 20 individual clones from each PT-PCR product were determined (cloning analysis). A pair of primers, M13m4 (+) and M13RV (−) shown in Table1, was used for colony direct sequencing.
Statistical Analysis
Comparisons between mean values, paired but not normally distributed, were assessed with the Wilcoxon rank sum test. Categorical data were compared by the Chi-square test or Fisher's exact test. All comparisons were conducted at the two-tailed 0.05 level of significance using Stat-View 5.0 software (SAS Institute, Tokyo, Japan).
Nucleotide Sequence Accession Numbers
The N-gene sequences determined in this study have been deposited in the DNA Data Bank of Japan (DDBJ) under accession numbers AB722450–AB723492.
RESULTS
Laboratory Diagnosis of HRSV Infection
Among the 1,735 clinical specimens, 1,690 were subjected to the rapid antigen detection assay, of which 1,204 (71.2%) tested positive for the HRSV antigen (Fig. 1). Early in the study, RT-PCR using Prs1 were conducted. Of the 1,557 samples subjected to RT-PCR using Prs1, 1,130 (72.6%) were positive for HRSV. On comparing the results of EIA and IC to those of RT-PCR, 159 samples were negative by RT-PCR using Prs1, although the viral antigen was detected by EIA or IC (Fig. 1). A portion (80/159) of these samples was re-examined by RT-PCR using another set of primers, Prs2. Using Prs2, 52 samples (65.0%) tested positive for HRSV RNA. Further, of the 486 samples that were negative for antigen detection, 58 and 112 samples were detected to be positive by PCR using Prs1 and Prs2, respectively. Consequently, a total of 1,312 lower respiratory tract infections were confirmed as HRSV infections in 1,010 patients, that is, positive by antigen detection and RT-PCR using Prs1 (1,045) or Prs2 (52), negative by antigen detection but positive by PT-PCR using Prs1 (58) or Prs2 (112), and only detected by RT-PCR using Prs1 (27) or Prs2 (18). The patients ranged in age from 5 days to 11 years (median, 18 months), and the duration analyzed was 20 epidemic years. The definition of 1 epidemic year was from September to August of the next year.
Of the 1,312 HRSV infections, 756 (57.6%) and 517 (39.4%) were caused by HRSV-A and HRSV-B, respectively. Through RFLP and cloning analysis, 39 (3.0%) HRSV infections were verified as dual infections with both HRSV-A and HRSV-B. Further, 617 of 756 (81.6%) HRSV-A infections, 487 of 517 (84.7%) HRSV-B infections, and 20 of 39 (51.3%) infections caused by both HRSV-A and HRSV-B were observed in patients aged less than 3 years. These results showed that HRSV-A and HRSV-B co-circulated continuously over 20 epidemic years, and the overall pattern of subgroup prevalence changed every 1 or 2 years (Fig. 2). From 1994 to 2005, 1 year of HRSV-A predominance was followed by 1 or 2 intervening years where the 2 subgroups presented in similar numbers or HRSV-B predominated.
Fig 2.
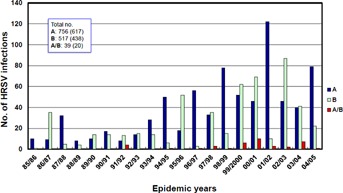
HRSV subgroup epidemiology. Distribution of HRSV subgroups circulating in Kawasaki, Japan, during 1985–2005. The number in parentheses indicates HRSV infections among patients aged less than 3 years. A, HRSV-A; B, HRSV-B; A/B, dual infection with HRSV-A and HRSV-B.
Rate of Repeated HRSV Infection
From the sequencing and cloning analyses, a total of 208 children were noted to have experienced multiple HRSV infections (2–9 times). Consequently, 510 infections (510/1,312 = 38.9%) were recorded as multiple infections observed in the 208 children. Among the 510 infections, 306, 166, and 38 infections were caused by HRSV-A, HRSV-B, and both subgroups, respectively (Table2). The rate of repeated HRSV-A infections among the total circulating HRSV-A infections (306/756 = 40.5%) was higher than that of HRSV-B infections (166/517 = 32.1%). The difference in the rate of HRSV-A and HRSV-B infections was statistically significant (P = 0.0029). Among the 208 children infected repeatedly with HRSV, 151 and 42 children were re-infected and infected 3 times, respectively. Further, 6, 3, and 3 children were infected 4, 5, and 6 times, respectively. Two children were infected 8 times, and 1 patient was infected 9 times (Table2).
