Abstract
Background
A ready-to-use betamethasone valerate 0.1% (BMV) dressing was found to be superior to placebo dressing and a reference 0.1% BMV cream in the treatment of patients with chronic plaque psoriasis (CPP).
Methods
This multicentre, prospective, randomized, investigator-blinded, controlled, non-inferiority trial compared the efficacy and safety of the BMV dressing to the calcipotriol–betamethasone dipropionate (CBD) ointment during a 4-week treatment of patients with mild to moderate CPP. The primary efficacy endpoint was the 4-item psoriasis total severity score (TSS-4) at week 4, and the associated non-inferiority margin was 1 point. Secondary outcome measures included the psoriasis global assessment (PGA) score and patients’ quality of life (QoL). Safety was assessed through adverse events (AE) reporting in each treatment group.
Results
Of 325 screened patients, 324 were randomized to BMV (N = 165) or CBD (N = 159), and were considered evaluable for the safety and intention-to-treat (ITT) efficacy analyses. Per protocol (PP) populations included 133 and 131 patients in the BMV and CBD groups respectively. The mean adjusted TSS-4 significantly decreased through the study from baseline in both groups. The PP (primary) analysis of week 4 data revealed a −0.288 (95% CI: −0.610 to 0.034) not significant between-group difference in adjusted means, demonstrating non-inferiority of BMV to CBD. Non-inferiority was also demonstrated in the ITT analysis. The PGA and other secondary outcomes were significantly improved from baseline in both groups at week 4. The QoL score was slightly better in the CBD group at week 4, but no difference was observed at follow-up. No safety or tolerability concerns were observed in either group.
Conflicts of interest
Centro Studi GISED, the centre led by LN, received a grant from IBSA Institut Biochimique SA. VF is an employee of IBSA Institut Biochimique SA.
Introduction
Chronic plaque psoriasis (CPP) is the most prevalent form of psoriasis, found in about 90% of subjects with the disease.1 Despite its often limited extent, CPP can profoundly impact patients’ quality of life (QoL) through social isolation, stigmatization, fear of other people's reactions, decreased levels of employment and psychological distress associated with the impaired response to treatment.2–4
Topical corticosteroids remain the primary treatment for steroid-responsive inflammatory skin diseases, including mild to moderate CPP, due to their anti-inflammatory, immunosuppressive, antiproliferative and vasoconstrictive properties.5 Occlusion with plastic film dressings is a widely accepted procedure to enhance their efficacy, especially in the treatment of psoriasis.6
A ready-to-use, cosmetically acceptable, self-adhering medicated plaster containing the active ingredient betamethasone 17-valerate (BMV) has been developed (Betesil®; IBSA-Institut Biochimique S.A, Pambio-Noranco, Switzerland). This 75 cm2 (75 × 100 mm) dressing contains 2.25 mg of BMV (0.1%) in the adhesive layer. Pharmacodynamic studies have shown that it has a vasoconstrictive and anti-inflammatory activity equivalent to occluded BMV 0.1% cream.7 Previous trials studying the treatment of psoriasis showed that it was safe and superior to a placebo dressing8 and BMV cream.9,10
The objective of this study was to compare short-term efficacy and safety of the BMV dressing to a fixed combination 50 μg–0.5 mg/g calcipotriol–betamethasone dipropionate ointment (CBD, Daivobet®/Dovobet®; LEO Pharma A/S, Ballerup, Denmark) in patients with mild to moderate CPP over a 4-week treatment period, a duration used in comparable trials.11–15 Efficacy was primarily evaluated by the psoriasis total severity score of four items (TSS-4). Safety and other efficacy measures were assessed as secondary outcomes. In addition, QoL was assessed with the dermatology life quality index (DLQI), a validated dermatology-specific QoL instrument.16,17
As the CBD fixed combination is a reference treatment for CPP, a non-inferiority design was used because the BMV dressing has theoretical and pragmatic advantages over traditional topical treatments (controlled dose and delivery, enhanced hydration and lack of greasiness).10 The non-inferiority margin of the primary efficacy outcome (TSS-4) was conservatively set at 1 point according to a literature search that showed:
A significant 2-point improvement on a 12-point TSS after 6–8 weeks of treatment with potent topical steroids vs. vehicle18
The antipsoriatic effects of the CBD ointment, as compared to either active ingredient in same vehicle, was approximately 1 point on the TSS after a 4-week treatment.19 The study was conducted under the provisions of the Declaration of Helsinki, and in accordance with the International Conference on Harmonisation Consolidated Guideline on Good Clinical Practice.
Methods
This multicentre, prospective, randomized, investigator- and assessor-blinded, controlled phase 4 non-inferiority trial involved 16 principal investigators/centres (1 in France, 5 in Italy and 10 in Poland). All patients signed an informed consent form and were enrolled from April 2010 to January 2011. After a baseline visit they attended control visits at weeks 1, 2, 3 and 4 while being treated with the study medications. After week 4, they entered an 8-week treatment-free follow-up period. Early entry into follow-up was possible if all target plaques were cleared or almost cleared before week 4. Patients attended an additional control visit during the follow-up in case of disease reappearance or significant worsening.
