Abstract
The Extracorporeal Treatments In Poisoning (EXTRIP) workgroup was formed to provide recommendations on the use of extracorporeal treatments (ECTR) in poisoning. Here, the workgroup presents its results for tricyclic antidepressants (TCAs). After an extensive literature search, using a predefined methodology, the subgroup responsible for this poison reviewed the articles, extracted the data, summarized findings, and proposed structured voting statements following a predetermined format. A two-round modified Delphi method was chosen to reach a consensus on voting statements and RAND/UCLA Appropriateness Method to quantify disagreement. Blinded votes were compiled, returned, and discussed in person at a meeting. A second vote determined the final recommendations. Seventy-seven articles met inclusion criteria. Only case reports, case series, and one poor-quality observational study were identified yielding a very low quality of evidence for all recommendations. Data on 108 patients, including 12 fatalities, were abstracted. The workgroup concluded that TCAs are not dialyzable and made the following recommendation: ECTR is not recommended in severe TCA poisoning (1D). The workgroup considers that poisoned patients with TCAs are not likely to have a clinical benefit from extracorporeal removal and recommends it NOT to be used in TCA poisoning.
The Extracorporeal Treatments In Poisoning (EXTRIP) workgroup is comprised of international experts representing diverse specialties and professional societies (Table S1) and was created to provide recommendations based on evidence (or, in its absence, consensus) on the use of extracorporeal treatments (ECTR) in poisoning (http://www.extrip-workgroup.org). Rationale, background, objectives, complete methodology, and the first poison recommendation have been previously published 1–3. The following text reviews the results and recommendations for tricyclic antidepressants (TCAs).
Pharmacology
TCAs have been in clinical use for the treatment of depression since the 1950s. Despite having been largely replaced by newer antidepressants, TCAs continue to be prescribed for a number of conditions, including major depressive disorder, chronic and neuropathic pain, attention deficit hyperactivity disorder, cycling vomiting, nocturnal enuresis, and obsessive-compulsive disorders 4–7. TCAs share a common three-ring structure and can be classified into tertiary amines (amitryptiline, clomipramine, doxepin, imipramine, trimipramine) and secondary amines (desipramine and nortriptyline) 8. The antidepressant effects of these drugs are largely the result of presynaptic reuptake inhibition of serotonin and norepinephrine. Other pharmacologic effects include competitive muscarinic and alpha-adrenergic antagonism and histamine inhibition, as well as GABA-A antagonism. TCAs produce cardiac sodium channel blockade and can be classified as having type IA antiarrhythmic properties.
TCAs are rapidly absorbed from the gastrointestinal tract, but because of their anticholinergic effects in overdose, decreased gastrointestinal motility can prolong the time to peak drug concentrations 9. TCAs are extensively bound to plasma proteins, mainly alpha-1 acid glycoprotein and lipoproteins. Due to their lipophilicity, free drug distributes rapidly into tissues with characteristically large volumes of distribution and long half-lives of elimination (Table1). Drug concentration in the myocardium and the brain has been reported to be 40–200 times greater than in plasma 10. TCAs undergo first-pass hepatic metabolism, and have a high endogenous clearance. Hepatic metabolism results in the generation of numerous metabolites, many with pharmacologic activity, most of which are eliminated in the urine 10,11.
Table 1.
Pharmacokinetic properties of TCA
| Drug | Bioavailability% | Protein Binding% | Plasma half-life (hours) | Active metabolites | Volume of distribution (l/kg) |
|---|---|---|---|---|---|
| Amitryptiline | 31–61 | 82–96 | 31–46 | Nortiptyline | 5–20 |
| Amoxapine | 46–82 | NA | 8.8–14 | NA | |
| Clomipramine | 36–62 | 90–98 | 22–84 | Desmethyl | 7–20 |
| Desipramine | 60–70 | 73–90 | 14–62 | 22–59 | |
| Dothiepin | 30 | 85 | 14–24 | Dothiepin-s-oxide | 11–78 |
| Doxepin | 13–45 | 80 | 8–24 | Desmethyl | 9–33 |
| Imipramine | 29–77 | 76–95 | 9–24 | Desipramine | 15–30 |
| Maprotiline | 79–87 | 88 | 27–50 | 16–32 | |
| Nortriptyline | 32–79 | 93–95 | 18–93 | 10-hydroxy | 21–57 |
| Protriptyline | 75–90 | 90–94 | 54–198 | 15–31 | |
| Trimipramine | 18–63 | 93–97 | 16–40 | 17–48 |
Adapted from Dziukas 1991, DeVane CL, Cyclic Antidepressants, In: Burton ME, Shaw LM, et al. Applied Pharmacokinetics and Pharmacodynamics: Principles of Therapeutic Drug Monitoring 2006; Lippincott Williams & Wilkins, pp 781–797.
Overview of Tricyclic Antidepressant Poisoning
TCAs continue to be a leading cause of mortality and morbidity in poisoned patients and are responsible for nearly half of all fatalities reported due to antidepressants 12.
The clinical features of TCA poisoning are largely an extension of their pharmacologic actions described above. Antihistaminic- and anticholinergic- mediated effects typically result in altered mental status ranging from agitation and delirium to central nervous system depression and coma. Other anticholinergic effects (dry flushed skin, tachycardia, ileus, mydriasis, urinary retention, and hyperthermia) are usually present. Seizures result from anticholinergic and GABA-A antagonism, while the cardiovascular effects are caused by muscarinic and alpha-adrenergic blockade. These effects are manifested by tachycardia, peripheral vasodilation, and hypotension. Sodium channel blockade and the resulting delayed depolarization can cause wide complex arrhythmias, AV conduction disturbances, and myocardial depression, which are the primary cause of death in TCA overdose. Most deaths occur in the prehospital environment and are reported in the first few hours after presentation 13,14.
Many exposures to TCA will result in benign courses requiring little or no treatment at all. The incidence of severe rhythm disturbances is rare, while hypotension and coma and seizures are more frequent 11,15,16. Predicting which patients will develop severe toxicity is still debated. A range of toxic doses of approximately 5–20 mg/kg is often cited, but the reported ingested dose of TCA is neither reliable nor a good predictor of outcome 14,17. Serum testing is not generally routinely available and concentrations are less predictive than electrocardiogram (ECG) findings to identify high-risk patients 18: QRS duration is a better predictor of seizures and ventricular arrhythmia than serum concentrations 19 and an R wave in aVR of more than 3 mm had a good predictive value for seizures or arrhythmias 20.
The management of patients poisoned with TCAs includes general proactive care directed at securing the patient's airway and treating seizures with benzodiazepines. Hypotension can be a consequence of decreased vascular resistance and should be initially treated with fluid challenges, or secondary to myocardial depression and arrhythmias. An ECG should be performed to identify hallmarks of TCA sodium channel blockade (ventricular arrhythmia, QRS widening, Brugada-like pattern, or terminal axis deviation of the QRS, e.g., prominent R wave in aVR). Gastrointestinal decontamination may be indicated if the patient presents early after ingestion, and is discussed more extensively elsewhere 21–23.
