Abstract
The last decade has been marked by tremendous progress in our understanding of the cell biology of mitochondria, with the identification of molecules and mechanisms that regulate their fusion, fission, motility, and the architectural transitions within the inner membrane. More importantly, the manipulation of these machineries in tissues has provided links between mitochondrial dynamics and physiology. Indeed, just as the proteins required for fusion and fission were identified, they were quickly linked to both rare and common human diseases. This highlighted the critical importance of this emerging field to medicine, with new hopes of finding drugable targets for numerous pathologies, from neurodegenerative diseases to inflammation and cancer. In the midst of these exciting new discoveries, an unexpected new aspect of mitochondrial cell biology has been uncovered; the generation of small vesicular carriers that transport mitochondrial proteins and lipids to other intracellular organelles. These mitochondrial-derived vesicles (MDVs) were first found to transport a mitochondrial outer membrane protein MAPL to a subpopulation of peroxisomes. However, other MDVs did not target peroxisomes and instead fused with the late endosome, or multivesicular body. The Parkinson's disease-associated proteins Vps35, Parkin, and PINK1 are involved in the biogenesis of a subset of these MDVs, linking this novel trafficking pathway to human disease. In this review, we outline what has been learned about the mechanisms and functional importance of MDV transport and speculate on the greater impact of these pathways in cellular physiology.
Keywords: mitochondria, Parkin, PINK1, quality control, vesicle transport
Introduction
Mitochondria are very complex organelles, housing hundreds of biochemical reactions from energy production to amino acid and lipid synthesis, to hormone production. These biochemical reactions involve substrates and products that flow between the many organelles within the cell. Rather than metabolite shuttling through free diffusion mechanisms, there is increasing evidence that direct interorganellar contacts are required. For example, elemental iron uptake into the mitochondria has been shown to require “kiss-and-run” contacts between the endosome and mitochondria (Zhang et al, 2005; Sheftel et al, 2007). Direct contacts between mitochondria and lipid droplets and peroxisomes are thought to facilitate fatty acid transport. The most advanced understanding of these contacts is between the ER and the mitochondria. It has long been known that ER is the source of lipids for mitochondrial biogenesis (Shiao et al, 1995) and that these contacts are important for cellular calcium homeostasis (Rizzuto et al, 1998). More recently, it was discovered that ER wrapping around the mitochondria marks the sites for mitochondrial division. A molecular understanding of these contacts has been advanced through studies in yeast and mammalian models (Csordas et al, 1999; de Brito & Scorrano, 2008; Kornmann et al, 2009) and is reviewed elsewhere. It is now clear that there is extensive biochemical cross talk between organelles, but the mechanisms are only beginning to emerge (Sheftel et al, 2007; Zehmer et al, 2009; Rowland & Voeltz, 2012; Mesmin et al, 2013).
This review will outline the emerging role of vesicular transport as another means of interorganellar communication. Mitochondrial-derived vesicles (MDVs) are generated through the selective incorporation of protein cargoes, which can be limited to the outer membrane, or can include outer, inner membrane, and matrix content, as illustrated in Fig 1 (Neuspiel et al, 2008; Soubannier et al, 2012a, 2012b). Ultrastructural analysis revealed their size to be relatively uniform, between 70 and 150 nm, and their scission does not require the established mitochondrial fission GTPase Drp1 (Neuspiel et al, 2008; Soubannier et al, 2012a, 2012b). Two distinct fates were identified for MDVs, with their targeting either to the late endosome/multivesicular body for degradation (Soubannier et al, 2012a), or to a subpopulation of peroxisomes (Neuspiel et al, 2008). Although this area of research is just emerging, this review will outline the molecular details of cargo selection, vesicle formation, and delivery, as well as the established and predicted impact of these pathways in cellular physiology.
Figure 1. Summary of MDVs cargo variability.
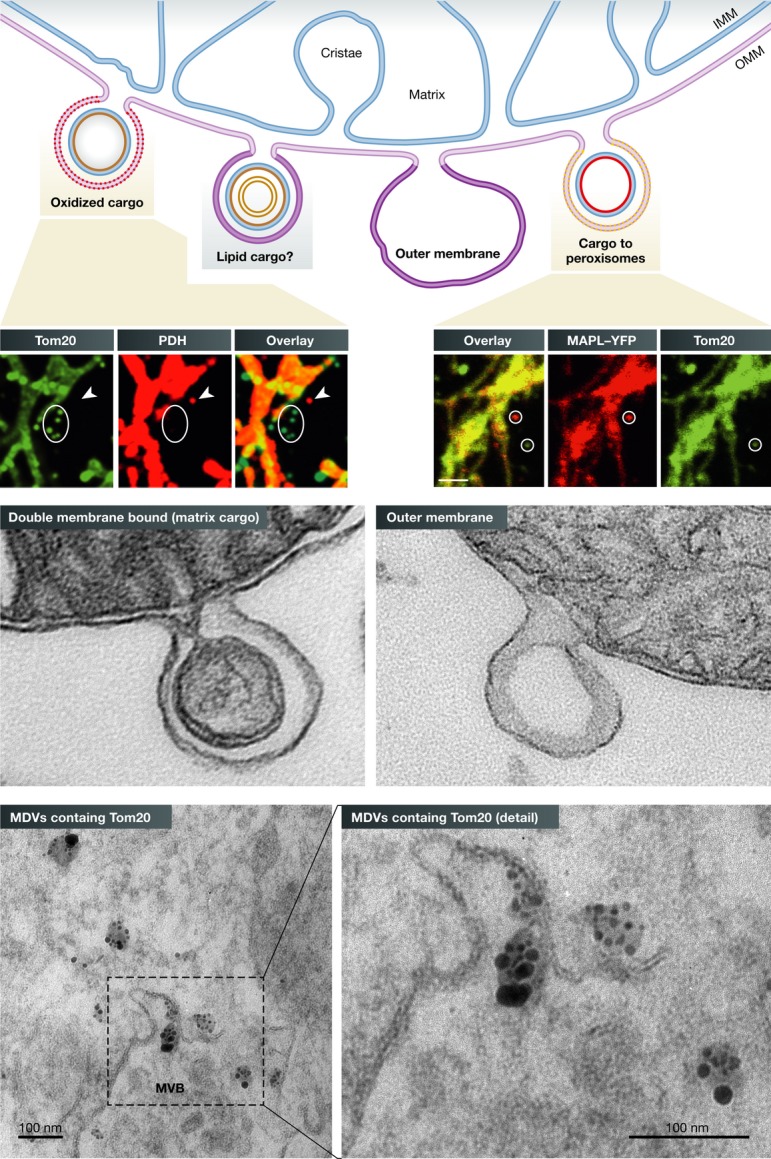
Immunofluorescent and EM images illustrate the diversity of cargo-selected MDVs. Immunofluorescent staining of Tom20 (an outer membrane protein) and pyruvate dehydrogenase (PDH, matrix protein) reveals a number of cargo-selected vesicular structures lying outside of the mitochondria (top left panels, circles versus arrowheads). Although Tom20 is absent from PDH-positive structures (arrowheads), EM and biochemical experiments confirm that these vesicles are double membrane bound. An example is shown to the left where both membranes are seen within the vesicle emerging from the intact mitochondria [with permission from Soubannier et al ( 2012b)]. Similar cargo selectivity is seen for MDVs carrying MAPL that target the peroxisomes [top right panel of immunofluorescent images, taken with permission from Neuspiel et al ( 2008)]. We also observe single membrane MDVs derived from just the outer mitochondrial membrane (EM panel on right side). Bottom electron microscopic pictures show MDVs containing Tom20 labeled by immunogold particles enter the multivesicular body [taken with permission from Soubannier et al ( 2012a)]. Scale bars in EM pictures represent 100 nm.
The selection of cargo for transport
Mitochondrial-vesicle transport carries cargo to peroxisomes and lysosomes. Cargo destined for the lysosomes is ultimately degraded (Soubannier et al, 2012a), and in vitro studies have shown enrichment of oxidized proteins within MDVs (Soubannier et al, 2012b). However, the purpose of vesicle delivery to the peroxisomes is unclear (Mohanty & McBride, 2013). Currently, only one protein is known to traffic to the peroxisomes, a membrane anchored protein ligase called MAPL (also called MULAN, MUL1, GIDE and HADES) (Neuspiel et al, 2008; Braschi et al, 2009). With just one known cargo, it is difficult to predict the mechanisms and principles that govern cargo selection. However, we can look to evolution to help generate testable, working hypotheses. We will first consider the mechanisms of cargo selection based on each target destination separately.
Cargo selection for transport to lysosomes
There are two primary pieces of evidence that contribute to our understanding of the nature of the cargo en route to the late endosome/lysosome. Firstly, MDVs generated in vitro from isolated mitochondria were shown to be enriched in oxidized protein, in a process that was stimulated by mitochondrial stress (Soubannier et al, 2012b). This reconstitution of MDV formation further revealed a selective incorporation of protein cargo based on the nature of the mitochondrial stress induced. For example, the generation of ROS in the reaction with xanthine oxidase/xanthine led to a stimulation of MDVs carrying the outer membrane pore protein VDAC, but generation of ROS within the mitochondria, upon addition of a complex III inhibitor antimycin A, led to MDVs carrying the complex III subunit core2, without any enrichment in VDAC (Soubannier et al, 2012b). These data suggest that potentially any cargo could be included within MDVs; assuming that they are first oxidized, which would “damage” the complex. This also suggests that oxidation may trigger aggregation or oligomerization, acting as a seed to initiate membrane curvature from the inside.
