Abstract
BACKGROUND
Semaphorins act as chemotactic cues for cell movement via their transmembrane receptors, plexins. Somatic missense mutations in the plexinB1 gene coupled with overexpression of the protein frequently occur in prostate tumors, indicating a role for plexinB1 in the pathogenesis of prostate cancer. However, the effect of semaphorin/plexin signaling is highly context dependent and whether plexinB1 acts as an inducer or inhibitor of prostate tumor progression in this context is not known.
METHODS
The response of prostate cancer cell lines to plexinB1 activation was assessed in migration, invasion, proliferation and protein phosphorylation assays. Expression was assessed by quantitative RTPCR and immunoblotting.
RESULTS
Different prostate cancer cell lines respond to Sema4D (the ligand for plexinB1) in diverse ways. Activation of endogenous plexinB1 enhances migration, invasion and anchorage-independent growth of LNCaP prostate cancer cells via activation of ErbB2 and Akt. In contrast, Sema4D-stimulation decreased the motility and proliferative capacity of PC3 cells. LNCaP has a missense mutation (Thr1697Ala) in the plexinB1 gene while LNCaP-LN3, a derivative of LNCaP, expresses high levels of wild-type plexinB1 only. Sema4D stimulation increases the motility and anchorage independent growth of both cell lines, showing that these responses are not dependent on the presence of the Thr1697Ala form of plexinB1. ErbB2 and plexinB1 are expressed in primary prostate epithelial cells.
CONCLUSIONS
PlexinB1 signals via ErbB2 to increase the invasive phenotype of prostate cancer cells. Both wild-type and mutant forms of plexinB1 are potential targets for anti-cancer therapy in prostate tumors that express ErbB2. Prostate 73:1326–1335, 2013. © 2013 The Authors. The Prostate published by Wiley Periodicals, Inc.
Keywords: semaphorin, ErbB2, c-Met, cell motility, invasion
INTRODUCTION
Semaphorins act as cell guidance cues through activation of their cell surface receptors neuropilins and plexins 1,2. Nine plexins, divided into four classes (A1-4, B1-3, C1, D1) 3 and 21 vertebrate semaphorins divided into five classes, have been identified, classified according to structure 4. Most class 3 semaphorins bind to a complex of neuropilin 1 or 2 and a plexin, whereas class 4 semaphorins bind to plexins directly 5. Plexins regulate several small GTPases, affecting the actin cytoskeleton and motility 6. Plexins also interact with various receptor tyrosine kinases. PlexinB1, a receptor for semaphorin (Sema) 4D, for example, signals via the small RhoGTPases RhoA 7, RhoD 8, Rac 9, Rnd 10, and R-Ras 11 and interacts with the receptor tyrosine kinases ErbB2 12 and c-Met 13.
Semaphorins have a role in many cancers, both affecting angiogenesis and interacting with the tumor cell directly 14–16. Some semaphorins act as tumor suppressor genes, for example expression of the Sema3B and 3F genes on chromosome 3p21 is lost by deletion and promoter methylation in lung cancer 17,18. Sema3F induces apoptosis and suppresses the formation of tumors in xenografts when re-expressed in tumor cells 19. Other semaphorins enhance tumor progression, for example Sema4D 20, Sema5A 21, and Sema3E 22,23 are overexpressed in some cancers and promote tumor growth in mouse models. The same semaphorin may have the dual effect of promoting tumor progression in some cancer types and antagonizing tumor progression in other cancer types. For example plexinB1 is overexpressed in some ovarian, breast 24, and prostate cancers 25 and knockdown of plexinB1 reduces metastasis in in vivo models of breast cancer 26. In contrast, expression of plexinB1 is lost in melanoma where it is thought to act as a tumor suppressor gene 27,28 and is reduced in renal cell cancer 29 and low proliferating ER positive breast cancer 30. The role of semaphorins/plexins in both normal and cancer cells is therefore highly context dependent.
We have previously found somatic missense mutations in the gene for plexinB1 in prostate cancer 25 with an increase in mutant copy number in metastases, and high levels of the plexinB1 protein in prostate tumors. The mutations in plexinB1 enhance adhesion, migration and invasion in vitro in HEK293 cells and inhibit COS7 cell collapse 25. The three mutations tested inhibit the R-RasGTPase activating protein (GAP) activity of plexinB1 and one or more inhibit RacGTP and Rnd1 binding, resulting in a loss of inhibition of migration. Two of the mutations tested, including the A5359G mutation found in the prostate cancer cell line LNCaP, increase RhoD binding. The finding of functionally significant mutations in the plexinB1 gene in prostate tumors and overexpression of the plexinB1 protein suggests that plexinB1 has a role in prostate cancer. However the mechanism by which plexinB1 contributes to prostate cancer progression and whether it acts as an inducer or inhibitor of tumor progression in this context is not known. Since the effect of semaphorin/plexin signaling is highly context dependent we sought to determine the role of plexinB1 in prostate cancer cells specifically.
MATERIALS AND METHODS
Cell Culture
Primary cultures of benign prostatic epithelial cells were grown from transurethral resection of the prostate. Tissue was digested overnight in L-15 medium with 5% (v/v) fetal calf serum (FCS) and 200 U/ml collagenase. Cells were grown in PrEGM (Clonetex) supplemented with Bulletkit® (Cambrex). Some cultures were immortalized with SV40 and htert and grown in PrEGM medium. Prostate cancer cell lines, all of human origin, were grown in RPMI (10% FCS). COS-7 cells were grown in DMEM (10% FCS). The prostate cancer cell lines were obtained from ATCC and were STR typed to confirm their identity.
Detection of Phosphorylated Proteins
Phosphorylated proteins were detected by western blotting with anti-phosphorylation antibodies.
