Abstract
Cyclosporine was proven efficacious in the treatment of feline hypersensitivity dermatitis. This target animal study was conducted to evaluate the safety, tolerability, and pharmacokinetics of ATOPICA for Cats® (cyclosporine oral solution, USP) MODIFIED following 6-month daily dosing in cats. Forty healthy cats (four cats/sex/group) received 0, 8 (1×), 16 (2×), 24 (3×), or 40 (5×) mg/kg cyclosporine once daily for 6 months (183 days). Body weight, food consumption, ophthalmoscopic, physical examinations including neurological assessments, blood pressure, electrocardiography, clinical pathology (hematology, coagulation, clinical chemistry, urinalysis), organ weights, and macroscopic and microscopic examinations were performed and assessed. In addition, blood concentrations of cyclosporine were measured at the pretreatment trough on Days 1, 2, 7, 14, 31, 91, 154, and 182, and post-treatment on Days 1, 31, and 182. Adverse effects possibly related to treatment included prolonged APTT and one report each of bone marrow hypocellularity and lymphoma; all occurred in cats treated with doses more than 16 mg/kg. There was no significant accumulation of cyclosporine beyond the first week of treatment. Results confirm that ATOPICA for Cats is safe and well tolerated in cats without unexpected accumulation beyond the first week of treatment when administered as directed.
Introduction
Cyclosporine (CsA), a selective immunosuppressor, is increasingly used in veterinary medicine to treat a variety of conditions in dogs and cats (Palmeiro, 2013). Recently cyclosporine oral solution received approval by the US Food and Drug Administration (US FDA) as ATOPICA for Cats® (cyclosporine oral solution, USP) MODIFIED (NADA #141-329; Novartis Animal Health US, Inc. (NAH), Greensboro, NC, USA) for the control of feline hypersensitivity dermatitis (HD) in cats. It is a cyclic polypeptide that exerts anti-inflammatory and antipruritic effects in the treatment of allergic dermatitis by preferentially inhibiting the activation of T-lymphocytes on antigenic stimulation through impairing the production of IL-2 and other T-cell-derived cytokines (Robson, 2003b). In addition, CsA has the capacity to inhibit the antigen-presenting function of the skin immune system and block the recruitment and activation of eosinophils, the production of cytokines by keratinocytes, the function of Langerhans cells, the degranulation of mast cells and therefore the release of histamine and pro-inflammatory cytokines (Robson, 2003b).
Adverse effects attributed to CsA use in humans include convulsions, fever, vomiting, diarrhea, high blood pressure, potassium retention, and kidney and liver dysfunction. In cats, few adverse reactions to CsA have been reported, with gastrointestinal signs (diarrhea, vomiting, and anorexia), the most frequently observed reactions (Robson, 2003a; Noli & Scarampella, 2006; Vercelli et al., 2006; Wisselink & Willemse, 2009; King et al., 2012). Similar dose-dependent, reversible adverse reactions are also observed in dogs. However, there are species differences (Robson, 2003a). For example, CsA is metabolized much more slowly by rat liver cells than by dog liver cells (Whalen et al., 1999). Further, unlike dogs, rats tend to accumulate the drug in plasma and tissues and thus are very susceptible to hepato- and nephrotoxicity, with significant mortality occurring at doses of 45 mg/kg/day (Ryffel, 1982). It has also been suggested that the blood concentration of cyclosporine may increase over time due to saturation of the tissue-binding sites (Vaden, 1997; Tanaka et al., 2000). Such accumulation could increase the risk of toxicity during long-term therapy.
This study was conducted to determine the safety and tolerability of a cyclosporine oral microemulsion formulation (ATOPICA for Cats) when repeatedly administered orally for 6 months to adult cats at high doses. Further, the pharmacokinetics of cyclosporine over the 6-month period was assessed to identify if unexpected accumulation occurs in cats.
Materials and Methods
Animals
Forty domestic short-hair cats (20 males and 20 females), approximately 6 months old and weighing between 1.86 and 5.00 kg on Day 1 prior to treatment, were enrolled in a 183-day (6 months) study. These laboratory purpose-bred cats were in good health as determined by a comprehensive physical examination (including neurological assessment) performed by a licensed veterinarian and the results of fecal examination for parasites, clinical pathology (hematology, clinical chemistry, urinalysis), and ophthalmological (performed by a board certified veterinary ophthalmologist), blood pressure and electrocardiographic examinations (a board certified veterinary cardiologist provided a qualitative, quantitative review and report).