The process of MDV formation has likely been conserved from archaebacteria, the mitochondrion's ancestors (Deatherage & Cookson, 2012). So, are there clues as to the cargo selection mechanisms within these ancient systems? All gram-negative bacteria, including Archae strains, shed vesicles, from those living within the soil to infectious strains like Helicobacter pylori, causing ulcers, or Treponema pallidum, the cause of syphilis (Kulp & Kuehn, 2010; Bonnington & Kuehn, 2014). These bacterial strains bud a variety of vesicles carrying specific cargoes, with unique tasks from the transport of virulence factors, or peptides that arrest the host cell cycle, to the generation of a biofilm. In addition, the quantity of bacterial protein incorporation into vesicles ranges from 0.1% to 8–12%, showing a remarkable > 100-fold variation in cargo incorporation (Soubannier et al, 2012b; Bonnington & Kuehn, 2014). Environmental changes in pH or nutrients are often a signal for bacterial vesicle secretion; however, the molecular mechanisms responsible for such varied cargo selection are not well understood (Deatherage & Cookson, 2012). Given the diversity and complexity of bacterial vesicle formation, we consider it unlikely that a single evolutionary set of machinery could be mapped to MDVs from the ancestral mechanisms of shedding. However, the utility of vesicles in all membrane systems has been demonstrated and mitochondria are no exception. Indeed, the lessons from bacteria help to frame our understanding of the use of vesicles as a highly selective way to sort mitochondrial proteins.
The second important finding was that the generation of MDVs destined for lysosomes required the protein kinase PINK1 and the cytosolic ubiquitin E3 ligase Parkin (McLelland et al, 2014). PINK1 and Parkin are both mutated in familial cases of Parkinson's disease (PD) (Trinh & Farrer, 2013) and were initially shown to act in a common pathway in mitochondrial quality control in Drosophila models of PD (Clark et al, 2006; Park et al, 2006; Yang et al, 2006). More recent work has shown that PINK1 is targeted to the mitochondria but is normally degraded very rapidly. During import, PINK1 is first cleaved by the matrix processing peptidases and PARL (Jin et al, 2010; Greene et al, 2012); however, almost all of the cleaved PINK1 is then released from the import channel and degraded in the cytosol through the N-end rule proteolytic pathway (Kondapalli et al, 2012; Lazarou et al, 2012; Yamano & Youle, 2013). Upon mitochondrial depolarization, the import machinery is inactivated and PINK1 becomes trapped either within the import channel or becomes anchored to the mitochondrial outer membrane near the import channel (Greene et al, 2012; Lazarou et al, 2012). This exposes the kinase domain to the cytosol where it phosphorylates ubiquitin and Parkin, leading to stable Parkin recruitment and activation at the mitochondrial surface (Kim et al, 2008; Shiba-Fukushima et al, 2012; Iguchi et al, 2013; Kane et al, 2014; Kazlauskaite et al, 2014). Parkin ubiquitinates a series of proteins on the mitochondrial surface, which are then recognized by autophagic adaptor proteins and delivered to the autophagosome (Narendra et al, 2008; Gegg et al, 2010; Lee et al, 2010; Matsuda et al, 2010; Tanaka et al, 2010; Chan et al, 2011; Chen & Dorn, 2013; Sarraf et al, 2013).
Given that PINK1 and Parkin are also required for MDV transport, we predict that the same mechanisms apply, but at a much more localized level (see model, Fig 2). The import channels are spatially restricted upon the mitochondrial surface, as shown with super-resolution microscopy or immunoelectron microscopy (Wurm et al, 2011). Protein misfolding in the matrix was recently shown to trigger mitophagy after long incubations (Jin & Youle, 2013), without any loss of electrochemical potential. Therefore, we consider that local protein aggregation at the import site, perhaps due to local oxidative damage, or complex assembly defects, would block the import process. Should the matrix chaperones become saturated, or cardiolipin become oxidized locally, then the inner membrane import channel may fail. Cardiolipin oxidizes to phosphatidic acid, a lipid known to alter membrane curvature (Yurkova et al, 2008; Donaldson, 2009; Horvath & Daum, 2013), and may help initiate the outward bending of the membrane. Upon complete depolarization or organelle dysfunction, the mechanism may switch from a local removal of a “patch” of mitochondrial content to the global arrest of PINK1 in all import channels, activation of the autophagic machinery, and entire engulfment of the organelle. This “patch” may not be strictly cargo selective, since whatever aggregated or oxidized proteins and lipids reside in proximity to an arrested import channel would be ejected. Supporting this concept, the kinetic analysis of events following treatment with antimycin A revealed the generation of MDVs at an early stage of ROS production, while global mitochondrial depolarization led to the kinetically slower process of mitophagy (McLelland et al, 2014). This indicates that MDVs are likely a first round of defense for the mitochondria to eject damaged proteins in order to avoid the complete failure of the organelle. This first response does not require the activation of autophagy machinery, as it occurs in the absence of Atg5, Rab9, or beclin (Soubannier et al, 2012a; McLelland et al, 2014).
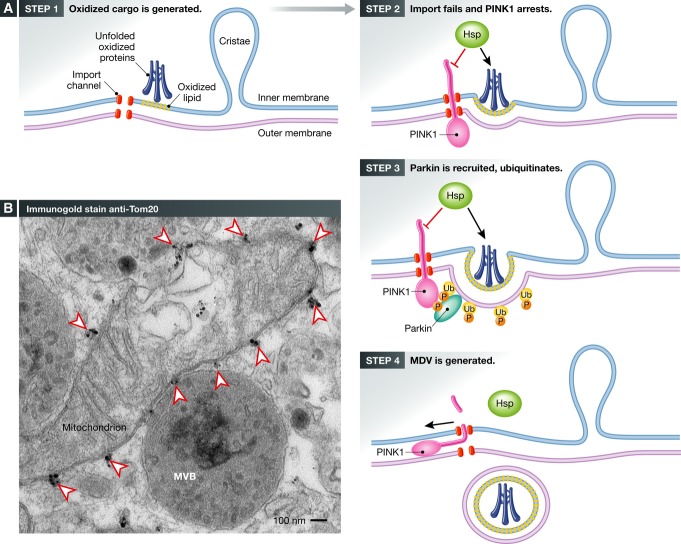
Figure 2. Working hypothesis for vesicle initiation by PINK1 and Parkin.
(A) Immunogold staining of endogenous Tom20 within COS7 cells reveals the regular spacing of the import channels indicated by arrowheads. Note the close tethering of three multivesicular bodies to the mitochondria. (B) An illustration of our working hypothesis of PINK1/Parkin-mediated MDV formation. In Step 1, unfolded, oxidized proteins within matrix, triggered by ROS or failure to assemble, leads to protein aggregation (blue). Oxidation of cardiolipin will generate PA, contributing to altered membrane curvature. In Step 2, protein aggregates may saturate chaperones, leading to a very localized failure to import at an individual channel. In addition, local oxidation of cardiolipin would further interfere with import channels. PINK1, which is rapidly imported, would then accumulate at these failed import channels. In Step 3, PINK1 phosphorylates both ubiquitin and the ubiquitin-like domain of Parkin, stabilizing the recruitment of activated Parkin. The ubiquitination activity of Parkin is required to generate MDVs, suggesting that domains on the surface may be cleared. In Step 4, a vesicle is formed and released in a process that will certainly involve a number of unidentified proteins. Future studies are needed to test this hypothesis and uncover the details governing the generation of MDVs.
A second argument in favor of the import channel acting as a sentinel for MDV formation is that import channels are restricted to the boundary membranes, where the inner and outer membrane are in close apposition to thread precursor proteins into the matrix and inner membrane. This explains how the two membranes may bud out together, since they would be locked in place by the arrested PINK1 precursor. It also lends insight into why PINK1 and Parkin are not required for the generation of MDVs that carry only outer membrane proteins like the import receptor Tom20. Ultrastructural analysis of mitochondrial single membrane vesicles reveal a more pleotropic appearance, rather like “blebs” than true, well-constructed vesicles (Fig 1, Soubannier et al, 2012b). The trigger for these vesicles may more closely mirror the bacterial mechanisms of outer membrane vesicle release, the mechanisms of which remain unclear. Indeed, despite the relatively greater abundance of these Tom20 outer membrane vesicles compared to, for example, MDVs carrying matrix pyruvate dehydrogenase that lack Tom20, to date no protein machineries required for their biogenesis have been identified.