TABLE II.
Patterns of Occurrence Among Children Infected Repeatedly With HRSV
| Patterns of occurrence in repeated infections | No. of patients | No. of specimens | HRSV subgroup | ||
|---|---|---|---|---|---|
| A | B | A/B | |||
| Twice | 151 | 302 | 186 | 103 | 13 |
| Three times | 42 | 126 | 72 | 48 | 6 |
| Four times | 6 | 24 | 16 | 4 | 4 |
| Five times | 3 | 15 | 8 | 2 | 5 |
| Six times | 3 | 18 | 11 | 3 | 4 |
| Eight times | 2 | 16 | 8 | 4 | 4 |
| Nine times | 1 | 9 | 5 | 2 | 2 |
| Total | 208 | 510 | 306a | 166a | 38 |
Difference between percentages of sample numbers of repeated cases for the total number of HRSV-A and HRSV-B cases is statistically significant (P = 0.0029). A/B indicates dual infection with HRSV-A and HRSV-B.
Subgroup Occurrence Among Children Re-Infected With HRSV
During the 20 epidemic years, 151 children experienced HRSV re-infections (302 infections; Table3). The patients had no underlying conditions such as congenital heart disease, immunodeficiencies, neuromuscular disorders, or chronic respiratory disorders, except previously diagnosed asthma (in 10 patients). Five children with low birth weights (<2,500 g) were included in the analysis, as they were not born prematurely, with a gestational age of at least ≥37 weeks. There were 16 (5.3%) cases of hospital admissions (16/302; hospitalization due to the first infection, 12; hospitalization due to a second case of infection, 4) in the group. In the second infection, 60 children were infected with a homologous subgroup, of which 50 and 10 re-infections by a homologous subgroup were caused by HRSV-A and HRSV-B, respectively. Re-infections with a heterologous subgroup were detected in 78 children, of whom 35 and 43 were first infected with HRSV-A and HRSV-B, respectively. Thirteen patients had dual infections with both subgroups in either the first or the second infection. Of the 151 patients, 133 (88.1%) were re-infected after more than 1 season and 18 patients (11.9%) within a single season (Table3). Re-infection with a homologous strain occurred more frequently with HRSV-A than HRSV-B (P = 0.0065). During the study period, the overall ratio of circulating HRSV-A/HRSV-B strains in the community was 3:2. Taking circulating HRSV into account, HRSV-A infections provided no protection from a second infection with a homologous strain (P = 0.91). However, patients with HRSV-B infections were protected significantly from a second homologous infection (P = 0.002). When the comparison was applied to children aged less than 3 years (total number of patients, 91; age range, 1–36 months; median, 15 months), the protection offered by HRSV-B against a second homologous infection was verified (P = 0.005). Sequence analysis indicated that 8 children were re-infected with an HRSV-A strain possessing the same nucleotide sequence in the N gene region (nucleotide positions 1548–1937); further, 4 children were infected with an HRSV-A strain and 1 child was infected with an HRSV-B strain possessing a very similar sequence in the N gene region (differing only in a single synonymous nucleotide substitution). The clinical features of 129 children could be traced (Table3).
TABLE III.
Subgroup Characteristics of HRSV Isolated From Re-Infected Children
| Patterns of occurrence | No. of re-infected children | Interval of re-infection | No. of children analyzed for clinical features | |||
|---|---|---|---|---|---|---|
| More than 1 separate season | Within a single season | |||||
| A–A* | 50 | 43 | 7 | 47 | ||
| A–B | 35 | 32 | 3 | 33 | ||
| B–B | 10 | 9 | 1 | 9 | ||
| B–A | 43 | 39 | 4 | 40 | ||
| A/B | 13 | 10 | First (5), Second (5) | 3 | First (2), Second (1) | |
| Total | 151 | 133 | 18 | 129 | ||
The first letter designates the subgroup responsible for the first HRSV infection, and the second letter, that for the second infection. A/B indicates that dual infection with both subgroups is included in the first or second infection. The numbers in parentheses indicate dual-infected HRSV samples in the first or second infection. The numbers in italics indicate re-infected children who were enrolled for the analysis of clinical features.