Although there is no specific antidote for TCA poisoning, there is a fundamental role for sodium bicarbonate in the treatment of symptomatic TCA-poisoned patients. Sodium bicarbonate may ameliorate hypotension due to volume and sodium loading, and improves myocardial conduction disturbances presumably by creating a sodium load and also by inducing alkalosis 24. Systemic alkalosis itself, achieved by hyperventilation, also improves hypotension and cardiac conduction disturbances. The beneficial effect of alkalosis is potentially due to increased protein binding thereby reducing free drug availability and altering the charge of the TCA-receptor complex 25–27. However, hypertonic sodium has also demonstrated benefit in few animal studies and isolated cases 25. Sodium bicarbonate combines the effect of sodium loading and alkalosis and remains the therapy of choice, especially for patients presenting with seizures, fluid-unresponsive hypotension, or typical ECG findings (ventricular dysrhythmia, QRS >100 ms, prominent R waves in aVR) 28. Induced hyperventilation can be considered in mechanically ventilated patients who cannot tolerate large fluid volumes. The combined effect of sodium bicarbonate and hyperventilation can result in profound alkalosis 28. Adverse effects of prolonged sodium bicarbonate therapy also include hypokalemia, hypocalcemia, impaired oxygen delivery by shifting the oxyhemoglobin dissociation curve to the left, and fluid overload. Hypotension that does not respond to adequate fluid resuscitation and bicarbonate should be treated with direct-acting vasopressors (e.g., norepinephrine).
Other experimental “rescue” treatments have been used in a limited number of critical cases unresponsive to the usual treatment. These include glucagon 29, lidocaine and magnesium sulfate 30, intra-aortic balloon pump, extracorporeal life support 31, and lipid emulsion therapy 32. Prolonged cardiac massage has also been reported to be successful after cardiac arrest in such patients 11,33.
Despite several anecdotal reports suggesting a benefit, current recommendations from widely consulted resources explicitly recommend against ECTR in TCA-poisoned patients 34–39. Some recent reviews and publications nevertheless advocate these therapies, including plasmapheresis, hemodialysis, and hemoperfusion for severely poisoned TCA patients 40–43. One guideline reviewing the management of tricyclic overdose does not comment on the therapeutic use of ECTR 44.
Methodology
A complete description of the methodology is provided elsewhere 2.
Articles from the literature search were obtained via the preliminary search database. Thereafter, a specific search retrieved other articles from Medline, Embase, Cochrane library (Review and Central), Conference proceedings/meeting abstracts of the EAPCCT and NACCT annual meetings, and Google Scholar. Finally, the bibliographies of all articles obtained were manually reviewed for completeness.
Search Strategy
We used the following search strategy in Medline (via PubMed), and adapted for the other databases: (tricyclic OR amitriptyline OR imipramine OR clomipramine OR doxepin OR trimipramine OR amoxapine OR desipramine OR nortriptyline OR protriptyline OR dibenzepin OR dothiepin OR maprotiline) AND (hemoperfusion OR haemoperfusion OR hemofiltration OR haemofiltration OR hemodialysis OR haemodialysis OR hemodiafiltration OR haemodiafiltration OR dialysis OR plasmapheresis OR plasma exchange OR exchange transfusion OR CRRT).
The designated subgroup completed the literature search, reviewed articles, extracted data, summarized findings, and proposed structured voting statements following a predetermined format, all of which was submitted to the workgroup. The benefit of the ECTR procedure was weighed against its cost, availability, alternative treatments, and its related complications. Level of evidence for clinical recommendations was determined by the subgroup and the appointed epidemiologist (Table S2). Dialyzability was determined by the workgroup following criteria listed in Table2. The strength of recommendations was evaluated by a two-round modified Delphi method for each proposed voting statement (Fig.1) and RAND/UCLA Appropriateness Method was used to quantify disagreement between voters. Blinded votes with comments were sent to the statistician, who then compiled and returned them to each participant. The workgroup met in person in June 2012 to discuss the evidence, exchange ideas, and debate statements. A second blinded vote was later submitted and results reflect the core of EXTRIP recommendations.
Table 2.
Criteria of dialyzability
| Dialyzabilitya | Primary criteria | Alternative criteria 1 | Alternative criteria 2 | Alternative criteria 3 |
|---|---|---|---|---|
| % Removedb | CLEC/CLTOT (%)c | T1/2 EC/T1/2 (%) | ReEC/ReTOT (%)c | |
| D, Dialyzable | >30 | >75 | <25 | >75 |
| M, Moderately dialyzable | >10–30 | >50–75 | >25–50 | >50–75 |
| S, Slightly dialyzable | ≥3–10 | ≥25–50 | ≥50–75 | ≥25–50 |
| N, Not dialyzable | <3 | <25 | >75 | <25 |
Applicable to all modalities of ECTR, including hemodialysis, hemoperfusion, hemofiltration.
Corresponds to% removal of ingested dose or total body burden in a 6-hour ECTR period.
Measured during the same period of time.
These criteria should only be applied if measured or calculated (not reported) endogenous half-life is >4 hours (otherwise, ECTR is considered not clinically relevant). Furthermore, the primary criterion is preferred for poisons having a large Vd (>5 l/kg). Obtained with permission from Clinical Toxicology.
Figure 1.
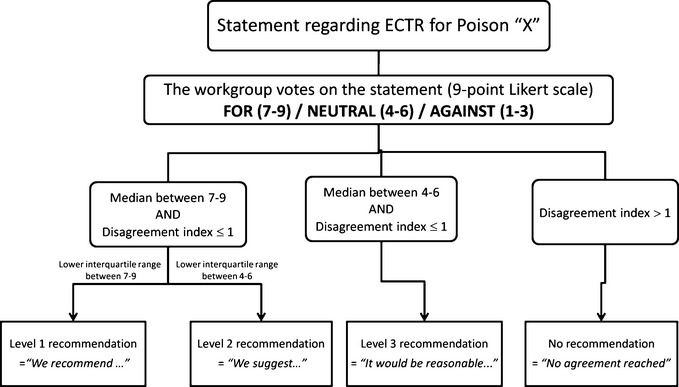
Delphi method (2 rounds) for each recommendation.
Results
Results of the literature search, last updated on November 1st 2013, are presented in Fig.2.
Figure 2.
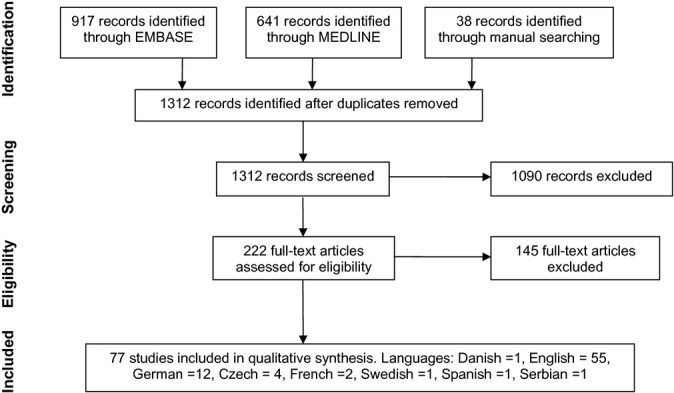
Results of search, selection, and inclusion of studies.
From the initial 1312 studies obtained, 77 studies met the inclusion criteria, including 1 observational study 45, 5 animal studies 46–50, 4 in vitro studies 51–54, 4 pharmacokinetic (PK) studies 55–58, and 63 case reports 40–42,59–118, 39 of which had sufficient toxicokinetic (TK) data. In total, 108 patients were included for analysis. No randomized trials were identified.