A role for Parkin in vesicle trafficking has been shown previously. In receptor-mediated endocytosis, a ubiquitin-interacting motif (UIM) within the adaptor protein Eps15 was shown to bind the ubiquitin-like domain (UBL) of Parkin (Fallon et al, 2006). This led to the monoubiquitination of Eps15 and inhibition of its capacity to recruit the endocytic machinery, thereby regulating its function as an adaptor for endocytosis of the EGF receptor (EGFR). In this way, by delaying EGFR endocytosis and degradation, Parkin can enhance signaling downstream of the receptor. Parkin was also shown to bind and monoubiquitinate the endocytic BAR domain protein endophilin A via a Ubl-SH3 interaction (Trempe et al, 2009). As BAR domains are involved in membrane remodeling and curvature, this finding further links Parkin to vesicle budding and trafficking machinery. It is unclear what the signal is to recruit and activate Parkin at the cell surface as it is unlikely to be PINK1, which is constitutively targeted to mitochondria. However, very recent data have shown that PINK1, upon stabilization at the mitochondrial outer membrane, phosphorylates ubiquitin at position S65 (Kane et al, 2014; Kazlauskaite et al, 2014; Koyano et al, 2014). Three independent studies demonstrated that phosphorylated ubiquitin efficiently activated Parkin ubiquitin ligase activity at the mitochondrial surface in acute settings of mitochondrial uncoupling. In this situation, there was a nearly stoichiometric phosphorylation of cellular ubiquitin, which is likely to reach other cellular ubiquitin targets. In this way, the generation of phosphor-ubiquitin at the mitochondrial surface could act as a signaling mechanism for a global cellular response to mitochondrial stress. Regardless of the mechanisms by which Parkin is activated in endocytosis, the data indicate that Parkin may have a multifaceted role in vesicle transport, at the plasma membrane, mitochondrial surface, and perhaps elsewhere. It will be particularly interesting to determine whether these or other adapters are involved in the membrane budding and trafficking involved in the biogenesis of the subset of MDVs involving PINK1 and Parkin at mitochondria. Thus, whereas Parkin clearly plays a role in mitophagy, it also has a steady-state role in the removal of selected, oxidized cargo in a pathway parallel to mitophagy.
Cargo selection for transport to peroxisomes
The fate of mitochondrial cargo transiting to the lysosome is to be degraded. However, it is much less obvious why there may be a need for vesicle transport to the peroxisomes. The only cargo identified to date transits to a subpopulation of peroxisomes, about 10–20% of the total peroxisomes in the cell (Neuspiel et al, 2008; Braschi et al, 2010). Immunogold analysis of MAPL-positive MDVs revealed the presence of two membranes (Neuspiel et al, 2008), leading us to consider that the cargo is not limited to outer membrane content. There is some information on the mechanisms of MAPL enrichment within peroxisome-bound MDVs. The retromer complex containing Vps35, Vps26, and Vps29 was identified as a MAPL binding partner in an affinity chromatography approach (Braschi et al, 2010). The retromer complex was first established as a coat-like complex that binds and enriches cargo into vesicles from the endosome for their return to the Golgi apparatus (Seaman et al, 1998; Arighi et al, 2004; Seaman, 2012). The retromer complex also binds to the sorting nexin family of proteins that contain a PX-BAR domain that facilitates membrane curvature required for vesicle formation. More recent experiments reveal a much broader role for the retromer complex in many transport pathways, where specificity is granted through the combinatorial use of different sorting nexin members, and variants of the retromer subunits (Rojas et al, 2007; Collins et al, 2008; Cullen & Korswagen, 2012). In each case, the Vps35 subunit of the retromer binds to cargo tails, hinting that transport to the peroxisome will be more signal specific compared with the mechanisms of transport to the lysosome. Silencing Vps35 blocked the delivery of MAPL to peroxisomes, confirming the functional requirement for this complex in MDV transport (Seaman, 2012). MAPL contains ubiquitin and SUMO E3 ligase activities within the cytosolic domain (Braschi et al, 2009), however mutations in the RING finger domain did not alter the delivery to the peroxisome (Neuspiel et al, 2008; Braschi et al, 2010), indicating that the SUMOylation/ubiquitination activity of MAPL are not mechanistically required for MDV formation. At this time, we consider that MAPL constitutes vesicle cargo that does not function in the generation of peroxisome-bound vesicles. Clearly, there is a great deal of work remaining to elucidate the extent of cargo incorporation and the role of the retromer complex in this pathway.
Vps35 participates in a variety of transport pathways throughout the cell. However, with mutations in Vps35 being recently linked to PD and Alzheimer's disease (Vilarino-Guell et al, 2011; Zimprich et al, 2011), its role in MDV transport has emerged as an intriguing functional arc that may link defects in Vps35 with mitochondrial dysfunction. Future work will determine whether an alteration in cargo delivery to peroxisomes may contribute to PD.
Mechanisms of MDV transport and delivery
As described above, we have identified three factors required for the generation of at least a subset of MDVs; those carrying matrix content for delivery to the lysosome (PINK1 and Parkin), and MDVs destined for the peroxisome (retromer complex). However, if we look to other vesicle transport paradigms, it is apparent that this is likely the tip of the iceberg. In addition to cargo selection mechanisms, MDV formation will require machineries that facilitate membrane curvature, potential coat complexes, incorporation of fusion machinery, and motility factors. MDVs are formed in the absence of the mitochondrial dynamin GTPase Drp1, indicating additional mechanisms are also required for the final scission event (Neuspiel et al, 2008; Soubannier et al, 2012a; McLelland et al, 2014). The independence of Drp1 is consistent with the diameter of the yeast mitochondrial dynamin (Dnm1) ring limited to 100 nm, which would be too large to constrict an MDV neck (Ingerman et al, 2005).
We may find clues as to the identity of MDV factors within the MitoCarta, an annotated map of the mitochondrial proteome (http://www.broadinstitute.org/pubs/MitoCarta/). For example, there are a number of vesicle-related proteins whose roles have not yet been characterized on the mitochondrial outer membrane. Two enzymes are predicted to modulate phosphatidylinositol phosphates (PIPs) on the surface; PI(4)-kinase IIIβ and splice variant of the PI(5)-phosphatase synaptojanin-2A (Nemoto et al, 2001). Although the presence of PIP-based microdomains on the mitochondria has not been studied intensively, PI(3)P domains were observed to form during mitophagy (Yang & Yang, 2013). PIP-related microdomains are known to recruit adaptor proteins that could favor membrane bending and vesicle generation (Mayinger, 2012). Consistent with adaptors that facilitate alterations in membrane curvature, another endophilin family member, endophilin B1 (Karbowski et al, 2004; Takahashi et al, 2005, 2007), and a mitochondrial phospholipase D, MitoPLD (Choi et al, 2006; Huang et al, 2011), may also modulate membrane dynamics at the outer membrane. Endophilin B1 has been implicated in the binding of Bax (Pierrat et al, 2001), beclin (Takahashi et al, 2007) and in the process mitochondrial fission (Karbowski et al, 2004). These lipid binding and modifying enzymes are all candidates for MDV transport machinery given the established roles for their activities in vesicle transport within the biosynthetic and endocytic pathways. Finally, several Rab GTPases have been shown to impact mitochondrial morphology, biogenesis or turnover, including Rab32, Rab11, Rab4, and Rab7 (Alto et al, 2002; Bui et al, 2010; Caza et al, 2013; Landry et al, 2014; Talaber et al, 2014; Yamano et al, 2014). Therefore, it would not be surprising if some of these small GTPases were involved in MDV transport as well.
It is also critical to learn how MDVs may fuse with their target organelle. A splice variant of VAMP1A, called VAMP1B, was identified in 1998 and contains a mitochondrial targeting sequence in place of the C-terminal tail anchor (Isenmann et al, 1998). VAMP1B is ubiquitously expressed, whereas VAMP1A variants are exclusive to neurons. The function of VAMP1B is currently unknown, but it is a prime candidate to mediate fusion events of MDVs with target organelles. Mitochondrial proteomic studies have not identified a t-SNARE or SNAP25 homologue, suggesting that the mitochondria may be unable to receive incoming vesicles.
Interestingly, a high-resolution proteome of a very divergent mitochondrion-related organelle, called a mitosome, from the parasite Giardia intestinalis was recently published (Jedelsky et al, 2011). Mitosomes have lost their mtDNA, as well as their capacity to respire, and have almost-unrecognizable import machinery. Their major role is in fact to generate iron sulfur clusters for distribution throughout the cell. Despite its divergence from a typical mitochondrion, the highly purified mitosome proteome included potential orthologues of the retromer component Vps35, an R-SNARE 3 (a v-SNARE) and VAP, a VAMP (vesicle associated membrane protein)-interacting protein (Jedelsky et al, 2011). Like mammalian mitochondria, Giardia mitosomes are also limited to v-SNAREs in the absence of t-SNAREs or SNAP orthologues. This provides a clue that even the simplest mitosome may sort cargo within vesicles for delivery within the cell, perhaps to distribute iron sulfur clusters to other organelles, or for degradation as we see in mammalian cells.
The physiological contribution of MDV transport to mitochondrial quality control
MDV transport to lysosomes adds a fourth mechanism to the paradigms of mitochondrial quality control. MDVs function alongside the actions of mitochondrial proteases, ubiquitin-mediated proteasomal degradation, and mitophagy. The unanswered question is to define the relative contributions and potential hierarchy of these 4 mechanisms (Fig 3). Mitochondrial proteases degrade unfolded and oxidized proteins within the matrix and intermembrane space (Tatsuta & Langer, 2009). In yeast, an in vitro peptide export assay indicated that mitochondrial proteases degrade between 6–12% of proteins per hour, consistent with proteases as a front line of mitochondrial quality control (Augustin et al, 2005). It is also possible that proteases may trim down complexes and cargoes, leaving more hydrophobic regions to be subsequently removed via MDVs, a possible example of the overlap among these pathways. Loss of mitochondrial proteases leads to various forms of neurodegeneration, including spastic paraplegia (Casari et al, 1998; Atorino et al, 2003; Nolden et al, 2005). For example, mutations in AFG3L2, an m-AAA protease within the inner membrane, are responsible for spinocerebellar ataxia 28 (SCA28) (Di Bella et al, 2010). In addition, mitochondrial proteases are required for the processing of PINK1 (Greene et al, 2012), further supporting the interdependence between multiple quality control pathways.
