Abstract
MALT1 paracaspase links signaling cascades emanating from adaptive or innate immune receptors to the canonical NF-κB pathway. Now, Jaworski et al (2014) investigate the physiological role of MALT1 protease activity in mice. Besides the expected requirement of MALT1 activity for immune activation, the study unveils a novel function for MALT1 activity for the development of peripheral tolerance. Thus, MALT1 protease can act immunogenic or tolerogenic, and this interplay will be highly relevant for the clinical development of MALT1 inhibitors.
See also: M Jaworski et al (December 2014)
The activation of the NF-κB family of transcription factors in immune cells represents a key event during the establishment of an immune response. Stimulation of B- or T-cell antigen receptors (BCR/TCR) or distinct innate immune receptors promotes the assembly of the CBM complex consisting of CARMA1(CARD11)-Bcl10-MALT1 or CARD9-BCL10-MALT1. Within the CBM complex, the MALT1 scaffold serves as a bridging factor to recruit the IκB kinase (IKK) complex and to activate canonical NF-κB signaling (reviewed in Thome, 2008). In addition, MALT1 also contains a very unique paracaspase domain whose proteolytic activity is activated upon TCR stimulation (Coornaert et al, 2008; Rebeaud et al, 2008). Pharmacological inhibition and genetic inactivation revealed that even though MALT1 proteolytic activity is dispensable for canonical NF-κB signaling, it is required for optimal T-cell activation (Rebeaud et al, 2008; Düwel et al, 2009). MALT1 deficiency was shown to protect mice from the induction of experimental autoimmune encephalomyelitis (EAE) in a murine multiple sclerosis model (MS) (Brüstle et al, 2012; Mc Guire et al, 2013). Further, constitutive MALT1 activity drives survival of ABC DLBCL, one of the most aggressive B-cell malignancies (Ferch et al, 2009; Hailfinger et al, 2009). Thus, MALT1 protease is a promising drug target to treat distinct lymphomas as well as autoimmune diseases.
To evaluate the contribution of MALT1 protease activity to the physiological role of MALT1, Thome and collaborators generated a mouse model expressing catalytically inactive MALT1 by replacing the active site cysteine with alanine (MALT1C472A/C472A) (Jaworski et al, 2014). As expected, MALT1C472A/C472A mice are characterized by profound defects in both the adaptive and the innate immune responses. Indeed, despite the fact that BCR and TCR-driven activation of IKK/NF-κB and JNK signaling is not relying on MALT1 protease activity, it is necessary for the establishment of effector functions in lymphocytes, natural killer (NK) and dendritic cells (DC). Just like in MALT1 KO mice, IL-2 production and proliferation of T cells are strongly compromised. MALT1 protease is also required for the development of marginal zone and B1 B cells, and T-cell-dependent or T-cell-independent IgM and IgG production upon immunization. Consequently, MALT1 inactivation protects mice in two T-cell-dependent autoimmune models from EAE induction as well as colitis.
Besides these expected phenotypes, MALT1C472A/C472A mice beyond the age of 6 weeks displayed swollen lymph nodes and an increase in the total number of B and T cells that was much more pronounced than in MALT1 deficient mice. T cells have an activated/effector phenotype and secrete higher levels of the Th1 and Th2 cytokines IFN-γ and IL-4. MALT1C472A/C472A mice develop spontaneous autoimmune gastritis, characterized by loss of animal weight and increased serum levels of IgE and IgG1. Thome and collaborators suggest that the autoimmune phenotype is caused by impaired peripheral tolerance (Fig1). MALT1C472A/C472A mice show a severe cell intrinsic defect in the development of FoxP3+ regulatory T cells (Tregs). Further, adoptive transfer of functional Tregs reduced T-cell activation and ameliorated the symptoms of autoimmune gastritis in MALT1C472A/C472A mice. These findings support a model in which the autoimmune phenotype in MALT1 protease defective mice is caused by residual immune activation that relies on the preserved MALT1 scaffolding function and the concomitant severe defect in peripheral tolerance as a result of reduced Treg numbers (Fig1). In contrast, MALT1 KO mice that are characterized by even a more severe decrease in the number of Tregs are protected from the development of autoimmune gastritis, because they also completely lack immune activation due to the loss of both scaffold and protease functions (Fig1). Hence, the autoimmune phenotype is caused by a partial TCR signaling defect in MALT1C472A/C472A mice that shifts the balance from tolerance to autoimmune activation.
Figure 1. Model for the function of MALT1 protease activity in balancing immune cell activation.