Cats were acclimated for approximately 3 weeks prior to study start, housed individually, each with perches, litter pans and enrichment toys, in an environmentally controlled room (room temperature: 64–84 °F; humidity: 30–70%; 12-h light/dark cycle) and were uniquely identified by microchip and ear tattoo. Lab Diet™ (Certified Feline Diet #5003; PMI Nutrition International, Inc., Richmond, IN, USA) and municipal tap water were available ad libitum, except during designated periods.
Study design
This study was a masked, randomized parallel design study with the individual cat as the experimental unit. The study was conducted in accordance with The United States FDA Good Laboratory Practice (GLP) Regulations, 21 CFR Part 58, the Target Animal Safety Guidelines for New Animal Drugs (CVM Guideline 33) and FDA Center for Veterinary Medicine (CVM) Guideline 104. The pharmacokinetic analysis of cyclosporine in blood samples and subsequent statistical evaluation were conducted in compliance with Swiss Ordinance relating to GLP and validated according to FDA guidance on Bioanalytical Method Validation, May 2001. All procedures were reviewed and approved by the local institution's animal care and use committee and were in compliance with NAH Animal Welfare Guidelines, the USDA Animal Welfare Act (2009) and the Public Health Service Policy on Humane Care and Use of Laboratory Animals (1986).
Investigational treatment administration
A final formulation of cyclosporine oral solution, USP MODIFIED (microemulsion solution at 100 mg/mL: ATOPICA for Cats, Novartis Animal Health) was used. Cats were blocked and randomized according to sex and body weight to either a negative control group receiving no treatment or to one of four treatment groups receiving either 1× (8 mg/kg/day), 2× (16 mg/kg/day), 3× (24 mg/kg/day) or 5× (40 mg/kg/day) the maximum therapeutic dose (8 mg/kg/day).
All cats were dosed once daily for 183 consecutive days (6 months), after fasting for 8 h and approximately 2 h before being fed; Day 1 was defined as the first day of treatment. Treatments were administered by unmasked individuals who were not involved in any other assessments. Cyclosporine microemulsion solution was administered into the back of the oral cavity and followed by 1 mL of tap water to ensure adequate ingestion. Doses were calculated based on the most recent body weight. The control group was sham dosed with an empty syringe followed by a 1 mL rinse of tap water in the same manner as the treated groups.
In vivo observations
Cats were observed twice a day throughout the study for morbidity, mortality, injury, and the availability of food and water. Baseline values for body weight and feed consumption were established during acclimation. During treatment administration, body weight was recorded and feed consumption was calculated on a weekly basis.
Detailed clinical observations by a trained technician were made twice daily, approximately 1 and 7 h after dosing. At a minimum, the following was evaluated at each observation: eyes, mucus membranes, respiratory system, circulatory system, autonomic and central nervous systems, somatomotor activity behavior pattern and gastrointestinal system (inappetence, emesis, stool abnormalities and diarrhea). Particular attention was given to the presence of clinical signs indicative of cyclosporine toxicity in cats (lethargy/depression, ataxia, imbalance, anorexia, vomiting, diarrhea, and gingival hypertrophy). A staff veterinarian was available, if necessary, to further investigate any abnormalities.
Ophthalmic examinations were performed by a veterinary ophthalmologist once during acclimation and again prior to necropsy. A licensed veterinarian completed a physical examination, including neurological assessment and blood pressure measurement, on each cat twice during acclimation and at least once monthly during the treatment phase. At a minimum, the following was evaluated at each examination: general health, behavior, body temperature, mucous membranes, integument, equilibrium/coordination, body condition, cardiovascular, respiratory, renal, urogenital, gastrointestinal, musculoskeletal, reproductive system and ocular. Prior to initiation of dosing and on Days 178 or 180, under sedation (acepromazine/ketamine), electrocardiography was performed by a board certified cardiologist. Standard ECGs (10 Lead) were recorded at 50 mm/sec. Using Lead II, the RR, PR, and QT intervals, and QRS duration were measured and recorded. Heart rate was calculated from the number of ECG complexes in 10 sec. At all times during the dosing period, it was possible to withdraw and euthanize any cat for humane reasons.
Clinical pathology
Blood samples for hematology, coagulation (activated partial thromboplastin time and prothrombin time) and clinical chemistry were collected from all cats (fasted overnight) during acclimation and approximately 24 h following the dosing on Days 7, 31, 91, 154, and 183. Urine was also collected on the same occasion by replacing litter with NoSorb® (Catco Inc., Cape Coral, FL, USA) for 16–24 h. Samples were handled and processed according to standard procedures by a diagnostic laboratory.