Figure 3. Outline of the 4 pathways of mitochondrial quality control.
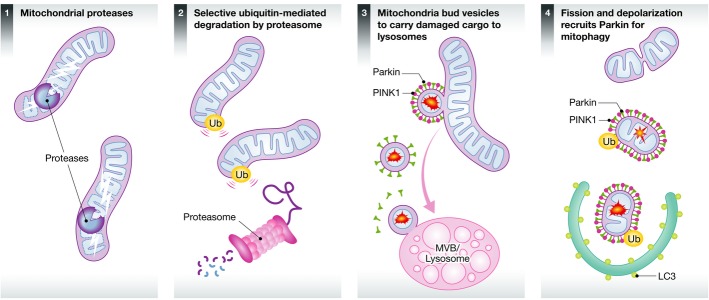
A schematic diagram depicting the presence of mitochondrial proteases within the mitochondrial matrix and intermembrane space, which likely acts as a first line of defense against unfolded and oxidized soluble proteins. Outer membrane proteins are instead removed from the mitochondria through a retrotranslocation pathway following ubiquitination. Degradation of these proteins is completed within the cytosolic proteasome, similar to the ER-associated degradation pathway. We propose that the third line of defense is the removal of mitochondrial patches through the generation of MDVs, which transit to the late endosome. Only upon complete mitochondrial dysfunction, or upon a failure of most import channels would the entire organelle be targeted to the autophagosome. Different tissues and cellular states may rely on each of these mechanisms to a variable degree, making it important to understand the levels of redundancy and overlap among these pathways.
Some outer membrane proteins are ubiquitinated and degraded by p97-dependent retrotranslocation and delivery to the cytosolic proteasome (Heo et al, 2010; Tanaka et al, 2010; Chan et al, 2011; Xu et al, 2011). This process efficiently removes surface proteins, which may be linked to quality control or to the selective removal of mitochondrial proteins in response to cellular signals (Neutzner et al, 2007). For example, the anti-apoptotic Bcl-2 family protein Mcl-1 is ubiquitinated by MULE during apoptosis, and its removal facilitates Bax activation and cell death (Warr et al, 2005; Zhong et al, 2005).
Mitophagy is an important mechanism to remove entirely dysfunctional mitochondria, whether linked to global protein misfolding or depolarization (Youle & Narendra, 2011). But what is the contribution of MDVs to steady-state quality control, and how does this compare with mitophagy? Unfortunately, since PINK1 and Parkin are required for both pathways, gene editing or siRNA approaches will not allow us to easily answer this. Loss of these genes in flies leads to a loss of dopaminergic neurons and flight muscle defects, consistent with their importance in mitochondrial quality control (Clark et al, 2006; Park et al, 2006; Yang et al, 2006). However, the loss of PINK1 or Parkin in mice leads to very mild phenotypes and a notable absence of neurodegeneration (Goldberg et al, 2003; Kitada et al, 2009). This hints at the existence of redundancies in both mitophagy and MDV formation in higher eukaryotes. Until we identify MDV-specific factors essential for their formation, we cannot determine the relative contributions in vivo. We do know that MDVs are released within 2–6 h following a mild stress like antimycin A, where mitophagy occurs between 12–24 h (McLelland et al, 2014). The prediction is that MDV formation protects the mitochondria from mitophagy by removing PINK1 and Parkin from each failed import channel. Any loss of MDV-specific machinery should therefore trigger premature mitophagy. Alternatively, the loss of MDVs may lead to increased global cellular damage since the mitochondria may need to deteriorate to an appropriate level in order to engage the mitophagy pathway. Deteriorating mitochondria would release increased levels of ROS and likely begin to consume cellular ATP in efforts to maintain their electrochemical potential.
Beyond a global consideration of MDVs acting upstream of mitophagy in quality control, there are a few clues that shed some light on the functional importance of MDV formation in mitochondrial homeostasis. Treating cultured cells with the lysosomal inhibitors bafilomycin A or pepstatin A/E-64D led to a significant accumulation of MDVs in the cytosol or within the multivesicular bodies (Soubannier et al, 2012a; McLelland et al, 2014). This demonstrated that MDV transport occurs in steady state, even in the absence of mitochondrial or redox-based stress. In addition, the amount of cargo released into MDVs using the in vitro budding assay was quantified, indicating that up to 4% of some proteins are ejected per hour (Soubannier et al, 2012b). While there are certainly limitations to these kinds of quantifications, it suggests that MDVs are transporting significant amounts of cargo in steady state.
In other approaches, a number of recent studies have quantified the half-lives of mitochondrial proteins under various conditions, from yeast to flies and mammalian tissues. The most striking observation from all of these studies is the extreme variety of half-lives of mitochondrial proteins—from minutes to weeks. For example, a recent study in mice demonstrated a threefold range in the turnover rates of different complex I subunits, indicating that mitochondrial proteins within the same macromolecular complex have differing half-lives (Kim et al, 2012). This is consistent with the existence of multiple, overlapping pathways for mitochondrial protein degradation. In an elegant study in Drosophila by the Pallanck lab, the accumulation of cellular proteins in flies lacking the core autophagy protein Atg5, compared with those lacking Parkin or PINK1, was quantified by mass spectrometry (Vincow et al, 2013). In the absence of Atg5, many cellular proteins—including ones targeted to mitochondria—accumulated, indicating that Atg5 was required for their turnover. There was a great deal of overlap between the Atg5 and Parkin accumulated proteomes, consistent with them functioning along the same pathway in mitophagy. However, there was a distinct subset of highly hydrophobic proteins of the mitochondrial inner membrane that accumulated specifically in the absence of Parkin (Vincow et al, 2013). This indicates that Parkin plays an additional role the selective removal of these proteins, most likely through vesicular carriers.
In yeast, the Abeliovich lab recently confirmed the selective degradation of mitochondrial content using a similar approach (Abeliovich et al, 2013). In this case, they observed a selective accumulation of proteins in stationary phase yeast upon the loss of Dnm1, the functional homologue of the fission GTPase Drp1. The authors concluded that mitophagy results in the selective removal of damaged content at very different rates and that this process requires ongoing fusion and fission events. Exactly how the cargo would be sorted into two halves of a dividing mitochondrion remains unclear. The authors proposed a type of “percolation” of specific proteins into large domains, perhaps based on aggregation. These large domains would eventually be separated from the functional organelle by dynamin-mediated constriction (Abeliovich et al, 2013). On the other hand, it is formally possible that yeast mitochondria bud vesicles in a Dnm1-dependent manner, where the lateral segregation of cargo may occur in an analogous manner to MDV transport in mammalian systems. There is a great deal of evolutionary distance between these two models, so these possibilities should not be ruled out without formally testing them with higher resolution imaging and biochemical analysis. Overall, quantitative mass spectrometry approaches are helping define the turnover of critically important mitochondrial proteins and lipids under a myriad of conditions.
The field of mitochondrial quality control has exploded with the causal links to many degenerative diseases. So do any of the recent studies support a role for MDVs in quality control? The most common approach is to use the protonophore CCCP to globally depolarize mitochondria and induce Parkin-dependent mitophagy. However, recent studies are expanding the analysis to more physiological systems, including the unfolded protein response (Jin & Youle, 2013), laser-induced damage (Yang & Yang, 2013), and the manipulation of mitochondrial proteases that interfere with PINK1 processing, such as the matrix processing protease (MPP) (Greene et al, 2012). Indeed, modest knockdown of the catalytic subunit MPPβ leads to PINK1 accumulation, Parkin recruitment, and mitophagy, without the widespread effects seen with CCCP on protein import and mitochondrial function (Greene et al, 2012). Using a different approach, the Youle lab employed a non-cleavable mutant of ornithine carbamyl transferase (Jin & Youle, 2013), first used to induce the mitochondrial unfolded protein response by Nick Hoogenraad (Zhao et al, 2002). Cells expressing this construct accumulated PINK1-YFP at specific foci along the mitochondrial tubules (Jin & Youle, 2013), presumably reflecting sites of failed import. Parkin was recruited to these sites as well, and after extended accumulation of unfolded proteins, respiratory competent mitochondria were cleared by mitophagy. Similarly, foci of Parkin and PI(3)P were observed along mitochondria following laser ablation (Yang & Yang, 2013), and these small fragments were targeted for mitophagy. In all these studies, the authors did not explore whether they were actually imaging MDVs as an early, targeted response to the stress. Certainly, if the magnitude of the damage is great enough, mitophagy will ensue, as is commonly observed. Interestingly, two tail-anchored mitochondrial membrane proteins, FKBP38 and Bcl-2, were shown to translocate to the endoplasmic reticulum to escape CCCP-induced mitophagy (Saita et al, 2013). The mechanism for this relocalization is not known, but the authors suggested the possibility of vesicle-based transport prior to mitochondrial removal by mitophagy. As the size of MDVs is smaller than the wavelength of light, routine confocal microscope approaches cannot distinguish between a mitochondrial fragment (˜400 nm diameter) and an MDV (˜100 nm) (Neuspiel et al, 2008; Soubannier et al, 2012a). Antibody staining can reveal the selection of specific mitochondrial cargo in these structures, and silencing Drp1 can help address the question of fission vs. budding. In the end, ultrastructural analysis is critical to directly visualize these events, something lacking from current studies. Some experimental considerations relevant to the analysis of cellular MDVs are listed in Box 1.
