Abstract
Background
Scabies has been estimated to affect approximately 300 million people worldwide each year. Scabies rates are high and pose a significant public health problem in Fiji. Community-based comparison treatment trials have not been undertaken. We estimated scabies prevalence and compared the efficacy and tolerability of mass drug administration (MDA) of benzyl benzoate lotion (BB) or oral ivermectin (IVM) in two villages in Fiji.
Methods
A prospective MDA trial was undertaken in two Fijian villages, comparing three daily applications of BB with single dose IVM or permethrin cream for those aged under two years. The therapies were offered to all community members regardless of the presence of scabies or its symptoms. The difference in prevalence was measured before and after the intervention and absolute risk reduction (ARR) and relative risk (RR) calculated.
Results
In the BB group, there were 572 eligible participants, of whom 435 (76%) enrolled and 201 (46%) returned for follow-up. In the IVM group, there were 667 eligible participants, of whom 325 (49%) enrolled and 126 (39%) returned. Scabies prevalence was lower after the intervention in both groups. It fell from 37.9 to 20.0% (ARR 18.0%; RR 0.52) in the BB group and from 23.7 to 9.5% (ARR 14.2%; RR 0.40) in the IVM group.
Conclusions
Our study provides proof of principle that MDA for scabies can reduce scabies prevalence at the community level, and that there was no significant difference in this trial between BB and oral IVM.
Introduction
Scabies, due to infestation with the Sarcoptes scabiei mite, has been estimated to affect approximately 300 million people worldwide each year. Its direct impact is itching, but secondary bacterial infection with Streptococci and Staphylococci is frequent and can lead to serious and potentially fatal complications, including invasive bacterial infections, renal failure, and chronic rheumatic heart disease.1–4.
The highest rates of scabies in the world are found in Pacific island countries.5 In a population-based survey in Fiji, 24% of participants had scabies, with a particularly high prevalence in young children (L. Romani, personal communication). A prospective study in Fijian schoolchildren documented scabies incidence at 51 cases per 100 person-years.6
Scabies treatment is primarily with application of topical agents, including benzyl benzoate and permethrin cream, which was introduced to Fiji in 2006 and is now the standard of care.7 As S. scabiei is transmitted by close body contact or shared objects, treatment of close contacts is also recommended. One oral agent, ivermectin (IVM), has been used as single dose scabies treatment, repeated at two weeks if symptoms persist.8–10
The frequency of reinfestation in endemic settings has led to consideration of mass drug administration (MDA) as a public health option for scabies control. Small, uncontrolled studies in closed communities such as prisons and aged care facilities have suggested that treatment of entire communities can sharply reduce the prevalence of infestation,8,11–13 using either topical treatment or oral IVM, the latter perhaps offering better adherence and fewer side effects.14 To assess the potential role of MDA in an open community setting in the general population, we studied oral vs. topical MDA in two Fijian villages.
Materials and methods
We performed a prospective trial comparing the efficacy and tolerability of two MDA treatment regimens for the control of scabies by offering treatment to all community members regardless of the presence of scabies or its symptoms. Hence, all family members of study participants, including asymptomatic family members of patients with scabies living in either village, were offered treatment immediately after examination and included in the study.
We conducted the study in two Fijian subdivisions (local administrative areas) in June and July 2004. The sites were selected by the Fiji Ministry of Health as having approximately the same number of inhabitants and being within a 2-hour drive of the capital, Suva, but relatively isolated from each other and from other communities. Allocation to treatment was decided by administrative chance based on the availability of medication at start point. In the Tailevu subdivision, three closely linked settlements (Sawakasa, Dakuinuku, and Lodoni, total population 572) were classified as one site. In the Rewa subdivision, Raralevu village was selected (total population 667). All Fijian nationals living in either of the two sites were considered eligible for the study and were enrolled after signing the consent forms, with parents signing for their children. Study participants were registered by name, age, and sex and were weighed at initial visit. After enrollment, participants were identified only by their numeric code and no further identifying data were used in the study.