Patients
Eligible subjects were outpatients of both genders, aged 18 years or more, affected by mild-to-moderate stable CPP for at least 12 months, not requiring systemic treatment, involving less than 10% of the body surface area (BSA) with at least two bilateral plaques on extending parts of the limbs, i.e. knees and/or elbows, measuring >10 cm2 and <75 cm2 (surface area equivalent of one BMV dressing). Main exclusion criteria were as follows: non-plaque forms of psoriasis; use of antipsoriatic treatments for a period before inclusion (topical: 2 weeks, topical retinoids and any systemic antipsoriatic product: 4 weeks, biological therapies modifying immune responses: 1 year) ascertained or presumptive hypersensitivity to the active principle and/or formulations’ ingredients; severe systemic diseases (e.g. cancer) or cardiac, renal or hepatic impairment.
Two to four BMV dressings were applied to target plaques once a day and had to be worn for at least 20 consecutive hours. The control CBD ointment was applied once a day on target plaques in adequate amounts. The maximum dose was 60 g/week (8.5 g/day).
Outcome measures
The primary efficacy outcome (endpoint) was the TSS-4 score assessed by the investigator at the end of treatment (week 4). The TSS-4 assesses signs (redness/erythema, scale/crusting and thickening/elevation) and symptoms (pruritus) on a 4-point scale (0 = absent, 1 = mild, 2 = moderate and 3 = severe). The score varies from 0 to 12.
Secondary efficacy variables were as follows:
TSS-4 at weeks 1–3 and at the follow-up visit, and its individual subscores at week 4
Psoriasis global assessment (PGA) score at weeks 1–4 assessed by investigators and patients on a 6-point scale (from 0 = clear to 5 = severe)
Patients’ evaluation of QoL by DLQI, calculated by summing the score of each individual heading (resulting in a maximum of 30 and a minimum of 0), at baseline, weeks 1–4 and follow-up visit; patients’ evaluation of DLQI individual headings (symptoms and feelings, daily activities, leisure, work and school, personal relationships and treatment) at baseline and at weeks 1–4
TSS of three items (TSS-3) at week 4 assessed by an independent, blinded assessor (experienced dermatologist) using digitalized photographs
Number of patients with disappearance of active lesions, based on (1) TSS-4 score ≤1; (2) TSS-3 score ≤1; (3) PGA score ≤1
Number of patients (among those having reached a complete remission) who experienced relapse/rebound during follow-up
Surface area of target plaques at week 4, based on digitalized analysis of standardized photographs by a blinded assessor; Patient's assessment of treatment acceptability/satisfaction, on a 0–100 mm visual analogue scale (VAS) (0 = not satisfied at all; 100 = extremely satisfied). Safety measures were adverse events (AEs), vital signs (heart rate and blood pressure) and patient exposure.
Statistics
The sample size calculation was based on the hypothesis that the BMV dressing was non-inferior to the control CBD ointment at week 4, assuming a 1-point non-inferiority margin on TSS-4, common standard deviation of 2.5 points, 90% power, a two-sided alpha level of 0.05 and a 95% confidence interval for the difference between means. Assuming a 10% drop-out rate, at least 300 patients total, 150 in each group, had to be recruited.
Patients were randomly assigned to a treatment group according to a computer-generated randomization list not accessible to investigators, generated in blocks of four with a balanced 1:1 ratio. Study investigators and independent assessors were blinded to treatment assignment. To minimize the risk of investigator unblinding, patients were instructed to remove the dressing at least 3 h before visits and to avoid applications of the control ointment before visits. Study coinvestigators always checked that the treatment sites were adequately cleaned before sending the patient to the blinded investigator for target plaque evaluation.
The following populations were considered for analysis: intention-to-treat population (ITT): all randomized patients who received at least one dose of study treatment; per protocol population (PP): all subjects in the ITT population who completed the treatment period, or discontinued the treatment period due to clearance of lesions, without major protocol deviations; safety population: all randomized patients who received at least one dose of study treatments. Missing values were replaced by the last observation carried forward (LOCF) method for up to visit 5 for the ITT and PP analyses. The primary and secondary efficacy variables were analysed in both PP and ITT populations. The primary assessment for non-inferiority was based on the PP analysis and verified by the ITT analysis.
Between-group comparisons for the primary efficacy outcome was performed using an ancova model with TSS-4 at week 4 as the dependent variable, treatment and centre as fixed effects and the baseline value of TSS-4 as a covariate. The significance of the difference between adjusted means was calculated using two-sided 95% confidence intervals (CIs) and P-values. As a sensitivity analysis, a comparison between treatment groups was performed for TSS-4 at weeks 1–4 using the non-parametric Wilcoxon rank-sum test.