Pharmacokinetic analysis
Blood samples were collected from fasted cats between 30 and 60 min prior to dosing on Days 1, 2, 7, 14, 31, 91, 154 and 182. In addition, on Days 1 and 31, blood was collected at 1 and 4 h after the dose, and on Day 182 blood was collected at 1, 2, 4, 8, and 24 h after the dose. Whole blood samples were stored frozen at approximately −70 °C and sent to Novartis Centre de Recherche Sante Animale SA, St-Aubin, Switzerland, for analysis by high performance liquid chromatography (HPLC) with mass selective detection (LC-MS) method validated for a range of approximately 3–250 ng/mL cyclosporine A. The analytical laboratory was blinded. Cyclosporine D (Novartis Pharma AG, Basel, Switzerland) was used as an internal standard. Spiked (fortified) specimens covering the analytical range of concentrations of cyclosporine A were prepared using untreated blood to establish the calibration curve by quadratic regression. This curve enabled the recovery corrected determination of cyclosporine A in unknown specimens. Recoveries of cyclosporine A and the internal standards were calculated for spiked blood specimens by comparison of peak areas to those collected for standard solutions without extraction. The lower limit of quantification (LLOQ) for cyclosporine A in this study was 2.95 ng/mL.
Necropsy and histopathology
Cats were euthanized by an intravenous overdose of sodium pentobarbital solution followed by exsanguination. A gross examination was conducted and full complement of organs, and tissues were collected under the supervision of a veterinary pathologist. Organs were removed, examined, weighed (paired organs were weighed together), and where indicated, placed in neutral-buffered formalin for fixation. Eyes and testes were fixed in modified Davidson's fixative, and lungs were infused with formalin. Representative samples of organs and tissues, as well as any gross lesions found, were prepared and processed using standard histological methods. Paraffin sections were stained with hematoxylin–eosin, and slides were examined by a veterinary pathologist using a 4-step grading system (trace, mild, moderate, and severe).
Statistical analysis
Summary statistics (mean and standard deviation) were provided for all variables. SAS (SAS Institute, Inc., Cary, NC, USA) was utilized for all analyses. Analysis of variance (anova) was conducted on any endpoint measured only once (organ weights). Endpoints measured multiple times during the study and having a pretreatment measurement (body weight, food consumption, blood pressure, clinical chemistry, hematology, coagulation, and urinalysis parameters) were analyzed using a repeated measures analysis of covariance (rmancova). Body weight change from baseline was analyzed using a repeated measures analysis of variance (rmanova). P-values of <0.10 were considered significant for the treatment fixed effect and treatment-by-time interaction; all other interactions were considered significant at P < 0.05.
Pharmacokinetic data were evaluated by noncompartmental analysis to determine Cmax, Tmax, and AUC0-last. T1/2 and AUC0-infinity were not estimated due to the chosen sampling scheme (too few sampling points after the last administration to estimate the terminal slope). Dose-dependent pharmacokinetic parameters (AUC and Cmax) were additionally normalized with respect to dose (Cmax_N and AUC0-last_N, respectively). After applying a log transformation, Tmax values were checked to see if they satisfied the normal distribution assumption. Summary statistics (arithmetic and geometric mean, median, standard deviation, coefficient of variation, minimum and maximum values) were provided.
Results
Observations
There were no treatment-related adverse findings from the clinical observations, physical examinations including neurological assessments, blood pressure, quantitative ECG parameters or ophthalmologic assessments, in any cat. Intermittent interventricular conduction disturbances were observed on the electrocardiogram in one 3× male and one 5× female during month 6 of treatment. In addition, in treated male cats, a dose-dependent increase in the frequency of soft feces was observed. There was one unscheduled death, a female dosed at 40 mg/kg/day, which was euthanized in extremis after 2 weeks of treatment for nonspecific clinical signs (see Postmortem findings).
Feed consumption
There was a significant treatment-by-time interaction in average food consumption. The treated groups had significantly increased food consumption compared to the control group on Weeks 12–16, 19, 21 and 26 for the 8 mg/kg/day group; Week 15 for the 16 mg/kg/day group; Weeks 15, 16, 18, 21, 25 and 26 for the 24 mg/kg/day group; and Weeks 15–19, 21, 24 and 25 for the 40 mg/kg/day group (see Table1). The increase in food consumption compared to controls correlated with body weight increases in all of the treated groups (see Table2). It was unclear whether the effect on food consumption was due to cyclosporine.
Table 1.
