Abstract
The in vivo assessment of epigenetic changes during mouse pancreatic beta-cell differentiation reveals surprising differences to directed, in vitro differentiation of human embryonic stem cells. New findings reported in this issue of The EMBO Journal further identify Ezh2 as a critical determinant of endocrine progenitor number and could instruct improved protocols for stem cell-based therapies.
See also: CR Xu et al (October 2014)
Epigenetic regulation of gene expression has emerged as an essential regulator of cell differentiation during embryogenesis and organ formation. Advances in stem cell technology have let to astonishing improvements in the generation of human cell surrogates with potential for therapeutic treatment. Despite impressive results regarding gene expression and functional capacities, concerns remain as to whether the current differentiation conditions truly and fully recapitulate the embryonic conditions and by extension, whether the final stem cell-derived end product is the full equivalent of the endogenous counterparts. Xu, Zaret and colleagues address some of these questions in the current issue of The EMBO Journal by isolating and analyzing small numbers of endodermal and pancreas progenitors at sequential stages of differentiation toward hormone-producing β-cells (Xu et al, 2014). Global epigenetic analysis for the repressive Histone H3 K27 tri-methylation mark (H3K27me3) revealed an unexpected global increase of repressive peaks during advanced stages of pancreas development in vivo (Fig1), with a concomitant loss of such marks at regulatory units of key progenitor transcription factors. Based on these findings and previous work from their laboratory (Xu, 2011), the authors demonstrate that precocious removal of H3K27me3 marks via reduction of EZH2 results in an increase in endocrine progenitor cells and consequently in more insulin-producing β-cells in vivo (Fig1).
Figure 1. Schematic of global H3K27 methylation marks during pancreas differentiation.
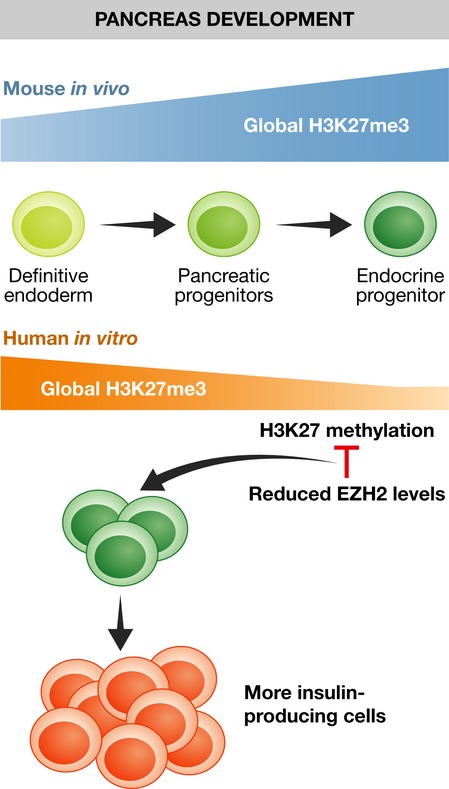
The number of H3K27 marks progressively increase during mouse pancreas development whereas little changes are observed during direct differentiation of hESC into pancreatic cells. Reducing H3K27 methylation by inhibition of EZH2 promotes the generation of insulin-producing cells.
Taken together, these findings question our current understanding of repressive epigenetic marks during human pancreas development and highlight important aspects of current approaches to generate functional human β-cells that need to be re-evaluated in light of the presented data. Our current knowledge of epigenetic changes during human pancreas development is based mainly on data derived from differentiation protocols of human embryonic stem cells (hESC) capable of giving rise to pancreas progenitors that differentiated into functional insulin-producing cells upon transplantation into surrogate animals (Schulz, 2012; Xie, 2013). While Xu et al show a progressive genome-wide increase in H3K27me3 marks during mouse pancreas development, direct differentiation of hESC into pancreatic cells under cell culture conditions exhibits little changes in the level of repressive marks from the gut tube to the pancreatic endoderm stage. This discrepancy between embryonic and hESC-derived cells raises the question of whether our inability to produce functional β-cells under cell culture conditions is due to aberrant epigenetic modifications. However, transplanted hESC-derived pancreas progenitors that are allowed to undergo maturation for months to form functional β-cells appear to possess even lower levels of repressive marks than immature β-like cells in vitro (Xie, 2013), possibly implying that reductions in repressive marks become cemented during later stages of differentiation, even upon transplantation. What might be responsible for the differential epigenetic regulation in embryonic cells compared to stem cell-derived counterparts? Xu and colleagues suggest that morphogenetic movements essential for the formation of endocrine cells that delaminate from an epithelial sheet during islet formation do not occur during in vitro differentiation of stem cells. In addition to the cell intrinsic properties that allow for cellular movement, the absence of non-endodermal supporting tissues in hESC cultures might also affect the epigenetic regulation of cell differentiation. Evidence for positive effects of mesenchymal factors during embryonic and hESC to pancreas development support this hypothesis (Landsman, 2011; Sneddon et al, 2012; Guo et al, 2013).