Antibodies
The following antibodies were used: anti-β-actin (Abcam, ab6276), anti-phospho-ErbB2, anti-phospho-Akt, Akt (R&D), ErbB2, phospho-Met (Millipore), Sema4D and plexinB1 (ECM Biosciences), and c-Met (c-28, Santa-Cruz).
Recombinant Sema4D
COS-7 cells transfected with Sema4D-AP or empty vector (control) were grown in serum free medium for 72 hr. The conditioned medium was collected and used directly or purified. Sema4D concentration was assessed by western blotting (Supplementary Fig. 2).
Fig 2.
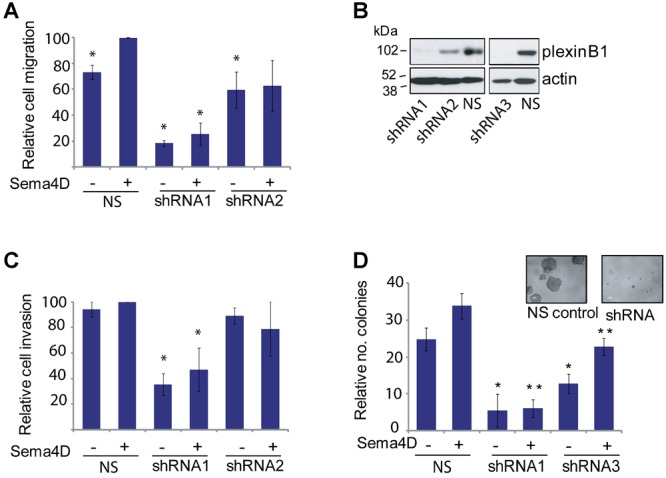
Effect of Sema4D stimulation on LNCaP prostate cancer cells. A: Sema4D increases the motility of LNCaP cells. Transwell migration assays of LNCaP cells with or without Sema4D and of LNCaP cells expressing two different shRNAs to plexinB1 (*P < 0.01 vs. LNCaP + Sema4D) (Student's t-test). Bars represent means ± SE. B: Knockdown of plexinB1 expression in LNCaP cells with three different lentivirally expressed shRNAs to plexinB1 or non-silencing (NS) control shRNA; western blot with anti-plexinB1 and actin antibodies. C: Knockdown of plexinB1 expression decreases the invasive capacity of LNCaP cells. Invasion assays of LNCaP cells with or without Sema4D and of LNCaP cells expressing shRNA to plexinB1 (*P < 0.05 vs. LNCaP + Sema4D, Student's t-test). D: Sema4D increases the capacity of LNCaP cells to grow under anchorage independent conditions. Knockdown of plexinB1 expression decreases anchorage independent growth (*P < 0.05 vs NS no Sema4D, **P < 0.05 vs NS + Sema4D, Student's t-test).
Immunoprecipitation
Lysates of transfected cells were incubated with 1 µg of selective antibody for 2 hr at 4°C. The antigen–antibody complex was incubated with Protein-G sepharose for 2 hr, washed three times and analyzed by immunoblotting.
RTPCR
RNA was reverse transcribed (Superscript III, Invitrogen) then amplified for 30–32 cycles using Taq polymerase (Thermo) and the following primers: 3S. AGG AGT GCC TCT CAC CCA GC; 3AS. GCC TGC TCA TCC AGC AGG TC. The amplified products were sequenced to verify their identity.
Quantitative RTPCR was performed using Brilliant III Ultra-Fast SYBR® Green QPCR Master Mix and the following primers for PLXNB1: S—TCTGCTCAGTGA CCTGGTTG, AS—CTACGGA GTCCCT CACGAAG. The following genes were used as controls (from qStandard): Beta-2-microglobulin (B2M): CTCTCTCTTTCTGGCCTGGAG, ACCCAGACACATAGCAATTCAG; glyceraldehyde-3-phosphate dehydrogenase (GAPDH): TGCACCACCAACTGCTTAGC, GGCATGGACTGTGGTCATGAG; succinate dehydrogenase complex, subunit A, flavoprotein (Fp), nuclear gene encoding mitochondrial protein (SDHA): AGAAGCCCTTTGAGGAGCA, CGATCACGGGTCTATATTCCAGA. Average of three experiments.
shRNA
shRNAs from Open Biosystems, target sets RHS4533-NM_001130082 for plexinB1 and RHS4533-NM_001005862 for ErbB2, both in pLKO.1 lentiviral vector, were used. Virus encoding shRNA to plexinB1 or ErbB2 or non-silencing control shRNA, produced in 293FT cells, was transduced into prostate cancer cells with polybrene (8 µg/ml). Infected cells were selected with puromycin. Knockdown of expression was confirmed by Western blotting.
Cell Motility
Transwell migration assays were performed using 24-well, 0.8 μm transwell chambers (BD) coated with fibronectin on the lower side. Serum-starved cells (2 × 104 per insert) were placed in the upper chamber and serum free RPMI ± Sema4D (100 ng/ml) in the lower chamber. After 6 hr, cells on underside were fixed, stained with crystal violet and counted.
Wound Healing Assays
Cells were grown to confluence in 24-well Essen ImageLock Plates and uniform scratch wounds made using a wound-making device (IncuCyte™). The relative migration of the cells was assessed by measuring the relative wound width using an IncuCyte™ live-cell imaging system at regular time intervals as indicated.
Cell Invasion
Serum-starved cells (6 × 104 cells per insert) in DMEM were placed in the upper chamber of 24-well, 0.8-µm BD Biocoat matrigel invasion chambers (BD Biosciences) with or without Sema4D conditioned medium. DMEM with 10% serum was placed in the lower chamber. The chambers were incubated at 37°C for 24 h. Cells on the upper side were removed, and those on the underside were fixed, stained with crystal violet, and counted.