Mean food consumption during 6 months of daily dosing with cyclosporine
| Mean food consumption (g/cat/day)* | ||||||
|---|---|---|---|---|---|---|
| Group number | Dose concentration (mg/kg/day) | Male (n = 4/group) | Female (n = 4/group) | |||
| Food consumption | % | Food consumption | % | |||
| 1 | Sham | 86.65 | NA | 70.27 | NA | |
| 2 | 8 (1×) | 95.47 | 10.2 | 78.90 | 12.3 | |
| 3 | 16 (2×) | 98.51 | 13.7 | 78.38 | 11.5 | |
| 4 | 24 (3×) | 103.84 | 19.8 | 75.52 | 7.5 | |
| 5 | 40 (5×) | 124.50 | 43.7 | 76.28 | 8.6 | |
NA, not applicable.
(%) Percentage difference from sham.
Statistical analysis: Pooled food consumption data had a statistically significant treatment-by-time interaction (P = 0.0001), treated groups were increased compared to controls on certain weeks (Weeks 12–16, 19, 21 and 26 for 8 mg/kg/day; Week 15 for 16 mg/kg/day; Weeks 15, 16, 18, 21, 25 and 26 for 24 mg/kg/day; and Weeks 15–19, 21, 24 and 25 for 40 mg/kg/day).
Table 2.
Mean body weights before and at the end of 6 months of daily dosing with cyclosporine
| Mean body weights (kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group number | Dose concentration (mg/kg/day) | Male (n = 4/group) | Female (n = 4/group) | ||||||
| Pretest | Study end | % | Pretest | Study end | % | ||||
| 1 | Sham | 3.773 | 4.675 | 23.9 | 2.375 | 2.843 | 19.7 | ||
| 2 | 8 (1×) | 3.605 | 5.043 | 39.9 | 2.660 | 3.600 | 35.3 | ||
| 3 | 16 (2×) | 3.748 | 5.230 | 39.5 | 2.668 | 3.548 | 32.9 | ||
| 4 | 24 (3×) | 3.655 | 5.495 | 50.3 | 2.683 | 3.613 | 34.7 | ||
| 5 | 40 (5×) | 3.963 | 6.028 | 52.1 | 2.695 | 3.593 | 33.3 | ||
(%) Percentage difference from pretest.
Statistical analysis pooled data: Body weight: P = 0.9264; Body weight gain: P = 0.8012.
Body weight
Body weights and body weight gains were slightly higher in treated animals than in controls throughout the study, although this was not statistically significant (Table2). It was unclear whether the affect on body weight was a direct effect of the cyclosporine or an indirect effect of increased food consumption.
Clinical pathology
There were no biologically relevant treatment-related effects on hematology, clinical chemistry, or urinalysis parameters at any dose in either sex. Prolonged APTT was seen at dose levels >1×, but this was considered unlikely to be biologically relevant (Table3).
Table 3.
Activated partial thromboplastin time (APTT) values with data from male and female cats pooled
| Variable | Group | Pretest | Overall | Day 8 | Day 32 | Day 92 | Day 155 | Day 184 | |
|---|---|---|---|---|---|---|---|---|---|
| APTT (sec) | Sham | Mean | 14.28 | 13.52 | 13.36 | 13.6 | 14.16 | 14.05 | 12.40 |
| SD | 1.58 | 1.32 | 1.14 | 1.17 | 1.42 | 1.42 | 0.89 | ||
| N | 8 | 40 | 8 | 8 | 8 | 8 | 8 | ||
| P-value | – | ||||||||
| 8 mg/kg/day | Mean | 14.58 | 14.48 | 14.59 | 14.76 | 15.06 | 14.99 | 13.00 | |
| SD | 1.84 | 1.9 | 2.32 | 2.02 | 1.77 | 1.7 | 1.12 | ||
| N | 8 | 40 | 8 | 8 | 8 | 8 | 8 | ||
| P-value | 0.1713 | ||||||||
| 16 mg/kg/day | Mean | 15.05 | 15.29 | 14.96 | 15.07 | 16.19 | 16.53 | 13.47 | |
| SD | 1.46 | 2.05 | 1.76 | 1.23 | 1.63 | 2.62 | 1.58 | ||
| N | 8 | 38 | 8 | 7 | 8 | 8 | 7 | ||
| P-value | 0.0248* | ||||||||
| 24 mg/kg/day | Mean | 14.51 | 14.97 | 14.49 | 15.46 | 14.98 | 15.84 | 14.14 | |
| SD | 2.17 | 1.78 | 1.73 | 1.82 | 0.96 | 2.66 | 1.10 | ||
| N | 8 | 39 | 8 | 7 | 8 | 8 | 8 | ||
| P-value | 0.0306* | ||||||||
| 40 mg/kg/day | Mean | 15.03 | 16.33 | 15.33 | 16.29 | 17.31 | 18.11 | 14.73 | |
| SD | 1.58 | 2.85 | 1.68 | 2.95 | 2.40 | 4.04 | 1.99 | ||
| N | 8 | 36 | 8 | 7 | 7 | 7 | 7 | ||
| P-value | 0.0007** |
Statistically significant at the 0.05, and 0.01 level, respectively.
