Abstract
Objective
We aimed to investigate the prevalence of depression in cancer patients assessed by diagnostic interviews and self-report instruments, and to study differences in prevalence between type of instrument, type of cancer and treatment phase.
Methods
A literature search was conducted in four databases to select studies on the prevalence of depression among adult cancer patients during or after treatment. A total of 211 studies met the inclusion criteria. Pooled mean prevalence of depression was calculated using Comprehensive Meta-Analysis.
Results
Hospital Anxiety and Depression Scale—depression subscale (HADS-D) ≥ 8, HADS-D ≥11, Center for Epidemiologic Studies ≥ 16, and (semi-)structured diagnostic interviews were used to define depression in 66, 53, 35 and 49 studies, respectively. Respective mean prevalence of depression was 17% (95% CI = 16–19%), 8% (95% CI = 7–9%), 24% (95% CI = 21–26%), and 13% (95% CI = 11–15%) (p < 0.001). Prevalence of depression ranged from 3% in patients with lung cancer to 31% in patients with cancer of the digestive tract, on the basis of diagnostic interviews. Prevalence of depression was highest during treatment 14% (95% CI = 11–17%), measured by diagnostic interviews, and 27% (95% CI = 25–30%), measured by self-report instruments. In the first year after diagnosis, prevalence of depression measured with diagnostic interviews and self-report instruments were 9% (95% CI = 7–11%) and 21% (95% CI = 19–24%), respectively, and they were 8% (95% CI = 5–12%) and 15% (95% CI = 13–17%) ≥ 1 year after diagnosis.
Conclusions
Pooled mean prevalence of depression in cancer patients ranged from 8% to 24% and differed by the type of instrument, type of cancer and treatment phase. Future prospective studies should disentangle whether differences in prevalence of depression are caused by differences in the type of instrument, type of cancer or treatment phase.
Introduction
In 2008, nearly 12.7 million new cases of cancer (excluding non-melanoma skin cancer) were diagnosed worldwide, and this number is expected to increase to 21.3 million by 2030 1. Of all cancer types, lung cancer (12.7%), breast cancer (10.9%), colorectal cancer (8%), stomach cancer (7.8%) and prostate cancer (7.1%) are the most common worldwide 1. Recent advances in diagnosis and treatment of cancer patients have led to improved survival rates.
Many cancer patients and survivors suffer from psychological problems, such as depression 2,3. This may interfere with the patient's ability to cope with the burden of the illness, it may decrease acceptance of treatment, extend hospitalization, reduce quality of life and increase suicide risk 4–6.
In the past decades, studies have evaluated the prevalence of depression in cancer patients. However, the overall prevalence rate of depression in cancer patients remains unclear; previous studies reported prevalence rates between 0% and 58% 7. Several factors may contribute to this wide range of prevalence rates, including: (i) the use of different instruments to assess depression with different psychometric properties; (ii) the use of different criteria to define depression; and (iii) differences between included cancer populations with respect to cancer type, stage and treatment modality 7,8. Recently, Mitchell et al. 9 conducted a meta-analysis on 66 studies to determine the prevalence of depression in cancer patients in oncological, hematological and palliative care settings. They reported a pooled prevalence of major depression in non-palliative care settings of 16.3% (95% CI = 13–20%), as measured via psychiatric interviews according to the DSM-IV criteria 10 or International Classification of Diseases 10 (ICD-10) 11.
The detection of depression in cancer patients is difficult. Depression can easily be overlooked because symptoms of cancer and its treatment resemble neurovegetative symptoms of depression, such as fatigue, loss of appetite and sleep disturbance 12. Nevertheless, because both physiological and psychological symptoms of depression can be diagnostically useful when looking for depression, excluding neurovegetative symptoms from depression assessment instruments may impair the ability to diagnose depression in cancer settings 13. Other difficulties to detect depression in cancer patients are the lack of specific skills to diagnose mental disorders 14, lack of time in busy oncological settings, and reluctance of the patient to discuss emotional well-being 14,15. In clinical practice, therefore, self-report instruments are often used to detect depressive symptoms and to assess severity of symptoms 16. Self-report instruments have the advantage of being quick, easy to administer, inexpensive and they rely on psychological and cognitive symptoms rather than physiological symptoms 8. No meta-analysis has been published to quantitatively summarize prevalence of depression in cancer patients as measured by self-report instruments and psychiatric interviews.
This study is a meta-analysis to estimate the prevalence of depression in patients during or after cancer treatment, as assessed by diagnostic interviews and self-report instruments. We distinguish between depressive disorders assessed using diagnostic interviews and symptom prevalence as measured by self-report instruments. Furthermore, we aim to examine whether the prevalence of depression differs by the type of instrument used to assess depression, the type of cancer and treatment phase.
Material and methods
Search strategy
A comprehensive literature search was performed up to December 2011 in four databases (PubMed, PsycINFO, EMBASE and CINAHL). Studies were identified by combining keywords and text words indicative of epidemiology (e.g., epidemiologic, epidemiological, epidemiol*, preval* and inciden*), depression (e.g., depressi*, depression emotion, distress, depressive disorder and major depression), and neoplasms (e.g., tumor, tumors, tumorous, tumour and carcino*). Pubmed was additionally scanned by using the following Mesh terms: ‘depression/epidemiology’, ‘psychological/epidemiology’, ‘depressive disorder/epidemiology’ and ‘neoplasms’. Detailed search profiles are available on request.