Dialyzability
Tricyclics are small molecules (between 200 and 400 Da) and can therefore cross any hemofilter or hemodialyzer, despite their extensive protein binding; this is confirmed by the many publications describing either a high extraction ratio, a significant reduction in TCA plasma concentrations, or a high plasma clearance during ECTR 55,78,91,95,107,119. However, based on their large VD, TCAs would be expected to be distributed extensively out of the vascular space; any reduction in plasma concentrations will therefore have an inconsequential effect on total body stores. Furthermore, significant redistribution from deeper compartments into plasma, commonly referred to as “rebound”, would be expected after any extracorporeal session.
For these reasons, according to EXTRIP criteria, the dialyzability of TCAs would be assumed to be poor, whatever extracorporeal modality is used and whatever the specific tricyclic studied. As described in Table2, the EXTRIP workgroup only reviewed publications in which the recovered amount of TCAs could be quantified; these are summarized in Table S3.
As expected, all of the articles that qualified for TK grading showed negligible TCA removal, despite high plasma clearance. This was true for all ECTR modalities: exchange transfusion 108,110, intermittent hemodialysis (IHD) 69,81,105, hemodialysis–hemoperfusion (HD–HP) 66, charcoal hemoperfusion (HP) 73,75,103,104, resin HP 83, liver dialysis 59, peritoneal dialysis 94,97,99,120, and continuous hemoperfusion 84.
For example, HD treatment for severe TCA poisoning in two reports failed to show any parent poison recovered in the dialysate 69,105. In another report of an imipramine overdose patient, HD recovered only 0.6% of the ingested dose 81. Even HP, which is in principle the ideal modality for protein-bound poisons, removed, in the best-case scenario, 7% of ingested dose during 6 hours of charcoal HP 103 and 2.7–6.2% in a series of eight cases treated with resin HP 83, despite extraction ratios nearing 100% and plasma clearance reaching 200 ml/minute 73,83. In another example, HD–HP was only able to remove 4 mg of amitriptyline in 4 hours despite a clearance of 72 ml/minute 66. Similarly, in a patient who overdosed with meprobate and amitriptyline treated with HP-HD for anuria, the author concluded that the removal of amitriptyline was inconsequential 76. Treatment with other less popular ECTR modalities confirmed the expected results based on TCA properties: in 2 cases of imipramine poisoning, exchange transfusion failed to recover more than 1% of the ingested dose 108,110. Four patients treated with peritoneal dialysis for amitriptyline, opipramol, and desipramine overdoses also had less than 1% of the estimated ingested dose removed 94,97,99,120. A hemodiabsorption technique also reported negligible removal of different TCAs 59.
Pharmacokinetic studies of TCA removal in nonpoisoned end-stage renal disease (ESRD) patients reveal similar results: in two studies, HD did not alter TCA kinetics 56,121. In another prospective study of five ESRD patients, a single HD session removed on average 37 μg in 4 hours, which was <1% of the administered oral dose of doxepin 57.
Although several of these papers are dated, results would not be expected to be significantly improved had more efficient technology been used (higher blood flows, higher efficiency filters), as extraction from earlier reports sometimes approach 100%. Again, the limiting factor appears to be the massive volume of distribution of TCAs and not extraction by the filter or adsorbent column. This can be illustrated by the following example: if a 60 kg patient ingests 2400 mg of amitryptiline (VD = 20 l/kg), assuming complete absorption and distribution, the plasma amitriptyline concentration will be 2,000 ng/ml. If charcoal HP is performed for 4 hours, with a blood flow equal to 350 ml/minute (or plasma flow = 200 ml/minute for a hematocrit of 40%), assuming in the best-case scenario an extraction ratio of 100%, the HP clearance will be 200 ml/minute. Therefore, 400 μg will be removed per minute, for a total removal of 96 mg over 4 hours. Thus, despite a high plasma clearance, HP will decrease the total body drug burden of the drug by less than 5%.
In those articles that satisfied criteria for dialyzability evaluation, most confirm very small amounts of TCA removed and are therefore categorized as “slightly dialyzable” or “not dialyzable” according to criterion 1 of the dialyzability grading. The EXTRIP workgroup concluded: TCAs are not dialyzable (Evidence = B)
Recommendations
Executive Summary
General: We recommend NOT to perform ECTR in patients with TCA poisoning.
Rationale
There are no randomized controlled trials or large observational series to analyze clinical outcome data of patients undergoing ECTR for TCA poisoning. One small retrospective observational study was identified in which five patients were treated with HP, and four were not 45: the fall in TCA serum concentrations was faster and the length of stay shorter in the HP group, although one patient in the HP group was not accounted for due to an extremely long and complicated stay. Unfortunately, the study was underpowered and statistical analysis impossible to perform.
In an animal model, a group treated with both HP and cardiopulmonary bypass was compared with another group only treated with cardiopulmonary bypass. When compared to the control group, HP did not improve hemodynamic instability and also did not remove more than 1–2% of administered dose 49.
The remaining evidence of a clinical effect of ECTR consists of case reports and case series that are often dated, that lacked control groups, had multiple confounders, heterogeneous treatments, and suffered from definite publication bias. The quality of evidence for all recommendation statements would therefore be graded as “very poor” 122.
It is interesting to note that 12 deaths were reported among the case reports included and that clinical improvement that occurred during or shortly after ECTR was reported in 68 of the 108 cases treated with any ECTR modality. Among the cases where improvement was reported, confounding therapies such as intubation, gastrointestinal decontamination, vasoactive drugs, and bicarbonate were consistently present. What effect, if any, was achieved by extracorporeal measures is therefore impossible to elucidate. Often the improvement reported some reversal of coma, while other more severe cardiovascular end-organ effects were not shown to dramatically improve during ECTR in a convincing time-related manner, such as the prompt ECG changes typically reported with sodium bicarbonate therapy. In fact, ECGs were rarely provided in the cases where improvement was reported, and sometimes ECG normalization only occurred days after ECTR had been completed 60.
Despite overwhelming TK evidence suggesting little to no significant enhancement of TCA elimination with ECTR, several authors still suggest a beneficial clinical effect of ECTR and have postulated several reasons for this: 1) TCA removal prior to distribution, 2) Protein binding alteration during ECTR, 3) Critical removal from the toxic compartments, and 4) Metabolic manipulation. These are presented here.
Early intervention with ECTR may clear TCA from plasma before it distributes to tissues and before it produces its toxic end-organ effects 60,78,79,83,117. However, it is also plausible that the rapid fall in TCA concentrations reported by these authors during early ECTR is more likely a result of TCA distribution than true drug removal. Furthermore, many of TCAs' end-organ toxic effects occur early after exposure, as has been shown in prospective series designed to evaluate the utility of ECG parameters 19,20. Due to the lack of availability and utility of serum drug concentrations to predict outcome as well as the difficulty in initiating ECTR during the supposed predistribution phase in a realistic time frame (which includes transfer to a specialized unit, organizing ECTR, and installation of a central catheter), such an attempt to either correct or prevent the appearance of life-threatening symptoms would not be realistic in most clinical contexts.
Other arguments made by authors who advocate the use of ECTR for patients with serious TCA poisoning involve the toxicokinetics of tricyclics in overdose. One hypothesis is that in a severely poisoned patient, hypotension contributes to decreased hepatic blood flow and tissue perfusion as well as acidosis, which in turn would favor a larger amount of free ionized drug by decreasing both protein binding and volume of distribution and prolonging half-life of elimination, making more drug available for extracorporeal elimination 60,61,83.