Plasma analyses
Pharmacokinetic parameters were calculated for cyclosporine profiles obtained on Day 182 at [h]: 0, 1, 2, 4, 8 and 24 (Table4). Increasing doses led to relatively lower Cmax,and AUC parameter estimates then predicted assuming dose proportionality (Fig.1). The estimated accumulation factors (R) after long-term dosing at 8 mg/kg/day, calculated as ratios of trough levels between Day 7 (i.e. at this time, steady-state is expected to be reached based on cyclosporine terminal half-life in cats (Mehl et al., 2003)) and subsequent study days with available trough samples were in general not significantly different from 1, indicating no further significant accumulation at 1× (Fig.2) with long-term exposure. An exception was the single R factor value for the ratio of the trough level between Day 7 and Day 154, which was R = 1.72.
Table 4.
Arithmetic mean (±standard deviation) pharmacokinetic parameters
| Group number | Dose concentration (mg/kg/day) | Tmax | Cmax | Cmax relative ratio | AUC | AUC relative bio-availability ratio |
|---|---|---|---|---|---|---|
| 2 | 8 (1×) | 1.3 ± 0.5 | 1.93 ± 0.60 | NA | 13.94 ± 6.53 | NA |
| 3 | 16 (2×) | 1.5 ± 0.5 | 3.64 ± 2.19 | 0.83*, † | 37.16 ± 31.70 | 1.05*, † |
| 4 | 24(3×) | 1.8 ± 0.5 | 4.03 ± 2.13 | 0.61* | 32.07 ± 15.68 | 0.70*, † |
| 5 | 40 (5×) | 1.4 ± 0.5 | 3.96 ± 3.32 | 0.33* | 28.70 ± 19.88 | 0.37* |
Cmax observed maximum cyclosporine concentration (day 182) (μg/mL).
Tmax observed time to reach Cmax (h).
AUC, area under the cyclosporine vs. time curve between time 0 and 24 h (Day 182) (hμg/mL).
Relative ratios are related to the 1× group and calculated using dose-normalized values.
NA, not applicable.
Statistical analysis: Significant difference of P ≤ 0.005.
Compared to 1× group
Compared to 5× group.
Figure 1.
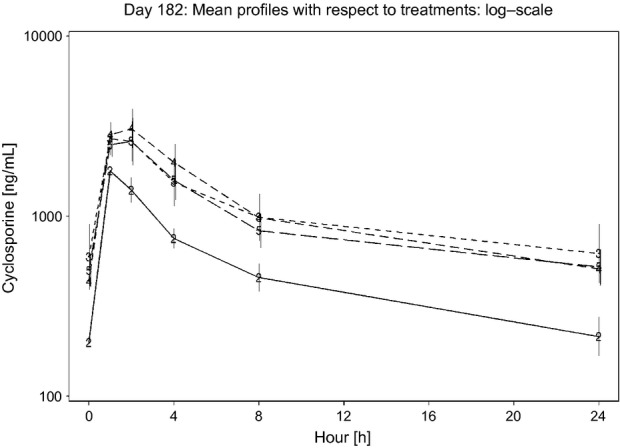
Day 182: Mean cyclosporine profiles with respect to treatment groups presented on a log scale. Treatment group 2 (8 mg/kg/day) is represented by a solid line; treatment groups 3 (16 mg/kg/day), 4 (24 mg/kg/day), and 5 (40 mg/kg/day) are represented as dotted lines. Vertical bars indicate ±SEM. Cmax and AUC were not dose-proportional.
Figure 2.
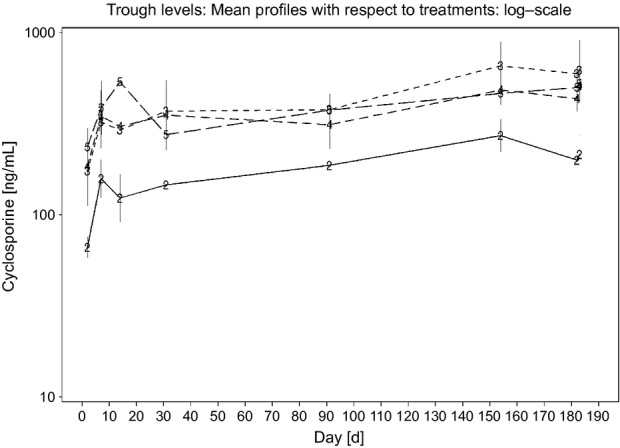
Mean trough cyclosporine profiles with respect to treatment. Treatment group 2 (8 mg/kg/day) is represented by a solid line; treatment groups 3 (16 mg/kg/day), 4 (24 mg/kg/day), and 5 (40 mg/kg/day) are represented as dotted lines. Vertical bars indicate ±SEM.