Inclusion and exclusion criteria
We included studies that: (i) reported the prevalence of depression in adult patients in non-palliative-care settings during or after cancer treatment; (ii) assessed depression by semi-structured or structured diagnostic interviews based on criteria by DSM-III(−R)/IV or ICD-10, or by self-report instruments with ‘good’ or ‘excellent’ psychometric quality as rated by Vodermaier et al. 8, that is, the Hospital Anxiety and Depression Scale—depression subscale (HADS-D) 17, the Center for Epidemiologic Studies—Depression Scale (CES-D) 18,19, Beck Depression Inventory (BDI), and Brief Symptom Inventory 20; (iii) defined depression as a ‘major depressive disorder’ based on criteria by DSM-III(−R)/IV or ICD-10, and as ‘increased risk of depression’ by self-report instruments; and (iv) were written in English.
We excluded studies examining psychometric properties of instruments; intervention studies including randomized controlled trials, reviews, case reports, reports on the prevalence of depression in palliative cancer patients; studies in which depression could not be distinguished from distress; and studies that only reported mean and standard deviations (SD) of the sum scores of outcome measures of depression instead of numbers or percentages of depressed patients.
Selection process and bias risk assessment
After eliminating duplicate studies of the identified references, the titles and available abstracts of the remaining studies were examined by three reviewers: A. K., L. B. and I. R. Studies that possibly met inclusion criteria, studies with no abstract and studies that could not clearly be excluded on the basis of the title and abstract were retrieved in full text and scrutinized more extensively for eligibility.
The bias risk of each study was assessed using a 13-item list adapted from existing criteria lists 21–23. As the prevalence of depression depends on the population under study, this list focused on: (i) the description of the study population and (ii) the representativeness of the study populations. Items for the description of the study population included sociodemographic characteristics (at least three of the following four: age, gender, marital status and education and employment or socioeconomic status), cancer type, tumor status, type of treatment, time since diagnosis, treatment phase, inclusion and exclusion criteria and information about (a history) of psychiatric problems of the participants. Items of the representativeness of the study population included sample size >100, presentation of participation or response rate, reasons for nonresponse or nonparticipation presented, comparison of characteristics of responders and non-responders, and consecutive sampling method. A positive score was given if the study provided adequate information regarding the item of concern. In case of incomplete or unclear information or a lack of description, a negative score was given. If a study referred to another publication describing relevant information about the first study, the additional publication was obtained to score the item of concern.
The reviewers A. K. and G. K. or I. R. independently performed the bias assessments. In case of disagreement between the two reviewers on assigning scores, a third reviewer (L. B.) was consulted to discuss the item of concern until consensus was reached. For each study, a total bias score was calculated by counting the number of criteria scored positively, divided by the total number of bias items (i.e., 13). A study was considered of low bias risk if the score was at least 9.75 (75%) of the total possible score and of medium bias risk if the score was between 6.5 and 9.75 (50–75%). A bias score lower than 6.5 (50%) was defined as high bias risk.
Data extraction
The reviewers A. K., I. R. and L. B. extracted the following data from the included studies: (i) mean/median age; (ii) sex; (iii) cancer type; (iv) time since diagnosis; (v) type of treatment; (vi) treatment phase: during treatment, <1 year after treatment, ≥1 year after treatment, and mixed phases; (vii) instrument for assessment of depression; and (viii) sample size and number of cases of depression, more specifically major depressive disorder as measured by diagnostic interviews, and increased risk for depression as measured by self-report instruments. Unclear data were discussed until consensus was reached.
Statistical analysis
To calculate pooled mean prevalence of depression, we used the computer program Comprehensive Meta-Analysis (version 2.2.064 by Borenstein et al., Biostat, Englewood (New Jersey, US), 2005.). As we expected considerable heterogeneity among the studies, we decided to calculate the mean point prevalence and 95% CI by using a random effects model. In the random effects model, it is assumed that the included studies are drawn from ‘populations’ of studies that differ from each other systematically (heterogeneity). In this model, the prevalence resulting from the included studies not only differs because of the random error within studies (fixed effects model) but also because of true variation in prevalence from one study to the next.
Pooled mean prevalence was calculated for instruments that were used more than 25 times in the total number of cohorts. In addition, we performed subgroup analyses, in which we tested whether there were significant differences in prevalence of depression between different types of: (i) depression measurement instruments; (ii) cancer type; and (iii) treatment phase (during diagnosis or treatment, <1 year posttreatment, and ≥1 year posttreatment). Because studies with high bias might lead to underestimation or overestimation of overall prevalence of depression, we excluded these studies from analysis. We used the mixed effects model, which pooled studies within subgroups with the random effects model but tested for significant differences between subgroups with the fixed effects model. We tested the heterogeneity under the fixed model, using the I2 statistic. I2 describes the variance between studies as a proportion of the total variance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity, with 0–25% as low, 25–50% as moderate and 50–75% as high heterogeneity 24. We also calculated the Q-statistic but only report whether this was significant or not. The p-values above 0.05 indicate that the total variance is due to variance within studies and not to variance between studies.
Results
Study selection
After removing duplicates, the literature searches yielded 2301 records. On the basis of the title and abstract, we excluded 1644 records that did not meet our inclusion criteria. Full text articles were retrieved for 657 potentially relevant records, of which 464 were excluded (Figure 1). Through reference search, an additional 18 studies were found eligible for inclusion. The 211 eligible studies described a total of 238 cohorts comprising 82,426 patients: 72 cohorts on cancer of the breast, 22 on cancer of the male genitalia, 21 on cancer of the head and neck, 16 on hematological malignancies, 15 on cancer of the female genitalia, 15 on cancer of the digestive tract, 10 on cancer of the respiratory tract, seven on cancer of the brain, three on cancer of the skin, two on cancer of the bone and soft tissue, two on cancer of the urinary tract, and two on cancer of the endocrine system. A mixed group was investigated in 51 cohorts.