Another hypothesis attempting to justify why ECTR might be effective despite removing only a negligible amount of drug suggests that increasing intercompartmental clearance with hemoperfusion facilitates redistribution of just enough drug away from the toxic compartments (i.e., cardiac receptors) to improve the cardiovascular status 83,123. The same authors admit that even if this were true, intercompartmental clearance would be reduced by the same hemodynamic conditions in severely poisoned patients.
The possibility that metabolic manipulation may have contributed to the effect of treatments with hemodialysis exists. Frank et al. described a very dramatic case of a patient treated after cardiac arrest due to doxepin with persistent cardiovascular instability, acidosis, and hypokalemia. They describe improvement during treatment with HP/HD and rapidly falling drug concentrations. The decision to use HP/HD in extremis in this case was partially motivated by the patient's hypokalemia and fear of fluid overload with prolonged bicarbonate infusion. Drug removal or clearance was not measured or calculated, and the improvement in this case may be largely due to the metabolic acidosis correction 61. Hemodialysis and hemofiltration would both expect to correct acidosis much quicker than bicarbonate infusion and could therefore contribute to clinical improvement despite a lack of meaningful TCA removal. Metabolic correction was not the intent of the authors in the remainder of cases reviewed, most of which used hemoperfusion. It is also possible that the outcome in this case was the natural course of the disease and other measures simultaneously administered; this type of dramatic improvement has in fact been reported in other patients not receiving ECTR 11,33.
For those cases with cardiovascular disturbances, several other measures are available. Considering the lack of significant TCA removal and unconfirmed clinical benefit, the use of ECTR is questionable. Furthermore, the application of ECTRs, even if they are usually considered generally safe, is not without cost and risks. In the present literature review, adverse effects of ECTRs, other than death, were reported: in subjects undergoing hemoperfusion, thrombocytopenia was reported in 25 patients, anemia in 12, bleeding or coagulation problems in 2, a clotting cartridge in 1, hypotension in 2, hypocalcemia in 5, and pulmonary edema in 1 67,71,75,78–80,83,87,91,96,98,104,107,109. Peritoneal dialysis-related peritonitis was reported in one patient, hyperglycemia in three cases, and hypothermia in one 99,100. Worsening acidosis was reported in one case treated with exchange transfusion 65.
Although some anecdotal reports have documented patient improvement, considering the lack of toxicokinetic benefit from most studies, the questionable clinical benefit, the absence of quality observational studies or trials, and the existence of efficacious alternative treatments, the EXTRIP workgroup strongly and unanimously recommended NOT proposing ECTR in TCA poisoning.
Conclusion
The EXTRIP workgroup presents here its recommendations for extracorporeal treatments in TCA poisoning. The workgroup recommends NOT performing ECTR for TCA poisoning.
Acknowledgments
The EXTRIP workgroup includes the following: Kurt Anseeuw, Ashish Bhalla, Emmanuel A Burdmann, Paul I Dargan, Brian S Decker, David S Goldfarb, Lotte C Hoegberg, David Juurlink, Martin Laliberté, Jan T Kielstein, Yi Li, Kathleen D Liu, Robert MacLaren, Robert Mactier, Bruno Mégarbane, James B Mowry, Véronique Phan, Darren M Roberts, Timothy J Wiegand, James F Winchester.
See online appendix Data S1 for additional acknowledgments.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Acknowledgements, financial disclosure, and competing interests.
Supporting societies.
Strength of recommendation and level of evidence scaling.
TK analysis of individual patients in which TCA removal was quantified.
References
- 1.Ghannoum M, Nolin TD, Lavergne V, Hoffman RS. Blood purification in toxicology: nephrology's ugly duckling. Adv Chronic Kidney Dis. 2011;18:160–166. doi: 10.1053/j.ackd.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Lavergne V, Nolin TD, Hoffman RS, Robert D, Gosselin S, Goldfarb DS, Kielstein JT, Mactier R, MacLaren R, Mowry JB, Bunchman TE, Juurlink D, Megarbane B, Anseeuw K, Winchester JF, Dargan PI, Liu KD, Hoegberg LC, Li Y, Calello D, Burdmann EA, Yates C, Laliberte M, Decker BS, Mello-Da-Silva CA, Lavonas E, Ghannoum M. The EXTRIP (Extracorporeal Treatments In Poisoning) workgroup: guideline methodology. Clin Toxicol. 2012;50:403–413. doi: 10.3109/15563650.2012.683436. [DOI] [PubMed] [Google Scholar]
- 3.Ghannoum M, Nolin TD, Goldfarb DS, Roberts DM, Mactier R, Mowry JB, Dargan PI, Maclaren R, Hoegberg LC, Laliberte M, Calello D, Kielstein JT, Anseeuw K, Winchester JF, Burdmann EA, Bunchman TE, Li Y, Juurlink DN, Lavergne V, Megarbane B, Gosselin S, Liu KD, Hoffman RS. Extracorporeal treatment for thallium poisoning: recommendations from the EXTRIP workgroup. Clin J Am Soc Nephrol. 2012;7:1682–1690. doi: 10.2215/CJN.01940212. [DOI] [PubMed] [Google Scholar]
- 4.Koszewska I, Rybakowski JK. Antidepressant-induced mood conversions in bipolar disorder: a retrospective study of tricyclic versus non-tricyclic antidepressant drugs. Neuropsychobiology. 2009;59:12–16. doi: 10.1159/000202824. [DOI] [PubMed] [Google Scholar]
- 5.Benbouzid M, Choucair-Jaafar N, Yalcin I, Waltisperger E, Muller A, Freund-Mercier MJ, Barrot M. Chronic, but not acute, tricyclic antidepressant treatment alleviates neuropathic allodynia after sciatic nerve cuffing in mice. Eur J Pain. 2008;12:1008–1017. doi: 10.1016/j.ejpain.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151:737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hejazi RA, Reddymasu SC, Namin F, Lavenbarg T, Foran P, McCallum RW. Efficacy of tricyclic antidepressant therapy in adults with cyclic vomiting syndrome: a two-year follow-up study. J Clin Gastroenterol. 2010;44:18–21. doi: 10.1097/MCG.0b013e3181ac6489. [DOI] [PubMed] [Google Scholar]
- 8.Fedi V, Guidi A, Altamura M. Tricyclic structures in medicinal chemistry: an overview of their recent uses in non-CNS pathologies. Mini Rev Med Chem. 2008;8:1464–1484. doi: 10.2174/138955708786786453. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis MR. Clinical pharmacokinetics of tricyclic antidepressant overdose. Psychopharmacol Bull. 1991;27:541–550. [PubMed] [Google Scholar]