Individual symptoms, TSS-4 subscores, TSS-3, PGA, surface area of target plaques, DLQI total score and subscores were summarized at weeks 1–4 by descriptive statistics. The mean and standard deviation (SD) of the changes from baseline (V1) were calculated with their 95% CIs. Between-group comparisons was based on an ANCOVA model with values at weeks 1, 2, 3 or 4 as the dependent variable, treatment and centre as fixed effects and the baseline value of the individual symptom of TSS, PGA and DLQI, respectively, as covariates. The differences between adjusted means were calculated with two-sided 95% CIs and P-values. Rates of disappearance of active lesions were compared by the Cochran–Mantel–Haenszel test, adjusting for the centre. Assessments of treatment satisfaction were summarized at each visit by descriptive statistics; means and SDs of the changes from baseline were calculated with their 95% CIs; between-group comparisons were based on an anova model with patient's assessment of treatment satisfaction at weeks 1, 2, 3 or 4 as the dependent variable, and treatment and centre as fixed effects. The number and percentage of patients with relapse/rebound during follow-up period were calculated by treatment group with their 95% CIs. Time to relapse/rebound was summarized by descriptive statistics. AEs were coded using the MedDRA dictionary. The system organ class (SOC) and preferred term (PT) were used for tabulation. Differences between groups were evaluated using the chi-squared test or Fisher's exact test.
Results
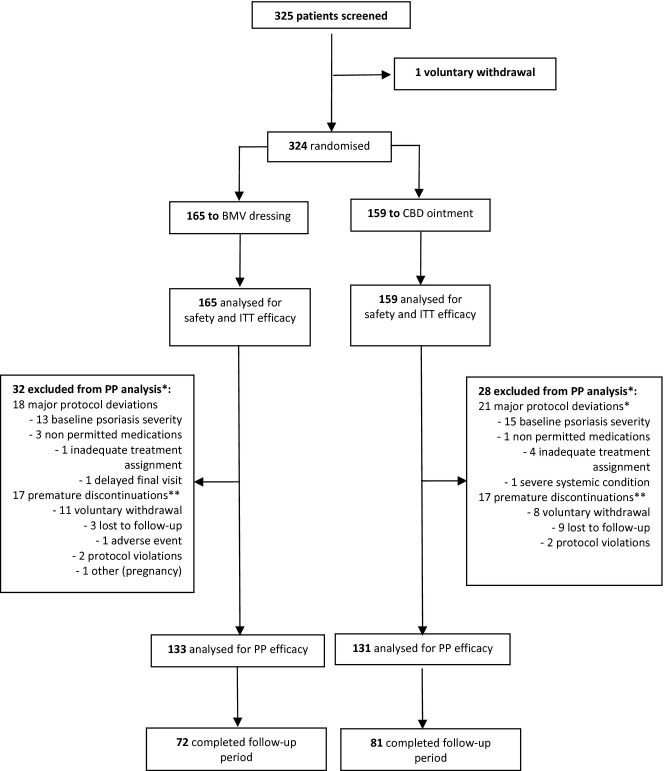
Of 325 screened patients, 324 were randomized to BMV (N = 165) or CBD (N = 159), and considered evaluable for both safety and ITT efficacy analyses. Thirty-two and 28 patients in the BMV and CBD groups, respectively, did not complete the study protocol due to premature discontinuation and/or major protocol deviations and were therefore excluded from the PP analysis. Patients with minor deviations were kept in the PP analysis. Thus, the PP primary efficacy outcome analysis included 133 and 131 patients in the BMV and CBD groups respectively (Fig.1).
Figure 1.
Patients flow through the study. *Some patients were excluded from PP for more than one reason. **10 patients, two in the BMV and eight in the CBD group, had their target plaques cleared at the time of premature withdrawal and were therefore considered completers and included in the PP analysis.
Major protocol deviations (18 and 21 patients in the BMV and CBD groups respectively) were mainly related to baseline psoriasis severity (either too mild or too severe according to inclusion criteria for 28 patients, 13 in the BMV and 15 in the CBD group). Other major protocol deviations included use of non-permitted medications prior to enrolment (four patients), study treatment incorrectly assigned (five patients), end of treatment control visit performed outside the allowed range (one patient), severe systemic medical condition incompatible with the study (one patient). In addition, 17 patients in each group prematurely discontinued treatment, mainly due to voluntary withdrawal (19 patients) and lost to follow-up (12 patients). Of these, 10 patients (two in the BMV group and eight in the CBD group) had their target plaques cleared at discontinuation, and were therefore considered to have completed the protocol and were used in the PP efficacy analysis. A single patient might have had more than one reason for exclusion from the PP analysis: for instance, two patients (one in each group) of the 34 who prematurely discontinued were also found to have major protocol deviations. Finally, 153 patients (72 and 81 in the BMV and CBD groups respectively) with cleared/almost cleared target plaques entered and completed the 8-week observational treatment-free follow-up period (Fig.1).