Postmortem findings
Postmortem findings were limited to the 40 mg/kg/day group (5×). There was one unscheduled death, a female dosed at 40 mg/kg/day, which was euthanized in extremis after 2 weeks of treatment for nonspecific clinical signs including recumbency, inappetence, dehydration, and decreased body weight (loss of approximately 18%). Pathology findings showed a healing rib fracture (estimated to be about 2 weeks old) and bone marrow hypocellularity characterized by a moderate reduction in the number of bone marrow cells of multiple lineages. Hematology parameters collected prior to euthanasia were not consistent with the observed bone marrow hypocellularity. The cat's trough cyclosporine concentration was 4858.8 ng/mL, approximately fivefold higher than any other cat in the 5× group. Malignant lymphoma involving the kidneys and a mesenteric lymph node was present in a male treated with 40 mg/kg/day. In addition, a female cat in this same group presented with abdominal fibroadenomatous nodules during the study.
Discussion
Although cyclosporine has been used in cats for some time, ATOPICA for Cats (cyclosporine oral solution, USP) MODIFIED has only recently been approved by the US FDA for the control of HD in cats of at least 6 months of age and 1.4 kg in body weight. As allergic dermatitis is a chronic condition, treatment at a dose of 7 mg/kg should be given for at least 4–6 weeks after which it can be tapered to maintain the desired therapeutic effect. Such long-term therapy raises concerns over toxicity, especially as it has been suggested that the blood concentration of CsA may increase over time due to saturation of the tissue-binding sites and adverse effects may be linked to high trough blood concentrations (>700 ng/mL) in cats also treated concomitantly with ketoconazole (Vaden, 1997; McAnulty & Lensmeyer, 1999). However, results from a field study with 100 client owned cats with HD showed that treatment with 7.0 mg/kg cyclosporine for up to 6 weeks was efficacious and well tolerated (King et al., 2012). The results of this present study confirm that CsA is safe and well tolerated by cats, even at multiples of the recommended dose of 7 mg/kg, given daily over a 6-month period.
Few adverse reactions to cyclosporine have been reported in cats treated for allergic dermatoses. Gastrointestinal signs (diarrhea, vomiting and anorexia) are the most frequently observed reactions (Robson, 2003a; Noli & Scarampella, 2006; Vercelli et al., 2006; Wisselink & Willemse, 2009; Heinrich et al., 2011; King et al., 2012). In a study conducted by Vercelli et al. (2006), mild gastrointestinal signs (intermittent soft feces or occasional vomiting) were observed in 4/23 cats during the first week of treatment with 5–14 mg/kg CsA dosed twice daily while Noli and Scarampella (2006) observed mild diarrhea in 1/10 cats following daily dosing with 3.6–8.3 mg/kg for 1 month. Also, following 4 weeks of treatment with 5 mg/kg CsA once daily, 11 of 18 treated cats experienced some form of reversible mild adverse reaction, including intermittent vomiting (three cats), diarrhea or loose stools (five cats), hyperactivity, increased appetite, and polydipsia (Wisselink and Willemse, 2009).
In the present study, following up to five times (5×), the approved dose administered daily over a 6-month period, there was an increase in the frequency and number of male cats with ‘soft feces’, but in contrast to some reported observations in the field, there was no vomiting or anorexia. Indeed in our study, as in the study conducted by Wisselink and Willemse (2009), food intake was generally increased in treated cats when compared to control cats, which likely contributed to the observed increase in body weight. Wisselink and Willemse (2009) also reported an increase in white blood cell counts, but suggested this may be due to the underlying inflammatory skin condition rather than due to CsA as it was also observed in the control prednisolone treated cats. Hematological parameters remained within normal ranges for treated cats within this current study. Activated partial thromboplastin time (APTT) was prolonged in cats administered at least twice the recommended dose of CsA, with the greatest increase observed in the cats receiving the highest dose following a minimum of 4 weeks of treatment. These changes may be test article-related; however, they were mild and not considered biologically relevant. The APTT is a performance indicator measuring the efficacy of both the ‘intrinsic’ and the common coagulation pathways. Prolonged APTT may indicate a coagulation factor deficiency, although it may also increase in liver disease, as the liver is the source of most coagulation factors (Couto, 1999). It is not clear why APTT was increased in this study; however, all liver parameters measured as part of the clinical pathology assessment were within normal ranges, and there were no macro- or microscopic hepatic changes suggesting liver toxicity.