- 10.Krishel S, Jackimczyk K. Cyclic antidepressants, lithium, and neuroleptic agents. Pharmacology and toxicology. Emerg Med Clin North Am. 1991;9:53–86. [PubMed] [Google Scholar]
- 11.Kerr GW, McGuffie AC, Wilkie S. Tricyclic antidepressant overdose: a review. Emerg Med J. 2001;18(4):236–241. doi: 10.1136/emj.18.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mowry JB, Spyker DA, Cantilena LR, Jr, Bailey JE, Ford M. 2012 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol (Phila) 2013;51:949–1229. doi: 10.3109/15563650.2013.863906. [DOI] [PubMed] [Google Scholar]
- 13.Callaham M, Kassel D. Epidemiology of fatal tricyclic antidepressant ingestion: implications for management. Ann Emerg Med. 1985;14:1–9. doi: 10.1016/s0196-0644(85)80725-3. [DOI] [PubMed] [Google Scholar]
- 14.Woolf AD, Erdman AR, Nelson LS, Caravati EM, Cobaugh DJ, Booze LL, Wax PM, Manoguerra AS, Scharman EJ, Olson KR, Chyka PA, Christianson G, Troutman WG American Association of Poison Control C. Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2007;45:203–233. doi: 10.1080/15563650701226192. [DOI] [PubMed] [Google Scholar]
- 15.Thorstrand C. Clinical features in poisonings by tricyclic antidepressants with special reference to the ECG. Acta Med Scand. 1976;199:337–344. doi: 10.1111/j.0954-6820.1976.tb06745.x. [DOI] [PubMed] [Google Scholar]
- 16.Hulten BA, Heath A. Clinical aspects of tricyclic antidepressant poisoning. Acta Med Scand. 1983;213:275–278. doi: 10.1111/j.0954-6820.1983.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 17.Crome P. Poisoning due to tricyclic antidepressant overdosage: clinical presentation and treatment. Med Toxicol Adv Drug Exp. 1986;1(4):261–285. doi: 10.1007/BF03259843. [DOI] [PubMed] [Google Scholar]
- 18.Bailey B, Buckley NA, Amre DK. A meta-analysis of prognostic indicators to predict seizures, arrhythmias or death after tricyclic antidepressant overdose. J Toxicol Clin Toxicol. 2004;42:877–888. doi: 10.1081/clt-200035286. [DOI] [PubMed] [Google Scholar]
- 19.Boehnert MT, Lovejoy FH., Jr Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313:474–479. doi: 10.1056/NEJM198508223130804. [DOI] [PubMed] [Google Scholar]
- 20.Liebelt EL, Francis PD, Woolf AD. ECG lead aVR versus QRS interval in predicting seizures and arrhythmias in acute tricyclic antidepressant toxicity. Ann Emerg Med. 1995;26:195–201. doi: 10.1016/s0196-0644(95)70151-6. [DOI] [PubMed] [Google Scholar]
- 21.Dargan PI, Colbridge MG, Jones AL. The management of tricyclic antidepressant poisoning: the role of gut decontamination, extracorporeal procedures and fab antibody fragments. Toxicol Rev. 2005;24:187–194. doi: 10.2165/00139709-200524030-00011. [DOI] [PubMed] [Google Scholar]
- 22.Chyka PA, Seger D, Krenzelok EP, Vale JA American Academy of Clinical T, European Association of Poisons C, Clinical T. Position paper: single-dose activated charcoal. Clin Toxicol (Phila) 2005;43:61–87. doi: 10.1081/clt-200051867. [DOI] [PubMed] [Google Scholar]
- 23.Bosse GM, Barefoot JA, Pfeifer MP, Rodgers GC. Comparison of three methods of gut decontamination in tricyclic antidepressant overdose. J Emerg Med. 1995;13:203–209. doi: 10.1016/0736-4679(94)00153-7. [DOI] [PubMed] [Google Scholar]
- 24.Blackman K, Brown SG, Wilkes GJ. Plasma alkalinization for tricyclic antidepressant toxicity: a systematic review. Emerg Med. 2001;13:204–210. doi: 10.1046/j.1442-2026.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- 25.Bradberry SM, Thanacoody HK, Watt BE, Thomas SH, Vale JA. Management of the cardiovascular complications of tricyclic antidepressant poisoning: role of sodium bicarbonate. Toxicol Rev. 2005;24:195–204. doi: 10.2165/00139709-200524030-00012. [DOI] [PubMed] [Google Scholar]
- 26.Hagerman GA, Hanashiro PK. Reversal of tricyclic-antidepressant-induced cardiac conduction abnormalities by phenytoin. Ann Emerg Med. 1981;10:82–86. doi: 10.1016/s0196-0644(81)80342-3. [DOI] [PubMed] [Google Scholar]
- 27.Sasyniuk BI, Jhamandas V. Mechanism of reversal of toxic effects of amitriptyline on cardiac Purkinje fibers by sodium bicarbonate. J Pharmacol Exp Ther. 1984;231:387–394. [PubMed] [Google Scholar]
- 28.Albertson TE, Dawson A, de Latorre F, Hoffman RS, Hollander JE, Jaeger A, Kerns WR, 2nd, Martin TG, Ross MP American Heart A, International Liaison Committee on R. TOX-ACLS: toxicologic-oriented advanced cardiac life support. Ann Emerg Med. 2001;37:S78–90. doi: 10.1067/mem.2001.114174. [DOI] [PubMed] [Google Scholar]
- 29.Teese S, Hogg K. Towards evidence based emergency medicine: best BETS from the Manchester General Infirmary. Glucagon in tricyclic overdose. Emerg Med J. 2003;20(3):264–265. doi: 10.1136/emj.20.3.264-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knudsen K, Abrahamsson J. Magnesium sulphate in the treatment of ventricular fibrillation in amitriptyline poisoning. Eur Heart J. 1997;18:881–882. doi: 10.1093/oxfordjournals.eurheartj.a015356. [DOI] [PubMed] [Google Scholar]
- 31.Daubin C, Quentin C, Goulle JP, Guillotin D, Lehoux P, Lepage O, Charbonneau P. Refractory shock and asystole related to tramadol overdose. Clin Toxicol. 2007;45(8):961–964. doi: 10.1080/15563650701438847. [DOI] [PubMed] [Google Scholar]
- 32.Jamaty C, Bailey B, Larocque A, Notebaert E, Sanogo K, Chauny JM. Lipid emulsions in the treatment of acute poisoning: a systematic review of human and animal studies. Clin Toxicol (Phila) 2010;48:1–27. doi: 10.3109/15563650903544124. [DOI] [PubMed] [Google Scholar]
- 33.Southall DP, Kilpatrick SM. Imipramine poisoning: survival of a child after prolonged cardiac massage. Br Med J. 1974;4:508. doi: 10.1136/bmj.4.5943.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley NA. In: Tricyclic Antidepressants. Dawson AH, editor. 2003. http://curriculum.toxicology.wikispaces.net/2.1.11.9.2.1+Tricyclic+Antidepressants,Wikitox, accessed January 8, 2014. [Google Scholar]
- 35.Liebelt EL. Cyclic antidepressants. In: Nelson LS, Lewis N, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, editors. Goldfrank's Toxicologic Emergencies. New York: McGraw-Hill; 2011. pp. 1049–1059. [Google Scholar]