Figure 1.
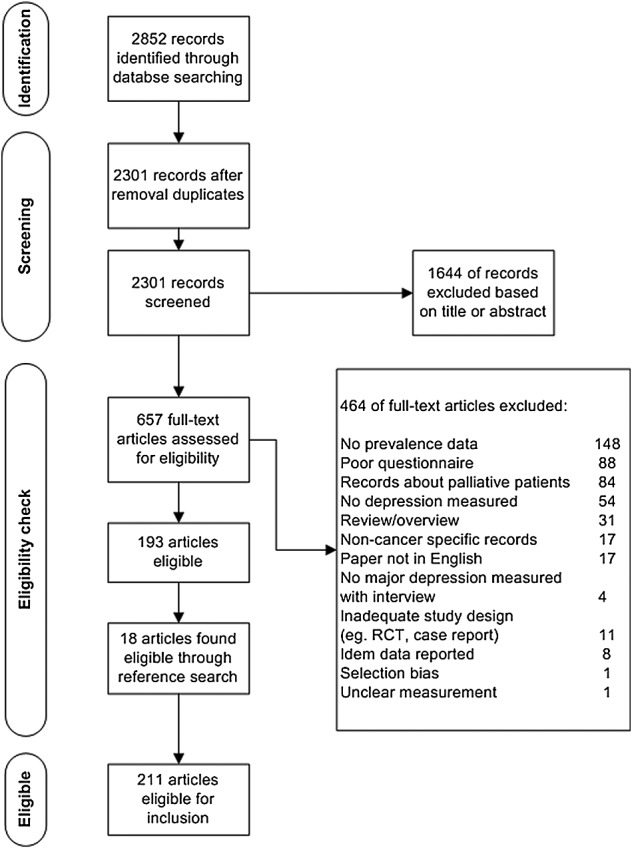
Selection of studies
Assessment of depression
A structured or semi-structured diagnostic interview (Table 1) was used 49 times in the 238 cohorts. Self-report instruments were used 267 times, of which the HADS-D with cut-off ≥8 was used 78 times, the HADS-D with cut-off ≥ 11 was used 59 times, and CES-D with cut-off ≥16 was used 38 times. Because they were used ≥25 times, diagnostic interviews, HADS-D (cut-offs ≥8 and ≥11), and CES-D (cut-off ≥ 16), which were embedded in 159 studies, were used for further analyses.
Table 1.
Number of times diagnostic interviews or self-report instruments were used in cohorts (n = 238)
| Diagnostic interviews and self-report instruments | N | ||
|---|---|---|---|
| All ratings | 316 | ||
| Self-report instruments | 267 | ||
| Diagnostic interviews | 49 | ||
| Diagnostic interviewsa only | N | ||
| All ratings | 49 | ||
| Interview DSM | 18 | ||
| SCID (DSM) | 16 | ||
| CIDI (ICD/DSM) | 3 | ||
| DIS DSM | 3 | ||
| MINI (DSM) | 3 | ||
| SADS RDC (similar to DSM) | 3 | ||
| MILP (DSM) | 1 | ||
| Mini-DIPS (ICD/DSM) | 1 | ||
| DQPD (ICD) | 1 | ||
| Self-report instrumentsb only | Cut-off | N | |
| All ratings | 269 | ||
| HADS-D | 153 | ||
| HADS-D ≥ 5 | 2 | ||
| HADS-D ≥ 7 | 2 | ||
| HADS-D ≥ 8 | 78 | ||
| HADS-D > 8 | 2 | ||
| HADS-D ≥ 10 | 2 | ||
| HADS-D ≥ 11 | 59 | ||
| HADS-D > 11 | 1 | ||
| HADS-D ≥ 15 | 3 | ||
| HADS-D ≥ 16 | 1 | ||
| HADS-D no cut-off | 3 | ||
| CES-D | 54 | ||
| CES-D ≥ 9 | 2 | ||
| CES-D ≥ 10 | 6 | ||
| CES-D > 10 | 2 | ||
| CES-D ≥ 15 | 1 | ||
| CES-D ≥ 16 | 38 | ||
| CES-D > 16 | 2 | ||
| CES-D ≥ 21 | 1 | ||
| CES-D ≥ 24 | 1 | ||
| CES-D no cut-off | 1 | ||
| BDI | 42 | BDI ≥ 5 | 2 |
| BDI ≥ 9 | 1 | ||
| BDI ≥ 10 | 7 | ||
| BDI ≥ 11 | 1 | ||
| BDI ≥ 13 | 2 | ||
| BDI ≥ 14 | 6 | ||
| BDI ≥ 15 | 3 | ||
| BDI ≥ 16 | 2 | ||
| BDI ≥ 17 | 3 | ||
| BDI ≥ 18 | 3 | ||
| BDI ≥ 19 | 3 | ||
| BDI ≥ 20 | 3 | ||
| BDI ≥ 22 | 1 | ||
| BDI ≥ 24 | 1 | ||
| BDI ≥ 25 | 1 | ||
| BDI ≥ 29 | 1 | ||
| BDI ≥ 30 | 2 | ||
| BSI | 18 | BSI 53 items | 15 |
| BSI 18 items | 3 |
Diagnostic interviews: DSM, Diagnostic and Statistical Manual of Mental Disorders; SCID, Structured Clinical Interview for DSM; CIDI, Composite International Diagnostic Interview; ICD-10, International Classification of Diseases; MILP, Monash Interview for Liaison Psychiatry; SADS, Schedule for Affective Disorders and Schizophrenia; RDC, Research Diagnostic Criteria; DQPD, Diagnostic Questionnaire for Depressive Patients (according to ICD-10).