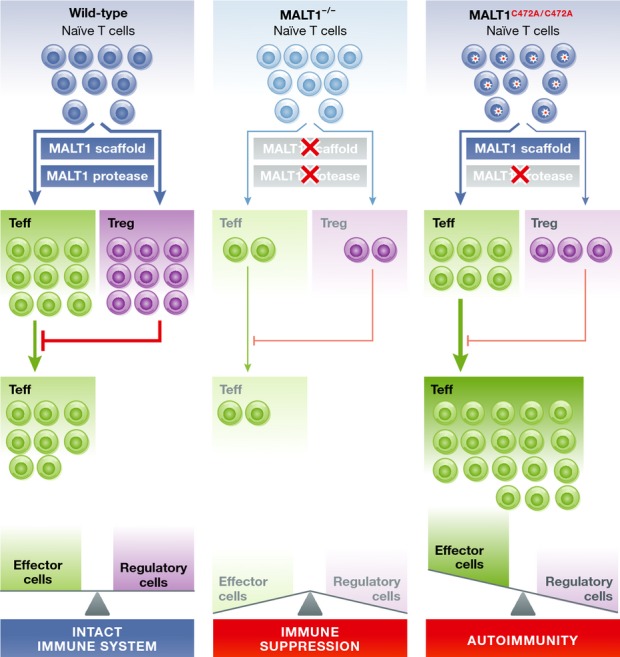
Naive T cells activated during an immune reaction differentiate toward effector T cells (Teffs) and suppressor regulatory T cells (Tregs), and both participate in a healthy immune reaction. MALT1 scaffold and protease activity maintains an equilibrium between Teffs and Tregs (wild-type mice). Upon destruction of MALT1 scaffold and protease functions in MALT1−/− mice, T-cell activation and generation of functional effector T cells as well as regulatory T cells is severely impaired, leading to immune suppression. In MALT1C472A/C472A mice which are defective only in the protease function of MALT1, the residual activation of naive T cells and the Teffs is no longer counterbalanced due to the strong accompanying reduction in Tregs. The loss of equilibrium in the T-cell compartments promotes the expansion of activated Teffs, which leads to autoimmunity.
The reported duality of the MALT1 protease to promote cell intrinsic T-cell effector functions and to suppress activated T cells in a Treg-dependent manner raises a number of interesting questions. Even though the adoptive transfer demonstrates the potential of Tregs to counteract the autoimmunity, it remains open, if the reduced number of Tregs is indeed sufficient to cause the inflammatory phenotype. In the adoptive transfer experiment, only very few Tregs are sufficient to rescue the autoimmune phenotype raising the possibility that MALT1C472A/C472A Tregs may be less functional. In addition, the increase in IFN-γ and IL-4 production in MALT1 protease mutant mice may also indicate that deregulated T-cell effector functions could also be involved in the early onset of gastritis, which is no longer counteracted due to the loss of peripheral T-cell tolerance. In this respect, it remains an unresolved question why the autoimmune phenotype is manifested as gastritis. One can speculate that the microenvironment of the gastric mucosa may support the onset of the disease, but it would be interesting if autoimmune reactions would further spread if the mice would survive beyond 15 weeks of age.
One obvious question is whether MALT1 protease activity is still a promising drug target for immunological diseases and in oncology. Jaworski et al show that MALT1C472A/C472A are protected from the induction of EAE and colitis, lending strong support to a possible clinical use of MALT1 protease inhibitors in the treatment of autoimmune diseases. In line with this, the MALT1 inhibitor mepazine was recently shown to attenuate EAE severity in mice (Mc Guire et al, 2014). However, it obviously needs to be evaluated whether therapeutic MALT1 inhibition could lead to a spontaneous autoimmune phenotype as observed in MALT1C472A/C472A mice. Administration of the MALT1 inhibitor mepazine in an acute EAE model did not affect the number of Tregs (Mc Guire et al, 2014). Even though more prolonged treatment protocols and the usage of potentially more effective MALT1 inhibitors will be required to come to a definite conclusion, the data indicate that a permanent genetic inactivation of MALT1 protease may not reflect the situation of MALT1 pharmacological inhibition. First, the strength of MALT1 inhibition may be quite different in a genetic model or upon inhibitor treatment. Second, in contrast to a MALT1 protease inhibitor that prevents active site accessibility and thus substrate recognition, an active site mutant may still bind substrates and could exert additional effects. Although Jaworski et al did not report on any dominant-negative effects of MALT1 C472A in heterozygous animals, a mouse model that prevents protease activation instead of destroying the cleavage site may more closely reflect the situation of a pharmacological inhibitor. This could be achieved, for example, by mutating the ubiquitin-acceptor site K644 that is required for MALT1 proteolytic activation (Pelzer et al, 2013). Third and most important, the mouse model abrogated MALT1 activity already in early embryogenesis before the immune system and peripheral tolerance have developed. Thus, inactivation in adult mice by an inducible system will be more reminiscent to MALT1 inhibitor treatment. Further, generation of conditional transgenic mice will be required to establish the role of MALT1 protease in Treg biology.
Besides its physiological role in the immune system, MALT1 function has been studied in the context of hematological malignancies and especially ABC DLBCL biology. The first small-molecule MALT1 paracaspase inhibitors have been identified that effectively kill ABC DLBCL tumors in a murine xenotransplantation model (Fontan et al, 2012; Nagel et al, 2012). As this tumor model required the usage of immune-deficient mice, effects on the immune compartment were not analyzed. However, higher numbers of Foxp3-positive cells correlated with an adverse clinical outcome in DLBCL cohort enriched for ABC DLBCL (Tzankov et al, 2008). Thus, in contrast to autoimmunity, inhibition of peripheral tolerance may even foster immune surveillance in lymphomas.