Participants were asked to complete questionnaires at enrollment and follow-up visit, in either English or Fijian, and assisted by the local community nurse if necessary. Each participant was examined by a healthcare professional with experience in scabies diagnosis and treatment and reviewed by a senior doctor if any skin lesions were seen. The diagnosis of scabies was made clinically, based on the presence of characteristic lesions with or without a history of itch. Suspected scabies lesions, either papules, pustules, or crusted lesions on the trunk and limbs, were counted and other dermatological conditions, such as boils and sores, eczema, and tinea were documented before and after treatment. If appropriate, oral antibiotics were given for secondarily infected lesions and therapy provided for other skin conditions. The extent of scabies was quantified as mild (10 lesions or fewer), moderate (11–49), severe (50 or more), or crusted (confluent lesions, too many to count).15–17 The diagnosis of infected lesions was made based on the presence of erythema, crusting, or pustules.
Interventions
All MDA study participants in the Rewa subdivision site (IVM group) aged 2 years and over with no medical contraindications received a single dose of oral IVM at 200 μg/kg body weight (Stromectol® 3 mg tablets; Merck Sharp & Dohme, South Granville, NSW, Australia), rounded to whole 3 mg IVM tablets. IVM was taken orally under direct observation, immediately after examination. Children aged <2 years received topical 5% permethrin cream (Lyderm®; Makans Drug & Pharmaceutical Supplies, Taravao, Fiji) to be applied from neck to toes. Pregnant (n = 2) and lactating women, and people who reported a history of neurological disease, such as stroke (n = 2) or neurofibromatosis (n = 1), were also given 5% permethrin cream.
At the time of the study, benzyl benzoate was the standard treatment for scabies in Fiji, available free through clinics, and both benzyl benzoate and permethrin were available for purchase from the private pharmacies. According to the national protocol, participants in the Tailevu subdivision site (benzyl benzoate, BB group) were given 25% topical benzyl benzoate lotion, diluted to 12.5% for children aged 2–12 years, and 8.3% for those aged less than 2 years. Adults were each provided with one 100 ml bottle of benzyl benzoate 25% and asked to apply it that night, and on two successive nights, from neck to toes, covering all areas. Participants were asked to leave it on for 24 hours, then wash and re-apply, following the recommended guidelines for benzyl benzoate in Fiji at that time.
Participants in both sites were provided with information about the medication they were offered and advised of the need to wash themselves, their bed linen, and clothes used within the previous 24 hours.
Follow-up assessment
In the BB group, the follow-up assessment took place 28 days after the initial visit, whereas in the IVM group it was at 24 days. At the follow-up visit, participants were re-examined; scabies lesions were counted and recent medical history recorded. If required, patients were given treatment for any persistent skin lesions.
All participants were specifically asked if they had experienced any adverse events apart from itch following treatment and if they had sought medical care for these events. In the self-administered post-treatment questionnaire, information was additionally collected on itch after treatment.
Statistical analysis
Data were entered into an SPSS database version 11.5 for Windows. Statistical analysis was conducted using STATA 12 (StataCorp LP, College Station, TX, USA) and IBM SPSS statistics Version 19 (IBM Company, Armonk, NY, USA). Differences in distributions of categorical variables were tested using a chi-squared test. Student's two-sample t-test was used to compare means. The effects of the interventions were measured by comparing the prevalence of scabies at follow-up with that before intervention by comparing the reduction of absolute risk with calculation of 95% confidence intervals (CI) and relative risk with 95% CIs between the two periods. For comparison of other skin diseases pre- and post-intervention, McNemar's test was used. Non-overlapping CIs were considered to be at the significance level.
Ethical approval
Ethics approval for the study was obtained from the Fiji National Research Ethics Review Committee and reviewed by St. Vincent's Hospital Ethics Committee in Sydney.
Results
Participation and follow-up rates
A higher proportion of the population was enrolled in the BB group than the IVM group (76% vs. 49% respectively, Fig.1). Otherwise, participant demographics at the two sites did not differ significantly in regards to sex, age, or weight at baseline (Table1). Of the 435 enrolled in the BB group, 201 (46%) returned for follow-up. In the IVM group, 325 were enrolled and 126 (39%) returned for follow-up.
Figure 1.