Self-report instruments: HADS-D, Hospital Anxiety and Depression Scale—Depression subscale; CES-D, Center for Epidemiological Studies—Depression Scale; BDI, Beck Depression Inventory; BSI, Brief Symptom Inventory.
Bias risk of the studies
The average bias score was 8.8 (SD 2.3) on a 13-point scale, and the scores ranged from 1 (highest bias risk) to 13 (lowest bias risk) (Figure 2(a)). Of the 159 assessed studies, 25 studies had a high bias risk, 67 studies had a medium bias risk, and another 67 had a low bias risk. More than 95% of the assessed studies reported cancer type, and inclusion and exclusion criteria (Figure 2(b)). Half of the studies provided information on ‘reasons for nonresponse or nonparticipation’ and ‘time since diagnosis’. A minority of the studies provided information on ‘comparison of characteristics between responders and nonresponders’ (27%) and ‘(history of) psychiatric problems’ (32%). The full bias risk assessment of the studies can be found in Table 4 (Supporting information).
Figure 2.
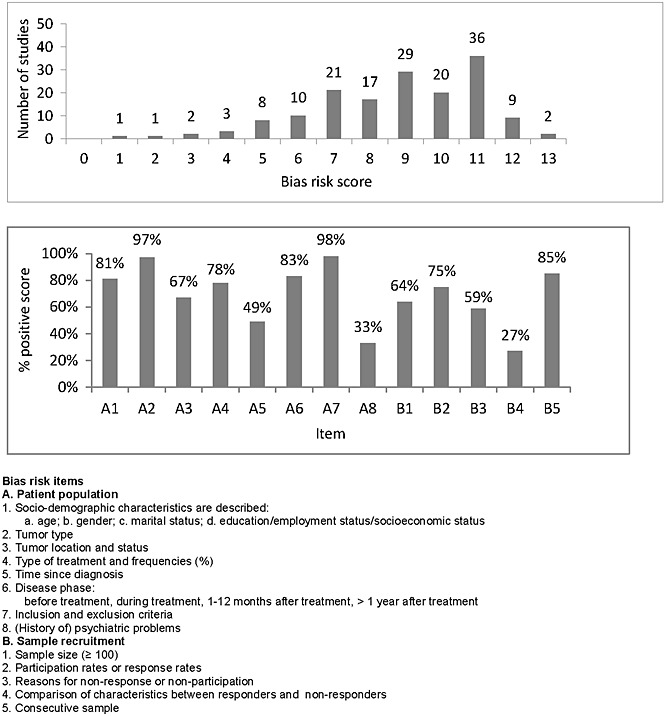
Bias risk assessment of 159 studies: number of studies per rating (a) and percentage of studies with a positive score at item level (b)
Prevalence of depression
Over all studies, pooled prevalence of major depressive disorder as measured by semi-structured and structured diagnostic interviews was 14% (95% CI = 11–16%). Pooled prevalence of depression was 18% (95% CI = 16–20%) in cohorts using HADS-D with cut-off ≥8, 7% (95% CI = 6–8%) in cohorts using HADS-D with cut-off ≥11, and 24% (95% CI = 21–26%) in cohorts using CES-D with cut-off ≥16 (Table 2). Characteristics of the analysed studies are shown in Table 3 (Supporting information).
Table 2.
Prevalence of depression
| Instrument | Number of cohorts | Total sample | Pooled mean | 95% CI | I2 | Between group difference |
|---|---|---|---|---|---|---|
| All cancer types | ||||||
| All studies | ||||||
| Diagnostic interviews | 49 | 8747 | 0.14 | 0.11–0.16 | 91.45 | |
| HADS-D ≥ 8 | 75 | 27,384 | 0.18 | 0.16–0.20 | 95.68 | |
| HADS-D ≥ 11 | 58 | 17,920 | 0.07 | 0.06–0.08 | 92.92 | |
| CES-D ≥ 16 | 38 | 6466 | 0.24 | 0.21–0.26 | 85.33 | |
| <0.001 | ||||||
| Studies of medium/low bias risk | ||||||
| Diagnostic interviews | 39 | 7322 | 0.13 | 0.11–0.15 | 92.08 | |
| HADS-D ≥ 8 | 68 | 26,132 | 0.17 | 0.16–0.19 | 96.02 | |
| HADS-D ≥ 11 | 49 | 16,011 | 0.08 | 0.07–0.09 | 93.54 | |
| CES-D ≥ 16 | 30 | 5583 | 0.24 | 0.21–0.26 | 86.37 | |
| <0.001 | ||||||
| Subgroup analyses per cancer type | ||||||
| Diagnostic interviewsa | ||||||
| Cancer type | ||||||
| Breast | 16 | 2297 | 0.11 | 0.08–0.16 | 88.77 | |
| Mixed | 11 | 3580 | 0.13 | 0.07–0.21 | 95.15 | |
| Head and neck | 5 | 591 | 0.11 | 0.03–0.34 | 95.11 | |
| Respiratory tract | 3 | 393 | 0.03 | 0.02–0.06 | 0.00 | |
| Hematological | 2 | 289 | 0.08 | 0.05–0.11 | 0.00 | |
| Brain | 1 | 89 | 0.28 | 0.20–0.38 | 0.00 | |
| Female genitalia | 1 | 83 | 0.23 | 0.15–0.33 | 0.00 | |
| <0.001 | ||||||
| Self-report instrumentsa, b | ||||||
| Cancer type | ||||||
| Breast | 27 | 8964 | 0.20 | 0.16–0.24 | 93.87 | |
| Mixed | 18 | 9530 | 0.25 | 0.21–0.30 | 96.60 | |
| Male genitalia | 14 | 7115 | 0.10 | 0.08–0.13 | 89.34 | |
| Head and neck | 11 | 1336 | 0.20 | 0.16–0.25 | 71.15 | |
| Hematological | 7 | 695 | 0.25 | 0.20–0.31 | 64.10 | |
| Female genitalia | 7 | 2381 | 0.26 | 0.18–0.35 | 94.17 | |
| Digestive tract | 6 | 577 | 0.27 | 0.18–0.37 | 92.13 | |
| Respiratory tract | 4 | 641 | 0.21 | 0.11–0.37 | 91.97 | |
| Bone and soft tissue | 1 | 36 | 0.33 | 0.21–0.48 | 0.00 | |