In order to ultimately define the functional contribution of MDV transport to mitochondrial quality control in vivo, we will continue to identify the core machinery required to generate and transport MDVs. We are currently utilizing the cell-free mitochondrial budding assay to generate vesicles in the presence of non-hydrolyzable GTP, for example, to stabilize potential coat proteins and other machinery. Mass spectrometry of isolated vesicles will help identify both the cargo and machinery. Lipidomics should lend insights into any lipid oxidation or enrichments that are likely to be very important cargoes as well. As the mechanisms gain in resolution, we will be able to directly assess the consequences on mitochondrial function and cell survival in different cell types and tissues.
Box 1. Experimental considerations for MDV analysis
– MDVs are visualized as small vesicular structures that show evidence of cargo selectivity. This is done using highly specific antibodies against endogenous mitochondrial proteins, or with a combination of transfected mitochondrial GFP-tagged constructs with antibodies to label a second or third mitochondrial protein. The limitation of antibody usage is the absolute dependence on the specificity and low or zero background signal. GFP-tagged and overexpressed proteins have not been efficient cargoes in general, perhaps because they are first targeted by proteases. We continue to evaluate mitochondrial content in MDVs and look to proteomics approaches for future analysis.
– To increase the ability to visualize MDVs, it is useful to minimize mitochondrial fragmentation by silencing the fission machinery, or expressing a dominant-negative Drp1. This results in hyperfused mitochondria, enabling the visualization of small, Drp-independent MDVs within the cell.
– To capture MDVs within the cytosol, prior to delivery to the lysosome, cells can be pre-treated with bafilomycin A. Alternatively, inhibition of lysosomal proteases with E64D/pepstatin A allows delivery to lysosomes, but blocks protein degradation. For transport to peroxisomes, the cargo is not degraded, so MAPL-positive MDVs en route to peroxisomes (or already within peroxisomes) is monitored by triple-labeling mitochondria, MAPL and peroxisomes. We do not yet have the tools to block the fusion of MAPL-positive MDVs with peroxisomes and accumulate them within the cytosol.
– Our experience with immunofluorescence approaches indicates a requirement for high percentages of PFA (5–6%), added directly to cells at 37°C without a PBS wash. This hints that the lipid/protein ratio in MDVs is not easily crosslinked and may be lost with low concentrations of fixative.
– The signal intensity of cargo within MDVs varies; Tom20-positive MDVs are brighter in Tom20 signal compared with Tom20 intensity within mitochondria, but PDH is less enriched in MDVs compared with the mitochondria. Given this variability, it is important to use an appropriate objective lens, preferably 100×, with the highest NA possible, at least a 1.4. Cameras for spinning confocal continue to improve, increasing the ability to visualize MDVs, and laser scanning confocal at high resolution also works well.
– Ultrastructural analysis is important to directly visualize MDVs within the cell using immunogold labeling. This is to confirm their size and differentiate them from Drp1-dependent fragments. The probabilities of capturing an unbudded MDV from the mitochondria is low unless cells are treated with stress agents like xanthine oxidaze/xanthine. Overexpressing MAPL in cells led to a dramatic stabilization of unbudded structures, suggesting that the GFP tag interfered with efficient removal. With the ongoing identification of new machinery required to generate MDVs, their silencing should help capture MDVs in various stages of formation. Tomography of tissues from disease animal models should also help visualize MDVs emerging in situ at high resolution.
The meaning of MDV transport to the peroxisome
Next to the ER, it can be argued that the peroxisome is the most closely linked organelle to the mitochondria (Mohanty & McBride, 2013). In mammalian cells, both organelles are responsible for the beta-oxidation of fatty acids, where peroxisomes selectively oxidize very long-chain fatty acids and perform alpha-oxidation reactions. Both organelles neutralize damaging oxidative by-products—peroxides (peroxisomes) or ROS (mitochondria)—and the biogenesis of both organelles is signaled by the peroxisome proliferator-activated receptor coactivator 1 alpha (PGC1α) pathway (Mohanty & McBride, 2013). The biogenesis and growth of both organelles is also coupled to the ER, which provides lipids to facilitate their growth. Finally, the organelle division machinery is shared, including the core fission GTPase Drp1 and its receptor Mff (Koch et al, 2003; Gandre-Babbe & van der Bliek, 2008). Indeed, membrane-anchored proteins like Mff are commonly localized to both peroxisomes and mitochondria, (Koch et al, 2005; Gandre-Babbe & van der Bliek, 2008). Most of these tail-anchored proteins appear to be imported into peroxisomes using the canonical, peroxisomal import machinery (Delille & Schrader, 2008).
We identified MAPL as a SUMO E3 ligase containing two transmembrane domains, with a C-terminal RING domain exposed to the cytosol and a approximately 40 kDa intermembrane space domain of unknown function (Li et al, 2008; Neuspiel et al, 2008; Zhang et al, 2008; Braschi et al, 2009; Jung et al, 2011). This protein has a unique evolutionary phylogeny, with the intermembrane space and transmembrane domains being conserved within distant bacteria, as well as plants (Andrade-Navarro et al, 2009). MAPL promotes the sumoylation of Drp1, stabilizing its recruitment to the mitochondria and promoting fission (Neuspiel et al, 2008; Braschi et al, 2009).
If peroxisomes can import their own proteins, why would MAPL require such a complex vesicular transport pathway? We are actively pursuing an answer to this question, so will only speculate here. Peroxisomes grow and divide as autonomous organelles. However, unlike the mitochondria, which multiply exclusively as a result of the enlargement and fission of existing organelles, peroxisomes can also be generated de novo (Dimitrov et al, 2013). Current evidence, primarily in yeast, supports a role for the ER in the generation of new peroxisomal precursor vesicles that may mature (Hoepfner et al, 2005), or fuse to form a mature organelle (Lam et al, 2010). The population of “young” versus “mature” peroxisomes varies depending on the cell type and growth conditions. As a regulator of Drp1, MAPL is likely to participate in peroxisomal biogenesis and division. The silencing of MAPL does not phenocopy Drp1, so it is not essential for mitochondrial fission, rather it can promote and stabilize Drp1 recruitment. It may be that MAPL is targeted to “young” or newly formed peroxisomes, which may have a higher rate of fission, or different regulatory mechanisms than the “older”, more mature peroxisomes.
A second reason to consider that MAPL-containing MDVs would target “young” peroxisomes is that old peroxisomes cannot fuse, only the ER-derived pre-peroxisomes are thought to be fusogenic (Dimitrov et al, 2013). Therefore, MDVs may deliver their cargo to the pool of nascent peroxisomes, and this cargo exchange may be necessary for peroxisomal maturation. As these peroxisomes grow and divide, MAPL would become diluted, or perhaps selectively degraded in some way. From an evolutionary perspective, it is interesting that most of the peroxisomal enzymes appear to derive from an archaebacterial origin (Gabaldon et al, 2006). This has led some to suggest that peroxisomes originated as specialized mitochondria responsible for the breakdown of very long-chain fatty acids (and a number of other biochemical processes) (Speijer, 2014). Perhaps, this ancient vesicular transport pathway allowed the selective segregation of potentially damaging (or highly ROS generating) mitochondrial pathways to a newly formed organelle within the primitive eukaryotic cell.
Supporting the idea that the mitochondria contribute to the generation of peroxisomes de novo is the fact that many peroxisomal membrane proteins default to the mitochondrial outer membrane in patient fibroblast cells lacking peroxisomes (Sacksteder et al, 2000; South et al, 2000; Kim et al, 2006; Toro et al, 2009). This is not an exclusive pathway, as other peroxins target the ER under these conditions (Kim et al, 2006; Toro et al, 2009; Yonekawa et al, 2011), as in yeast. To date, this mitochondrial default pathway in mammalian cells has not been considered physiologically meaningful. However, this observation is consistent with the mitochondria as a contributor to peroxisomal biogenesis. Indeed, a recent study from the Erdman laboratory showed that a yeast mutant strain lacking Pex3 was rescued by ectopically targeting Pex3 to the mitochondria, resulting in the biogenesis of functional peroxisomes in cells that previously lacked these organelles (Rucktaschel et al, 2010). This indicates that the machinery exists to generate peroxisomes from mitochondria, even in yeast. We are currently exploring whether the retromer subunit Vps35 may bind to mitochondrial-localized peroxins like Pex3. Upon transfection of Pex3-GFP, some cells show high levels of mitochondrial targeting even when peroxisomes are functioning normally (Kim et al, 2006). It is also possible to test whether the retromer is required to generate new peroxisomes in patient fibroblasts upon rescue with the missing peroxin. Approaches like this should help define the functional contribution of MDV transport to peroxisomes.
What mitochondrial cargo do peroxisomes require that cannot be simply imported directly from the cytosol? These organelles share many metabolites, including heme, which is generated from iron sulfur clusters in the mitochondria and required for peroxisomal enzymes like catalase (Lazarow & de Duve, 1973; Stehling et al, 2014). Phospholipids like phosphatidylethanolamine, which is used to generate plasmalogens (Braverman & Moser, 2012), and perhaps even very long-chain fatty acids that may be misdirected to the mitochondria could be carried more easily within MDVs rather than via a soluble, cytosolic intermediate. With the identification of the retromer complex as requisite for this vesicle transport pathway, we can dissect the functional consequences on peroxisomal function when Vps35 is lost (Braschi et al, 2010). For example, if heme is a cargo, then loss of Vps35 may lead to oxidative stress within peroxisomes through catalase dysfunction. This would be particularly dangerous for neurons, since it is established that patients suffering from peroxisomal disorders present with a primarily neurological phenotype (Powers, 2001). Patients suffering from Zellweger syndrome carry mutations in core peroxisomal import proteins and therefore lack peroxisomes. The primary manifestation of the disease is a loss of myelination and neuronal cell death through the accumulation of very long-chain fatty acids and ROS production (Barry & O'Keeffe, 2013). Although primarily a disease of infants, an analysis of peroxisomal dysfunction in age-related diseases like PD is currently gaining momentum (Fransen et al, 2013). In general terms, it is clear that the mitochondria and peroxisomes are intimately linked and that the dysfunction of one can lead to the dysfunction of the other (Baumgart et al, 2001).