There was no significant difference between the two groups, in both ITT and PP populations, for basic demographic data (age, gender and body mass index), medical history and concomitant diseases, previous and concomitant medications, history of previous psoriasis and psoriatic treatments and current psoriatic condition (target lesions area and surface, total area of psoriatic lesions, TSS-4 and TSS-3 total scores and individual subscores, PGA mean scores and range, total DLQI score and subscores) (Table1).
Table 1.
Patients baseline characteristics (safety/ITT population)
| BMV dressing (N = 165) | CBD ointment (N = 159) | |
|---|---|---|
| Age (years), mean (SD) | 47.5 (14.6) | 46.7 (13.6) |
| Male/Female (%) | 61.8/38.2 | 62.3/37.7 |
| Psoriasis total surface area (cm2) | ||
| Mean (SD) | 162.8 (170.2) | 176.8 (208.3) |
| Median (range) | 106.0 (17.3–1132.0) | 111.0 (23.0–1132.0) |
| Target lesions surface area (cm2) | ||
| Mean (SD) | 76.0 (51.1) | 72.5 (45.0) |
| Median (range) | 66.5 (10.0–288.0) | 65.0 (20.7–230.0) |
| Target lesions localization (% patients) | ||
| Elbow | 49.7% | 49.1% |
| Knee | 11.5% | 10.1% |
| Elbow and knee | 38.8% | 40.9% |
| Total TSS-4 | ||
| Mean (SD) | 6.70 (1.48) | 6.61 (1.51) |
| Median (range) | 7.00 (4.0–11.0) | 6.50 (4.0–10.5) |
| Total TSS-3 | ||
| Mean (SD) | 4.73 (1.58) | 4.44 (1.43) |
| Median (range) | 4.75 (0.0–8.25) | 4.50 (1.0–8.0) |
| PGA score, assessed by | ||
| Investigator | ||
| Mean (SD) | 3.08 (0.64) | 3.08 (0.68) |
| Median (range) | 3.00 (1.0–5.0) | 3.00 (1.0–5.0) |
| Patient | ||
| Mean (SD) | 2.97 (0.91) | 2.92 (1.11) |
| Median (range) | 3.00 (−2.0–5.0) | 3.00 (−2.0–5.0) |
| Total DLQI | ||
| Mean (SD) | 8.58 (5.94) | 8.52 (5.89) |
| Median (range) | 8.00 (0.0–26.0) | 7.00 (0.0–25.0) |
BMV, betamethasone valerate dressing; CBD, calcipotriol–betamethasone dipropionate ointment; DLQI, dermatology life quality index; PGA, psoriasis global assessment; ITT, intention to treat; TSS, total severity score.
The mean TSS-4 significantly decreased from baseline to week 4 in both groups, and the mean change was comparable in the two groups (BMV: −4.69; 95% CI: −5.08 to −4.30; CBD: −4.75; 95% CI: −5.07 to −4.44). The adjusted mean scores at week 4 were 1.981 in the BMV group and 1.693 in the CBD group. The difference between means was −0.288 (95% CI: −0.610 to 0.034) (Table2). The two-sided 95% CI for the difference between adjusted means lied entirely to the right of the non-inferiority margin (−1), thus showing that BMV was non-inferior to CBD. The difference between groups was not statistically significant (P = 0.079) (Fig.2). The ITT analysis found a significant −0.539 difference between adjusted means (P = 0.001; Table3) but again, the two-sided 95% CI (−0.862 to −0.216) lied entirely to the right of the non-inferiority margin, confirming the non-inferiority of BMV to CBD.
Table 2.