- 36.Tricyclic Antidepressants. POISINDEX®. Greenwood Village: Thomson Reuters (Healthcare); 2014. ystem http://www.thomsonhc.com, accessed January 8. [Google Scholar]
- 37.Tricyclic Antidepressants. Toxinz. 2014. http://www.toxinz.com/Spec/2303299, accessed January 8. [Google Scholar]
- 38.Salhanick SD. Tricyclic antidepressant poisoning. In: Traub SJ, Grayzel J, editors. UpToDate. 2014. http://www.uptodate.com/contents/tricyclic-antidepressant-poisoning?source=search_result&search=tricyclic+overdose&selectedTitle=1∼31, accessed January 8, [Google Scholar]
- 39.Tsai V. Tricyclic Antidepressant Toxicity. 2014. http://emedicine.medscape.com/article/819204-overview, accessed January 8. [Google Scholar]
- 40.Ozayar E, Degerli S, Gulec H. Hemodiafiltration: a novel approach for treating severe amitriptyline intoxication. Toxicol Int. 2012;19:319–321. doi: 10.4103/0971-6580.103682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sari I, Turkcuer I, Erurker T, Serinken M, Seyit M, Keskin A. Therapeutic plasma exchange in amitriptyline intoxication: case report and review of the literature. Transfus Apher Sci. 2011;45:183–185. doi: 10.1016/j.transci.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Mutlu M, Karaguzel G, Bahat E, Aksoy A, Guven B, Dilber B, Dilber E. Charcoal hemoperfusion in an infant with supraventricular tachycardia and seizures secondary to amitriptyline intoxication. Hum Exp Toxicol. 2011;30:254–256. doi: 10.1177/0960327110369822. [DOI] [PubMed] [Google Scholar]
- 43.Winchester JF, Boldur A, Oleru C, Kitiyakara C. Use of dialysis and hemoperfusion in treatment of poisoning. In: Daugirdas JT, Blake PG, Ing TS, editors. Handbook of Dialysis. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 300–320. [Google Scholar]
- 44.Body R, Bartram T, Azam F, Mackway-Jones K. Guidelines in Emergency Medicine Network (GEMNet): guideline for the management of tricyclic antidepressant overdose. Emerg Med J. 2011;28:347–368. doi: 10.1136/emj.2010.091553. [DOI] [PubMed] [Google Scholar]
- 45.Heise G, Schoebel FC, Grabensee B, Heering P. Amitriptyline intoxication—the place of hemoperfusion [German] Intensivmedizin und Notfallmedizin. 2000;37(5):475–481. [Google Scholar]
- 46.Heath A, Lofstrom B, Martensson E. Lidocaine and amitriptyline interaction during experimental haemoperfusion. Hum Toxicol. 1984;3:165–171. doi: 10.1177/096032718400300302. [DOI] [PubMed] [Google Scholar]
- 47.Meineke I, Schmidt W, Nottrott M, Schroder T, Hellige G, Gundert-Remy U. Modelling of non-linear pharmacokinetics in sheep after short-term infusion of cardiotoxic doses of imipramine. Pharmacol Toxicol. 1997;80:266–271. doi: 10.1111/j.1600-0773.1997.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 48.Harvey M, Cave G, Hoggett K. Correlation of plasma and peritoneal diasylate clomipramine concentration with hemodynamic recovery after intralipid infusion in rabbits. Acad Emerg Med. 2009;16(2):151–156. doi: 10.1111/j.1553-2712.2008.00313.x. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt W, Nottrott M, Meineke I, Schroder T, Muller S, Hellige G. Imipramine poisoning in an animal model-treatment with single cardiopulmonary bypass support or additional resin-haemoperfusion? Br J Anaesth. 1997;79(SUPPL. 2):32. [Google Scholar]
- 50.Asbach HW, Holz F, Mohring K, Schuler HW. Lipid hemodialysis versus charcoal hemoperfusion in imipramine poisoning. Clin Toxicol. 1977;11:211–219. doi: 10.3109/15563657708989834. [DOI] [PubMed] [Google Scholar]
- 51.Derzsiova K, Mydlik M, Petrikova V, Molcanyiova A. Hemoperfusion of amitriptyline and nortriptylin—an in vitro study [Czech] Aktuality v Nefrologii. 2005;11(1):6–10. [Google Scholar]
- 52.Monhart V, Balikova M, Tlustakova M. Hemoperfusion sorbents of Czechoslovak make, and their sorption characteristics for some drugs [Czech] Casopis lekaru ceskych. 1984;123(35):1091–1095. [PubMed] [Google Scholar]
- 53.Asbach HW, Mohring K, Holz F, Schuler HW, Herrmann B, Faigle JW. Haemodialysis in imipramine poisoning? An experimental study. Klin Wochenschr. 1976;54:83–87. doi: 10.1007/BF01468773. [DOI] [PubMed] [Google Scholar]
- 54.Decker WJ, Combs HF, Treuting JJ, Banez RJ. Dialysis of drugs against activated charcoal. Toxicol Appl Pharmacol. 1971;18:573–578. doi: 10.1016/s0041-008x(71)80010-8. [DOI] [PubMed] [Google Scholar]
- 55.Unterecker S, Muller P, Jacob C, Riederer P, Pfuhlmann B. Therapeutic drug monitoring of antidepressants in haemodialysis patients. Clinical Drug Investig. 2012;32:539–545. doi: 10.1007/BF03261907. [DOI] [PubMed] [Google Scholar]
- 56.Dawlilng S, Lynn K, Rosser R, Braithwaite R. The pharmacokinetics of nortriptyline in patients with chronic renal failure. Br J Clin Pharmacol. 1981;12:39–45. doi: 10.1111/j.1365-2125.1981.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faulkner RD, Senekjian HO, Lee CS. Hemodialysis of doxepin and desmethyldoxepin in uremic patients. Artif Organs. 1984;8:151–155. doi: 10.1111/j.1525-1594.1984.tb04264.x. [DOI] [PubMed] [Google Scholar]
- 58.Lieberman JA, Cooper TB, Suckow RF, Steinberg H, Borenstein M, Brenner R, Kane JM. Tricyclic antidepressant and metabolite levels in chronic renal failure. Clin Pharmacol Ther. 1985;37:301–307. doi: 10.1038/clpt.1985.44. [DOI] [PubMed] [Google Scholar]
- 59.Ash SR, Levy H, Akmal M, Mankus RA, Sutton JM, Emery DR, Scanlon JC, Blake DE, Carr DJ. Treatment of severe tricyclic antidepressant overdose with extracorporeal sorbent detoxification. Adv Ren Replace Ther. 2002;9:31–41. doi: 10.1053/jarr.2001.30475. [DOI] [PubMed] [Google Scholar]
- 60.Donmez O, Cetinkaya M, Canbek R. Hemoperfusion in a child with amitriptyline intoxication. Pediatr Nephrol. 2005;20:105–107. doi: 10.1007/s00467-004-1654-2. [DOI] [PubMed] [Google Scholar]
- 61.Frank RD, Kierdorf HP. Is there a role for hemoperfusion/hemodialysis as a treatment option in severe tricyclic antidepressant intoxication? Int J Artif Organs. 2000;23:618–623. [PubMed] [Google Scholar]
- 62.Islek I, Degim T, Akay C, Turkay A, Akpolat T. Charcoal haemoperfusion in a child with amitriptyline poisoning. Nephrol Dial Transplant. 2004;19:3190–3191. doi: 10.1093/ndt/gfh232. [DOI] [PubMed] [Google Scholar]
- 63.Maclaren G, Butt W, Cameron P, Preovolos A, McEgan R, Marasco S. Treatment of polypharmacy overdose with multimodality extracorporeal life support. Anaesth Intensive Care. 2005;33:120–123. doi: 10.1177/0310057X0503300118. [DOI] [PubMed] [Google Scholar]