An alternative fate for MAPL-containing MDVs may be to a specialized functional population of peroxisomes. For example, it is possible that peroxisomes may not all be identical; some may specialize in bile acid synthesis, some in long-chain beta-oxidation, and others in plasmalogen synthesis. It is difficult to envision how the import machinery would select for a subset of functional enzymes. On the other hand, the delivery of an MDV to a peroxisome would change the content by supplying metabolite substrates or enzymes, thereby actively generating a functionally distinct class of peroxisome. Although some evidence supports the existence of different populations of peroxisomes in isolated tissue, identified by altered buoyant densities (Schrader et al, 1994), the functional implications of these different pools have not been established. Again, once the fusion machinery and cargo are identified, we can directly test these hypotheses.
Other fates for MDVs?
The fate of mitochondrial cargo entering the late endosome/multivesicular body is primarily to be degraded, since the inhibition of lysosomal proteases led to an accumulation of mitochondrial content (Soubannier et al, 2012a; McLelland et al, 2014). However, there may be other fates of this cargo. The multivesicular body can be routed to the cell surface, where its limiting membrane can fuse with the plasma membrane (Raposo & Stoorvogel, 2013). This leads to the release of its internal contents—intralumenal vesicles (exosomes), which can contain protein and microRNAs, as well as vacuolar proteases—that can have broad-ranging effects on neighboring cells; from microRNA-based reprogramming to cancer cell migration (Raposo & Stoorvogel, 2013). Proteomic analysis of exosome content, compiled within the “Vesiclepedia” (http://www.microvesicles.org) indicates that up to 10% of secreted proteins are mitochondrial (Choi et al, 2013; Burke et al, 2014), although it is not known whether this is functionally significant. Autophagosomes can also be a source of unconventional secretion, which can include a subset of leaderless cytosolic proteins (Zhang & Schekman, 2013), with some evidence of transit through the recycling endosome (Duran et al, 2010; Manjithaya et al, 2010). The most robust example of secreted autophagosomes occurs during erythrocyte development when the cytosolic organelles are ejected to form the red cell (Ney, 2011). MDV transport to the late endosome in steady state would predict the presence of selected mitochondrial content in exosomes under most conditions (Fig 2). Therefore, both mitophagy and MDV transport to the late endosome provide a means for the secretion of mitochondrial content from the cell.
The steady-state presence of mitochondrial content in exosomes may have some important physiological consequences. Under the conditions we have examined so far, we have shown that MDVs can contain at least three of the five complexes of the electron transport chain. Complexes III and IV both include subunits encoded by the mitochondrial DNA, which are translated with a formylated initiating methionine, conserved from bacterial translation (Kozak, 1983). The presence of extracellular formylated proteins or peptides can launch an immune response, whether of bacterial or mitochondrial origin (Zhang et al, 2010). This is because non-methylated DNA (Pollack et al, 1984) and formylated peptides bind and activate Toll-like receptor 9 leading to cytokine release and lymphocyte infiltration (Zhang et al, 2010). This pathway was shown to become activated within the endosome of cells lacking the lysosomal DNAseII (Oka et al, 2012). The subsequent accumulation of mtDNA within the autophagosome eventually escaped into the endosomal compartments, activating the receptors in the lumen of the endosome. Therefore, whether secreted through exosomes, or accumulated within the endosome, mitochondrial content has the potential to launch an inflammatory response.
Another possible link between MDVs, exosomes and disease may involve α-synuclein. α-synuclein is an aggregation-prone cytosolic protein important in the pathogenesis of sporadic PD. Moreover, mutations in α-synuclein, like those in Parkin and PINK1, lead to a familial form of PD, albeit a dominant iteration of the disease (Klein & Westenberger, 2012). Secretion and cell-to-cell transfer of α-synuclein has been shown both in cells and in vivo (Lee et al, 2004, 2005, 2008a, 2008b; Desplats et al, 2009; Luk et al, 2012a, 2012b), and exosomes constitute a proposed method of propagation (Emmanouilidou et al, 2010; Kong et al, 2014; Lee et al, 2014). While a functional role for α-synuclein has been proposed in the assembly of presynaptic SNARE complexes (Chandra et al, 2005; Burre et al, 2010; Diao et al, 2013), α-synuclein has also been shown to function at mitochondria. A number of studies have identified α-synuclein as present either within mitochondria (Cole et al, 2008; Devi et al, 2008), or localized to mitochondrial-associated membranes of the ER (Guardia-Laguarta et al, 2014) and have implicated it in binding to anionic and cardiolipin-containing membranes (Nakamura et al, 2011; Zigoneanu et al, 2012; Diao et al, 2013) and in promoting Drp1-independent fission (a hallmark of MDV formation) in cells (Kamp et al, 2010; Nakamura et al, 2011). Thus, the incorporation of α-synuclein into multivesicular bodies via MDVs represents an intriguing candidate mechanism for its exosomal secretion, with additional implications for PD.
Final thoughts
With advances in the mechanistic characterization of MDV transport, we hope the field will be encouraged to carefully examine these structures within various physiological paradigms. There are many lines of evidence supporting the importance of MDV transport in mitochondrial cell biology; from an unbiased evolutionary consideration of the process (Kulp & Kuehn, 2010; Jedelsky et al, 2011), to their potential roles in peroxisomal function (Neuspiel et al, 2008; Braschi et al, 2010) and mitochondrial quality control (Soubannier et al, 2012a, 2012b; McLelland et al, 2014). MDVs can be observed by monitoring multiple mitochondrial markers by confocal microscopy, particularly in cells where Drp1 function is lost (Box 1). With the resulting hyperfused reticulum, these small vesicles are much more noticeable using high-resolution imaging techniques. Technically, there are still some limitations, primarily in the limited assortment of antibodies. There are approximately 1,000 proteins in the mitochondria, and we can only follow a handful using immunofluorescence approaches. In addition, we have noted that MDVs are somewhat fragile and not as easily fixed compared with the mitochondrial tubules (we routinely fix with 5–6% paraformaldehyde, for example). This likely reflects differences in lipid and protein composition within MDVs. In future studies, it will be important to examine the functional contribution of MDVs in the physiology of specific tissues, particularly neurons, given the apparent links to Parkinson's disease.
The mitochondria are the most recent eukaryotic organelle from which vesicle transport has been observed. However, there is some irony in the knowledge that this process has clearly been conserved from the organelle's humble origins as archaebacteria. It leads us to wonder what other fascinating and unexpected cellular pathways are waiting to be discovered as we dig deeper into the mechanisms and meaning of mitochondrial vesicles.
Funding
Canadian Institutes of Health Research (to EAF and HMM). Heart and Stroke Foundation of Ontario (to HMM).