Primary and secondary efficacy outcome measures (PP population)
| BMV dressing | CBD ointment | Significance for the difference | |
|---|---|---|---|
| TSS-4 mean total score | |||
| Week 1 | 4.240 | 4.179 | P = 0.708 |
| Week 2 | 3.380 | 3.098 | P = 0.062 |
| Week 3 | 2.752 | 2.550 | P = 0.164 |
| Week 4 (primary endpoint) | 1.981 | 1.693 | P = 0.079 |
| 3-month follow-up | 2.773 | 3.401 | P = 0.101* |
| TSS-4 mean subscores at week 4 | |||
| Redness/erythema | 0.907 | 0.877 | P = 0.619 |
| Scale/crusting | 0.444 | 0.306 | P = 0.015 |
| Thickening/elevation | 0.540 | 0.444 | P = 0.158 |
| Pruritus | 0.092 | 0.059 | P = 0.302 |
| PGA mean score: Investigator rated | |||
| Week 1 | 2.422 | 2.385 | P = 0.607 |
| Week 2 | 2.081 | 2.020 | P = 0.427 |
| Week 3 | 1.876 | 1.847 | P = 0.707 |
| Week 4 | 1.401 | 1.344 | P = 0.543 |
| PGA mean score: Patient rated | |||
| Week 1 | 2.323 | 2.298 | P = 0.781 |
| Week 2 | 1.978 | 1.903 | P = 0.428 |
| Week 3 | 1.777 | 1.675 | P = 0.268 |
| Week 4 | 1.479 | 1.297 | P = 0.090 |
| DLQI mean total score | |||
| Week 1 | 5.914 | 5.366 | P = 0.154 |
| Week 2 | 4.787 | 4.381 | P = 0.296 |
| Week 3 | 3.880 | 3.531 | P = 0.334 |
| Week 4 | 3.466 | 2.743 | P = 0.044 |
| 3-month follow-up | 3.096 | 4.353 | P = 0.146† |
| TSS-3 mean total score | |||
| Week 4 | 2.481 | 2.292 | P = 0.225 |
| Target plaques surface area at week 4 (cm2) | 25.267 | 21.318 | P = 0.050 |
| Success/disappearance of active lesions at week 4 (% of patients) | |||
| PGA (investigator rated) | 52.6% | 56.5% | P = 0.329‡ |
| TSS-4 (investigator rated) | 51.9% | 57.3% | P = 0.201‡ |
| TSS-3 (assessor rated) | 21.8% | 22.9% | P = 0.797‡ |
| Relapse/rebound during follow-up | 17.3% | 19.8% | NA |
| Patients’ acceptability/satisfaction at week 4, VAS (mm) | 76.9 | 79.6 | P = 0.285§ |
Number of observations available for TSS-4 at 3-month follow-up: N = 67 in each group.
Number of observations available for DLQI at 3-month follow-up: N = 66 and 76, in BMV and CBD group respectively.
Cochran–Mantel–Haenszel test, stratified by centre.
anova model, with treatment and centre as fixed effects.
Data are adjusted means from ancova model with the parameter as dependent variable, treatment and centre as fixed effects and baseline value as covariate, unless otherwise specified, with relevant P-value for between treatment groups comparison.
BMV, betamethasone valerate dressing; CBD, calcipotriol–betamethasone dipropionate ointment; DLQI, dermatology life quality index; PGA, psoriasis global assessment; PP, per protocol; TSS, total severity score; VAS, visual analogue scale; NA, not available.
Figure 2.
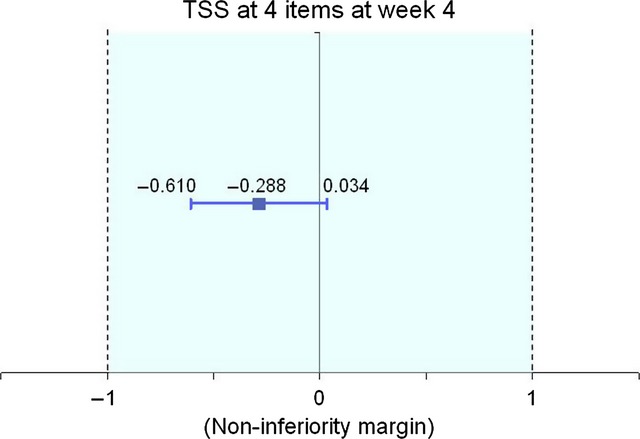
Efficacy: primary outcome measure (TSS-4 score) assessment at week 4.
Table 3.