Anchorage Independent Growth
Cells (2 × 103 cells/ml) were grown in 0.34% agarose in serum-free conditioned medium with or without Sema4D, over a base agarose layer of 0.7% agarose, for 3 weeks. Colonies were viewed down a microscope and measured and counted.
Proliferation Assay
Proliferation in the presence of Sema4D conditioned medium or control conditioned medium, was assessed using a Cell Proliferation Kit II (XTT) according to manufacturer's instructions (Roche) using 2 × 103 cells per well of 96-well plate.
DNA Sequencing
cDNA was amplified by PCR and subjected to Sanger sequencing as described in Ref. 25.
RESULTS
Expression of PlexinB1 and Sema4D in Prostate Cancer Cell Lines
In order to understand the role of plexinB1 in prostate cancer we investigated the expression of endogenous plexinB1 and Sema4D in prostate cancer cells. Prostate cancer cell lines express plexinB1 mRNA (Fig. 1A) and protein (Fig. 1B) with high levels of protein expression in LNCaP, LNCaP-LN3, and VCaP and low levels in PC3 cells. Quantitative RTPCR showed that plexinB1 mRNA levels were significantly higher in LNCaP-LN3 cells than in LNCaP cells from which they were derived (Fig. 1A, ii). Sema4D is expressed by LNCaP, LNCaP-LN3 VCaP, and DU145 (Fig. 1A and B). but not PC3.
Fig 1.
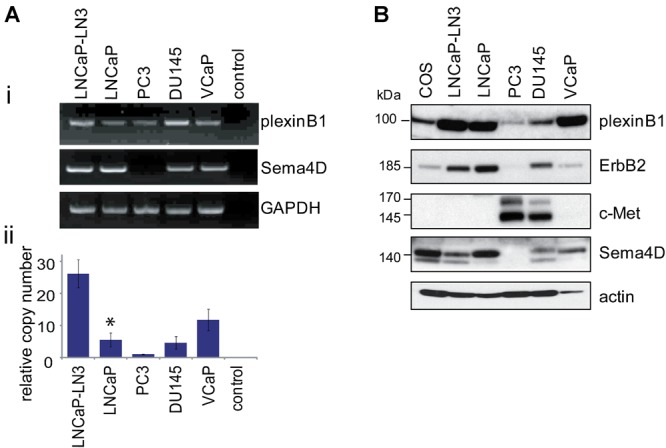
Endogenous expression of plexinB1, Sema4D, ErbB2, and c-Met in prostate cancer cells. A: (i) Endogenous expression of plexinB1 and Sema4D mRNA in prostate cancer cell lines detected by RTPCR. ii: Endogenous expression of plexinB1 in prostate cancer cell lines detected by quantitative real time RTPCR, *P < 0.05 versus LNCaP-LN3 (Student's t-test). B: Endogenous expression of indicated proteins in prostate cancer cell lines, detected by immunoblotting.
The effect of Sema4D stimulation on cell motility is partly dependent on which co-receptors are expressed with plexinB1 by the responding cell. Sema4D enhances cell motility of breast cancer cell lines expressing plexinB1 and ErbB2 and decreases motility in cells expressing plexinB1 and c-Met and the effect is reversed by switching expression of ErbB2 and c-Met 31. We assessed the levels of expression of these receptor tyrosine kinases in prostate cells. LNCaP and LNCaP-LN3 express high levels of ErbB2 and negligible levels of c-Met whereas PC3 expresses high levels of c-Met and low levels of ErbB2 and DU145 expresses similar levels of both proteins (Fig. 1B and Supplementary Fig. 1).
Sema4D Stimulation Increases the Motility, Invasive Capacity, and Anchorage Independent Growth of the Prostate Cancer Cell Line LNCaP
Treatment with exogenously applied Sema4D significantly increased the motility of LNCaP cells in transwell migration assays (Fig. 2A) and in wound healing assays (Supplementary Fig. 3) measuring single and collective cell migration respectively. LNCaP cells in which plexinB1 expression had been knocked down by two different shRNAs (Fig. 2A and B) showed reduced motility compared to LNCaP cells expressing a non-silencing shRNA control. The motility of cells expressing shRNA1 was reduced relative to cells expressing non-silencing shRNA even in the absence of Sema4D, probably because LNCaP expresses endogenous Sema4D (Fig. 1). The reduction in motility of cells expressing shRNA2 was less than for those expressing shRNA1 as some residual plexinB1 expression remains in shRNA2-infected cells (Fig. 2B). The observed Sema4D-induced increase in motility is therefore dependent on plexinB1 expression. Sema4D also increased the capacity of LNCaP cells to invade through matrigel (Fig. 2C). A reduction in invasive capacity was observed in LNCaP cells in which plexinB1 expression was knocked down (Fig. 2C).
LNCaP cells form colonies when grown in anchorage independent conditions, a phenotype associated with tumorigenicity. Treatment of LNCaP with Sema4D increased anchorage independent growth and reducing the level of plexinB1 protein expression by shRNA resulted in a significant decrease in anchorage independent growth, showing that expression of plexinB1 is required for growth of LNCaP cells in these conditions (Fig. 2D).
Sema4D Stimulation Decreases the Motility and Proliferation of the Prostate Cancer Cell Line PC3
In contrast to LNCaP, stimulation of PC3 cells with Sema4D reduced cell motility in transwell assays (Fig. 3A). Knockdown of plexinB1 expression with shRNA (Fig. 3A, ii) inhibited this response. Stimulation of DU145 cells with Sema4D had little effect on motility (data not shown).
Fig 3.