- 64.Marbury T, Mahoney J, Fuller T, Juncos L, Cade J. Treatment of amitriptyline overdosage with charcoal hemoperfusion (abstract) Kidney Int. 1977;12:465. [Google Scholar]
- 65.Robins MH. Survival following massive intoxication with Tofranil (imipramine hydrochloride) J Am Osteopath Assoc. 1971;70:898–902. [PubMed] [Google Scholar]
- 66.Sevela K, Samkova H, Matyas V. [Hemoperfusion and hemodialysis in acute amitriptyline poisoning] Vnitr Lek. 1987;33:1072–1077. [PubMed] [Google Scholar]
- 67.Kobr J, Sasek L, Pizingerova K. Intentional poisoning with lethal dose of imipramine in a 14-year boy [Czech] Cesko-Slovenska Pediatrie. 2005;60(4):213–218. [Google Scholar]
- 68.Jorgensen KA, Christensen KN, Pedersen RS, Klitgaard NA. Hemoperfusion in deliberate drug poisoning. Review and 2 case reports [Danish] Ugeskr Laeger. 1978;140(17):960–963. [PubMed] [Google Scholar]
- 69.Bailey RR, Sharman JR, O'Rourke J, Buttimore AL. Haemodialysis and forced diuresis for tricyclic antidepressant poisoning. Br Med J. 1974;4:230–231. doi: 10.1136/bmj.4.5938.230-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bayrakci B, Unal S, Erkocoglu M, Gungor HY, Aksu S. Case reports of successful therapeutic plasma exchange in severe amitriptyline poisoning. Ther Apher Dial. 2007;11:452–454. doi: 10.1111/j.1744-9987.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 71.Bek K, Ozkaya O, Mutlu B, Dagdemir A, Sungur M, Acikgoz Y, Islek I, Baysal K. Charcoal haemoperfusion in amitriptyline poisoning: experience in 20 children. Nephrology (Carlton) 2008;13:193–197. doi: 10.1111/j.1440-1797.2008.00922.x. [DOI] [PubMed] [Google Scholar]
- 72.Belen B, Akman A, Yuksel N, Dilsiz G, Yenicesu I, Olgunturk R. A case report of amitriptyline poisoning successfully treated with the application of plasma exchange. Ther Apher Dial. 2009;13:147–149. doi: 10.1111/j.1744-9987.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 73.Bloodworth L, Wilson A, Collins P, Rainford DJ. Severe dothiepin intoxication—a report of two cases. Postgrad Med J. 1984;60:442–444. doi: 10.1136/pgmj.60.704.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Celik U, Celik T, Avci A, Annagur A, Yilmaz HL, Kucukosmanoglu O, Topaloglu AK, Daglioglu N. Metabolic acidosis in a patient with type 1 diabetes mellitus complicated by methanol and amitriptyline intoxication. Eur J Emerg Med. 2009;16:45–48. doi: 10.1097/MEJ.0b013e3283034245. [DOI] [PubMed] [Google Scholar]
- 75.Comstock TJ, Watson WA, Jennison TA. Severe amitriptyline intoxication and the use of charcoal hemoperfusion. Clin Pharm. 1983;2:85–88. [PubMed] [Google Scholar]
- 76.De Broe ME, Verpooten BA, Van Haesebrouck B. Recent experience with prolonged hemoperfusion-hemodialysis treatment. Artif Organs. 1979;3:188–190. doi: 10.1111/j.1525-1594.1979.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 77.de Groot G, Maes RA, van Heijst AN. A toxicological evaluation of hemoperfusion using pharmacokinetic principles. Dev Toxicol Environ Sci. 1980;8:387–395. [PubMed] [Google Scholar]
- 78.Diaz-Buxo JA, Farmer CD, Chandler JT. Hemoperfusion in the treatment of amitriptyline intoxication. Trans Am Soc Artif Intern Organs. 1978;24:699–703. [PubMed] [Google Scholar]
- 79.Durakovic Z, Plavsic F, Ivanovic D, Gasparovic V, Gjurasin M. Resin hemoperfusion in the treatment of tricyclic antidepressant overdose. Artif Organs. 1982;6:205–207. doi: 10.1111/j.1525-1594.1982.tb04084.x. [DOI] [PubMed] [Google Scholar]
- 80.Hals PA, Jacobsen D. Resin haemoperfusion in levomepromazine poisoning: evaluation of effect on plasma drug and metabolite levels. Hum Toxicol. 1984;3:497–503. doi: 10.1177/096032718400300604. [DOI] [PubMed] [Google Scholar]
- 81.Harthorne JW, Marcus AM, Kaye M. Management of massive imipramine overdosage with mannitol and artificial dialysis. N Engl J Med. 1963;268:33–36. doi: 10.1056/NEJM196301032680108. [DOI] [PubMed] [Google Scholar]
- 82.Heath A, Delin K, Eden E, Martensson E, Selander D, Wickstrom I, Ahlmen J. Hemoperfusion with Amberlite resin in the treatment of self-poisoning. Acta Med Scand. 1980;207:455–460. doi: 10.1111/j.0954-6820.1980.tb09754.x. [DOI] [PubMed] [Google Scholar]
- 83.Heath A, Wickstrom I, Martensson E, Ahlmen J. Treatment of antidepressant poisoning with resin hemoperfusion. Hum Toxicol. 1982;1:361–371. doi: 10.1177/096032718200100401. [DOI] [PubMed] [Google Scholar]
- 84.Iversen BM, Willassen YW, Bakke OM. Charcoal haemoperfusion in nortriptyline poisoning. Lancet. 1978;1:388–389. doi: 10.1016/s0140-6736(78)91112-1. [DOI] [PubMed] [Google Scholar]
- 85.Kolsal E, Tekin IO, Piskin E, Aydemir C, Akyuz M, Cabuk H, Eldes N, Numanoglu V. Treatment of severe amitriptyline intoxication with plasmapheresis. J Clin Apher. 2009;24:21–24. doi: 10.1002/jca.20185. [DOI] [PubMed] [Google Scholar]
- 86.Koppel C, Wiegreffe A, Tenczer J. Clinical course, therapy, outcome and analytical data in amitriptyline and combined amitriptyline/chlordiazepoxide overdose. Hum Exp Toxicol. 1992;11:458–465. doi: 10.1177/096032719201100604. [DOI] [PubMed] [Google Scholar]
- 87.McAlpine SB, Calabro JJ, Robinson MD, Burkle FM., Jr Late death in tricyclic antidepressant overdose revisited. Ann Emerg Med. 1986;15:1349–1352. doi: 10.1016/s0196-0644(86)80623-0. [DOI] [PubMed] [Google Scholar]
- 88.Oreopoulos DG, Lal S. Recovery from massive amitriptyline overdosage. Lancet. 1968;2:221. doi: 10.1016/s0140-6736(68)92657-3. [DOI] [PubMed] [Google Scholar]
- 89.Pedersen RS, Jorgensen KA, Olesen AS, Christensen KN. Charcoal haemoperfusion and antidepressant overdose. Lancet. 1978;1:719–720. doi: 10.1016/s0140-6736(78)90833-4. [DOI] [PubMed] [Google Scholar]
- 90.Pedersen RS. Hemoperfusion in tricyclic antidepressant poisoning. Lancet. 1980;1:154–155. doi: 10.1016/s0140-6736(80)90636-4. [DOI] [PubMed] [Google Scholar]
- 91.Pentel PR, Bullock ML, DeVane CL. Hemoperfusion for imipramine overdose: elimination of active metabolites. J Toxicol Clin Toxicol. 1982;19:239–248. doi: 10.3109/15563658209025728. [DOI] [PubMed] [Google Scholar]
- 92.Ryan R, 3rd, Wians FH, Jr, Stigelman WH, Jr, Clark H, McCurdy F. Imipramine poisoning in a child: lack of efficacy of resin hemoperfusion. Pediatr Emerg Care. 1985;1:201–204. doi: 10.1097/00006565-198512000-00008. [DOI] [PubMed] [Google Scholar]