Subgroup Occurrence Among Children Infected 3 Times
During the study period, 42 children were infected with HRSV 3 times (126 infections; Table4). Among these, all possible patterns of occurrence were observed. The patients had no underlying conditions except previously diagnosed asthma (in 8 patients) and a low birth weight (in 2 patients). Overall, hospitalization was required in 9 of 126 cases of infections (7.1%; first infection, 6; second infection, 2; third infection, 1). Among the 42 children, 19 (17 + 2) (45.2%) had experienced HRSV infections multiple times (2 or 3 times) within a single epidemic year. The clinical characteristics of 29 children were analyzed (Table4).
TABLE IV.
Subgroup Characteristics of HRSV Isolated From Children Infected 3 Times
| Patterns of occurrence | No. of children infected 3 times | Interval of repeated infection 3 times | No. of children analyzed for clinical features | ||||
|---|---|---|---|---|---|---|---|
| More than one separate season | Two infections within a single season | Three infections within a single season | |||||
| A–A–A* | 5 | 2 | 2 | 1 | 4 | ||
| A–A–B | 4 | 1 | 2 | A–A (2)a | 1 | 4 | |
| A–B–A | 8 | 5 | 3 | A–B (2), B–A (1) | 0 | 7 | |
| A–B–B | 1 | 1 | 0 | 0 | 0 | ||
| B–B–B | 2 | 1 | 1 | 0 | 1 | ||
| B–B–A | 3 | 1 | 2 | B–A (2) | 0 | 2 | |
| B–A–B | 3 | 2 | 1 | A–B (1) | 0 | 1 | |
| B–A–A | 10 | 7 | 3 | B–A (3) | 0 | 10 | |
| A/B | 6 | 3 | First (1)b, Second (2) | 3 | Second (1), Third (2) | 0 | |
| Total | 42 | 23 | 17 | 2 | 29 | ||
The first letter designates the subgroup responsible for the first infection; the second letter, that for the second infection; and the third letter, that for the third infection. A/B indicates dual infection in the first, second, or third infection. The numbers in parentheses indicate the sample numbers for each pattern of occurrence in 2 infections within a single seasona, and dual-infected HRSV samples in the first, second, or third infectionb. The numbers in italics indicate children infected 3 times who were enrolled for the analysis of clinical features.
On comparing the first and second infections among patients infected 3 times, the number of patients infected from HRSV-A to HRSV-A (A–A), from HRSV-B to HRSV-B (B–B), from HRSV-A to HRSV-B (A–B), and from HRSV-B to HRSV-A (B–A) were 8, 3, 7, and 11, respectively. Similarly, on comparing the first and third infections, there were 11 (A–A), 2 (B–B), 4 (A–B), and 12 (B–A) children in the various subgroup infection patterns. Likewise, on comparing the second and third infection, 14 (A–A), 1 (B–B), 5 (A–B), and 9 (B–A) children were classified into the various subgroup infection patterns (Table5). Taking circulating HRSV into account, comparisons among the first and second, the first and third, and the second and third homologous infections revealed that HRSV-A or HRSV-B infections conferred no immunity against the same subgroup. However, considering all the patients, independent of the temporal patterns of repetition, HRSV-B infections provided significant protection from a second infection by a homologous strain (P = 0.003). Similarly, HRSV-B infections in children aged less than 3 years (total number of patients, 15; age range, 2–36 months; median, 17 months) also provided significant protection against another homologous infection (P = 0.003).
TABLE V.