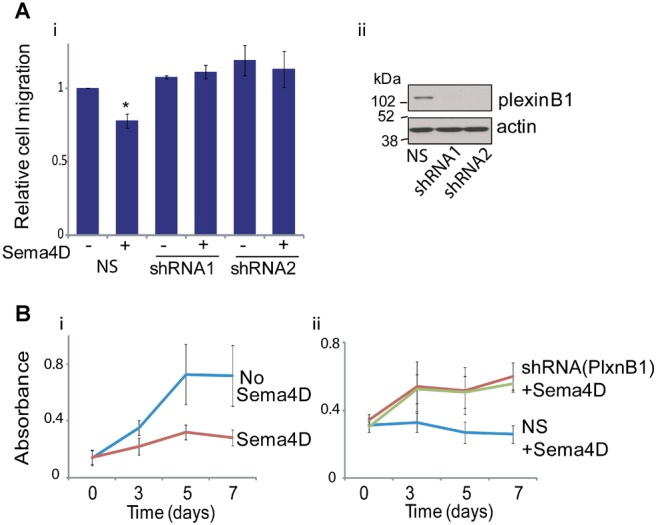
Effect of Sema4D stimulation on PC3 prostate cancer cells. A: Sema4D decreases motility of PC3 cells. i: Transwell motility assays of PC3 cells treated with Sema4D expressing non-silencing (NS) or shRNA to plexinB1 (*P < 0.05 vs. PC3 cells without Sema4D, Student's t-test). ii: Western blot of lysates from PC3 cells infected with lentivirus expressing non-silencing (NS) or plexinB1-specific shRNA. B: Sema4D decreases proliferation of PC3 cells. i: XTT assays of PC3 cells treated with or without Sema4D. ii: XTT assays of PC3 cells infected with lentivirus expressing non-silencing (NS) or shRNA to plexinB1, treated with Sema4D.
Sema4D also decreased the proliferation of PC3 cells under normal in vitro growth conditions (Fig. 3B, i). Knockdown of plexinB1 expression in PC3 cells reversed the Sema4D-induced decrease in proliferation (Fig. 3B, ii).
Sema4D Stimulation Increases the Motility and Anchorage Independent Growth of the Prostate Cancer Cell Line LNCaP-LN3
LNCaP cells are heterozygous for a missense mutation (A5359G, Thr1697Ala) in the plexinB1 gene, and express mutant and wild-type (WT) plexinB1 at a ratio of 3:1 25. Exogenous expression of this mutant form of plexinB1 in HEK293 cells increases motility relative to WT plexinB1 25. To determine if the increase in motility and invasion in LNCaP cells is dependent on the mutant form of plexinB1, we performed motility assays using LNCaP-LN3 32, a derivative of LNCaP which has lost the mutant copies of plexinB1 and expresses WT plexinB1 only (Fig. 4A). As for LNCaP, treatment with Sema4D increased the motility of LNCaP-LN3 (Fig. 4B). This response was inhibited by knockdown of plexinB1 expression by shRNA (Fig. 4B and C). These results suggest that activation of both WT and mutant plexinB1 increases the motility of LNCaP cells.
Fig 4.
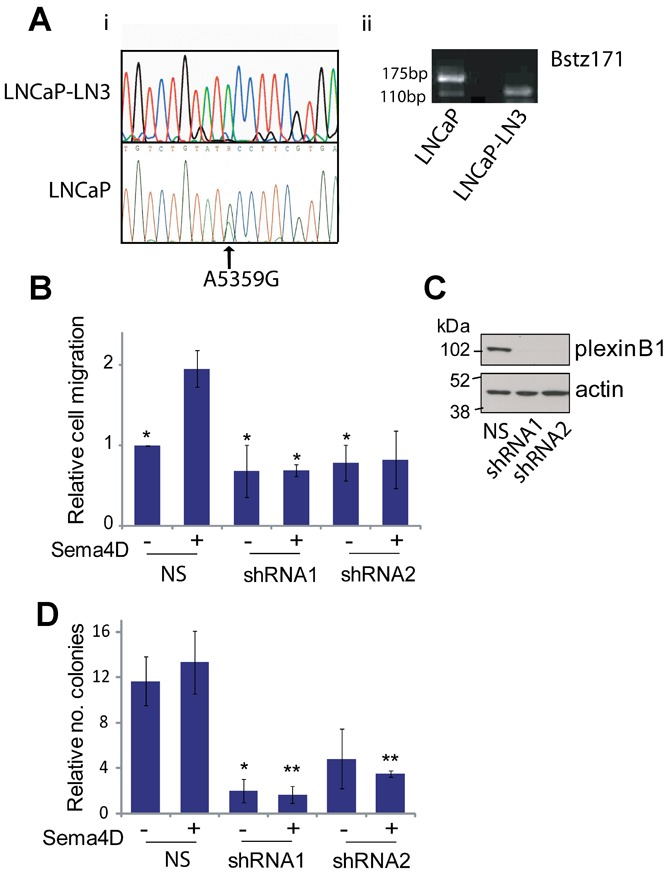
Effect of Sema4D stimulation on LNCaP-LN3 prostate cancer cells. A: LNCaP-LN3 expresses wild type plexinB1. i: cDNA sequence of LNCaP-LN3 (top panel) and LNCaP (bottom panel) around region of A5359G (Thr1697Ala) mutation (arrow). ii: Agarose gel of PCR products digested with Bstz171. The A5359G mutation, which destroys a Bstz171 site, is represented by the top band (170 bp). B: Sema4D increases motility of LNCaP-LN3 cells. Transwell migration assays of LNCaP-LN3 cells with or without Sema4D expressing control non-silencing (NS) shRNA or 2 different shRNAs to plexinB1 (*P < 0.05, versus LNCaP-LN3(NS) + Sema4D, Student's t-test). Bars represent means ± SE. C: Knockdown of plexinB1 expression in LNCaP-LN3 cells with 2 different lentivirally expressed shRNAs to plexinB1; western blot with anti-plexinB1 or anti-actin antibodies. NS, non-silencing shRNA. D: Knockdown of plexinB1 expression in LNCaP-LN3 decreases anchorage independent growth (*P < 0.05 vs. NS no Sema4D, **P < 0.05 vs. NS+ Sema4D Student's t-test).