Primary and secondary efficacy outcome measures (ITT population)
| BMV dressing | CBD ointment | Significance for the difference | |
|---|---|---|---|
| TSS-4 mean total score | |||
| Week 1 | 4.348 | 4.232 | P = 0.440 |
| Week 2 | 3.558 | 3.136 | P = 0.005 |
| Week 3 | 2.878 | 2.513 | P = 0.012 |
| Week 4 (primary endpoint) | 2.215 | 1.679 | P = 0.001 |
| 3-month follow-up | 2.215 | 1.676 | P = 0.001* |
| TSS-4 mean subscores at week 4 | |||
| Redness/erythema | 0.974 | 0.873 | P = 0.084 |
| Scale/crusting | 0.499 | 0.289 | P < 0.001 |
| Thickening/elevation | 0.603 | 0.437 | P = 0.009 |
| Pruritus | 0.142 | 0.073 | P = 0.072 |
| PGA mean score: Investigator rated | |||
| Week 1 | 2.446 | 2.394 | P = 0.420 |
| Week 2 | 2.135 | 2.018 | P = 0.097 |
| Week 3 | 1.927 | 1.784 | P = 0.057 |
| Week 4 | 1.454 | 1.295 | P = 0.073 |
| PGA mean score: Patient rated | |||
| Week 1 | 2.362 | 2.304 | P = 0.474 |
| Week 2 | 2.041 | 1.894 | P = 0.100 |
| Week 3 | 1.823 | 1.643 | P = 0.052 |
| Week 4 | 1.558 | 1.267 | P = 0.007 |
| DLQI mean total score | |||
| Week 1 | 6.046 | 5.781 | P = 0.462 |
| Week 2 | 5.041 | 4.590 | P = 0.232 |
| Week 3 | 3.949 | 3.603 | P = 0.315 |
| Week 4 | 3.717 | 2.908 | P = 0.022 |
| 3-month follow-up | 3.000 | 4.529 | P = 0.076† |
| TSS at 3 items mean total score | |||
| Week 4 | 2.665 | 2.309 | P = 0.016 |
| Target plaques surface area at week 4 (cm2) | 27.687 | 21.281 | P = 0.002 |
| Success/disappearance of active lesions at week 4 (% of patients) | |||
| PGA (investigator rated) | 46.1 | 55.3 | P = 0.057‡ |
| TSS-4 (investigator rated) | 45.5 | 56.6 | P = 0.039‡ |
| TSS-3 (assessor rated) | 17.6 | 23.3 | P = 0.185‡ |
| Relapse/rebound during follow-up | 14.5% | 19.5% | ND |
| Patients’ acceptability/satisfaction at week 4, VAS (mm) | 74.945 | 79.634 | P = 0.043§ |
Number of observations available for TSS-4 at 3-month follow-up: N = 72 and 81, in BMV and CBD group respectively.
Number of observations available for DLQI at 3-month follow-up: N = 71 and 81, in BMV and CBD group respectively.
Cochran–Mantel–Haenszel test, stratified by centre.
anova model, with treatment and centre as fixed effects.
Data are adjusted means from ancova model with the parameter as dependent variable, treatment and centre as fixed effects and baseline value as covariate, unless otherwise specified, with relevant P-value between treatment groups comparison.
BMV, betamethasone valerate dressing; CBD, calcipotriol–betamethasone dipropionate ointment; DLQI, dermatology life quality index; PGA, psoriasis global assessment; ITT, intention to treat; TSS, total severity score; VAS, visual analogue scale; ND, not done.
Secondary efficacy measures were significantly reduced or improved from baseline at all postbaseline time points, in both groups for both the PP and ITT analyses. The PP analysis found no significant difference between the BMV and CBD groups according to most variables, including (Table2):
TSS-4 total score on weeks 1–3, and follow-up
TSS-4 subscores for erythema, elevation and pruritus
PGA scores evaluated by investigators and patients
disappearance of the active lesions at week 4, whether assessed on PGA or TSS-4 by investigators, or on TSS-3 by assessors
DLQI scores at weeks 1–3 and 3-month follow-up
relapse/rebound during follow-up rates and median times to occurrence (56 vs. 57 days in the BMV and CBD groups respectively);TSS-3 total score at week 4. Significant differences favouring CBD ointment were occasionally observed at some time points during the treatment period (e.g. DLQI score and target plaques area at week 4), but they were transient (Table2).
The ITT analysis found more significant differences favouring CBD ointment (Table3) at week 4, which included scale/crusting and thickening/elevation subscores, patient-rated (but not investigator-rated) PGA, DLQI score, TSS-3 score, target plaques surface, investigator-rated TSS-4-based clearance rate and patients’ satisfaction measured by VAS. The response also was more rapid in the CBD ointment group according to TSS-4 reduction from week 1 onward.
In both treatment groups, the patients’ improvement during the study period was less marked according to photographic assessment of TSS-3 by an independent assessor than according to clinical evaluation of TSS-4 by the blinded investigators.
The mean exposure period was 26.3 days in the BMV group and 26.6 days in the CBD group. The number of treatment-emergent AEs (TEAEs; 20 in both groups) and the proportion of patients with TEAEs (8.48% in the BMV group and 9.43% in the CBD group) were comparable in both groups. No signs of skin atrophy on treated areas were noted in either group. Nasopharyngitis (two patients in the BMV and four in the CBD group) was the most common TEAE. Only one patient in the BMV group reported an adverse drug-related reaction (burning sensation). One non-treatment-related serious AE (stroke) was reported in one patient in the BMV group. This patient was the only one who discontinued prematurely the study due to a TEAE. No clinically relevant changes from baseline of vital signs were observed in either treatment group.
Discussion
This prospective, multicentre, randomized, assessor-blind study showed that the BMV dressing was non-inferior to the CBD ointment in the treatment of patients with mild to moderate CPP, according to both PP and ITT analyses of the primary endpoint (TSS-4 score at week 4). A statistically significant difference was noted between the two groups in the ITT analysis, but it did not reach the conservatively predetermined non-inferiority margin (1-point TSS-4 score), and thus may be considered to have limited, if any, clinical relevance. Overall, patients’ psoriasis and QoL significantly improved from baseline to the end of treatment in both groups.