Taken together, the study by Jaworski et al underscores that deciphering the exact mechanism of autoimmune protection versus induction will be relevant for therapeutic targeting of the MALT1 protease in the context of autoimmune diseases as well as lymphomas.
References
- Brüstle A, Brenner D, Knobbe CB, Lang PA, Virtanen C, Hershenfield BM, Reardon C, Lacher SM, Ruland J, Ohashi PS, Mak TW. The NF-κB regulator MALT1 determines the encephalitogenic potential of Th17 cells. J Clin Invest. 2012;122:4698–4709. doi: 10.1172/JCI63528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coornaert B, Baens M, Heyninck K, Bekaert T, Haegman M, Staal J, Sun L, Chen ZJ, Marynen P, Beyaert R. T cell antigen receptor stimulation induces MALT1 paracaspase–mediated cleavage of the NF-κB inhibitor A20. Nat Immunol. 2008;9:263–271. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- Düwel M, Welteke V, Oeckinghaus A, Baens M, Kloo B, Ferch U, Darnay BG, Ruland J, Marynen P, Krappmann D. A20 negatively regulates T cell receptor signaling to NF-kappaB by cleaving Malt1 ubiquitin chains. J Immunol. 2009;182:7718–7728. doi: 10.4049/jimmunol.0803313. [DOI] [PubMed] [Google Scholar]
- Ferch U, Kloo B, Gewies A, Pfänder V, Düwel M, Peschel C, Krappmann D, Ruland J. Inhibition of MALT1 protease activity is selectively toxic for activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2009;206:2313–2320. doi: 10.1084/jem.20091167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan L, Yang C, Kabaleeswaran V, Volpon L, Osborne MJ, Beltran E, Garcia M, Cerchietti L, Shaknovich R, Yang SN, Fang F, Gascoyne RD, Martinez-Climent JA, Glickman JF, Borden K, Wu H, Melnick A. MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell. 2012;22:812–824. doi: 10.1016/j.ccr.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailfinger S, Lenz G, Ngo V, Posvitz-Fejfar A, Rebeaud F, Guzzardi M, Penas E-MM, Dierlamm J, Chan WC, Staudt LM, Thome M. Essential role of MALT1 protease activity in activated B cell-like diffuse large B-cell lymphoma. Proc Natl Acad Sci USA. 2009;106:19946–19951. doi: 10.1073/pnas.0907511106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski M, Marsland BJ, Gehrig J, Held W, Favre W, Luther SA, Perroud M, Golshayan D, Gaide O, Thome M. Malt1 protease inactivation efficiently dampens immune responses but causes spontaneous autoimmunity. EMBO J. 2014;33:2765–2781. doi: 10.15252/embj.201488987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Guire C, Wieghofer P, Elton L, Muylaert D, Prinz M, Beyaert R, van Loo G. Paracaspase MALT1 deficiency protects mice from autoimmune-mediated demyelination. J Immunol. 2013;190:2896–2903. doi: 10.4049/jimmunol.1201351. [DOI] [PubMed] [Google Scholar]
- McGuire C, Elton L, Wieghofer P, Staal J, Voet S, Demeyer A, Nagel D, Krappmann D, Prinz M, Beyaert R, van Loo G. Pharmacological inhibition of MALT1 protease activity protects mice in a mouse model of multiple sclerosis. J Neuroinflammation. 2014;11:124. doi: 10.1186/1742-2094-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel D, Spranger S, Vincendeau M, Grau M, Raffegerst S, Kloo B, Hlahla D, Neuenschwander M, Peter von Kries J, Hadian K, Dörken B, Lenz P, Lenz G, Schendel DJ, Krappmann D. Pharmacologic inhibition of MALT1 protease by phenothiazines as a therapeutic approach for the treatment of aggressive ABC-DLBCL. Cancer Cell. 2012;22:825–837. doi: 10.1016/j.ccr.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Pelzer C, Cabalzar K, Wolf A, Gonzalez M, Lenz G, Thome M. The protease activity of the paracaspase MALT1 is controlled by monoubiquitination. Nat Immunol. 2013;14:337–345. doi: 10.1038/ni.2540. [DOI] [PubMed] [Google Scholar]
- Rebeaud F, Hailfinger S, Posevitz-Fejfar A, Tapernoux M, Moser R, Rueda D, Gaide O, Guzzardi M, Iancu EM, Rufer N, Fasel N, Thome M. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat Immunol. 2008;9:272–281. doi: 10.1038/ni1568. [DOI] [PubMed] [Google Scholar]
- Thome M. Multifunctional roles for MALT1 in T-cell activation. Nat Rev Immunol. 2008;8:495–500. doi: 10.1038/nri2338. [DOI] [PubMed] [Google Scholar]
- Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]