Sema4D increased the anchorage independent growth of LNCaP-LN3 and knockdown of plexinB1 expression by shRNA resulted in a significant decrease in anchorage independent growth, similar to LNCaP (Fig. 4D).
Interaction of PlexinB1 with ErbB2 in Prostate Cancer Cell Line LNCaP
LNCaP and LNCaP-LN3 cells express high levels of plexinB1, Sema4D and ErbB2 (Fig. 1). Since plexinB1 interacts with ErbB2 12, Sema4D may act as a pro-migratory cue to LNCaP cells through the plexinB1-mediated activation of ErbB2. To test this hypothesis we investigated whether plexinB1 interacts with ErbB2 in LNCaP cells. Co-immunoprecipitation of endogenous plexinB1 with endogenous ErbB2 and vice versa demonstrated that plexinB1 and ErbB2 physically interact with each other in LNCaP cells (Fig. 5A). Furthermore, stimulation of LNCaP and LNCaP-LN3 with Sema4D resulted in phosphorylation of endogenous ErbB2 (Fig. 5B and D) and endogenous Akt (Fig. 5C and E), a signaling factor downstream of ErbB2.
Fig 5.
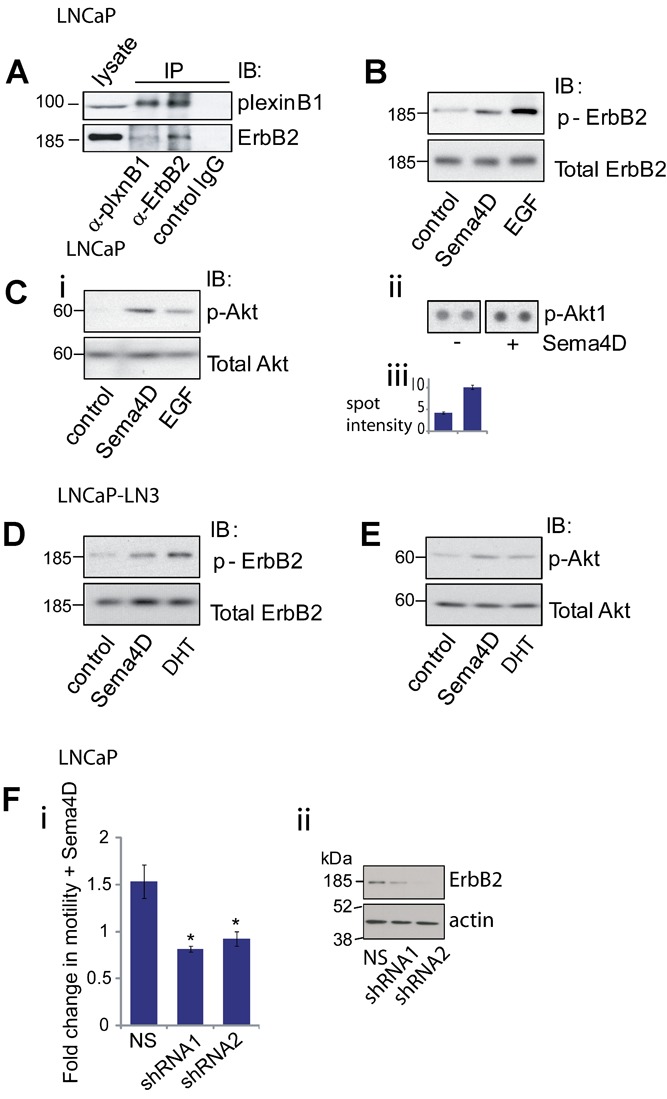
PlexinB1-ErbB2 signaling in LNCaP and LNCaP-LN3 cells. A: PlexinB1 interacts with ErbB2 in LNCaP cells. Lysates of LNCaP were co-immunoprecipitated with plexinB1, ErbB2, or control antibody and pulled down proteins were detected by Western blotting. B: Sema4D treatment induces phosphorylation of endogenous ErbB2 in LNCaP cells. LNCaP cells were stimulated for 20 min with control or Sema4D conditioned medium or EGF (500 ng/ml). ErbB2 phosphorylation was detected by Western blotting. C: Sema4D treatment induces phosphorylation of Akt in LNCaP cells. LNCaP cells were stimulated for 20 min with control or Sema4D conditioned medium or EGF (500 ng/ml). Akt phosphorylation was detected by Western blotting (i) or on an antibody array (ii and iii). D: Sema4D treatment induces phosphorylation of endogenous ErbB2 in LNCaP-LN3 cells. LNCaP-LN3 cells were stimulated for 20 min with control or Sema4D conditioned medium or DHT (1 nM). ErbB2 phosphorylation was detected by Western blotting. E: Sema4D treatment induces phosphorylation of Akt in LNCaP-LN3 cells. LNCaP-LN3 cells were stimulated for 20 min with control or Sema4D conditioned medium or DHT (1 nM). Akt phosphorylation was detected by Western blotting. F: Knockdown of ErbB2 expression in LNCaP cells blocks Sema4D-induced migration. i: Transwell motility assay of LNCaP cells expressing non-silencing (NS) shRNA or two different shRNAs to ErbB2. Migration of Sema4D-stimulated cells relative to unstimulated cells (*P < 0.01 (Student's t-test). ii: Western blot of lysates from LNCaP cells expressing non-silencing (NS) or ErbB2 specific shRNAs.