| Endocrine system | 1 | 136 | 0.17 | 0.12–0.24 | 0.00 | |
| Urinary tract | 1 | 102 | 0.16 | 0.10–0.24 | 0.00 | |
| Skin | 1 | 202 | 0.07 | 0.04–0.11 | 0.00 | |
| <0.001 | ||||||
| Subgroup analyses per treatment phase | ||||||
| Diagnostic interviewsa | ||||||
| Acute phase | 11 | 1379 | 0.14 | 0.11–0.17 | 92.98 | |
| <1 year posttreatment | 9 | 1138 | 0.09 | 0.07–0.11 | 67.48 | |
| ≥1 year posttreatment | 7 | 1195 | 0.08 | 0.05–0.12 | 86.35 | |
| <0.001 | ||||||
| Self-report instrumentsa, b | ||||||
| Acute phase | 38 | 8134 | 0.27 | 0.25–0.30 | 92.42 | |
| <1 year posttreatment | 32 | 7198 | 0.21 | 0.19–0.24 | 89.20 | |
| ≥1 year posttreatment | 27 | 11,206 | 0.15 | 0.13–0.17 | 94.62 | |
| <0.001 | ||||||
CI, confidence interval. I2, the percentage of total variation across the studies that is due to heterogeneity rather than chance.
Outliers Montazeri et al. 2004, Mhaidat et al. 2009 and Tavoli et al. 2007 are left out of analysis.
Diagnostic interviews/self-report instruments: only medium and low bias risk studies.
Self-report instruments: HADS-D ≥ 8 + CES-D ≥ 16.
After excluding studies with high bias risk (n = 25), we found a pooled prevalence of major depressive disorder as measured by semi-structured and structured diagnostic interviews of 13% (95% CI = 11–15%) (Table 2). Pooled prevalence of depression was 17% (95% CI = 16–19%) in cohorts using HADS-D with cut-off ≥8, 8% (95% CI = 7–9%) in cohorts using HADS-D with cut-off ≥11, and 24% (95% CI = 21–26%) in cohorts using CES-D with cut-off ≥16. Heterogeneity was high, ranging from 86 to 96% (Table 2).
On the basis of diagnostic interviews, the prevalence of depression ranged from 3% in patients with lung cancer to 28% in patients with cancer of the brain (Table 2). On the basis of self-report instruments (HADS-D with cut-off ≥8 and CES-D with cut-off ≥16) prevalence of depression ranged from 7% in patients with skin cancer to 31% patients with cancer of the digestive tract. Heterogeneity was high, ranging from 64 to 97% (Table 2).
Regarding treatment phase, as measured by diagnostic interviews, we found the highest prevalence of depression in the acute phase of disease with a pooled prevalence of 14% (95% CI = 11–17%) against a pooled prevalence of 9% (95% CI = 7–11%) in the first year posttreatment and 8% (95% CI = 5–12%) 1 year or more posttreatment. On the basis of self-report instruments, we also found the highest prevalence of depression in the acute phase of disease, with a pooled prevalence of 27% (95% CI = 25–30%). Pooled prevalence in the first year posttreatment was 21% (95% CI = 19–24%) and it was 15% (95% CI = 13–17%) after the first year. Heterogeneity was high (68–95%, Table 2).
Discussion
In this meta-analysis, we found pooled mean prevalence of depression to be 8–24% in cancer patients in non-palliative-care settings during or after treatment, and the prevalence differed by the type of instrument used to measure depression, cancer type and treatment phase. Prevalence of major depressive disorder appeared to be 13% as measured by DSM or ICD. In an earlier meta-analysis, Mitchell et al. 9 reported a pooled prevalence of 16.3% (95% CI = 13–20%) among 66 studies using diagnostic interviews. This small difference may be caused by the fact that we searched four databases and included more recently conducted studies up to December 2011, and we did not include studies examining psychometric properties of instruments, nor did we include studies with a high bias score in our analysis. In addition, we included 13 papers that were not included by Mitchell et al. 25–37.