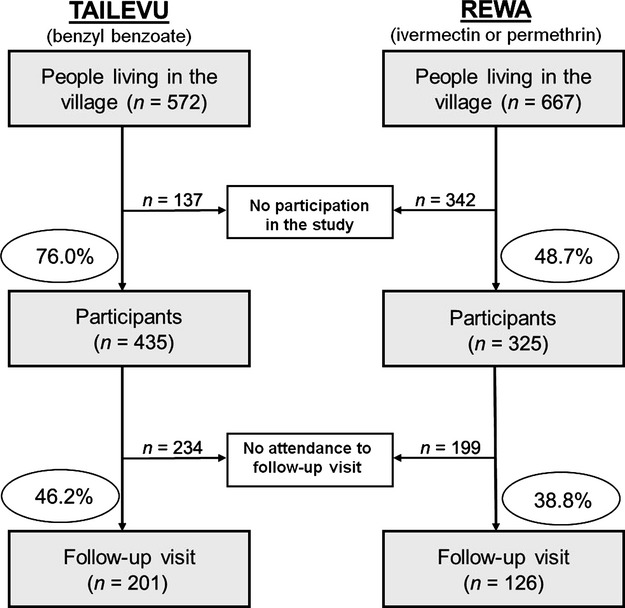
Flow chart of participation and follow-up rate at each study site
Table 1.
Demographics characteristics of baseline study populations
| Characteristics | Overall n (%) | BB n (%) | IVM n (%) | P-value |
|---|---|---|---|---|
| All participants | 760 (100.0) | 435 (57.2) | 325 (42.8) | – |
| Sex | ||||
| Male | 353 (46.4) | 194 (44.6) | 159 (48.9) | 0.257 |
| Female | 407 (53.6) | 241 (55.4) | 166 (51.1) | |
| Ethnicity | ||||
| Fijian | 524 (98.5) | 278 (97.5) | 246 (99.6) | 0.053b |
| Othersa | 8 (1.5) | 7 (2.5) | 1 (<1) | |
| Median age (IQR) | 16 (8–43) | 16 (8–41) | 16 (8–44) | 0.795c |
| <5 years | 100 (13.2) | 55 (12.6) | 45 (13.8) | 0.785b |
| 5–14 years | 264 (34.7) | 153 (35.2) | 111 (34.2) | |
| 15–29 years | 134 (17.6) | 81 (18.6) | 53 (16.3) | |
| 30+ years | 262 (34.5) | 146 (33.6) | 116 (35.7) | |
BB, benzyl benzoate group; IVM, ivermectin group.
Includes six Indian–Fijian (all in Tailevu) and two European (one in Tailevu and one in Rewa).
Chi-squared test.
Wilcoxon rank-sum test.
Specifically there was significantly higher follow-up in children under the age of five years in the BB group (45.5%) than in the IVM group (20.0%) (P = 0.01; OR 3.3; 95% CI 1.4–8.2), in contrast to those aged 5–14 years, where 54.2 and 70.3% attended follow-up (P = 0.01; OR 0.5; 95% CI 0.3–0.8). In people aged 15–29 years, the difference was highest, 29.6% attending for follow-up in the BB and 9.4% in the IVM group (P = 0.01; OR 4.0; 95% CI 1.4–11.4).
At the baseline visit, differences were noted between the two groups, in particular scabies prevalence was higher in the BB group (37.9%) compared to the IVM group (23.7%) (Table2). Infected scabies was also more common in the BB group. Overall, the severity of scabies at baseline, defined by the number of lesions, did not significantly differ between the two villages. Many study participants had other skin conditions at baseline, overall more common in the IVM group (36.6%) compared to the BB group (26.7%).
Table 2.
Dermatologic conditions detected at initial examination and at follow-up
| Baseline | Follow-up | |||||
|---|---|---|---|---|---|---|
| Overall n = 760 | BB n = 435 | IVM n = 325 n = 325 | Overall n = 327 | BB n = 201 | IVM n = 126 | |
| Scabies | 242 (31.8) | 165 (37.9) | 77 (23.7) | 52 (15.9) | 40 (19.9) | 12 (9.5) |
| Number of lesions | ||||||
| ≤10 | 111 (45.9) | 65 (39.4) | 46 (59.7) | 25 (48.1) | 18 (45.0) | 7 (58.3) |
| 11–49 | 74 (30.6) | 50 (30.3) | 24 (31.2) | 21 (40.4) | 16 (40.0) | 5 (41.7) |
| ≥50 | 16 (6.6) | 9 (5.5) | 7 (9.1) | 4 (7.7) | 4 (10.0) | 0 (0.0) |
| Crusteda | 4 (1.6) | 4 (2.4) | 0 (0.0) | 2 (3.8) | 2 (5.0) | 0 (0.0) |
| Number not recorded | 37 (15.3) | 37 (22.4) | 0 (0.0) | – | – | – |
| Infected scabies | 85 (11.2) | 68 (15.6) | 17 (5.2) | 10 (3.1) | 8 (4.0) | 2 (1.6) |
| Boils/abscess | 27 (3.6) | 7 (1.6) | 20 (6.2) | 18 (5.5) | 10 (5.0) | 8 (6.4) |
| Eczema/dermatitis | 46 (6.1) | 23 (5.3) | 23 (7.1) | 19 (5.8) | 10 (5.0) | 9 (7.1) |
| Tinea | 144 (19.0) | 71 (16.3) | 73 (22.5) | 72 (22.0) | 41 (20.4) | 31 (24.6) |
| Other skin disease | 25 (3.2) | 20 (4.6) | 5 (1.5) | 14 (4.3) | 7 (3.5) | 7 (5.6) |
BB, benzyl benzoate group; IVM, ivermectin group.