Sema4D-Stimulated Increase in Motility in LNCaP Cells is Dependent on ErbB2
To determine if the increase in motility observed in LNCaP cells in response to Sema4D stimulation is dependent on ErbB2 signaling, motility assays were performed on LNCaP cells in which ErbB2 expression had been knocked down by shRNA. Knockdown of ErbB2 expression resulted in a decrease in Sema4D-induced motility (Fig. 5F), demonstrating that Sema4D-induced motility in LNCaP is dependent on ErbB2 expression.
Interaction of c-Met with PlexinB1 in PC3 Cells
Sema4D/plexinB1 signaling acts as an anti-migratory cue to PC3 cells (Fig. 3A) which express high levels of c-Met (Fig. 1). The opposite response of LNCaP and PC3 to Sema4D stimulation may be accounted for by differences in the balance of ErbB2 and c-Met expression. To determine if plexinB1 interacts with c-Met in PC3 cells, co-immunoprecipition experiments were performed. Endogenous c-Met co-immunoprecipitated with endogenous plexinB1 in PC3 cells showing that c-Met binds to plexinB1 in the PC3 cell line (Fig. 6A). Sema4D treatment of PC3 cells did not however result in phosphorylation of c-Met (Fig. 6B) and no evidence for dephosphorylation of c-Met following Sema4D treatment was found (data not shown).
Fig 6.
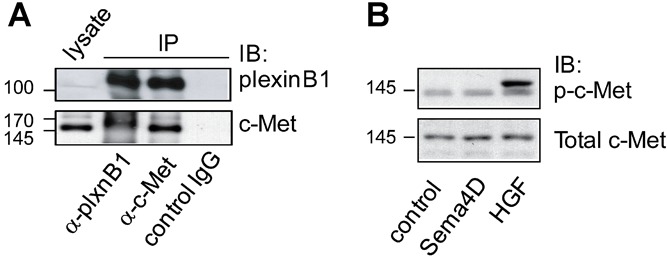
PlexinB1 interacts with c-Met in PC3 cells. A: Lysates of PC3 cells were co-immunoprecipitated with plexinB1, c-Met or control antibody and pulled down proteins were detected by Western blotting. B: Sema4D treatment does not induce c-Met phosphorylation in PC3 cells. PC3 cells were stimulated for 20 min with control or Sema4D conditioned medium or HGF (50 ng/ml) and c-Met phosphorylation was detected by Western blotting.
Expression of PlexinB1, Sema4D, ErbB2, and c-Met in Primary Prostate Cells
Sema4D can act as a pro-migratory cue to prostate cancer cell lines expressing plexinB1 and ErbB2 and has the opposite effect in others. To establish the expression patterns of these proteins in primary prostate epithelial cells we performed immunoblotting of lysates of cells from prostate tissue. We found that plexinB1 is expressed in primary cultures of cells from benign prostatic epithelium (Fig. 7A) and in immortalized benign prostatic epithelial cells (Fig. 7B). Two of the three primary cultures of benign prostate epithelial cells tested expressed ErbB2, the other expressed c-Met (Fig. 7A). All seven immortalized benign prostate epithelial cells expressed ErbB2 and one expressed c-Met (Fig. 7B).
Fig 7.
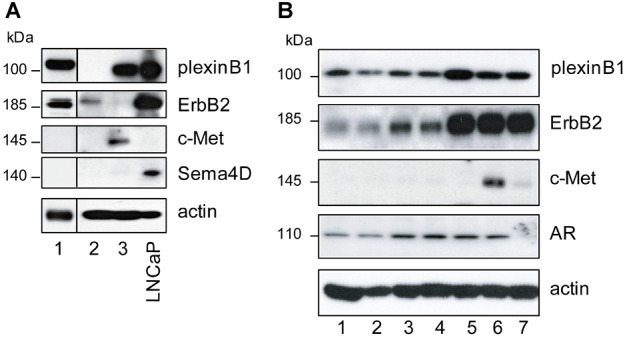
Endogenous expression of plexinB1, Sema4D, ErbB2, and c-Met in primary prostate cells. A: Endogenous expression of indicated proteins in primary cultures of benign prostatic epithelium from three different individuals. B: Endogenous protein expression in immortalized benign prostatic epithelial cells from seven different individuals. AR, androgen receptor.
DISCUSSION
The presence of mutations and high expression levels of plexinB1 in prostate tumors points to a role for this signaling pathway in prostate cancer. The response of a cell to Sema4D/plexinB1 signaling is highly context dependent, differing according to which co-receptors are expressed by the responding cell. The aim of this study was to determine the role of plexinB1/Sema4D signaling in prostate cancer cells specifically.
We found that all prostate cancer cell lines tested and most primary prostate tissue samples express plexinB1 and most express the ligand for plexinB1, Sema4D. If cleaved and shed by these cells, endogenous Sema4D may activate plexinB1 by an autocrine mechanism. Sema4D may also inhibit plexinB1 expressed in the same cell by cis inhibition as described in ephrin, Notch and Sema6A/plexinA4 signaling systems 33. Furthermore, the prostate cancer cells LNCaP, LNCaP-LN3, and PC3 were responsive to Sema4D stimulation.
Our results suggest that both mutation and overexpression of plexinB1 contribute to prostate cancer progression. Firstly, the related cell lines LNCaP and LNCaP-LN3 which express mutant (Thr1697Ala) or higher levels of wild-type plexinB1 respectively, responded to Sema4D stimulation in a similar way. Secondly, in primary prostate cancer tissue, plexinB1 mutations were present in a low proportion of the DNA copies analyzed 25 yet the majority of tumor cells in each tumor showed high levels of plexinB1 protein expression.