Clearly, the prevalence of a major depressive disorder in cancer patients is much higher compared with the 4% found in the general population 38. Prevalence of depression differed substantially according to the diagnostic instrument used and was substantially higher when self-report instruments were used as compared with the diagnostic instruments. An explanation for this difference might be that diagnostic interviews are standardized tools and use more stringent criteria according to DSM or ICD for clinical depression than self-report instruments. Self-report instruments are designed to measure an increased risk for or severity of depression instead of diagnosing a depressive disorder 39,40. Therefore, we note that the use of self-report instruments might overestimate the presence of depression and consequently overrate patients' need for psychological treatment.
Conversely, in patients with symptoms of depression, assessment by diagnostic interviews may lead to under-recognition of unmet needs for psychological support, as some oncologists may be insufficiently skilled to identify psychological distress and perceived social support in patients 14,41. Under-recognition may result in undertreatment, as two-thirds of screen positive cases may develop a full-blown depression if left untreated 42. Furthermore, standardized diagnostic interviews are time-consuming and thus relatively expensive, which hampers routine implementation in busy oncological settings. Consequently, Vodermaier et al. 8 previously recommended implementing routine self-report for symptoms of depression in cancer patients using valid and reliable self-report instruments, such as the HADS-D, the CES-D or the BDI. These recommendations are supported by Mitchell et al. 43, who also advised using a two-step procedure incorporating both screening (ruling out non-cases) and case-finding (ruling in probable cases) by two stem questions. For the use of these self-report instruments, no specific skills are required, and in case an increased risk of depression is detected, the patient should be able to be referred to a specialized psychosocial care provider.
We found differences in the prevalence of depression across patients treated for different cancer types. Although the prevalence of depression appeared to be highest in patients with cancer of the digestive tract, the brain, female genitalia and patients with hematological malignancies, the limited number of studies per cancer type and small sample sizes of specific cancer types hamper us to draw firm conclusions. Differences in prevalence of depression were not only found between patients treated for different cancer types, but also within patient populations treated for the same cancer type. For example, our results from a relatively large group of breast cancer patients, including 11,182 patients from 43 cohorts, showed pooled prevalence of depression of 11% (95% CI = 8–16%) as measured by diagnostic interviews and of 20% (95% CI = 16–24%) as measured by self-report instruments. These results are in accordance with the findings of Fann et al. 44, reporting the prevalence of major depressive disorder as measured by structured interviews among breast cancer patients ranging from 5% to 15%. Also, Massie et al. 7 reported wide ranges in the prevalence of depression in breast cancer patients, that is, from 1.5% to 46%. Wide ranges in prevalence of depression within groups of patients with similar diagnosis may be caused by the time-point of measurement, type of cancer treatment, number of side effects of cancer treatment, and gender 45,46. Unfortunately, in the current study, we were unable to identify the influences of these factors on prevalence rates.
Further, our findings show that prevalence of depression assessed by both diagnostic interviews and self-report instruments was highest in the acute phase of the disease (14% and 27%, respectively), and decreases afterwards. A similar drop has been found in early breast cancer patients by Burgess et al. 3 and Lee et al. 47. Burgess et al. showed a point prevalence of depression, anxiety or both of 33% at diagnosis, 24% at 3 months after diagnosis, and 15% at 1 year after treatment. Lee et al. showed a point prevalence of depression of 7% preoperatively, 8% at 3 months postoperatively, and 2% at 1 year after treatment. Other prospective studies showed that there are distinct patterns regarding the course of psychological distress, ranging from resilience (no distress before or after treatment), recovery (elevated distress followed by return to normal), delayed recovery and persisting distress 48–50. In the present study, we could not determine a clear pattern of depression rates at the different treatment phases of specific cancer types because we were unable to disentangle whether differences in prevalence rates were due to treatment phase or to types of cancer included. Future prospective trials should obtain insight in the course of depression at the different treatment phases of explicit cancer types, using preferably one standardized instrument to assess depression.
Bias risk score
Of the 159 assessed studies, 84% had a medium and low bias risk, and 16% a high bias risk. The majority (73%) of the studies did not compare characteristics of responders and nonresponders, limiting insight in the generalizability of the results. In addition, only one third of the studies reported information on history of psychiatric problems. A history of depression increases the risk of recurrence of a depressive episode 51.
We excluded high bias risk studies from subgroup analysis, because these studies might lead to overestimation or underestimation of overall prevalence of depression. We found a minimal difference in prevalence of depression between analysis of all studies and analysis of medium and low bias risk studies only. Nevertheless, we recommend that criteria and demands regarding population and sample recruitment in studies should be standardized and followed conscientiously in future research in order to secure psychometrical quality of trials. Larger samples of specific cancer types at a precise point in cancer treatment should be examined, and information on history of depression is preferable. Also, data on sample recruitment should be collected and reported rigorously.
Strength and limitations
A strength of this study is the inclusion of both diagnostic interviews and self-report instruments. Because both instruments are frequently used in cancer research and clinical practice for the assessment of depression, we thought it was important to analyse both assessment methods. Another strength was the inclusion of a bias assessment and our focus on medium and low bias risk studies. Nevertheless, heterogeneity was high, and it remained high after analysing subgroups of different instruments, cancer types and treatment phase. Also, the equal weighing of the 13 items of the bias list may be to some extent arbitrary. The small number of cohorts hampered us to disentangle differences in prevalence caused by cancer type and differences caused by treatment phase, which may reduce the heterogeneity of studies. Also, we were unable to study influences of other variables such as age, gender or type of treatment because of lack of information in the majority of the included studies.
To determine major depression, ICD-10 uses similar criteria as DSM-III(−R)/IV, but adding higher threshold categories, which might have resulted in lower prevalences of major depression when assessed by ICD-10.