Due to the different nature of the investigational products (dressing vs. ointment), a double-blind design was not applicable. The 10% drop-out rate observed in the study was acceptable, considering both the medical condition studied and the topical route of the treatments. The significant decrease in psoriasis scores from baseline to week 4, and the between-groups differences found in efficacy analyses demonstrate our study's sensitivity.
The comparison between the BMV dressing and CBD ointment is challenging as the former has a single active ingredient (betamethasone 17-valerate), whereas the latter has two active ingredients (betamethasone dipropionate and calcipotriol), which have been shown to be significantly more effective than either ingredient alone.13,20 Accordingly, our study found several significant differences indicating CBD ointment's more rapid and robust effect during the treatment period, especially in the ITT analysis and on the hyperkeratotic component of psoriasis (scale/crusting). However, it should be stressed that these findings do not invalidate the BMV dressing non-inferiority conclusion based on primary efficacy analyses. Typically, the significant differences found in the ITT analysis were not confirmed in the PP analysis. Moreover, the statistically significant differences often appeared to be of limited clinical relevance. For example, considering that the mean number of target plaques was 2.5 and the difference in target plaque surface area between the treatment groups at week 4 was 3.9 and 6.4 cm2 in the PP and ITT populations, respectively, the mean additional benefit of CBD over BMV was 2 cm2 (1.5–2.5 cm2) per target plaque.
It was surprising to note in both treatment groups that the photographic assessment of target plaque improvement by distant assessors displayed notably inferior results than those of the clinical assessments of the same plaques by blinded investigators. As this finding mainly depended on ratings of the ‘erythema/redness’ subscore by distant assessors, we believe that it may have been caused by systematic alteration of colour reproduction on the digitalized photographs.
The 8-week follow-up treatment-free phase was included in the study according to current recommendations to observe relapse-free rates and durations. Among patients having reached target plaques clearance, relapse/rebound rates during follow-up were low and comparable in the two groups (14.5–17% vs. 19.5–20% in the BMV and CBD groups respectively). There was no difference in median time to relapse/rebound. A comparable 14% relapse rate was observed during a 12-week follow-up period in a previous randomized trial comparing the same BMV 0.1% dressing to a BMV 0.1% cream.10
Both treatments were safe. Rates of TEAEs were low (<10%), similar in the two groups and consistent with the established safety profile of both products. All TEAEs were mild or moderate. We also found no indication that BMV dressing had atrophogenic potential, in accordance with previous clinical results.9,10,21
An important result of this study is the favourable impact on patients’ QoL, measured by the validated DLQI instrument. DLQI scores were significantly improved at the end of the study with both treatments (5.33-point and 5.62-point reductions in the BMV and CBD group respectively) with a significant difference favouring CBD at week 4, but not at weeks 1–3, nor at the 3-month follow-up. Interestingly, the significant QoL improvement was preserved during the 8-week follow-up observational period in patients with complete remission of the disease in the BMV group (mean DLQI: 3.60 ± 4.70; median: 2.00), whereas in the CBD group a more rapid worsening was reported (mean DLQI: 4.53 ± 4.54; median: 3.00). Consistently, slightly better scores for the main efficacy variables (TSS-4, TSS-3, investigator- and patient-rated PGA) were reported at the end of the 8-week observational phase in the BMV group than the CBD group.
Our results are consistent with those of previous studies of the same products. In patients with mild to moderate CPP localized at elbows and/or knees, the BMV dressing was found to clear target plaques in 53% and 60% of patients after 3 and 5 weeks of treatment respectively.10 The BMV reduced the TSS score by −5.36 after a 4-week treatment in patients with baseline psoriasis severity similar to our patients (mean baseline TSS score: 6.91).22 Studies of the CBD fixed combination ointment over a 4-week period showed efficacy results that are consistent with the findings of this study.11–14,18
Conclusion
BMV dressing is non-inferior to CBD ointment in patients with mild to moderate CPP. Both treatments significantly improve patients’ psoriasis and QoL.
Acknowledgments
We wish to thank the principal investigators and site staff of all the research centres who participated in the study. We thank the staff of CROS NT S.r.l., Verona (I), in charge of the statistical analysis and the staff of CROMSOURCE S.r.l., Verona (I) for the study management and study sites monitoring activities. We thank Stefano Rovati and Arturo Lanzarotti (IBSA Institut Biochimique SA) and Sophie Azzaro (Laboratoires Genévrier SA) for critical review of the manuscript. We also acknowledge the writing and editorial assistance provided by Bruno Trumbic (Cap Evidence, Paris [F]), and funded by Laboratoires Genévrier SA, for the preparation of this manuscript.
This study was sponsored by IBSA Institut Biochimique SA and Laboratoires Genévrier SA.