Confluence of lesions precluded accurate counting.
Scabies prevalence after mass drug administration
Comparing the prevalence of scabies before and after MDA, the reduction in prevalence was significant in both groups but similar in magnitude (Table3). After MDA, scabies lesions were present in 40 of 201 people examined (20.0%) in the BB group and 12 of 126 examined (9.5%) in the IVM group. The absolute risk reduction in the BB group was 18% (95% CI 10.5–24.8) with a relative risk of 0.52 (95% CI 0.39–0.71), whereas in the IVM group the absolute risk reduction was 14.2% (95% CI 6.5–20.5) with a relative risk of 0.40 (95% CI 0.23–0.71). Similar reduction in prevalence was observed consistently across all age groups.
Table 3.
Scabies positivity rate before and after mass drug administration in the two groups overall and by age group
| BB | IVM | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 435) n (%) | Follow-up (n = 201) n (%) | Absolute risk reduction% (95% CI) | Relative risk (95% CI) | Baseline (n = 325) n (%) | Follow-up (n = 126) n (%) | Absolute risk reduction% (95% CI) | Relative risk (95% CI) | |
| Scabies | 165 (37.9) | 40 (20.0) | 18.0 (10.5–24.8) | 0.52 (0.39–0.71) | 77 (23.7) | 12 (9.5) | 14.2 (6.5–20.5) | 0.40 (0.23–0.71) |
| Age | ||||||||
| <5 years | 38/55 (69.1) | 9/25 (36.0) | 33.1 (10.0–52.1) | 0.51 (0.30–0.91) | 17/45 (37.8) | 0/9 (0.0) | 37.8 (5.3–52.4)a | – |
| 5–14 years | 71/153 (46.4) | 20/83 (24.1) | 22.3 (10.0–33.5) | 0.52 (0.34–0.77) | 18/111 (16.2) | 11/78 (14.1) | 2.1 (8.9–12.1) | 0.91 (0.43–1.67) |
| 15–29 years | 23/81 (28.4) | 4/24 (16.7) | 11.7 (10.0–26.3) | 0.59 (0.23–1.43) | 15/53 (28.3)b | 0/5 (0.0) | 28.3 (16.4–41.6) | – |
| 30+ years | 33/146 (22.6) | 7/69 (10.1) | 12.4 (1.3–21.5) | 0.45 (0.21–1.0) | 27/116 (23.28) | 1/34 (3.0) | 20.3c (7.6–29.1) | 0.13 (0.02–0.91) |
BB, benzyl benzoate group; IVM, ivermectin group.
Permethrin was used in <2 year olds; however, no significant difference was found.
Permethrin was used in two pregnant women with no scabies at start, no follow-up visit.
Permethrin was used in three cases with a history of neurological disease, two had scabies at start, none of the three at follow-up.
Other skin conditions were noted in 32.3% (65 of 201) of participants in the BB group and in 42.1% (53 of 126) in the IVM group after treatment. There was no significant difference in the change in prevalence of other skin conditions at baseline and follow-up in either group overall; however, diagnoses of boils and sores increased in the BB group from 1.0 to 5.0% (P = 0.04) and fungal diseases in the IVM group from 15.1 to 24.6% (P = 0.02) in participants who attended both visits.