We acknowledge that cut-off points are to some extent arbitrary, and prevalence of depression depends on the chosen cut-off point(s) per questionnaire. An exploration at symptom level would have been preferable. Unfortunately, the prevalence of depression in the analysed studies was mostly reported as exceeding a certain cut-off point rather than a score on the individual symptoms of depression. For that reason, we could not perform a depression analysis at symptom level.
For the pooled analysis, we focussed on the HADS-D and the CES-D, because prevalence of depression assessed by these instruments were reported more than 25 times in the total number of cohorts. Because the BDI and the Brief Symptom Inventory were not reported sufficiently (n < 25) in the total number of cancer patient cohorts, we could not incorporate these instruments in the pooled analysis.
Conclusion
Pooled mean prevalence of depression in cancer patients during or after treatment ranged between 8% and 24% and depended on the instruments used, type of cancer and treatment phase. The use of self-report instruments may overestimate the presence of depression. Future prospective trials investigating the prevalence of depression in cancer patients using valid and reliable instruments are needed.
Acknowledgments
The study is funded by The Netherlands Organisation for Health Research and Development, grant-number 300020012. We would like to thank search specialist R. H. J. Otten, MSc for supporting the database searches. The contribution of Dr. L. M. Buffart was supported by a fellowship granted by the EMGO Institute for Health and Care Research. A related randomized controlled trial has passed Ethical Committee Review.
Conflict of interest
The authors declare that they have no competing interests.
Supporting Information
Supporting information may be found in the online version of this article.
Supplementary
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. IARC CancerBase No. 10 [Internet] Lyon, France: International Agency for Research on Cancer; 2010. GLOBOCAN 2008 v 1.2, cancer incidence and mortality worldwide. Available from: http://globocan.iarc.fr, accessed on 23/01/2012. [Google Scholar]
- 2.Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249:751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 3.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prieto JM, Blanch J, Atala J, et al. Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol. 2002;20:1907–1917. doi: 10.1200/JCO.2002.07.101. [DOI] [PubMed] [Google Scholar]
- 5.Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet. 2000;356:1326–1327. doi: 10.1016/S0140-6736(00)02821-X. [DOI] [PubMed] [Google Scholar]
- 6.Yousaf U, Christensen ML, Engholm G, Storm HH. Suicides among Danish cancer patients 1971–1999. Br J Cancer. 2005;92:995–1000. doi: 10.1038/sj.bjc.6602424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 8.Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464–1488. doi: 10.1093/jnci/djp336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12:160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. In Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 11.WHO. In The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization; 1993. [Google Scholar]
- 12.Newport DJ, Nemeroff CB. Assessment and treatment of depression in the cancer patient. J Psychosom Res. 1998;45:215–237. doi: 10.1016/s0022-3999(98)00011-7. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell AJ, Lord K, Symonds P. Which symptoms are indicative of DSMIV depression in cancer settings? An analysis of the diagnostic significance of somatic and non-somatic symptoms. J Affect Disord. 2012;138:137–148. doi: 10.1016/j.jad.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counselling? Br J Cancer. 2001;84:179–185. doi: 10.1054/bjoc.2000.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vachon ML. Caring for the caregiver in oncology and palliative care. Semin Oncol Nurs. 1998;14:152–157. doi: 10.1016/s0749-2081(98)80021-1. [DOI] [PubMed] [Google Scholar]
- 16.Sharp LK, Lipsky MS. Screening for depression across the lifespan: a review of measures for use in primary care settings. Am Fam Physician. 2002;66:1001–1008. [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 19.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 20.Derogatis LR. In Brief Symptom Inventory: Administration, Scoring and Procedures Manual. 3. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 21.Luppa M, Sikorski C, Luck T, et al. Age- and gender-specific prevalence of depression in latest-life—systematic review and meta-analysis. J Affect Disord. 2010 doi: 10.1016/j.jad.2010.11.033. S0165-0327(10)00730-5 [pii]; doi: 10.1016/j.jad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41:2613–2619. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Chinapaw MJ, Proper KI, Brug J, van Mechelen W, Singh AS. Relationship between young peoples' sedentary behaviour and biomedical health indicators: a systematic review of prospective studies. Obes Rev. 2011;12:e621–e632. doi: 10.1111/j.1467-789X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akechi T, Okamura H, Yamawaki S, Uchitomi Y. Why do some cancer patients with depression desire an early death and others do not? Psychosomatics. 2001;42:141–145. doi: 10.1176/appi.psy.42.2.141. [DOI] [PubMed] [Google Scholar]
- 26.Akechi T, Okamura H, Nishiwaki Y, Uchitomi Y. Psychiatric disorders and associated and predictive factors in patients with unresectable nonsmall cell lung carcinoma: a longitudinal study. Cancer. 2001;92:2609–2622. doi: 10.1002/1097-0142(20011115)92:10<2609::aid-cncr1614>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Dausch BM, Compas BE, Beckjord E, et al. Rates and correlates of DSM-IV diagnoses in women newly diagnosed with breast cancer. J Clin Psychol Med Settings. 2004;11:159–169. [Google Scholar]