Hypertension is another common adverse reaction to CsA in people, and hypertension has been recorded in renal transplanted cats treated with CsA (Robson, 2003a). However, in the current study, there was no observed effect on blood pressure, even in 3× and 5× groups. One 3× male and one 5× female during month six of treatment experienced intermittent interventricular conduction disturbances. This did not result in any clinical signs; however, as an intermittent interventricular conduction disturbance is not reported as a normal variant in cats, a test article effect is possible. In this study, one female cat in the 5× dose group was euthanized due to welfare reasons. The euthanasia was considered to be the result of a decline in health possibly related to a previous trauma rather than to the administration of CsA. Malignant lymphoma was present in one 5× cat. While cyclosporine does not induce tumors, it does inhibit T-lymphocytes and therefore treatment with cyclosporine may increase the susceptibility to infection and the development of neoplasia. There are reports in the human literature regarding the role of immunosuppression in the development of lymphoproliferative disease in transplant patients due to Epstein–Barr virus (Tanner & Alfieri, 2001). However, lymphoma is common in cats, and additional studies are needed to determine whether there is any link between CsA treatment and the occurrence of feline lymphoma. In addition, one female cat dosed with 40 mg/kg CsA developed palpable abdominal nodules on Day 133. The animal was anesthetized, and mammary gland biopsies containing the nodules were obtained on Day 135. Microscopic examination of the fixed hematoxylin and eosin-stained paraffin sections was consistent with a fibroadenomatous change, which is a spontaneous change in young intact cats and can be related to higher levels of circulating progesterone (de las Mulas et al., 2000).
In this study, dose-normalized peak plasma concentrations and plasma drug concentration-time curves were not dose proportional at dose levels greater than the recommended dose. Relative to Day 7 (when drug blood concentrations are expected to be at steady-state), accumulation factors were generally not different than one, indicating no significant unexpected accumulation with long-term dosing of cyclosporine. This is in contrast to rats, where there is significant accumulation in plasma and tissues (Ryffel, 1982). Nondose proportionality is linked to one or several nonlinear processes and is a known property of cyclosporine pharmacokinetics (Mueller et al., 1994). No specific experiments have been performed by the authors to identify the cause of this nonlinearity in cats. One hypothesis is nonlinear absorption related to high local concentrations at high doses leading to incomplete solubilization, or saturation of enzymes and transporters. A benefit of this under-proportionality is the lower than expected exposure of cats in the event of accidental overdosing.
In conclusion, these data demonstrate that ATOPICA for Cats (cyclosporine oral solution, USP) MODIFIED is safe and well tolerated in cats without unexpected accumulation beyond the first week of treatment when administered as directed.
References
- Couto CG. Clinical approach to bleeding dog or cat. Veterinary Medicine. 1999;May:450–459. [Google Scholar]
- FDA Center for Veterinary Medicine (CVM) Guidance for Industry 104: Content and Format of Effectiveness and Target Animal Safety Technical Sections and Final Study Reports for Submission to the Division of Therapeutic Drugs for Non-Food Animals. July 2001.
- Guidance for industry, Bioanalytical method validation, May 2001:US Department of Health and Human Service, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) and Center for Veterinary Medicine (CVM), 7500 Standish Place, Rockville, MD 20855, USA.