References
- 1.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 2.Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339–342. [PubMed] [Google Scholar]
- 3.Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752–756. doi: 10.1001/archderm.139.6.752. [DOI] [PubMed] [Google Scholar]
- 4.Finlay AY. Psoriasis from the patient's point of view. Arch Dermatol. 2001;137:352–353. [PubMed] [Google Scholar]
- 5.Del Rosso J, Friedlander SF. Corticosteroids: options in the era of steroid-sparing therapy. J Am Acad Dermatol. 2005;53:S50–S58. doi: 10.1016/j.jaad.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Volden G, Kragballe K, van de Kerkhof PC, Aberg K, White RJ. Remission and relapse of chronic plaque psoriasis treated once a week with clobetasol propionate occluded with a hydrocolloid dressing versus twice daily treatment with clobetasol propionate alone. J Dermatolog Treat. 2001;12:141–144. doi: 10.1080/09546630152607862. [DOI] [PubMed] [Google Scholar]
- 7.Seidenari S. Double blind, double placebo study in human volunteers. Modena, Italy: Università degli Studi di Modena e Reggio Emilia; 2001. Comparative study of the vasoconstrictor and anti-inflammatory activity (“blanching test”) of IBSA TAPE (betamethasone valerate 0.1% medicated plaster) vs. betamethasone valerate cream. [Google Scholar]
- 8.Seidenari S. Studio in doppio cieco. Modena, Italy: Università degli Studi di Modena e Reggio Emilia; 2003. Studio comparativo di IBSA TAPE (betametasone valerato cerotto), betametasone valerato e clobetasolo propionato crema, nel test di placca psoriasica. [Google Scholar]
- 9.Pacifico A, Daidone R, Peris K. A new formulation of an occlusive dressing containing betamethasone valerate 0.1% in the treatment of mild to moderate psoriasis. J Eur Acad Dermatol Venereol. 2006;20:153–157. doi: 10.1111/j.1468-3083.2006.01387.x. [DOI] [PubMed] [Google Scholar]
- 10.Naldi L, Yawalkar N, Kaszuba A, et al. Efficacy and safety of the betamethasone valerate 0.1% plaster in mild-to-moderate chronic plaque psoriasis: a randomized, parallel-group, active-controlled, phase III study. Am J Clin Dermatol. 2011;12:191–201. doi: 10.2165/11539780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Douglas WS, Poulin Y, Decroix J, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol. 2002;82:131–135. doi: 10.1080/00015550252948194. [DOI] [PubMed] [Google Scholar]
- 12.Guenther L, van de Kerkhof PC, Snellman E, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double-blind, vehicle-controlled clinical trial. Br J Dermatol. 2002;147:316–323. doi: 10.1046/j.1365-2133.2002.04967.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389–393. doi: 10.1159/000066440. [DOI] [PubMed] [Google Scholar]
- 14.Papp KA, Guenther L, Boyden B, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J Am Acad Dermatol. 2003;48:48–54. doi: 10.1067/mjd.2003.130. [DOI] [PubMed] [Google Scholar]
- 15.Traulsen J, Hughes-Formella BJ. The atrophogenic potential and dermal tolerance of calcipotriol/betamethasone dipropionate ointment compared with betamethasone dipropionate ointment. Dermatology. 2003;207:166–172. doi: 10.1159/000071788. [DOI] [PubMed] [Google Scholar]
- 16.Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 17.Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The dermatology life quality index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035. doi: 10.1111/j.1365-2133.2008.08832.x. [DOI] [PubMed] [Google Scholar]
- 18.Mason J, Mason A, Cork MJ. Topical Preparations for the Treatment of Psoriasis in Primary Care: A Systematic Review. Heslington, United Kingdom: Centre for Health Services Research, University of Newcastle upon Tyne; 2002. [Google Scholar]
- 19.van de Kerkhof PC, Hoffmann V, Anstey A, et al. A new scalp formulation of calcipotriol plus betamethasone dipropionate compared with each of its active ingredients in the same vehicle for the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. Br J Dermatol. 2009;160:170–176. doi: 10.1111/j.1365-2133.2008.08927.x. [DOI] [PubMed] [Google Scholar]
- 20.Mason AR, Mason J, Cork M, Dooley G, Edwards G. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2009:CD005028. doi: 10.1002/14651858.CD005028.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Queille-Roussel C. Evaluation of the Skin Atrophogenic Potential of IBSA TAPE (Betamethasone 0.1% Tape) Versus Betamethasone Valerate 0.12% Creams in Normal Healthy Volunteers. Nice, France: Centre de Pharmacologie Clinique Appliquée à la Dermatologie; 2002. [Google Scholar]
- 22.Ortonne JP. An Exploratory, Open-Label, Intra-Individual, Active-Controlled Study Comparing the Efficacy and Safety of BETESIL® Versus DAIVOBET® for the Treatment of Chronic Plaque Psoriasis. Nice, France: Centre de Pharmacologie Clinique Appliquée à la Dermatologie; 2010. [Google Scholar]