Tolerability and acceptability of treatments
Nineteen of 200 people (9.5%) reported having had adverse events (apart from itch exacerbation) after treatment in the BB group whereas only three of 126 (2.4%) did in the IVM group (P = 0.01, OR 4.3, 95% CI 1.25–14.86). The reported side effects included stinging and burning in the BB group and lethargy and giddiness in the IVM group. No study participants presented for medical care related to adverse events, and community-wide no hospitalizations or deaths occurred during the study period. Mean age in those who reported adverse events was not significantly different between the two villages, 26 years in both groups. No side effects in the IVM group occurred in children under the age of 10 years. In the BB group, 38 of 192 (19.8%, 95% CI 14.4–26.1) experienced worsening of their itch after treatment, compared to four of 111 (3.6%, 95% CI 1.0–9.0) in the IVM group.
Discussion
This is the first comparative study of MDA for scabies in a general population setting and sets a precedent for possibly conducting general community MDA.
Our study showed that MDA for scabies using either topical or oral medication reduced scabies prevalence by a factor of up to 60%. Although the relative reduction in risk was higher for MDA of IVM (60%), compared to benzyl benzoate (48%), the difference was not statistically significant. Both strategies had a low rate of adverse events, but IVM was better tolerated, particularly in terms of stinging and burning. The high scabies prevalence at baseline in the BB group of 38%, and IVM of 24% with frequent secondary bacterial infection, as has been described in other studies, indicates that scabies is a major health issue in Fiji.6,18
Scabies was found to be a major public health problem in our two examined Fijian villages, with particularly high rates among children in both villages. In our study, 69% of all children examined under the age of 5 years had scabies in Tailevu, compared to 43% in Rewa. Subsequently, scabies prevalence rates of 18% in schoolchildren and 14% in infants were reported in Fiji (in 2006/2007).6 The infection rates found in our study in Fiji were much higher than in other community-based studies in Brazil, Timor-Leste, the Solomon Islands, Vanuatu, and Kenya.19–23 A study conducted in Papua, New Guinea, found higher scabies prevalence than in our study overall (87% in one village and 52% in another); however, numbers of examined people in this study were low.24
Several previous non-comparative studies have shown a marked reduction in scabies prevalence following MDA. These include an MDA of topical permethrin in the San Blas islands of the Republic of Panama25 and an MDA of IVM on five isolated islands in the Solomon Islands.21 In these studies, the prevalence of scabies fell from 33 to 25%, respectively, to <1% at three years. However, both studies employed regular follow-up and retreatment to maintain the sustained reduction in prevalence. Whether the reduction seen in this study could be maintained beyond one month without regular follow-up and re-treatment is not known. Our study is the first comparative study of MDA for scabies at the community level for all ages. A previous comparative trial conducted in a closed population of 84 children living in an urban hostel of Delhi found that MDA IVM (two doses) was more effective than individual treatment with permethrin (single application) for symptomatic patients. In the first six months of the study, permethrin was used, and there were 22 cases, while after MDA IVM, there was only one case of scabies detected in the subsequent six months.12
MDA has been documented as being effective for treating endemic scabies in the institutional setting for both children and adults but has never been tested in a comparative study in a general population previously.7,10,11 Our findings provide support for an MDA treatment approach rather than an individual-based approach for communities with a high prevalence of scabies, similar to that used in institutional settings.
There are, however, several limitations to our study. The first is that follow-up was conducted only at 24–28 days after the initial administration of treatment due to operational constraints, and therefore the longer-term impact of MDA on scabies could not be assessed.
There were also considerable differences in participation rates between the two groups; 76% in the BB group and 49% in the IVM group. This may have diminished the apparent effect of the IVM MDA because of a higher risk of reinfestation by non-treated community members in this arm, as those treated may have been exposed to untreated non-participants in their community and hence have had a higher rate of reinfestation. The reasons for the differences in participation may be due to the BB group village being more isolated and that scabies was identified very strongly by this community as a serious and significant health issue (K. Haar, personal communication). Further, there was a high loss to follow-up in this study, 54% in one group and 61% in the other. This may have been because people that were successfully treated did not return for follow-up (K. Haar, personal communication), and particularly those with persisting skin conditions such as tinea, boils, and sores returned. This factor would have underestimated the efficacy of MDA in both arms of the study. Follow-up was particularly low in “working-age” people, with only 9% of participants aged 15–29 years attending in the IVM group. The relatively high follow-up rate of 70% among children aged 5–14 years in the IVM group can be attributed to the research team visiting the school to examine trial participants there. Insufficient communication with the village health workers of the importance of all study participants returning for the second village visit, regardless of their outcome, may have contributed to the poor follow-up rates. To improve community-based studies and to enhance participation and follow-up rates, timely planning and detailed written information with precise dates, locations, and instructions should be provided to all community leaders and participants.