Comparison of Clinical Features Among Children With HRSV Re-Infection and Who Were Infected 3 Times
| Patterns of occurrence | Re-infection | Repeated infection 3 times | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of children | Fever | Dyspnoea | No. of children | Fever | Dyspnoea | |||||
| Mean no. of febrile days ± SD | P-Value | Rate of dyspnoea | P-Value | Mean no. of febrile days ± SD | P-Value | Rate of dyspnoea | P-Value | |||
| A–A* | 47 | F = 2.1 ± 0.3 | 0.95 | F = 28/47** | 0.004 | 33 (8, 11, 14) | Fs = 1.1 ± 0.3 | 0.23 | Fs = 16/33 | 0.45 |
| S = 2.1 ± 0.3 | S = 14/47 | St = 1.6 ± 0.3 | St = 20/33 | |||||||
| B–B | 9 | F = 2.4 ± 0.6 | 0.89 | F = 5/9 | 0.61 | 6 (3, 2, 1) | Fs = 1.8 ± 0.9 | 0.58 | Fs = 2/6 | >0.99 |
| S = 2.6 ± 0.6 | S = 7/9 | St = 1.0 ± 0.7 | St = 2/6 | |||||||
| A–B | 33 | F = 2.4 ± 0.3 | 0.31 | F = 16/33 | >0.99 | 16 (7, 4, 5) | Fs = 0.7 ± 0.3*** | 0.04 | Fs = 7/10 | 0.46 |
| S = 1.9 ± 0.3 | S = 16/33 | St = 2.1 ± 0.5 | St = 4/10 | |||||||
| B–A | 40 | F = 2.1 ± 0.3 | 0.26 | F = 24/40 | 0.5 | 32 (11, 12, 9) | Fs = 2.6 ± 0.3 | 0.33 | Fs = 15/32 | >0.99 |
| S = 2.5 ± 0.3 | S = 21/40 | St = 1.7 ± 0.3 | St = 15/32 | |||||||
| Total | 129 | 87 (29 × 3) | ||||||||
The first letter designates the subgroup responsible for the first HRSV infection (in the re-infection) or the second infection (in the repeated infection 3 times); and the second letter, that for the second (re-infection) or third infection (repeated infection 3 times). The letter “F” indicates the first HRSV infection, “S” and “s” indicate the second, and “T” and “t” indicate the third infection. The numbers in parentheses indicate the number of patients between the first and second infection, the first and third, and the second and third, respectively. The Wilcoxon rank-sum test was used for comparing the mean duration of fever in each infection, and Fisher's exact test or Chi-square test was used for comparing the rate of dyspnoea at each infection. **, ***The differences are statistically significant.
Clinical Features Among Children Re-Infected and Infected 3 Times
To assess the alleviation of clinical symptoms in the second or third infection among children re-infected or infected 3 times, the mean number of febrile days and respiratory difficulty were compared between the first and second infections, the first and third, and the second and third infections (Table5).
Among the re-infected patients, a reduction in dyspnoeic attack rates was observed between the first and second HRSV-A infections (P = 0.004) (Table5). Other homologous or heterologous subgroup re-infections were not significantly different for these clinical characteristics even on comparing the infections occurring at an interval of more than 1 epidemic or within a single epidemic. Similarly, a reduction in dyspnoeic attack rates was observed between the first and second HRSV-A infections in the younger age groups (≤3 years; P = 0.016). In contrast, when the younger age groups (≤3 years) infected with HRSV-B were re-infected with HRSV-A, an increase in the number of febrile days was observed.
Among patients infected repeatedly 3 times, all the patients were summed up according to the temporal patterns of repetition (i.e., the first and second, the first and third, and the second and third infections). The alleviation of clinical symptoms was compared among all the patients with these 4 infection patterns (i.e., A–A, B–B, A–B, and B–A; Table5). No significant alleviation of clinical symptoms, such as the duration of fever or rate of dyspnoea, was observed in any of the 4 patterns of occurrence. On the contrary, the mean duration of fever in the infecting pattern from HRSV-A to HRSV-B (A–B) was longer in the second or third infection (P = 0.04). The alleviation of these symptoms was not verified; however, the number of febrile days was increased in children belonging to the younger age groups who were infected with HRSV-B after infection with HRSV-A.
Clinical Course of Children Infected More Than 4 Times
Fifteen children were diagnosed with HRSV infections more than 4 times. Among these 15 children, 6, 3, 3, and 2 patients as well as 1 patient had a history of HRSV infections 4, 5, 6, 8, and 9 times, respectively. Consequently, 82 HRSV infections were observed in these 15 children. The patients had no underlying conditions, including previously diagnosed asthma and a low birth weight. There were five (6.1%) hospital admissions (5/82; hospitalization due to the first infection, 4; hospitalization due to second infection, 1). The patients' ages ranged from 1 month to 11 years (median, 31.5 months).
All the infections, except 2, were subjected to cloning analysis, which indicated that 19 infections (23.8%) were dual infections of HRSV-A and HRSV-B. Various random patterns of occurrence were observed in the repeated infections. All 15 children had experienced HRSV infections multiple times within the same epidemic season, and 4 of the 15 children showed 3–5 separate as well as different subgroup infections within a single season. However, there was no apparent decrease in the duration of fever for each infective episode in these patients. On the other hand, respiratory difficulties tended to recur even after multiple infections in 9 of the 15 children. The remaining children had showed no dyspnoeic attacks from the very first infection.