Another interesting aspect of the study by Xu and co-authors pertains to the finding that repression of EZH2 levels appears to be beneficial in increasing the formation of endocrine progenitors during stem cell differentiation. Intriguing data are presented that suggest that reducing, but not abolishing, levels of EZH2 would be beneficial to generate more β-cells. Additional experiments in other systems will be required to confirm this observation, in particular with regard to the potentially exciting discovery that lower levels of EZH2 might function in an INK4A/ARF independent manner.
In summary, Xu et al point out critical differences in repressive epigenetic dynamics between mouse and human pancreas development. The exact mechanisms for this discrepancy, however, remain unresolved. Simple species differences between transgenic mice and human stem cell-derived pancreas cells cannot be excluded, including the fact that human endocrine and β-cells differentiate with a much longer time frame than their rodent counterparts. The exciting observation suggesting modulation of the levels of the methyltransferase EZH2 as a means to increase endocrine progenitor numbers that result in β-cells with potentially improved function emphasizes the need for a more comprehensive understanding of the epigenetic changes associated with cell differentiation. It appears that obtaining such detailed knowledge will be instrumental in succeeding to generate functional cell types in vitro, including insulin-producing β-cells.
Acknowledgments
Work on stem cell differentiation in the laboratory of MH is funded by a grant from the Leona M. and Harry B. Helmsley Charitable Trust (2012PG-T1D017). HAR is supported by a JDRF postdoctoral fellowship (3-2012-266).
References
- Guo T, Landsman L, Li N, Hebrok M. Factors expressed by murine embryonic pancreatic mesenchyme enhance generation of insulin-producing cells from hESCs. Diabetes. 2013;16:1581–1592. doi: 10.2337/db12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L, Nijagal A, Whitchurch TJ, VanderLaan RL, Zimmer WE, MacKenzie TC, Hebrok M. Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS Biol. 2011;9:e1001143. doi: 10.1371/journal.pbio.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TC, Young HY, Agulnick AD, Badin MJ, Baetge EE, Bang AG, Bhoumik A, Cepa I, Cesario RM, Haakmeester C, Kadoya K, Kelly JR, Kerr J, Martinson LA, McLean AB, Moorman MA, Payne JK, Richardson M, Ross KG, Sherrer ES, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon JB, Borowiak M, Melton DA. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature. 2012;491:765–768. doi: 10.1038/nature11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Everett LJ, Lim H, Patel NA, Schug J, Kroon E, Kelly OG, Wang A, D'Amour KA, Robins AJ, Won K, Kaestner KH, Sander M. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Stem Cell. 2013;12:224–237. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CR, Cole PA, Meyers DJ, Kormish J, Dent S, Zaret KS. Chromatin ‘prepattern’ and histone modifiers in a fate choice for liver and pancreas. Science. 2011;332:963–966. doi: 10.1126/science.1202845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CR, Li LC, Donahue G, Ying L, Zhang YW, Gadue P, Zaret KS. Dynamics of genomic H3K27me3 domains and role of EZH2 during pancreatic endocrine specification. EMBO J. 2014;33:2157–2170. doi: 10.15252/embj.201488671. [DOI] [PMC free article] [PubMed] [Google Scholar]