Author contributions
HMM wrote the manuscript, AS, GLM and EAF contributed to the writing, the development of the concepts presented, and the generation of the figures.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abeliovich H, Zarei M, Rigbolt KT, Youle RJ, Dengjel J. Involvement of mitochondrial dynamics in the segregation of mitochondrial matrix proteins during stationary phase mitophagy. Nat Commun. 2013;4:2789. doi: 10.1038/ncomms3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto NM, Soderling J, Scott JD. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Navarro MA, Sanchez-Pulido L, McBride HM. Mitochondrial vesicles: an ancient process providing new links to peroxisomes. Curr Opin Cell Biol. 2009;21:560–567. doi: 10.1016/j.ceb.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atorino L, Silvestri L, Koppen M, Cassina L, Ballabio A, Marconi R, Langer T, Casari G. Loss of m-AAA protease in mitochondria causes complex I deficiency and increased sensitivity to oxidative stress in hereditary spastic paraplegia. J Cell Biol. 2003;163:777–787. doi: 10.1083/jcb.200304112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin S, Nolden M, Muller S, Hardt O, Arnold I, Langer T. Characterization of peptides released from mitochondria: evidence for constant proteolysis and peptide efflux. J Biol Chem. 2005;280:2691–2699. doi: 10.1074/jbc.M410609200. [DOI] [PubMed] [Google Scholar]
- Barry DS, O'Keeffe GW. Peroxisomes: the neuropathological consequences of peroxisomal dysfunction in the developing brain. Int J Biochem Cell Biol. 2013;45:2012–2015. doi: 10.1016/j.biocel.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Baumgart E, Vanhorebeek I, Grabenbauer M, Borgers M, Declercq PE, Fahimi HD, Baes M. Mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model for Zellweger syndrome (PEX5 knockout mouse) Am J Pathol. 2001;159:1477–1494. doi: 10.1016/S0002-9440(10)62534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnington KE, Kuehn MJ. Protein selection and export via outer membrane vesicles. Biochim Biophys Acta. 2014;1843:1612–1619. doi: 10.1016/j.bbamcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi E, Zunino R, McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009;10:748–754. doi: 10.1038/embor.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi E, Goyon V, Zunino R, Mohanty A, Xu L, McBride HM. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol. 2010;20:1310–1315. doi: 10.1016/j.cub.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Bui M, Gilady SY, Fitzsimmons RE, Benson MD, Lynes EM, Gesson K, Alto NM, Strack S, Scott JD, Simmen T. Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem. 2010;285:31590–31602. doi: 10.1074/jbc.M110.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M, Choksawangkarn W, Edwards N, Ostrand-Rosenberg S, Fenselau C. Exosomes from myeloid-derived suppressor cells carry biologically active proteins. J Proteome Res. 2014;13:836–843. doi: 10.1021/pr400879c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casari G, De Fusco M, Ciarmatori S, Zeviani M, Mora M, Fernandez P, De Michele G, Filla A, Cocozza S, Marconi R, Durr A, Fontaine B, Ballabio A. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell. 1998;93:973–983. doi: 10.1016/s0092-8674(00)81203-9. [DOI] [PubMed] [Google Scholar]
- Caza TN, Fernandez DR, Talaber G, Oaks Z, Haas M, Madaio MP, Lai ZW, Miklossy G, Singh RR, Chudakov DM, Malorni W, Middleton F, Banki K, Perl A. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis. 2013;73:1888–1897. doi: 10.1136/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dorn GW., II PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8:1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL. Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res. 2008;314:2076–2089. doi: 10.1016/j.yexcr.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BM, Norwood SJ, Kerr MC, Mahony D, Seaman MN, Teasdale RD, Owen DJ. Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic. 2008;9:366–379. doi: 10.1111/j.1600-0854.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol. 2012;14:29–37. doi: 10.1038/ncb2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delille HK, Schrader M. Targeting of hFis1 to peroxisomes is mediated by Pex19p. J Biol Chem. 2008;283:31107–31115. doi: 10.1074/jbc.M803332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bella D, Lazzaro F, Brusco A, Plumari M, Battaglia G, Pastore A, Finardi A, Cagnoli C, Tempia F, Frontali M, Veneziano L, Sacco T, Boda E, Brussino A, Bonn F, Castellotti B, Baratta S, Mariotti C, Gellera C, Fracasso V. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet. 2010;42:313–321. doi: 10.1038/ng.544. [DOI] [PubMed] [Google Scholar]
- Diao J, Burre J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Sudhof TC, Brunger AT. Native alpha-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife. 2013;2:e00592. doi: 10.7554/eLife.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov L, Lam SK, Schekman R. The role of the endoplasmic reticulum in peroxisome biogenesis. Cold Spring Harb Perspect Biol. 2013;5:a013243. doi: 10.1101/cshperspect.a013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG. Phospholipase D in endocytosis and endosomal recycling pathways. Biochim Biophys Acta. 2009;1791:845–849. doi: 10.1016/j.bbalip.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol. 2010;188:527–536. doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M, Rouleau G, Thorarinsdottir T, Brice A, van Bergen En Henegouwen PM, Fon EA. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- Fransen M, Nordgren M, Wang B, Apanasets O, Van Veldhoven PP. Aging, age-related diseases and peroxisomes. Subcell Biochem. 2013;69:45–65. doi: 10.1007/978-94-007-6889-5_3. [DOI] [PubMed] [Google Scholar]
- Gabaldon T, Snel B, van Zimmeren F, Hemrika W, Tabak H, Huynen MA. Origin and evolution of the peroxisomal proteome. Biol Direct. 2006;1:8. doi: 10.1186/1745-6150-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 2012;13:378–385. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S. Alpha-synuclein is localized to mitochondria-associated ER membranes. J Neurosci. 2014;34:249–259. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JM, Livnat-Levanon N, Taylor EB, Jones KT, Dephoure N, Ring J, Xie J, Brodsky JL, Madeo F, Gygi SP, Ashrafi K, Glickman MH, Rutter J. A stress-responsive system for mitochondrial protein degradation. Mol Cell. 2010;40:465–480. doi: 10.1016/j.molcel.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122:85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Huang H, Gao Q, Peng X, Choi SY, Sarma K, Ren H, Morris AJ, Frohman MA. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev Cell. 2011;20:376–387. doi: 10.1016/j.devcel.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi M, Kujuro Y, Okatsu K, Koyano F, Kosako H, Kimura M, Suzuki N, Uchiyama S, Tanaka K, Matsuda N. Parkin-catalyzed ubiquitin-ester transfer is triggered by PINK1-dependent phosphorylation. J Biol Chem. 2013;288:22019–22032. doi: 10.1074/jbc.M113.467530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenmann S, Khew-Goodall Y, Gamble J, Vadas M, Wattenberg BW. A splice-isoform of vesicle-associated membrane protein-1 (VAMP-1) contains a mitochondrial targeting signal. Mol Biol Cell. 1998;9:1649–1660. doi: 10.1091/mbc.9.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedelsky PL, Dolezal P, Rada P, Pyrih J, Smid O, Hrdy I, Sedinova M, Marcincikova M, Voleman L, Perry AJ, Beltran NC, Lithgow T, Tachezy J. The minimal proteome in the reduced mitochondrion of the parasitic protist Giardia intestinalis. PLoS ONE. 2011;6:e17285. doi: 10.1371/journal.pone.0017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SM, Youle R. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy. 2013;9:1750–1757. doi: 10.4161/auto.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Bae S, Lee JY, Woo SR, Cha HJ, Yoon Y, Suh KS, Lee SJ, Park IC, Jin YW, Lee KH, An S, Lee JH. E3 ubiquitin ligase Hades negatively regulates the exonuclear function of p53. Cell Death Differ. 2011;18:1865–1875. doi: 10.1038/cdd.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T, Giese A, Beyer K, Eimer S, Winklhofer KF, Haass C. Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 2010;29:3571–3589. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205:143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol. 2004;166:1027–1039. doi: 10.1083/jcb.200407046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, Muqit MM. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J. 2014;460:127–139. doi: 10.1042/BJ20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PK, Mullen RT, Schumann U, Lippincott-Schwartz J. The origin and maintenance of mammalian peroxisomes involves a de novo PEX16-dependent pathway from the ER. J Cell Biol. 2006;173:521–532. doi: 10.1083/jcb.200601036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Park J, Kim S, Song S, Kwon SK, Lee SH, Kitada T, Kim JM, Chung J. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- Kim TY, Wang D, Kim AK, Lau E, Lin AJ, Liem DA, Zhang J, Zong NC, Lam MP, Ping P. Metabolic labeling reveals proteome dynamics of mouse mitochondria. Mol Cell Proteomics. 2012;11:1586–1594. doi: 10.1074/mcp.M112.021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Tong Y, Gautier CA, Shen J. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J Neurochem. 2009;111:696–702. doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Westenberger A. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Thiemann M, Grabenbauer M, Yoon Y, McNiven MA, Schrader M. Dynamin-like protein 1 is involved in peroxisomal fission. J Biol Chem. 2003;278:8597–8605. doi: 10.1074/jbc.M211761200. [DOI] [PubMed] [Google Scholar]