DISCUSSION
This study was conducted in an ambulatory care setting but not with birth cohort monitoring. Previous studies have demonstrated that over two-thirds of children were infected with HRSV within their first year of life [Glezen et al., 1986]. It was also reported that several neonates were completely asymptomatic although infected with HRSV [Hall et al., 1979]. Therefore, it is likely that a considerable number of the patients in this study had already experienced HRSV infections before their first symptomatic episode. Nevertheless, the data in this study showed that HRSV lower respiratory tract infections occurred repeatedly in the same individual at a rate of 20.6% (208/1,010), which was higher than those reported by 2 previous studies as 9.0% and 7.8% [Zlateva et al., 2007; Yamaguchi et al., 2011]. The higher rate of detection of recurrent HRSV infections in this study as compared to those in previous studies may be attributed partly to the differences in the detection methods used. Two different RT-PCR primer sets (Prs1 and Prs2) and antigen detection assays were used in this study, whereas in the other report, HRSV infections were detected using a single RT-PCR protocol targeting the G gene, which is more variable than the N gene targeted by the present RT-PCR protocols [Yamaguchi et al., 2011]. In fact, the first RT-PCR (Prs1) failed to detect a substantial number of HRSV infections, which were identified by the second RT-PCR (Prs2) and antigen detection assays. The present analysis supports the need to identify HRSV infections using more than 2 diagnostic methods, since the number of infections might otherwise be overlooked. These data thus demonstrated that repeated HRSV infections occur more frequently in Japan than indicated by the previous study.
From 1994 to 2005, a cyclic pattern of subgroup prevalence was observed in the present study. Each HRSV-A predominant year was followed by 1 or 2 epidemic years with relatively equal proportions of the 2 subgroups or HRSV-B predominance. Similar cyclic patterns have been reported in Belgium [Zlateva et al., 2007] and in Rochester, New York [Hall et al., 1990]; however, the patterns differed among the reports. The former study was performed using 2 kinds of RT-PCR procedures and the latter using an immunofluorescent test with monoclonal antibodies. Epidemiological studies around the world have disclosed HRSV-A predominance [Sullender, 2000]. However, the high total HRSV-B detection rate of 44% in Zlateva's study compared with 39% in this study; the 29% HRSV-B detection rate in Hall's study might be due to the methods used and/or the appropriate selection of RT-PCR primers for HRSV-B, since HRSV-B has greater variability in terms of stop codon usage and insertion or deletion than does HRSV-A [Matheson et al., 2006]. Therefore, the different detection rates for HRSV-B using various procedures could influence the results of molecular epidemiology.
In this study, no significant difference was observed in the rate of repeated HRSV-A infections by a homologous subgroup as compared with those by a heterologous subgroup. A birth-cohort study conducted over 2 consecutive years showed that re-infections with the same strain possessing the G gene sequence occurred in infants even within the same epidemic year [Scott et al., 2005]. This observation is thus consistent with the data recorded in the present study. In addition, this study confirmed that the rate of re-infection with HRSV-A was higher than that with HRSV-B, which has also been shown previously [Yamaguchi et al., 2011]. Importantly, HRSV-B re-infection provided protection from a second infection with a homologous subgroup among the re-infected children and among the children infected with HRSV 3 times, even on comparison with children from the younger age groups (≤3 years). This might reflect the 2 distinct lineages of divergent evolution with extensive genetic and serologic differences between HRSV-A and HRSV-B [Matheson et al., 2006]. In addition, this might also explain the cyclic pattern of subgroup prevalence.
Many children who suffered from multiple infections within a short period of time were observed in this study; some of these were infected more than 3 times within the same season, suggesting that some individuals, such as children infected more than 4 times, are highly susceptible to HRSV. Individual host genetic factors are considered important to the immunopathogenesis of HRSV infections [Miyairi and DeVincenzo, 2008; Oshansky et al., 2009]. This influence involves a complex interaction of age-related immunity, previous HRSV experience on priming immune-developmental genetic processes, genes protecting against viral entry in the early phase, and genes modifying later immunopathology. The results in the present study might reflect the differences among individual host genetic factors that determine the susceptibility to HRSV. For highlighting the importance of host genetic factors, patients with multiple HRSV infections appeared to be roughly divisible into 2 groups; those suffering dyspnoeic attacks even after many HRSV infections, and those with no respiratory difficulties from the start. The clinical features of patients presenting with recurrent dyspnoeic attack after HRSV infections could possibly be diagnosed with asthma later in their clinical course.
A previous study showed that the incidences of lower respiratory tract infections, bronchiolitis, and otitis media with effusion were reduced on the third but not second infection of HRSV, and that age at infection influenced the disease severity [Henderson et al., 1979]. The clinical features of repeated HRSV infections, such as the number of febrile days and the presence of dyspnoea, were compared among individuals. In addition, these clinical features were compared among individuals infected with the different subgroups; this analysis was not performed in the previous studies. Among children infected repeatedly with homologous or heterologous HRSV, a reduction in neither the febrile period nor the rate of respiratory difficulty was observed at the second or third infection, even on comparison with children from the younger age group (≤3 years). The only difference noted was a reduced rate of dyspnoeic attacks among the group re-infected with the same HRSV-A, which might indicate that individuals in this group showed a lower immunopathologic response to HRSV-A than the individuals in the other group. In contrast, among the patients infected 3 times, a longer duration of febrile period was noted in the second infection, with infection from HRSV-A to HRSV-B, as compared with the first infection. Similarly, among the re-infected younger age group, infection from HRSV-B to HRSV-A was associated with a longer duration of febrile period. HRSV can occur together with other respiratory viruses, especially adenoviruses [De Paulis et al., 2011]. Although it is possible that other microorganisms modulated the clinical manifestations of HRSV infections at least in some patients in this study, it has been previously reported that viral co-infections do not appear to affect the severity of HRSV infections in hospitalized infants [De Paulis et al., 2011].
The cloning procedure was applied mainly to samples obtained from children with multiple HRSV infections; therefore, a high rate (23.8%) of dual infections was identified. The total rate of dual infections was 3.0%, which was still a relatively high rate of incidence as compared to that reported by an earlier study (0.6%) [Zlateva et al., 2007]. It is possible that more dual infections could be detected by applying cloning procedures to all the samples. The sequence of HRSV-B displays greater variability than that of HRSV-A, which could lead to lower detection rates for HRSV-B and affect the discovery of dual infections, if the primers used to detect HRSV-B are not appropriate for the prevalent strains. It would be worthwhile to examine the exact incidence of dual infections, the manner in which individual clinical symptoms are affected by dual infections, and the evolutionary interaction of HRSV-A and HRSV-B inside the human body.
Recently, it was demonstrated that severe fatal lower respiratory tract infections caused by HRSV are characterized by the absence of a pulmonary cytotoxic lymphocyte response, robust viral loads, and an apoptotic crisis, based on an analysis of autopsy specimens from Chilean infants in the absence of mechanical ventilation [Welliver et al., 2007]. This has cast some doubt on the immunopathogenesis of HRSV disease. The results of the present study may provide additional insights into the wide variations in the severity of HRSV disease, which are affected by the age at infection as well as by genetic polymorphisms among races, individual host genetic factors and socioeconomic conditions [Miyairi and DeVincenzo, 2008; Oshansky et al., 2009]. The susceptibility to HRSV and the disease severity or pattern may vary and depend upon individual factors. If certain genetic factors can predict HRSV disease severity, these findings can lead to either a new vaccination strategy that induces the formation of antibodies blocking the CX3C–CX3CR1 interaction of G protein [Zhang et al., 2010] or antiviral compounds derived from plant lectins [Ooi et al., 2010] as an individualized treatment option.
Acknowledgments
The authors thank Dr. Yoshinao Takeuchi for many helpful discussions and his critical comments on the original version of the manuscript. The authors also thank Dr. Kouji Kurauchi for his assistance in statistical analysis, Ms. Yoshie Motegi for her skilled technical assistance and Ms. Emiko Mori for her assistance with collecting clinical samples.
REFERENCES
- De Paulis M, Gilio AE, Ferraro AA, Ferronato AE, do Sacramento PR, Botosso VF, de Oliveira DB, Marinheiro JC, Hársi CM, Durigon EL, Vieira SE. Severity of viral coinfection in hospitalized infants with respiratory syncytial virus infection. J Pediatr (Rio J) 2011;87:307–313. doi: 10.2223/JPED.2100. [DOI] [PubMed] [Google Scholar]
- Galiano MC, Palomo C, Videla CM, Arbiza J, Melero JA, Carballal G. Genetic and antigenic variability of human respiratory syncytial virus (groups A and B) isolated over seven consecutive seasons in Argentina (1995–2001) J Clin Microbiol. 2005;43:2266–2273. doi: 10.1128/JCM.43.5.2266-2273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- Hall CB, Kopelman AE, Douglas RG, Jr, Geiman JM, Meagher MP. Neonatal respiratory syncytial virus infection. N Engl J Med. 1979;300:393–396. doi: 10.1056/NEJM197902223000803. [DOI] [PubMed] [Google Scholar]
- Hall CB, Walsh EE, Schnabel KC, Long CE, McConnochie KM, Hildreth SW, Anderson LJ. Occurrence of groups A and B of respiratory syncytial virus over 15 years: Associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis. 1990;162:1283–1290. doi: 10.1093/infdis/162.6.1283. [DOI] [PubMed] [Google Scholar]
- Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- Henderson FD, Collier AM, Clyde WA, Jr, Denny FW. Respiratory syncytial-virus infections, reinfections and immunity: A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;339:237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Matheson JW, Rich FJ, Cohet C, Grimwood K, Huang QS, Penny D, Hendy MD, Kirman JR. Distinct patterns of evolution between respiratory syncytial virus subgroups A and B from New Zealand isolates collected over thirty-seven years. J Med Virol. 2006;78:1354–1364. doi: 10.1002/jmv.20702. [DOI] [PubMed] [Google Scholar]
- Miyairi I, DeVincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008;21:686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson MA, Belshe RB, Orvell C, Norrby E. Subgroup characteristics of respiratory syncytial virus strains recovered from children with two consecutive infections. J Clin Microbiol. 1987;25:1535–1539. doi: 10.1128/jcm.25.8.1535-1539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi LS, Ho WS, Ngai KL, Tian L, Chan PK, Sun SS, Ooi VE. Narcissus Tazetta Lectin shows strong inhibitory effects against respiratory syncytial virus, influenza A (H1N1, H3N2, H5N1) and B viruses. J Biosci. 2010;35:95–103. doi: 10.1007/s12038-010-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshansky CM, Zhang W, Moore E, Tripp RA. The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiol. 2009;4:279–297. doi: 10.2217/fmb.09.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott RH, Kim HW, Arrobio JO, Hodes DS, Murphy BR, Brandt CR, Camargo E, Chanock RM. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973;98:289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- Scott PD, Ochola R, Ngama M, Okiro EA, James Nokes D, Medley GF, Cane PA. Molecular analysis of respiratory syncytial virus reinfections in infants from Coastal Kenya. J Infect Dis. 2005;193:59–67. doi: 10.1086/498246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullender WM, Mufson MA, Prince GA, Anderson LJ, Wertz GW. Antigenic and genetic diversity among the attachment proteins of group A respiratory syncytial viruses that have caused repeat infection in children. J Infect Dis. 1998;178:925–932. doi: 10.1086/515697. [DOI] [PubMed] [Google Scholar]
- Sullender WM. Respiratory syncytial virus genetic and antigenic diversity. Clin Microbiol Rev. 2000;13:1–15. doi: 10.1128/cmr.13.1.1-15.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris M. Pattern of respiratory syncytial virus epidemics in Finland: Two-year cycles with alternating prevalence of groups A and B. J Infect Dis. 1991;163:464–469. doi: 10.1093/infdis/163.3.464. [DOI] [PubMed] [Google Scholar]
- Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC., Sr Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Sano Y, Dapat IC, Saito R, Suzuki Y, Kumaki A, Shobugawa Y, Dapat C, Uchiyama M, Suzuki H. High frequency of repeated infections due to emerging genotypes of human respiratory syncytial viruses among children during eight successive epidemic seasons in Japan. J Clin Microbiol. 2011;49:1034–1040. doi: 10.1128/JCM.02132-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui I, Hoshi A, Shigeta Y, Takami T, Nakayama T. Detection of human respiratory syncytial virus sequences in peripheral blood mononuclear cells. J Med Virol. 2003;70:481–489. doi: 10.1002/jmv.10421. [DOI] [PubMed] [Google Scholar]
- Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, Jones LP, Tripp RA. Vaccination to induce antibodies blocking the CX3C–CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol. 2010;84:1148–1157. doi: 10.1128/JVI.01755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlateva KT, Vijgen L, Dekeersmaeker N, Naranjo C, Van Ranst M. Subgroup prevalence and genotype circulation patterns of human respiratory syncytial virus in Belgium during ten successive epidemic seasons. J Clin Microbiol. 2007;45:3022–3030. doi: 10.1128/JCM.00339-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