Mutations in primary tumors were only detected following SSCP analysis and laser capture microdissection 25,34, suggesting, as has been previously found in prostate cancer, a high degree of intratumor genetic heterogeneity, with the mutations in plexinB1 conferring a selective advantage to small clones of cells in the primary cancers. The proportion of copies of mutant DNA in the samples increased from primary to lymph node and bone metastases.
The Thr1697Ala mutation found in LNCaP increases RhoD binding to plexinB1 35 and inhibits the R-RasGAP activity of plexinB1 25, promoting cell migration in HEK293 cells. It is not known if this mutation affects ErbB2-mediated phosphorylation of the nearby Y1708 residue which is required for PLCγ binding and Rho activation 36. Both overexpression and mutation of plexinB1 is expected to result in an increase in RhoD binding and sequestration, leading to an increase in motility and therefore both changes are expected to confer a competitive advantage to prostate tumor cells.
In contrast to LNCaP and LNCaP-LN3, stimulation of PC3 cells with Sema4D decreases cell migration and reduces proliferation. Sema4D/plexinB1-mediated activation of c-Met has been shown to both promote and inhibit migration in other cell types 31,37 and to increase or decrease c-Met phosphorylation 13,28. PC3 cells respond to Sema4D in a similar way to certain melanoma cells in which introduction of plexinB1 decreases migration and proliferation and decreases HGF induced c-Met phosphorylation 28. PlexinB1 expression is lost in melanoma and plexinB1 acts as a tumor suppressor gene in this type of cancer 27,28. PC3 may exemplify a subset of prostate tumors in which plexinB1 has a role in antagonizing tumor progression.
Late stage prostate tumors show low level overexpression of ErbB2 and ErbB2 expression is correlated with poor outcome and high Gleason score 38, although the ErbB2 gene is not amplified in prostate cancer. Expression of ErbB2 as well as plexinB1 was observed in all seven samples of immortalized prostate epithelial cells and two of the primary cultures. Androgen receptor expression, which is high in late stage prostate cancer, suppresses the expression of c-Met 39. In this background of high ErbB2 expression and low c-Met expression in late stage prostate cancer, overexpression and/or mutation of plexinB1 may promote prostate cancer progression.
CONCLUSIONS
PlexinB1 signals via ErbB2 to enhance the invasive phenotype of prostate cancer cells.
Both wild-type and mutant plexinB1 are potential targets for anti-cancer therapy in prostate tumors that express ErbB2.
Acknowledgments
We thank Dr Patricia De Winter for help with the qRTPCR.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
Endogenous expression of ErbB2 and c-Met.
Sema4D in conditioned medium.
Sema4D increases motility of LNCaP cells in wound healing assays. i: Migration of LNCaP cells ± Sema4D, assessed by a wound healing assay. The relative wound width measured every 4 hr using an IncuCyte™ live-cell imaging system. ii: Relative wound width at 52 hr, *P < 0.05. iii: Images from representative wound healing assay of LNCaP cells at 0, 24, 48, and 72 hr.
REFERENCES
- 1.Neufeld G, Kessler O. The semaphorins: Versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 2.Tamagnone L, Comoglio PM. To move or not to move. EMBO Rep. 2004;5:356–361. doi: 10.1038/sj.embor.7400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 4.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 5.Neufeld G, Lange T, Varshavsky A, Kessler O. Semaphorin signaling in vascular and tumor biology. Adv Exp Med Biol. 2007;600:118–131. doi: 10.1007/978-0-387-70956-7_10. [DOI] [PubMed] [Google Scholar]
- 6.Hota PK, Buck M. Plexin structures are coming: Opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci. 2012;69:3765–3805. doi: 10.1007/s00018-012-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- 8.Tong Y, Chugha P, Hota PK, Alviani RS, Li M, Tempel W, Shen L, Park HW, Buck M. Binding of Rac1, Rnd1, and RhoD to a novel Rho GTPase interaction motif destabilizes dimerization of the plexin-B1 effector domain. J Biol Chem. 2007;282:37215–37224. doi: 10.1074/jbc.M703800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vikis HG, Li W, Guan KL. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 2002;16:836–845. doi: 10.1101/gad.966402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oinuma I, Katoh H, Harada A, Negishi M. Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by Plexin-B1 and induces cell contraction in COS-7 cells. J Biol Chem. 2003;278:25671–25677. doi: 10.1074/jbc.M303047200. [DOI] [PubMed] [Google Scholar]
- 11.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 12.Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol. 2004;165:869–880. doi: 10.1083/jcb.200312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- 14.Kigel B, Varshavsky A, Kessler O, Neufeld G. Successful inhibition of tumor development by specific class-3 semaphorins is associated with expression of appropriate semaphorin receptors by tumor cells. PLoS ONE. 2008;3:e3287. doi: 10.1371/journal.pone.0003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ch'ng ES, Kumanogoh A. Roles of Sema4D and Plexin-B1 in tumor progression. Mol Cancer. 2010;9:251. doi: 10.1186/1476-4598-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaur P, Bielenberg DR, Samuel S, Bose D, Zhou Y, Gray MJ, Dallas NA, Fan F, Xia L, Lu J, Ellis LM. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin Cancer Res. 2009;15:6763–6770. doi: 10.1158/1078-0432.CCR-09-1810. [DOI] [PubMed] [Google Scholar]