- Koch A, Yoon Y, Bonekamp NA, McNiven MA, Schrader M. A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2005;16:5077–5086. doi: 10.1091/mbc.E05-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapalli C, Kazlauskaite A, Zhang N, Woodroof HI, Campbell DG, Gourlay R, Burchell L, Walden H, Macartney TJ, Deak M, Knebel A, Alessi DR, Muqit MM. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012;2:120080. doi: 10.1098/rsob.120080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, Farghaian H, Cole AR, Lay PA, Sue CM, Cooper AA. Parkinson's disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum Mol Genet. 2014;23:2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, Matsuda N. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983;47:1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Yoda N, Schekman R. A vesicle carrier that mediates peroxisome protein traffic from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2010;107:21523–21528. doi: 10.1073/pnas.1013397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry MC, Champagne C, Boulanger MC, Jette A, Fuchs M, Dziengelewski C, Lavoie JN. A functional interplay between the small GTPase Rab11a and mitochondria-shaping proteins regulates mitochondrial positioning and polarization of the actin cytoskeleton downstream of Src family kinases. J Biol Chem. 2014;289:2230–2249. doi: 10.1074/jbc.M113.516351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22:320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow PB, de Duve C. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J Cell Biol. 1973;59:507–524. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008a;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee SJ. Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008b;372:423–428. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Bae EJ, Lee SJ. Extracellular alpha–synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10:92–98. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A, Chanda SK, Batalov S, Joazeiro CA. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the Organelle's dynamics and signaling. PLoS ONE. 2008;3:e1487. doi: 10.1371/journal.pone.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012a;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012b;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjithaya R, Anjard C, Loomis WF, Subramani S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol. 2010;188:537–546. doi: 10.1083/jcb.200911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayinger P. Phosphoinositides and vesicular membrane traffic. Biochim Biophys Acta. 2012;1821:1104–1113. doi: 10.1016/j.bbalip.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33:282–295. doi: 10.1002/embj.201385902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesmin B, Antonny B, Drin G. Insights into the mechanisms of sterol transport between organelles. Cell Mol Life Sci. 2013;70:3405–3421. doi: 10.1007/s00018-012-1247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, McBride HM. Emerging roles of mitochondria in the evolution, biogenesis, and function of peroxisomes. Front Physiol. 2013;4:268. doi: 10.3389/fphys.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, Sesaki H, Cheng Y, Finkbeiner S, Nussbaum RL, Masliah E, Edwards RH. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem. 2011;286:20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto Y, Wenk MR, Watanabe M, Daniell L, Murakami T, Ringstad N, Yamada H, Takei K, De Camilli P. Identification and characterization of a synaptojanin 2 splice isoform predominantly expressed in nerve terminals. J Biol Chem. 2001;276:41133–41142. doi: 10.1074/jbc.M106404200. [DOI] [PubMed] [Google Scholar]
- Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol. 2008;18:102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Neutzner A, Youle RJ, Karbowski M. Outer mitochondrial membrane protein degradation by the proteasome. Novartis Found Symp. 2007;287:4–14. discussion 14–20. [PubMed] [Google Scholar]
- Ney PA. Normal and disordered reticulocyte maturation. Curr Opin Hematol. 2011;18:152–157. doi: 10.1097/MOH.0b013e328345213e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolden M, Ehses S, Koppen M, Bernacchia A, Rugarli EI, Langer T. The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria. Cell. 2005;123:277–289. doi: 10.1016/j.cell.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Pierrat B, Simonen M, Cueto M, Mestan J, Ferrigno P, Heim J. SH3GLB, a new endophilin-related protein family featuring an SH3 domain. Genomics. 2001;71:222–234. doi: 10.1006/geno.2000.6378. [DOI] [PubMed] [Google Scholar]
- Pollack Y, Kasir J, Shemer R, Metzger S, Szyf M. Methylation pattern of mouse mitochondrial DNA. Nucleic Acids Res. 1984;12:4811–4824. doi: 10.1093/nar/12.12.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers JM. Normal and defective neuronal membranes: structure and function: neuronal lesions in peroxisomal disorders. J Mol Neurosci. 2001;16:285–287. doi: 10.1385/JMN:16:2-3:285. discussion 317–221. [DOI] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rojas R, Kametaka S, Haft CR, Bonifacino JS. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol Cell Biol. 2007;27:1112–1124. doi: 10.1128/MCB.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucktaschel R, Halbach A, Girzalsky W, Rottensteiner H, Erdmann R. De novo synthesis of peroxisomes upon mitochondrial targeting of Pex3p. Eur J Cell Biol. 2010;89:947–954. doi: 10.1016/j.ejcb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Sacksteder KA, Jones JM, South ST, Li X, Liu Y, Gould SJ. PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis. J Cell Biol. 2000;148:931–944. doi: 10.1083/jcb.148.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saita S, Shirane M, Nakayama KI. Selective escape of proteins from the mitochondria during mitophagy. Nat Commun. 2013;4:1410. doi: 10.1038/ncomms2400. [DOI] [PubMed] [Google Scholar]
- Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496:372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Baumgart E, Volkl A, Fahimi HD. Heterogeneity of peroxisomes in human hepatoblastoma cell line HepG2. Evidence of distinct subpopulations. Eur J Cell Biol. 1994;64:281–294. [PubMed] [Google Scholar]
- Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN. The retromer complex – endosomal protein recycling and beyond. J Cell Sci. 2012;125:4693–4702. doi: 10.1242/jcs.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftel AD, Zhang AS, Brown C, Shirihai OS, Ponka P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood. 2007;110:125–132. doi: 10.1182/blood-2007-01-068148. [DOI] [PubMed] [Google Scholar]
- Shiao YJ, Lupo G, Vance JE. Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J Biol Chem. 1995;270:11190–11198. doi: 10.1074/jbc.270.19.11190. [DOI] [PubMed] [Google Scholar]
- Shiba-Fukushima K, Imai Y, Yoshida S, Ishihama Y, Kanao T, Sato S, Hattori N. PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci Rep. 2012;2:1002. doi: 10.1038/srep01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, McBride HM. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012a;22:135–141. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Soubannier V, Rippstein P, Kaufman BA, Shoubridge EA, McBride HM. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS ONE. 2012b;7:e52830. doi: 10.1371/journal.pone.0052830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South ST, Sacksteder KA, Li X, Liu Y, Gould SJ. Inhibitors of COPI and COPII do not block PEX3-mediated peroxisome synthesis. J Cell Biol. 2000;149:1345–1360. doi: 10.1083/jcb.149.7.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speijer D. Reconsidering ideas regarding the evolution of peroxisomes: the case for a mitochondrial connection. Cell Mol Life Sci. 2014;71:2377–2378. doi: 10.1007/s00018-013-1507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehling O, Wilbrecht C, Lill R. Mitochondrial iron-sulfur protein biogenesis and human disease. Biochimie. 2014;100:61–77. doi: 10.1016/j.biochi.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Karbowski M, Yamaguchi H, Kazi A, Wu J, Sebti SM, Youle RJ, Wang HG. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol. 2005;25:9369–9382. doi: 10.1128/MCB.25.21.9369-9382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mul JJ, Pledger WJ, Wang HG. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaber G, Miklossy G, Oaks Z, Liu Y, Tooze SA, Chudakov DM, Banki K, Perl A. HRES-1/Rab4 promotes the formation of LC3(+) autophagosomes and the accumulation of mitochondria during autophagy. PLoS ONE. 2014;9:e84392. doi: 10.1371/journal.pone.0084392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T, Langer T. AAA proteases in mitochondria: diverse functions of membrane-bound proteolytic machines. Res Microbiol. 2009;160:711–717. doi: 10.1016/j.resmic.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Toro AA, Araya CA, Cordova GJ, Arredondo CA, Cardenas HG, Moreno RE, Venegas A, Koenig CS, Cancino J, Gonzalez A, Santos MJ. Pex3p-dependent peroxisomal biogenesis initiates in the endoplasmic reticulum of human fibroblasts. J Cell Biochem. 2009;107:1083–1096. doi: 10.1002/jcb.22210. [DOI] [PubMed] [Google Scholar]
- Trempe JF, Chen CX, Grenier K, Camacho EM, Kozlov G, McPherson PS, Gehring K, Fon EA. SH3 domains from a subset of BAR proteins define a Ubl-binding domain and implicate parkin in synaptic ubiquitination. Mol Cell. 2009;36:1034–1047. doi: 10.1016/j.molcel.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat Rev Neurol. 2013;9:445–454. doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, Behrouz B, Melrose HL, Hentati E, Puschmann A, Evans DM, Conibear E, Wasserman WW, Aasly JO, Burkhard PR, Djaldetti R. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89:162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci USA. 2013;110:6400–6405. doi: 10.1073/pnas.1221132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr MR, Acoca S, Liu Z, Germain M, Watson M, Blanchette M, Wing SS, Shore GC. BH3-ligand regulates access of MCL-1 to its E3 ligase. FEBS Lett. 2005;579:5603–5608. doi: 10.1016/j.febslet.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Wurm CA, Neumann D, Lauterbach MA, Harke B, Egner A, Hell SW, Jakobs S. Nanoscale distribution of mitochondrial import receptor Tom20 is adjusted to cellular conditions and exhibits an inner-cellular gradient. Proc Natl Acad Sci USA. 2011;108:13546–13551. doi: 10.1073/pnas.1107553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Peng G, Wang Y, Fang S, Karbowski M. The AAA-ATPase p97 is essential for outer mitochondrial membrane protein turnover. Mol Biol Cell. 2011;22:291–300. doi: 10.1091/mbc.E10-09-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano K, Youle R. PINK1 is degraded through the N-end rule pathway. Autophagy. 2013;9:1–12. doi: 10.4161/auto.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano K, Fogel AI, Wang C, van der Bliek AM, Youle RJ. Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. Elife. 2014;3:e01612. doi: 10.7554/eLife.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JY, Yang WY. Bit-by-bit autophagic removal of parkin-labelled mitochondria. Nat Commun. 2013;4:2428. doi: 10.1038/ncomms3428. [DOI] [PubMed] [Google Scholar]
- Yonekawa S, Furuno A, Baba T, Fujiki Y, Ogasawara Y, Yamamoto A, Tagaya M, Tani K. Sec16B is involved in the endoplasmic reticulum export of the peroxisomal membrane biogenesis factor peroxin 16 (Pex16) in mammalian cells. Proc Natl Acad Sci USA. 2011;108:12746–12751. doi: 10.1073/pnas.1103283108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkova IL, Stuckert F, Kisel MA, Shadyro OI, Arnhold J, Huster D. Formation of phosphatidic acid in stressed mitochondria. Arch Biochem Biophys. 2008;480:17–26. doi: 10.1016/j.abb.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Zehmer JK, Huang Y, Peng G, Pu J, Anderson RG, Liu P. A role for lipid droplets in inter-membrane lipid traffic. Proteomics. 2009;9:914–921. doi: 10.1002/pmic.200800584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AS, Sheftel AD, Ponka P. Intracellular kinetics of iron in reticulocytes: evidence for endosome involvement in iron targeting to mitochondria. Blood. 2005;105:368–375. doi: 10.1182/blood-2004-06-2226. [DOI] [PubMed] [Google Scholar]
- Zhang B, Huang J, Li HL, Liu T, Wang YY, Waterman P, Mao AP, Xu LG, Zhai Z, Liu D, Marrack P, Shu HB. GIDE is a mitochondrial E3 ubiquitin ligase that induces apoptosis and slows growth. Cell Res. 2008;18:900–910. doi: 10.1038/cr.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Schekman R. Cell biology, unconventional secretion, unconventional solutions. Science. 2013;340:559–561. doi: 10.1126/science.1234740. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Zigoneanu IG, Yang YJ, Krois AS, Haque E, Pielak GJ. Interaction of alpha-synuclein with vesicles that mimic mitochondrial membranes. Biochim Biophys Acta. 2012;1818:512–519. doi: 10.1016/j.bbamem.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]