Another confounder to our study was that the villages were not equal in terms of access to water supply and to primary healthcare. The BB group had a communal water source useable only during the day and single dirt road access, which was sometimes impassable during the wet season. In the IVM group, continuous water supply and plumbing to individual residences was common, with tar road village access, and they were located closer to the local community hospital. Although this situation has improved in the intervening years, assessment of these factors in future studies may determine their role.
One limitation of the study is that it was not truly randomized; however, allocation to treatment was decided by administrative chance, based on the availability of medication at start point. In any future study, we would recommend that true randomization is undertaken.
The protocol of applying benzyl benzoate for three consecutive nights was the Fiji Ministry of Health Guidelines for the treatment of scabies at that time. One potential source of bias could be the correct application, which we tried to rule out through specific questions post-treatment. However, we did not control the application per se, nor collect empty bottles. Permethrin was unavailable through the Government healthcare system at the time of the study. Although this protocol allowed IVM to be compared with the standard of care at that time, permethrin cream subsequently became the standard of care and therefore for any future study should include permethrin cream. Ongoing studies in Fiji indicate that the overall prevalence of scabies in the country has not changed since this study was performed (L. Romani, personal communication), suggesting that the introduction of permethrin cream for individual case management has had little impact on disease burden. Therefore, further investigation into population-based control methods are as pressing today as they were when this study was undertaken in 2004.
Our study provides proof of principle that MDA for scabies can reduce scabies prevalence at the community level. A larger study is needed that has longer and more complete follow-up, which includes a cost-effectiveness analysis and compares MDA to the more conventional approach of treating symptomatic cases and their contacts. Without these data, scabies will continue to be an endemic problem in many tropical developing countries.
Acknowledgments
The authors would like to thank the Scientific Research Fund of the Australasian College of Dermatologists, and the estate of Albert W. Johnston and Buster Grant for funding support, and the study participants, the turanga-ni-koros, village elders, community nurses, and volunteer village health workers for their cooperation. Also thanks to Dr. Timaima Tuiketei, Dr. Lepani Waqatakirewa, Dr. Ilisapeci Kubuabola-Samisoni, Jope Davetanivalu, Peter Zinck, Vasiti Nawadra, and the staff from the Tamavua Twomey Hospital. The authors would also like to thank A/Prof. Richard Hillman, Prof. Jonathan Carapetis, and Drs. Delwyn Dyall-Smith and Robert Ma for their preliminary guidance with the project and manuscript review.
References
- 1.Chosidow O. Scabies. N Engl J Med. 2006;354:1718–1727. doi: 10.1056/NEJMcp052784. [DOI] [PubMed] [Google Scholar]
- 2.Svartman M, Finklea JF, Earle DP, et al. Epidemic scabies and acute glomerulonephritis in Trinidad. Lancet. 1972;1:249–251. doi: 10.1016/s0140-6736(72)90634-4. [DOI] [PubMed] [Google Scholar]
- 3.Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767–1774. doi: 10.1016/S0140-6736(06)68772-2. [DOI] [PubMed] [Google Scholar]
- 4.Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis. 2012;25:145–153. doi: 10.1097/QCO.0b013e3283511d27. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Epidemiology and Management of Common Skin Diseases in Children in Developing Countries. Geneva: World Health Organisation; 2005. [Google Scholar]