- 93.Sert A, Aypar E, Odabas D, Aygul MU. Temporary cardiac pacemaker in the treatment of junctional rhythm and hypotension due to imipramine intoxication. Pediatr Cardiol. 2011;32:521–524. doi: 10.1007/s00246-011-9914-y. [DOI] [PubMed] [Google Scholar]
- 94.Sunshine P, Yaffe SJ. Amitriptyline poisoning. Clinical and pathological findings in a fatal case. Am J Dis Child. 1963;106:501–506. [PubMed] [Google Scholar]
- 95.Trafford A, Horn C, Sharpstone P, O'Neal H, Evans R. Hemoperfusion in acute drug toxicity. Clin Toxicol. 1980;17:547–556. doi: 10.3109/15563658008990005. [DOI] [PubMed] [Google Scholar]
- 96.Trafford JA, Jones RH, Evans R, Sharp P, Sharpstone P, Cook J. Haemoperfusion with R-004 Amberlite resin for treating acute poisoning. Br Med J. 1977;2:1453–1456. doi: 10.1136/bmj.2.6100.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bickel MH, Brochon R, Friolet B, Herrmann B, Stofer AR. Clinical and biochemical results of a fatal case of desipramine intoxication. Psychopharmacologia. 1967;10(5):431–436. doi: 10.1007/BF00403984. [DOI] [PubMed] [Google Scholar]
- 98.Engstrom JW, Young J, Rennie WA, Kennedy TP. Noncardiogenic pulmonary edema after charcoal hemoperfusion. South Med J. 1985;78(5):611–613. doi: 10.1097/00007611-198505000-00025. [DOI] [PubMed] [Google Scholar]
- 99.Halle MA, Collipp PJ. Amitriptyline hydrochloride poisoning. Unsuccessful treatment by peritoneal dialysis. N Y State J Med. 1969;69(11):1434–1436. [PubMed] [Google Scholar]
- 100.Royds RB, Knight AH. Tricyclic antidepressant poisoning. The Practitioner. 1970;204(220):282–286. [PubMed] [Google Scholar]
- 101.Sheppard C. Cardiopulmonary bypass support for tricyclic poisoning: a case report. J Extra Corpor Technol. 1995;27(1):49–53. [Google Scholar]
- 102.Shigemura J, Kuwahara T, Nomura S, Yokoyama A, Uemura H. Successful treatment of rhabdomyolysis and acute renal failure following amoxapine overdose. Int J Psychiatry Clin Pract. 2001;5(4):287–290. doi: 10.1080/13651500152733071. [DOI] [PubMed] [Google Scholar]
- 103.Bismuth C, Chollet A. Hemoperfusion through activated charcoal [French] Medecine et Hygiene. 1979;37(1346):3058–3062. [Google Scholar]
- 104.Lambert H, Laprevote-Heully MC, Manel J. Results of the utilization of hemoperfusion in the treatment of acute intoxications. 23 observations [French] Annales Medicales de Nancy et de l'Est. 1981;20(AUG.-SEPT):935–946. [Google Scholar]
- 105.Bauditz W, Bartelheimer HK. [Hemodialysis treatment of a 3 1-4-year-old child with imipramine poisoning] Arch Toxikol. 1970;26:133–141. [PubMed] [Google Scholar]
- 106.Busch-Petersen D, Tiess D, Gulzow HU, Bremer H. [Clinical aspects and treatment of acute imipramine (Melipramine) poisoning in childhood] Kinderarztl Prax. 1974;42:74–78. [PubMed] [Google Scholar]
- 107.Hofmann V, Riess W, Descoeudres C, Studer H. [The problem of hemoperfusion in poisonings: ineffectiveness in maprotiline poisoning] Schweiz Med Wochenschr. 1980;110:291–294. [PubMed] [Google Scholar]
- 108.Louis C, Olbing H, Bohlmann HG, Philippou A, Heimsoth V. [Therapy of imipramine poisoning in the child] Dtsch Med Wochenschr. 1970;95:2078–2082. doi: 10.1055/s-0028-1108783. [DOI] [PubMed] [Google Scholar]
- 109.Sakka SG, Kuethe F, Demme U, Huttemann E. [Intoxication with a tricyclic antidepressant] Anaesthesist. 2007;56:581–586. doi: 10.1007/s00101-007-1191-z. [DOI] [PubMed] [Google Scholar]
- 110.Sidiropoulos D, Bickel MH. [Fatal poisoning with a small dose of Imipramine in an infant] Schweiz Med Wochenschr. 1971;101:851–854. [PubMed] [Google Scholar]
- 111.Weigert S, Schroter K, Gorisch V. Imipramine (melipramine) poisoning in childhood. Z Arztl Fortbild (Jena) 1972;66:562–568. [PubMed] [Google Scholar]
- 112.Brodersen HP, Glitz HH, Minderjahn KP, Larbig D. The treatment of intoxications with carbamazepine, lithium and doxepin [German]. Therapeutische Aspekte Von Intoxikationen Mit Carbamazepine, Lithium Und Doxepin. Intensivmedizin und Notfallmedizin. 1986;23:273–276. [Google Scholar]
- 113.Gutschmidt HJ, Burck HC, Laessing C. Physostigmine salicylate therapy instead of hemoperfusion in amitriptyline poisoning [German] Intensivmedizin. 1982;19(6):264–267. [Google Scholar]
- 114.Schnert W. Physostigmine in treatment of severe poisoning with tricyclic antidepressants [German] Intensivmedizin und Notfallmedizin. 1987;24(6):296–300. [Google Scholar]
- 115.Vlachoyannis J, Schneider H, Hoppe D. Combined treatment of an amitryptiline intoxication by hemoperfusion and forced diuresis [German] Intensivmedizin. 1982;19(6):268–269. [Google Scholar]
- 116.Durakovic Z, Gasparovic V, Ivanovic D. Use of hemoresin hemoperfusion in the treatment of antidepressant overdose [Serbian] Lijec Vjesn. 1982;104(1):16–18. [PubMed] [Google Scholar]
- 117.Marquez del Cid J, Vergara Chozas JM, Crespo S, Meca Rovayo ML. The efficacy of hemoperfusion in acute poisoning by tricyclic antidepressants [Spanish] Med Clin. 1991;97(14):555. [PubMed] [Google Scholar]
- 118.Colleen I, Lindberg U. A case of imipramine poisoning in a 2-year old boy treated with peritoneal dialysis [Swedish] Lakartidningen. 1967;64(34):3278–3279. [PubMed] [Google Scholar]
- 119.Heath A, Wickstrom I, Ahlmen J. Haemoperfusion in tricyclic antidepressant poisoning. Lancet. 1980;1:155. [PubMed] [Google Scholar]
- 120.Schober JG, Mantel K. Fatal poisoning with thymoleptics in infancy [German] Monatsschrift fur Kinderheilkunde. 1970;118(6):340–341. [PubMed] [Google Scholar]
- 121.Dawling S, Lynn K, Rosser R, Braithwaite R. Nortriptyline metabolism in chronic renal failure: metabolite elimination. Clin Pharmacol Ther. 1982;32:322–329. doi: 10.1038/clpt.1982.167. [DOI] [PubMed] [Google Scholar]
- 122.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr, Zaza S. Grading quality of evidence and strength of recommendations. Br Med J. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gibson TP. Hemoperfusion for drug intoxication: what is it, what does it do, and how much do we know about it? Pharmacy Int. 1981;2(1):14–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Acknowledgements, financial disclosure, and competing interests.
Supporting societies.
Strength of recommendation and level of evidence scaling.
TK analysis of individual patients in which TCA removal was quantified.