- 17.Castro-Rivera E, Ran S, Thorpe P, Minna JD. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci USA. 2004;101:11432–11437. doi: 10.1073/pnas.0403969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroki T, Trapasso F, Yendamuri S, Matsuyama A, Alder H, Williams NN, Kaiser LR, Croce CM. Allelic loss on chromosome 3p21.3 and promoter hypermethylation of semaphorin 3B in non-small cell lung cancer. Cancer Res. 2003;63:3352–3355. [PubMed] [Google Scholar]
- 19.Xiang R, Davalos AR, Hensel CH, Zhou XJ, Tse C, Naylor SL. Semaphorin 3F gene from human 3p21.3 suppresses tumor formation in nude mice. Cancer Res. 2002;62:2637–2643. [PubMed] [Google Scholar]
- 20.Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci USA. 2006;103:9017–9022. doi: 10.1073/pnas.0508825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadanandam A, Varney ML, Singh S, Ashour AE, Moniaux N, Deb S, Lele SM, Batra SK, Singh RK. High gene expression of semaphorin 5A in pancreatic cancer is associated with tumor growth, invasion and metastasis. Int J Cancer. 2010;127:1373–1383. doi: 10.1002/ijc.25166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- 23.Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, Risio M, Trusolino L, Weitz J, Schneider M, Mazzone M, Comoglio PM, Tamagnone L. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest. 2010;120:2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valente G, Nicotra G, Arrondini M, Castino R, Capparuccia L, Prat M, Kerim S, Tamagnone L, Isidoro C. Co-expression of plexin-B1 and Met in human breast and ovary tumours enhances the risk of progression. Cell Oncol. 2009;31:423–436. doi: 10.3233/CLO-2009-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong OG, Nitkunan T, Oinuma I, Zhou C, Blanc V, Brown RS, Bott SR, Nariculam J, Box G, Munson P, Constantinou J, Feneley MR, Klocker H, Eccles SA, Negishi M, Freeman A, Masters JR, Williamson M. Plexin-B1 mutations in prostate cancer. Proc Natl Acad Sci USA. 2007;104:19040–19045. doi: 10.1073/pnas.0702544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worzfeld T, Swiercz JM, Looso M, Straub BK, Sivaraj KK, Offermanns S. ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Invest. 2012;122:1296–1305. doi: 10.1172/JCI60568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argast GM, Croy CH, Couts KL, Zhang Z, Litman E, Chan DC, Ahn NG. Plexin B1 is repressed by oncogenic B-Raf signaling and functions as a tumor suppressor in melanoma cells. Oncogene. 2009;28:2697–2709. doi: 10.1038/onc.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens L, McClelland L, Fricke A, Williamson M, Kuo I, Scott G. Plexin B1 suppresses c-Met in melanoma: A role for plexin B1 as a tumor-suppressor protein through regulation of c-Met. J Invest Dermatol. 2010;130:1636–1645. doi: 10.1038/jid.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez Roman JJ, Garay GO, Saenz P, Escuredo K, Sanz IC, Gutkind S, Junquera C, Simon L, Martinez A, Fernandez Luna JL, Val-Bernal JF. Plexin B1 is downregulated in renal cell carcinomas and modulates cell growth. Transl Res. 2008;151:134–140. doi: 10.1016/j.trsl.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Rody A, Karn T, Ruckhaberle E, Hanker L, Metzler D, Muller V, Solbach C, Ahr A, Gatje R, Holtrich U, Kaufmann M. Loss of Plexin B1 is highly prognostic in low proliferating ER positive breast cancers—Results of a large scale microarray analysis. Eur J Cancer. 2009;45:405–413. doi: 10.1016/j.ejca.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem. 2008;283:1893–1901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- 32.Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, Fidler IJ. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–1636. [PubMed] [Google Scholar]
- 33.Yaron A, Sprinzak D. The cis side of juxtacrine signaling: A new role in the development of the nervous system. Trends Neurosci. 2012;35:230–239. doi: 10.1016/j.tins.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Balakrishnan A, Penachioni JY, Lamba S, Bleeker FE, Zanon C, Rodolfo M, Vallacchi V, Scarpa A, Felicioni L, Buck M, Marchetti A, Comoglio PM, Bardelli A, Tamagnone L. Molecular profiling of the “plexinome” in melanoma and pancreatic cancer. Hum Mutat. 2009;30:1167–1174. doi: 10.1002/humu.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou C, Wong OG, Masters JR, Williamson M. Effect of cancer-associated mutations in the PlexinB1 gene. Mol Cancer. 2012;11:11. doi: 10.1186/1476-4598-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swiercz JM, Worzfeld T, Offermanns S. Semaphorin 4D signaling requires the recruitment of phospholipase C gamma into the plexin-B1 receptor complex. Mol Cell Biol. 2009;29(23):6321–6334. doi: 10.1128/MCB.00103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23:5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- 38.Minner S, Jessen B, Stiedenroth L, Burandt E, Kollermann J, Mirlacher M, Erbersdobler A, Eichelberg C, Fisch M, Brummendorf TH, Bokemeyer C, Simon R, Steuber T, Graefen M, Huland H, Sauter G, Schlomm T. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res. 2010;16:1553–1560. doi: 10.1158/1078-0432.CCR-09-2546. [DOI] [PubMed] [Google Scholar]
- 39.Verras M, Lee J, Xue H, Li TH, Wang Y, Sun Z. The androgen receptor negatively regulates the expression of c-Met: Implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67:967–975. doi: 10.1158/0008-5472.CAN-06-3552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Endogenous expression of ErbB2 and c-Met.
Sema4D in conditioned medium.
Sema4D increases motility of LNCaP cells in wound healing assays. i: Migration of LNCaP cells ± Sema4D, assessed by a wound healing assay. The relative wound width measured every 4 hr using an IncuCyte™ live-cell imaging system. ii: Relative wound width at 52 hr, *P < 0.05. iii: Images from representative wound healing assay of LNCaP cells at 0, 24, 48, and 72 hr.