- Heinrich NA, McKeever PJ. Eisenschenk MC. Adverse events in 50 cats with allergic dermatitis receiving ciclosporin. Veterinary Dermatology. 2011;22:511–520. doi: 10.1111/j.1365-3164.2011.00983.x. [DOI] [PubMed] [Google Scholar]
- King S, Favrot C, Messinger L, Nuttall T, Steffan J, Forster S. Seewald W. A randomized double-blinded placebo-controlled study to evaluate an effective ciclosporin dose for the treatment of feline hypersensitivity dermatitis. Veterinary Dermatology. 2012;23:440–e84. doi: 10.1111/j.1365-3164.2012.01086.x. [DOI] [PubMed] [Google Scholar]
- McAnulty JF. Lensmeyer GL. The effects of ketoconazole on the pharmacokinetics of cyclosporine A in cats. Veterinary Surgery. 1999;28:448–455. doi: 10.1111/j.1532-950x.1999.00448.x. [DOI] [PubMed] [Google Scholar]
- Mehl ML, Kyles AE, Craigmill AL, Epstein S. Gregory CR. Disposition of cyclosporine after intravenous and multi-dose oral administration in cats. Journal of Veterinary Pharmacology and Therapeutics. 2003;26:349–354. doi: 10.1046/j.1365-2885.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- Mueller EA, Kovarik JM, van Bree JB, Tetzloff W, Grevel J. Kutz K. Improved dose linearity of cyclosporine pharmacokinetics from a microemulsion formulation. Pharmaceutical Research. 1994;11:301–304. doi: 10.1023/a:1018923912135. [DOI] [PubMed] [Google Scholar]
- de las Mulas JM, Millan Y, Bautista MJ, Perez J. Carrasco L. Oestrogen and progesterone receptors in feline fibroadenomatous change: an immunohistochemical study. Research in Veterinary Science. 2000;68:15–21. doi: 10.1053/rvsc.1999.0327. [DOI] [PubMed] [Google Scholar]
- Noli C. Scarampella F. Prospective open pilot study on the use of ciclosporin for feline allergic skin disease. Journal of Small Animal Practice. 2006;47:434–438. doi: 10.1111/j.1748-5827.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- Palmeiro BS. Cyclosporine in Veterinary Dermatology. The Veterinary Clinics of North America. Small Animal Practice. 2013;43:153–171. doi: 10.1016/j.cvsm.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Public Health Service Policy on Humane Care and Use of Laboratory Animals. National Institutes of Health (US) Office of Protection from Research Risks. 1986.
- Robson D. Review of the pharmacokinetics, interactions and adverse reactions of cyclosporine in people, dogs and cats. Veterinary Record. 2003a;152:739–748. doi: 10.1136/vr.152.24.739. [DOI] [PubMed] [Google Scholar]
- Robson D. Review of the properties and mechanisms of action of cyclosporine with an emphasis on dermatological therapy in dogs, cats and people. Veterinary Record. 2003b;152:768–772. doi: 10.1136/vr.152.25.768. [DOI] [PubMed] [Google Scholar]
- Ryffel B. Experimental toxicological studies with cyclosporin A. In: White DJG, editor. Cyclosporin A. Amsterdam: Elsevier Biomedical Press; 1982. pp. 45–75. [Google Scholar]
- Swiss Ordinance relating to Good Laboratory Practice, adopted May 18, 2005 [RS 813.112.1] and based on The OECD Principles of Good Laboratory Practice, as revised in 1997 and adopted on November 26, 1997 by decision of the OECD Council C (97) 186/Final.
- Tanaka C, Kawai R. Rowland M. Dose-dependent pharmacokinetics of cyclosporine A in rats: events in tissues. Drug Metabolism Disposition. 2000;28:582–589. [PubMed] [Google Scholar]
- Tanner JE. Alfieri C. The Epstein-Barr virus and post-transplant lymphoproliferative disease: interplay of immunosuppression, EBV, and the immune system in disease pathogenesis. Transplant Infectious Disease. 2001;3:60–69. doi: 10.1034/j.1399-3062.2001.003002060.x. [DOI] [PubMed] [Google Scholar]
- Target Animal Safety Guidelines for New Animal Drugs, Center for Veterinary Medicine (CVM), Food and Drug Administration, June 1989, and U.S. Food and Drug Administration (CVM Guideline 33)
- United States Department of Agriculture. Title 9 Code of Federal Regulations. 2009. Animal Welfare 7 U.S.C. 2131-2159; 7 CFR 2.22, 2.80, and 371.7.
- Vaden SL. Cyclosporine and tacrolimus. Seminars in Veterinary Medicine and Surgery (Small Animal) 1997;12:161–166. doi: 10.1016/s1096-2867(97)80028-x. [DOI] [PubMed] [Google Scholar]
- Vercelli A, Raviri G. Cornegliani L. The use of oral cyclosporin to treat feline dermatoses: a retrospective analysis of 23 cases. Veterinary Dermatology. 2006;17:201–206. doi: 10.1111/j.1365-3164.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Whalen RD, Tata PN, Burckart GJ. Venkataramanan R. Species differences in the hepatic and intestinal metabolism of cyclosporine. Xenobiotica. 1999;29:3–9. doi: 10.1080/004982599238777. [DOI] [PubMed] [Google Scholar]
- Wisselink MA. Willemse T. The efficacy of cyclosporine A in cats with presumed atopic dermatitis: a double blind, randomised prednisolone-controlled study. The Veterinary Journal. 2009;180:55–59. doi: 10.1016/j.tvjl.2007.11.018. [DOI] [PubMed] [Google Scholar]