- 6.Steer AC, Jenney AWJ, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;87:173–179. doi: 10.1371/journal.pntd.0000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steer AC, Tikoduadua LV, Manalac EM, et al. Validation of an integrated management of childhood illness algorithm for managing common skin conditions in Fiji. Bull World Health Organ. 2009b;87:173–179. doi: 10.2471/BLT.08.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leppard B, Naburi AE. The use of ivermectin in controlling an outbreak of scabies in a prison. Br J Dermatol. 2000;143:520–523. doi: 10.1111/j.1365-2133.2000.03704.x. [DOI] [PubMed] [Google Scholar]
- 9.Meinking TL, Taplin D, Hermida JL, et al. The treatment of scabies with ivermectin. N Engl J Med. 1995;333:26–30. doi: 10.1056/NEJM199507063330105. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan JR, Watt G, Barker B. Successful use of ivermectin in the treatment of endemic scabies in a nursing home. Australas J Dermatol. 1997;38:137–140. doi: 10.1111/j.1440-0960.1997.tb01130.x. [DOI] [PubMed] [Google Scholar]
- 11.Paasch U, Haustein UF. Management of endemic outbreaks of scabies with allethrin, permethrin, and ivermectin. Int J Dermatol. 2000;39:463–470. doi: 10.1046/j.1365-4362.2000.00990.x. [DOI] [PubMed] [Google Scholar]
- 12.Abedin S, Narang M, Gandhi V, et al. Efficacy of permethrin cream and oral ivermectin in treatment of scabies. Indian J Pediatr. 2007;74:915–916. doi: 10.1007/s12098-007-0168-x. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Lucho VE, Fallon F, Caputo C, et al. Role of prolonged surveillance in the eradication of nosocomial scabies in an extended care Veterans Affairs medical center. Am J Infect Control. 1995;23:44–49. doi: 10.1016/0196-6553(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 14.Brooks PA, Grace RF. Ivermectin is better than benzyl benzoate for childhood scabies in developing countries. J Paediatr Child Health. 2002;38:401–404. doi: 10.1046/j.1440-1754.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 15.Elmogy M, Fayed H, Marzok H, et al. Oral ivermectin in the treatment of scabies. Int J Dermatol. 1999;38:926–928. doi: 10.1046/j.1365-4362.1999.00865.x. [DOI] [PubMed] [Google Scholar]
- 16.Dourmishev A, Serafimova D, Dourmishev L. Efficacy and tolerance of oral ivermectin in scabies. J Eur Acad Dermatol Venereol. 1998;11:247–251. [PubMed] [Google Scholar]
- 17.Chouela EN, Abeldano AM, Pellerano G, et al. Equivalent therapeutic efficacy and safety of ivermectin and lindane in the treatment of human scabies. Arch Dermatol. 1999;135:651–655. doi: 10.1001/archderm.135.6.651. [DOI] [PubMed] [Google Scholar]
- 18.Steer A, Jenney AJW, Oppedisano F, et al. High burden of invasive β-haemolytic streptococcal infections in Fiji. Epidemiol Infect. 2008;136:621–627. doi: 10.1017/S095026880700917X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heukelbach J, Wilcke T, Winter B, et al. Epidemiology and morbidity of scabies and pediculosis capitis in resource-poor communities in Brazil. Br J Dermatol. 2005;153:150–156. doi: 10.1111/j.1365-2133.2005.06591.x. [DOI] [PubMed] [Google Scholar]
- 20.dos Santos MML, Amaral S, Harmen SP, et al. The prevalence of common skin infections in four districts in Timor-Leste: a cross sectional survey. BMC Infect Dis. 2009;10:61. doi: 10.1186/1471-2334-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence G, Leafasia J, Sheridan J, et al. Control of scabies, skin sores and haematuria in children in the Solomon Islands: another role for ivermectin. Bull World Health Organ. 2005;83:34–42. [PMC free article] [PubMed] [Google Scholar]
- 22.Harris M, Nako D, Hopkins T, et al. Skin infections in Tanna, Vanuatu in 1989. P N G Med J. 1992;35:137–143. [PubMed] [Google Scholar]
- 23.Schmeller W, Dzikus A. Skin diseases in children in rural Kenya: long-term results of a dermatology project within the primary health care system. Br J Dermatol. 2001;144:118–124. [PubMed] [Google Scholar]
- 24.Bockarie MJ, Alexander NDE, Kazura JW, et al. Treatment with ivermectin reduces the high prevalence of scabies in a village in Papua New Guinea. Acta Trop. 2000;75:127–130. doi: 10.1016/s0001-706x(99)00087-x. [DOI] [PubMed] [Google Scholar]
- 25.Taplin D, Porcelain SL, Meinking TL, et al. Community control of scabies: a model based on use of permethrin cream. Lancet. 1991;337:1016–1018. doi: 10.1016/0140-6736(91)92669-s. [DOI] [PubMed] [Google Scholar]