- 28.Fritzsche K, Struss Y, Hammel A, Bertz H, Stein B. Relationship between psychosocial distress, treatment need and use of psychotherapeutic interventions within a psychosomatic liaison service in hematological oncology. Onkologie. 2004;27:457–461. doi: 10.1159/000080364. [DOI] [PubMed] [Google Scholar]
- 29.Gil F, Costa G, Perez FJ. Does chemotherapy reduce stress? Palliat Support Care. 2010;8:455–460. doi: 10.1017/S1478951510000337. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert J, Haman KL, Dietrich MS, Blakely RD, Shelton RC, Murphy BA. Depression in patients with head and neck cancer and a functional genetic polymorphism of the serotonin transporter gene. Head Neck. 2012;34:359–364. doi: 10.1002/hed.21744. [DOI] [PubMed] [Google Scholar]
- 31.Grassi L, Malacarne P, Maestri A, Ramelli E. Depression, psychosocial variables and occurrence of life events among patients with cancer. J Affective Disord. 1997;44:21–30. doi: 10.1016/s0165-0327(97)01445-6. [DOI] [PubMed] [Google Scholar]
- 32.Kissane DW, Grabsch B, Love A, Clarke DM, Bloch S, Smith GC. Psychiatric disorder in women with early stage and advanced breast cancer: a comparative analysis. Aust N Z J Psychiatry. 2004;38:320–326. doi: 10.1080/j.1440-1614.2004.01358.x. [DOI] [PubMed] [Google Scholar]
- 33.McCaffrey JC, Weitzner M, Kamboukas D, Haselhuhn G, LaMonde L, Booth-Jones M. Alcoholism, depression, and abnormal cognition in head and neck cancer: a pilot study. Otolaryngology - Head & Neck Surgery. 2007;136:92–97. doi: 10.1016/j.otohns.2006.06.1275. [DOI] [PubMed] [Google Scholar]
- 34.Palmer SC, Kagee A, Coyne JC, DeMichele A. Experience of trauma, distress, and posttraumatic stress disorder among breast cancer patients. Psychosom Med. 2004;66:258–264. doi: 10.1097/01.psy.0000116755.71033.10. [DOI] [PubMed] [Google Scholar]
- 35.Pirl WF, Greer J, Temel JS, Yeap BY, Gilman SE. Major depressive disorder in long-term cancer survivors: analysis of the National Comorbidity Survey Replication. J Clin Oncol. 2009;27:4130–4134. doi: 10.1200/JCO.2008.16.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popoola AO, Adewuya AO. Prevalence and correlates of depressive disorders in outpatients with breast cancer in Lagos, Nigeria. Psycho-Oncology. 2012;21:675–679. doi: 10.1002/pon.1968. doi: 10.1002/pon.1968. [DOI] [PubMed] [Google Scholar]
- 37.Schrier M, Amital D, Arnson Y, et al. Association of fibromyalgia characteristics in patients with non-metastatic breast cancer and the protective role of resilience. Rheumatol Int. 2011 doi: 10.1007/s00296-011-2104-7. [DOI] [PubMed] [Google Scholar]
- 38.Waraich P, Goldner EM, Somers JM, Hsu L. Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry. 2004;49:124–138. doi: 10.1177/070674370404900208. [DOI] [PubMed] [Google Scholar]
- 39.Trask PC. Assessment of depression in cancer patients. J Natl Cancer Inst Monogr. 2004:80–92. doi: 10.1093/jncimonographs/lgh013. [DOI] [PubMed] [Google Scholar]
- 40.Akechi T, Ietsugu T, Sukigara M, et al. Symptom indicator of severity of depression in cancer patients: a comparison of the DSM-IV criteria with alternative diagnostic criteria. Gen Hosp Psychiatry. 2009;31:225–232. doi: 10.1016/j.genhosppsych.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists' recognition of depression in their patients with cancer. J Clin Oncol. 1998;16:1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- 42.Weissman MM, Neria Y, Gameroff MJ, et al. Positive screens for psychiatric disorders in primary care: a long-term follow-up of patients who were not in treatment. Psychiatr Serv. 2010;61:151–159. doi: 10.1176/appi.ps.61.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell AJ, Meader N, Davies E, et al. Meta-analysis of screening and case finding tools for depression in cancer: evidence based recommendations for clinical practice on behalf of the Depression In Cancer Care consensus group. J Affect Disord. 2012;140:149–160. doi: 10.1016/j.jad.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 44.Fann JR, Thomas-Rich AM, Katon WJ, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30:112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seedat S, Scott KM, Angermeyer MC, et al. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66:785–795. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MS, Love SB, Mitchell JB, et al. Mastectomy or conservation for early breast cancer: psychological morbidity. Eur J Cancer. 1992;28A:1340–1344. doi: 10.1016/0959-8049(92)90514-3. [DOI] [PubMed] [Google Scholar]
- 48.Lam WW, Bonanno GA, Mancini AD, et al. Trajectories of psychological distress among Chinese women diagnosed with breast cancer. Psycho-Oncology. 2010;19:1044–1051. doi: 10.1002/pon.1658. [DOI] [PubMed] [Google Scholar]
- 49.Verdonck-de Leeuw IM, de Bree R, Keizer AL, et al. Computerized prospective screening for high levels of emotional distress in head and neck cancer patients and referral rate to psychosocial care. Oral Oncol. 2009;45:e129–e133. doi: 10.1016/j.oraloncology.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Dunn J, Ng SK, Holland J, et al. Trajectories of psychological distress after colorectal cancer. Psycho-Oncology. 2012;22:1759–1765. doi: 10.1002/pon.3210. [DOI] [PubMed] [Google Scholar]
- 51.Fava GA, Park SK, Sonino N. Treatment of recurrent depression. Expert Rev Neurother. 2006;6:1735–1740. doi: 10.1586/14737175.6.11.1735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary


