Abstract
In the seed, a fundamental transition between embryo and vegetative phases of plant development is coordinated by the interaction between the AFL and VAL sub-clades of the plant specific B3 domain transcription factor family. The AFL B3 factors together with LEC1-type HAP3 transcription factors promote embryo maturation; whereas the VAL B3 factors repress the LEC1/AFL (LAFL) network during seed germination. Recent advances reveal that genes in key developmental programs and hormone signaling pathways are downstream targets of the LAFL network highlighting the central role of the LAFL network in integration of intrinsic developmental and hormonal signals during plant development. The VAL B3 proteins are proposed to mediate repression by recruiting a histone deacetylase complex (HDAC) to LAFL genes that contain the Sph/RY cis-element recognized by AFL and VAL B3-DNA-binding domains. In addition to VAL B3 factors, epigenetic mechanisms are implicated in maintaining repression of LAFL network during vegetative development. WIREs Dev Biol 2014, 3:135–145. doi: 10.1002/wdev.126
INTRODUCTION
The evolution of the seed was a key adaption that contributed to the success and diversification of the land plants. Regulation of seed formation and the critical transition between seed and seedling phases of plant development is controlled in part through concerted alterations in the biosynthetic and signaling pathways for major plant hormones including auxin, abscisic acid (ABA), and gibberellins (GA). The plant-specific B3 domain transcription factors were first discovered as mutants of maize [viviparous1 (vp1)]1 and Arabidopsis [abscisic acid insensitive 3 (abi3)]2 that alter ABA signaling in the developing seed. In Arabidopsis, seed development is regulated by a network of transcription factors that includes the AFL clade of B3 domain proteins [ABI3,2 FUSCA3 (FUS3),3 and LEAFY COTYLEDON 2 (LEC2) 4] (Figure 1) and two LEC1-type HAP3 family CCAAT-box binding factors, LEC15 and LEC1-LIKE (L1L).6 Together these genes comprise the LAFL transcription factor network. The program for seed development is refined by mutual interactions of LAFL genes combined with inputs from various hormone, sugar, and light signaling pathways.7–9 Key downstream targets of the LAFL network include genes that control major hormone metabolism and signaling pathways, as well as other transcription factor networks that program the transcriptome of the developing seed. Genetic analyses show that this elaborate program must be repressed during germination of the seed in order for the embryo to complete a transition to the vegetative phase of the plant life cycle. The VAL/HSI B3 factors [VAL1 (HSI2), VAL2 (HSL1), and VAL3 (HSL2)] which form a sister-clade to the AFL subfamily (Figure 1),10,11 play a central role in coordinating repression of the LAFL network during seed germination through recruitment of chromatin remodeling complexes.
Figure 1.
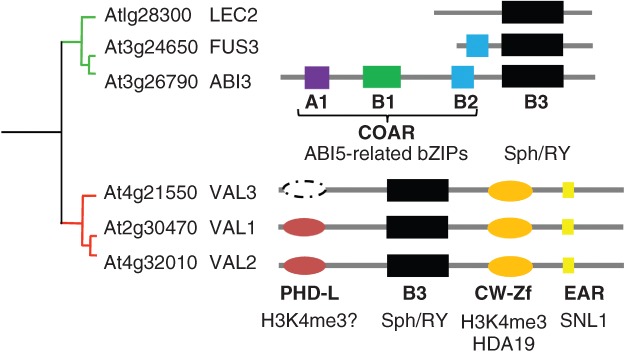
Domain architectures of AFL and VAL B3 transcription factors. The AFL and VAL groups are sister clades in the ABI3/VP1 family of B3 proteins in Arabidopsis. AFL and VAL proteins have distinct domain architectures: B3 domain (dark), B1 (green), B2 (blue), A1 (purple), PHD-L domain (red), CW domain (orange), and EAR motif (yellow). ABI3 has an N-terminal co-activator/co-repressor (COAR) domain that physically interacts with ABI5-related-bZIP factors. VAL3 has an incomplete PHD-L domain (dashed circle). AFL B3 domains specifically bind to the Sph/RY motif (CATGCA), and VAL B3 domains are proposed to bind the same motif. PHD and CW-Zf domains are identified as the histone modification readers that recognize the H3K4me3 mark. VAL2 CW-Zf interacts with HDA19 to repress target gene transcription (see text). EAR motif may mediate the interaction of VAL1 with co-repressor SNL1 (see text).
THE LAFL TRANSCRIPTION FACTOR NETWORK
Genetic analyses show that the LAFL network is organized by complex mutual interactions among the LAFL genes (Figure 2). In this respect, the network is neither strictly hierarchical nor linear. While LEC1 can activate ABI3, FUS3, and LEC2 expression14,16; ectopic expression of LEC2 is sufficient to up-regulate LEC1 and FUS3 in vegetative tissue.13 ABI3 and FUS3 in turn are regulated by mutual positive interactions.14 Moreover, L1L was shown to be regulated by FUS3 in a transcriptome analysis.15 While the molecular basis for the genetic interactions among LAFL factors is not yet fully understood, recent insights have been gained through ChIP (chromatin immunoprecipitation)-on-chip analyses. For example, L1L was identified as a potential direct target of LEC128; whereas, FUS3 physically interacts with regulatory regions of the LEC1, FUS3, and ABI3 genes22; and, FUS3 was identified as a putative ABI3 target.21
Figure 2.
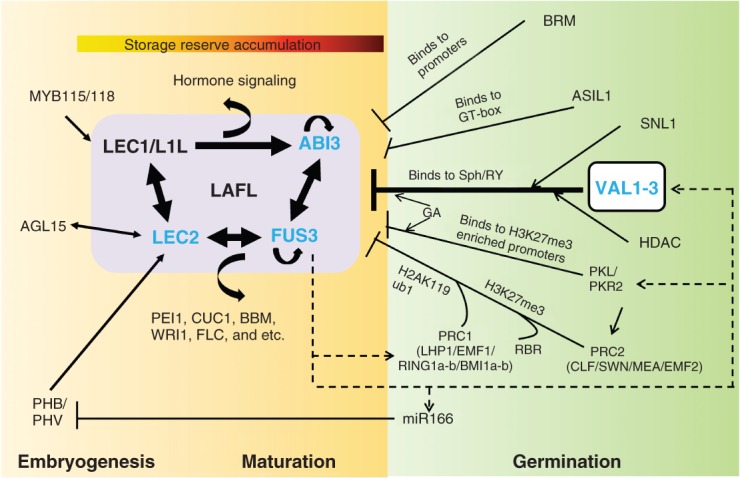
LAFL and VAL networks regulate the seed to seedling phase transition. Spatial and temporal patterns of LAFL gene expression are refined by mutual interactions.12–15 Important direct targets of LAFL factors include ( 1) SSP 12,16–18 and LEA genes,19,20 (2) transcription factor genes that control seed specific processes including PEI1,15,21 CUC1,22 BBM,22 WRI122–24 and FLC,15,25 and (3) genes that function in major hormone metabolism and signaling pathways.13,15,22,26–29 AGL15,30,31 PHB/PHV,32 and MYB115/118 33are proposed to act upstream of LAFL network. The LAFL network is repressed by VAL B3 factors and other repressors during seed germination to enable the transition to seedling development. VAL factors play a central role in repression of LAFL network in part by binding to the Sph/RY motif and recruiting an HDAC.10,11,34,35 The interaction of VAL B3 factors with HDAC may be mediated by the co-repressor SNL1.36 PRC2 proteins (CLF/SWN/MEA/EMF2) add H3K27me3 marks to LAFL genes.37,38 Acting in concert with PRC2, PRC1 proteins (LHP1/ EMF1/RING1a-b/BMI1a-b) bind to H3K27me3 marks and deposit H2AK119ub1 to maintain a stable repressed state of LAFL genes.37,39,40 The CHD3 chromatin remodeling factors, PKL and PKR2, can indirectly promote H3K27me3 modification by up-regulating genes encoding PRC2 proteins.41 PKL is also present in the promoter region of LEC1, LEC2 and FUS3 genes that are enriched for H3K27me3.42 RBR can interact with the promoter of ABI3, and is required for establishing H3K27me3 modification by cooperating with PRC2.43,44 The SNF2 chromatin remodeling ATPase, BRM, can repress seed maturation genes by physically interacting with their promoters.45,46 The plant-specific trihelix factor, ASIL1, contributes to repression of LAFL genes by binding to the GT element (CGTGATT).47 miR166 indirectly represses LEC2 transcription by targeting upstream PHB and PHV.32 GA signaling enhances VAL and PKL repression of the LAFL network.10,48 Negative feedback loops: LAFL factors (FUS3 and LEC1) up-regulate VAL1, RING1b, miR166, and PKL.22,24 Black lines with arrows indicate activation, and black lines ending with bars indicate repression. Inferred functions with less experimental evidence are indicated by dashed lines.
The LAFL transcription factor network regulates diverse seed-specific processes including deposition of storage reserves (starch, storage proteins, and lipids), acquisition of desiccation tolerance, developmental arrest of the embryo, and dormancy.12,14,16,49–51, Important direct targets of LAFL factors include (1) seed storage protein (SSP) and late-embryogenesis-abundant (LEA) genes, (2) genes encoding transcription factors that control lipid biosynthesis and other seed specific processes (Figure 2 and Table1), and (3) genes that function in hormone metabolism and signaling pathways (Table2).
Table 1.
Key Developmental Genes Regulated by the LAFL Network
| AGI Code | Gene Name | Protein Family | Cis-Element Enriched in LAFL Bound Promoter | Up-Regulated in val1 val2 | LEC1 | LEC2 | FUS3 | ABI3 | References | Potential Function (TAIR) |
|---|---|---|---|---|---|---|---|---|---|---|
| AT5G47670 | L1L | HAP3 | Sph/RY; CCAAT box | √ | √ | √ | 10,15,18,24,28 | Regulator of embryo development | ||
| AT1G28300 | LEC2 | B3 | Sph/RY | √ | √ | 10,12 | Plays critical roles during early and late embryo development | |||
| At3g26790 | FUS3 | B3 | Sph/RY | √ | √ | √ | √ | √ | 10,12–14,21,22,24 | Regulator of gene expression during late embryogenesis |
| AT3G24650 | ABI3 | B3 | Sph/RY; ABRE | √ | √ | √ | √ | √ | 10,12,14,22,24 | Regulator of the transition between embryo maturation and early seedling development |
| AT2G30470 | VAL1/HSI2 | B3 | Sph/RY | √ | 15,22 | Repression of seed maturation program during germination | ||||
| AT5G07500 | PEI1 | Zf | Sph/RY | √ | √ | √ | 10,15,21 | Required for heart-stage embryo formation | ||
| AT3G15170 | CUC1 | NAC | Sph/RY | √ | √ | 10,22 | Shoot apical meristem formation and auxin-mediated lateral root formation | |||
| AT5G17430 | BBM | AP2 | — | √ | √ | 10,22 | Promotes cell proliferation, differentiation and morphogenesis, especially during embryogenesis | |||
| AT3G54320 | WRI1 | AP2 | Sph/RY; ABRE | √ | √ | √ | √ | 10,15,22–24 | Control of lipid biosynthetic and metabolic processes | |
| AT5G10140 | FLC | MADS box | Sph/RY | √ | √ | 10,15 | A repressor of floral transition | |||
| AT5G13790 | AGL15 | MADS box | Sph/RY | √ | √ | √ | 10,15,22,26 | Embryonic and post embryonic development | ||
| AT3G27785 | MYB118 | MYB | — | √ | 21 | Regulates the embryonic pathway by up-regulating LEC1 | ||||
| AT1G03770 | RING1b | PRC1 | Sph/RY | √ | √ | 10,22 | Core component of Polycomb Repressive Complex1 (PRC1). Interacts physically with CLF and LHP1 and function together to repress target gene expression. | |||
| AT2G46685 | MIR166 | — | Sph/RY | √ | 22 | Encodes a microRNA that targets several HD-ZIPIII family members including PHV and PHB |
Table 2.
Overview of Hormone Pathway Genes Regulated by the LAFL Network
| Hormone | Pathway | AGI | Gene | LEC1 | LEC2 | FUS3 | Method | References |
|---|---|---|---|---|---|---|---|---|
| ABA | Biosynthesis | AT1G30100 | NCED5 | √ | Cc | 22 | ||
| AT3G24220 | NCED6 | √ | M | 15 | ||||
| AT1G78390 | NCED9 | √ | M | 15 | ||||
| AT1G52340 | ABA2 | √ | M | 15 | ||||
| Signaling | AT3G44460 | bZIP67 | √ | M | 15 | |||
| AT2G41070 | bZIP12/EEL | √ | √ | M, Cc, Q, E | 15,22,26 | |||
| AT1G42990 | bZIP60 | √ | Cc | 28 | ||||
| AT3G58120 | bZIP61 | √ | Cc | 22 | ||||
| GA | Biosynthesis | AT1G05160 | CYP88A3 | √ | M | 15 | ||
| AT1G80340 | GA3OX2 | √ | √ | Q,E | 27 | |||
| AT4G21690 | GA3OX3 | √ | Cc | 22 | ||||
| AT1G80330 | GA3OX4 | √ | M | 15 | ||||
| AT5G51810 | GA20OX2 | √ | M | 15 | ||||
| AT5G07200 | GA20OX3 | √ | M | 15 | ||||
| Catabolism | AT1G47990 | GA2OX4 | √ | Cc | 22 | |||
| AT5G56300 | GAMT2 | √ | Cc | 22 | ||||
| Auxin | Biosynthesis | AT4G13260 | YUC2 | √ | √ | M, C, Q | 13,15 | |
| AT5G11320 | YUC4 | √ | √ | M, C, Q | 13,15 | |||
| AT1G04180 | YUC9 | √ | Cc | 22 | ||||
| AT1G48910 | YUC10 | √ | √ | M, C, Q | 15,28 | |||
| AT5G20960 | AAO1 | √ | M | 15 | ||||
| AT3G44300 | NIT2 | √ | M | 15 | ||||
| Catabolism | AT5G55250 | IAMT1 | √ | Cc | 22 | |||
| AT1G44350 | ILL6 | √ | Cc | 22 | ||||
| Signaling | AT3G62980 | TIR1 | √ | Cc | 28 | |||
| AT5G62000 | ARF2 | √ | Cc | 22 | ||||
| AT1G30330 | ARF6 | √ | Cc | 22 | ||||
| AT1G19220 | ARF19 | √ | Cc | 22 | ||||
| AT4G14560 | IAA1 | √ | Q | 13 | ||||
| AT1G04550 | IAA12 | √ | Cc | 22 | ||||
| AT1G04250 | IAA17 | √ | √ | Cc, Q | 13,22 | |||
| AT3G04730 | IAA16 | √ | Cc | 28 | ||||
| AT3G15540 | IAA19 | √ | Cc, Q | 28 | ||||
| AT3G62100 | IAA30 | √ | M, Q | 26 | ||||
| AT3G17600 | IAA31 | √ | √ | M | 15,26 | |||
| BR | Biosynthesis | AT3G50660 | DWF4 | √ | Cc, Q | 28 | ||
| AT4G36380 | ROT3 | √ | M | 15 | ||||
| AT3G30180 | BR6OX2 | √ | M | 15 | ||||
| Catabolism | AT2G36800 | DOGT1 | √ | Cc, Q | 28 | |||
| Signaling | AT1G19350 | BES1 | √ | Cc, Q | 28 | |||
| AT3G61460 | BRH1 | √ | Cc, Q | 28 | ||||
| CK | Biosynthesis | AT1G68460 | IPT1 | √ | M | 15 | ||
| AT1G25410 | IPT6 | √ | M | 15 | ||||
| Catabolism | AT1G75450 | CKX5 | √ | Cc | 22 | |||
| Ethylene | Biosynthesis | AT1G01480 | ACS2 | √ | M | 15 | ||
| AT2G22810 | ACS4 | √ | Q | 13 | ||||
| AT4G11280 | ACS6 | √ | M, Q | 29 | ||||
| Signaling | AT4G17500 | ERF1 | √ | M, Q | 29 | |||
| AT5G47220 | ERF2 | √ | M | 29 | ||||
| AT1G28360 | ERF12 | √ | Cc | 22 | ||||
| AT5G61600 | ERF104 | √ | M | 29 | ||||
| AT1G25560 | EDF1 | √ | M, Q | 29 | ||||
| AT1G68840 | EDF2 | √ | M, Q | 29 | ||||
| AT1G13260 | EDF4 | √ | M, Q | 29 | ||||
| AT5G25190 | ESE3 | √ | M | 29 | ||||
| JA | Catabolism | AT1G19640 | JMT | √ | M | 15 | ||
| Signaling | AT1G19180 | JAZ1 | √ | Cc | 22 | |||
| AT1G72450 | JAZ6 | √ | Cc | 28 | ||||
Method: C, ChIP; Cc, ChIP-on-chip; M, Microarray; Q, Quantitative PCR; E, Electrophoretic mobility shift assay.
LAFL ACTIVATION OF SSP AND LEA GENES
Gene activation by AFL B3 factors is mediated by the Sph/RY cis-element (CATGCA) that is specifically recognized by the B3-DNA-binding domain.26,52–55 Ectopic expression of ABI3 or FUS3 in vegetative tissues causes activation of SSP genes, such as 2S albumin storage protein 3 (At2S3) and Cruciferin C (CRC).16,17 The LEC1 HAP3 factor activates CRC expression indirectly through regulation of AFL B3 factors,12 as well as via a direct interaction with the ABA-response element (ABRE) binding factor basic-leucine-zipper protein 67 (bZIP67).18 An important subset of LAFL regulated genes, including LEA genes, which have both Sph/RY and ABRE motifs in their promoters, are regulated by a combinatorial interaction between ABI3 and ABI5-related bZIP transcription factors.19,20 Hence, coupling of the LAFL network to ABA signaling is mediated by physical interaction of the N-terminal COAR (co-activator/co-repressor) domain of ABI3 with ABI5 and related bZIP factors. 19,20 ABREs are also found in the promoters of other target genes of LAFL factors (Table1), suggesting that other components of the LAFL network are potentially co-regulated by ABA.21,22,28 In addition, elegant studies in Phaseolus vulgaris have delineated the role of histone modifications in transcriptional activation of the phaseolin gene by ABI3 ortholog PVALF and ABA.56
LAFL ACTIVATION OF DOWNSTREAM TRANSCRIPTION FACTOR NETWORKS
Recent studies reveal that combinatorial interactions of LAFL factors up-regulate a diverse array of downstream transcription factor networks (Table1). These include Zinc finger (Zf) factor PEI1, NAC factor CUP-SHAPED COTYLEDON 1 (CUC1), APETALA2 (AP2) family factor BABY BOOM (BBM), and WRINKLED (WRI1). PEI1 is a potential direct target of ABI321 that is also up-regulated in response to FUS3 over-expression.15 CUC1, BBM, and WRI1 are identified as targets of FUS3.22 WRI1 is up-regulated by LEC124 and LEC2.23 As summarized in Table1, the downstream transcription factors in turn regulate critical pathways in seed development. Additional targets of FUS3 include FLOWERING LOCUS C (FLC),15 a key regulator of flowering and vegetative phase transition,25 as well as diverse NAC, MYB, bHLH, WRKY, bZIP, and Homebox family genes.22
LAFL REGULATION OF MULTIPLE HORMONE SIGNALING PATHWAYS
A key function of the LAFL network is re-programming of the major plant hormone signaling pathways in the seed. A set of target genes of LEC1, LEC2, and FUS3 that are implicated in ABA, GA, auxin, brassinosteroid (BR), cytokinin (CK), ethylene, and jasmonic acid (JA) metabolism and signaling pathways is summarized in Table 2.13,15,22,26–29 Table2 highlights the central role of FUS3 in coordinating developmental regulation of hormone signaling. For example, FUS3 establishes the critical balance between dormancy and seed germination inducing signals by simultaneously regulating biosynthesis and turnover of ABA and GA in the seed.27,49 While LEC2 also contributes to regulation of ABA, GA, and ethylene biosynthesis pathways13,26; LEC128 and LEC213,26 principally regulate auxin signaling through activation of YUCCA and IAA genes. By contrast, as noted above, ABI3 has a unique role in integrating ABA signaling with the LAFL network through interactions mediated by its N-terminal COAR domain with bZIP factors.19,20 Interestingly, LAFL factors also play a role in postembryonic plant development by coordinating hormone signaling networks. For instance, FUS3 was shown to regulate vegetative phase transitions (juvenile to adult phase) by controlling the ethylene-responsive gene expression.29 In addition, LEC1 was found to be involved in regulation of hypocotyl elongation-related functions by targeting genes in auxin, BR, and light signaling networks.28 Therefore, the LAFL network participates in integration of hormonal and intrinsic developmental signals during seed development and other developmental stages. The implications of LAFL regulation of CK and JA signaling pathways remain to be determined.
REGULATION OF THE LAFL NETWORK
Genes implicated in activation of the LAFL network early in seed development (Figure 2 and Table1) include the MADS-box factor AGAMOUS-LIKE15 (AGL15),30,31 HD-ZIPIII family factors PHABULOSA (PHB) and PHAVOLUTA (PHV),32 and MYB115/118.33 LEC1 and LEC2 are up-regulated in transgenic plants over-expressing of AGL15.30 Moreover, AFL B3 genes were identified as direct targets of AGL15.31 While these lines of evidence indicate that AGL15 acts upstream of the AFL B3 network, AGL15 is also regulated by LAFL factors. For example, AGL15 was identified as a direct target of FUS322 and its expression is induced by LEC2.26 LAFL factors are activated in vegetative tissues by over-expression of adaxial/abaxial polarity genes PHB and PHV, and PHB has been shown to physically associate with the LEC2 promoter.32 In addition, LEC1 is up-regulated by over-expression of MYB115 or MYB118.33 Interestingly, ectopic expression of ABI3 in transgenic seedlings also up-regulates MYB118 transcription in presence of ABA.21 These findings indicate that upstream regulators and LAFL factors mutually regulate each other. To varying degrees, ectopic expression of individual LAFL genes and upstream regulators can induce expression of embryonic traits in vegetative tissues.4–6,17,30,32,33,49
REPRESSION OF THE LAFL NETWORK DURING GERMINATION
Genetic studies show that repression of the LAFL embryonic pathway during germination is necessary to enable the transition to seedling development. Key pathways that maintain repression of the LAFL network in the embryo prior to its transition to seedling development are summarized in Figure 2. The corresponding mutants commonly display embryonic traits during vegetative development though with variable penetrance (Table3). In addition, the subset of genes in the LAFL network that are de-repressed differ among mutants (Table3). Genes implicated in direct repression of the LAFL network include the VAL B3 factors, chromatin modifiers, and trihelix factors (Table3), whereas, other mechanisms such as the miRNA (miR166) pathway most likely act indirectly via silencing of upstream regulator PHB and PHV.32
Table 3.
Mutants Causing Ectopic Embryonic Traits and Up-Regulation of LAFL Network during Vegetative Development
| Mutant or RNAi | Protein Family | Embryonic Trait | EC Penetrance | Up-Regulation of LAFL Factors | References |
|---|---|---|---|---|---|
| val1 val2 | B3 | EC1 and arrested growth | High | LAFL | 10,11 |
| HDA6/HDA19 RNAi | HDAC | ELS and arrested growth | n.a. | LEC1, FUS3, and ABI3 | 34 |
| clf swn | PcG | EC3 and arrested growth | No data | LEC1, LEC2 and FUS3 | 41,62 |
| Atring1a Atring1b | PcG | EC4 and arrested growth | intermediate | LAFL | 39 |
| Atbmi1a Atbmi1b | PcG | EC4 and arrested growth | Intermediate | LAFL | 39 |
| pkl | CHD3 | EC2 | Low | LEC1, LEC2, and FUS3 | 48,57 |
| pkl pkr2 | CHD3 | EC2 | Intermediate | LEC1, FUS3, ABI3 | 41 |
| RBR RNAi or RBR overexpression | RB | ECP and arrested growth | n.a. | ABI3 and LEC2 (induced by sucrose) | 43 |
| Brm | SWI/SNF | Arrested growth | n.a. | FUS3 | 46 |
| asil1 | Trihelix | Arrested growth | n.a | LAFL | 47 |
ELS, embryo-like structure; ECP, embryonic cell proliferation; Penetrance: low, 10–30%; intermediate, 30–70%; high, 70–100%. n.a., not applicable. Embryonic traits: EC, embryonic callus.
All mutants accumulate SSPs and lipids.
Shoot and root.
Primary root tip.
Shoot.
Cotyledon and root.
REPRESSION OF THE LAFL NETWORK BY VAL B3 DOMAIN FACTORS
Repression of the LAFL network is mediated by a family of VAL B3 domain factors that are closely related to the AFL B3 factors (Figures 1 and 2, Table3).10,11 No other mutants implicated in repression display full activation of LAFL network and the extent of embryonic seedling phenotypes observed in the val1 val2 mutant (Table3). GA signaling can enhance the repression of LAFL network by VAL factors.10 Although the DNA binding specificity of VAL B3 domain has not been directly determined, transcriptomics analyses of val mutants are consistent with the hypothesis that the VAL B3 domain binds the same Sph/RY motif recognized by the AFL B3 domain.10 In addition, VAL proteins contain conserved PHD-L (plant homeodomain-like) Zf, CW-Zf, and EAR [ethylene response factor (ERF)-associated repression] domains (Figure 1). The CW-Zf domain of VAL1 was shown to interact with the histone 3 lysine 4 trimethylation (H3K4me3) marks.58 Although the PHD domain has been shown to be H3K4me3 reader,59 the specificity of the divergent VAL PHD-L domain is not yet known. A mutation in VAL1 PHD-L domain leads to de-repression of seed-specific genes, including FUS3 and AGL15 confirming that the PHD-L domain has a critical role in VAL mediated transcriptional repression.60 EAR motifs mediate transcriptional repression through interacting with co-repressors, such as SIN3 (SWI-independent 3) and TOPLESS (TPL), to recruit a histone deacetylase complex (HDAC) to target genes.61 VAL1 was identified as a SIN3-LIKE 1 (SNL1) interacting protein in a yeast two-hybrid (Y2H) assay36; however, it is not yet confirmed that the EAR motif is necessary for this interaction. Many genes up-regulated in the val1 val2 double mutant are also identified as direct targets of LAFL factors (Table1), suggesting that VAL may directly target LAFL factors to shut off the network upon germination. This hypothesis is supported by a recent study showing that HDA19 interacts directly with the CW-Zf domain of VAL2 to repress expression of LEC1, LEC2, and other seed maturation genes.35 HDA6 and HDA19 were also shown to act redundantly to repress of ABI3, FUS3, and LEC1 expression in the leaf tissues (Table3).34 Hence, one possible mechanism underlying VAL B3-mediated transcriptional repression is that VAL proteins recruit an HDAC to target genes that contain Sph/RY-motifs recognized by the B3 domain and specific chromatin marks recognized by the PHD-L and CW-Zf domains.
REPRESSION OF THE LAFL NETWORK BY CHROMATIN MODIFICATIONS
Chromatin modifications are emerging as a key mechanism for maintaining repression of the LAFL network during vegetative development. At least three distinct interacting chromatin modification systems are implicated in repression of the LAFL network (Table3): (1) polycomb repressive complex 2 (PRC2), (2) polycomb repressive complex 1 (PRC1), and (3) CHD3 (chromodomain, helicase/ATPase, and DNA binding domain) and SWI/SNF (SWITCH/SUCROSE NONFERMENTING) families of chromatin remodeling factors.
PRC2 proteins [CURLY LEAF (CLF), SWINGER (SWN), and MEDEA (MEA)] add H3K27me3 marks at repressed loci.40 Consistent with PRC2 involvement in repression of LAFL network, LEC1, LEC2, FUS3, and ABI3 genes have H3K27me3 marks in vegetative tissues,37,38 and FUS3 has been identified as a direct target of MEA.62 However, the mechanisms for PRC2 recruitment to target loci remain unclear in plants. Recent work identifying a cis-element, repressive LEC2 element (RLE), that is required for H3K27me3 modification and transcriptional repression of LEC2 during vegetative growth,63 sheds new light on the mechanism of PRC2 recruitment. Other proteins that partner with PRC2 include RETINOBLASTOMA-RELATED PROTEIN (RBR) which interacts with the MULTICOPYSUPPRESSOR OF IRA1 (MSI1) component of PRC2.44 RBR interacts with the promoter of LAFL member ABI3, and is required for establishing H3K27me3 modification.43
Acting in concert with PRC2, PRC1 proteins including LIKE HETEROCHROMATIN PROTEIN1 (LHP1), EMBRYONIC FLOWER 1 (EMF1), RING1a-b and BMI1a-b recognize the H3K27me3 marks and induce histone 2A lysine 119 mono-ubiquitination (H2AK119ub1) to maintain a stable repressed state of target loci.37,39,40 Consistent with the up-regulation of LAFL genes observed in PRC1 mutants, LEC2, FUS3, and ABI3 were identified as direct targets of EMF1 in ChIP analyses.37
In addition to the PRC complexes, CHD3 and SWI/SNF families of chromatin remodeling ATPases encoded by the PICKLE (PKL), PICKLE-RELATED 2 (PKR2), and BRAHAM (BRM) genes, respectively, are implicated in repression of the LAFL network. Recent studies suggest that PKL regulation of LAFL genes is mediated by interaction with PRC2.41,42 For instance, during seed germination, PKL is bound to the promoter regions of LEC1, LEC2, and FUS3 genes that are enriched for H3K27me3 modification.42 In addition, PKL and PKR2 may indirectly promote H3K27me3 modification at target loci by controlling the expression of PRC2 genes including EMF2, CLF, and SWN.41 BRM in turn contributes to repression of FUS346 and ABA-response factor ABI545 in leaf tissues where it physically interacts with target promoters.
Other potential players include a plant specific trihelix factor, ARABIDOPSIS 6b-INTERACTING PROTEIN LIKE1 (ASIL1)47, which binds to a GT cis-element (CGTGATT) found in promoters of LAFL genes where it frequently overlaps ABRE and Sph/RY elements recognized by bZIP and B3 proteins, respectively.
While the VAL B3 proteins evidently play a central role in mediating repression of the LAFL network during germination through recruitment of an HDAC; it is still elusive how VALs physically and functionally interact with other chromatin modification pathways. Interestingly, VAL1, RING1b, and miR166 were shown to be direct targets of FUS3,22 and PKL expression is enhanced when LEC1 is over-expressed,24 which suggest that LAFL factors (mainly FUS3) have a role in controlling the feedback regulation of the network (Table1 and Figure 2). Consistent with this hypothesis, PKR2 and RING1b are up-regulated in val1 val2 seedlings.10
CONCLUSION
Recent findings advance our understanding of the role of LAFL network in integrating the complex hormonal and intrinsic developmental signals that control seed development. While the resulting seed is superbly adapted for enabling propagation of the seed plants in diverse environments, a massive reprogramming of the transcriptome and attendant hormone signaling pathways is evidently required before the plant can resume vegetative development. We propose that repression is initiated by recruitment of an HDAC to genes that contain a combination of active chromatin marks recognized by PHD-L and CW-Zf domains and the Sph/RY motif recognized by the B3-DNA-binding domain. However, key predictions of this model including the binding specificities of the VAL B3 and PHD-L domains remain to be tested.
Acknowledgments
Our work was supported by grants from the National Science Foundation (Grant DBI: 1116561) and U.S. Department of Agriculture (Grant 2011-67013-30082).
REFERENCES
- 1.McCarty DR, Hattori T, Carson CB, Vasil V, Lazar M, Vasil IK. The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell. 1991;66:895–905. doi: 10.1016/0092-8674(91)90436-3. [DOI] [PubMed] [Google Scholar]
- 2.Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992;4:1251–1261. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luerssen H, Kirik V, Herrmann P, Miséra S. FUSCA3 encodes a protein with a conserved VP1/AB13-like B3 domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. Plant J. 1998;15:755–764. doi: 10.1046/j.1365-313x.1998.00259.x. [DOI] [PubMed] [Google Scholar]
- 4.Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci U S A. 2001;98:11806–11811. doi: 10.1073/pnas.201413498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lotan T, Ohto M, Yee KM, West MA, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998;93:1195–1205. doi: 10.1016/s0092-8674(00)81463-4. [DOI] [PubMed] [Google Scholar]
- 6.Kwong RW, Bui AQ, Lee H, Kwong LW, Fischer RL, Goldberg RB, Harada JJ. LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. Plant Cell. 2003;15:5–18. doi: 10.1105/tpc.006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez L, Van Wuytswinkel O, Castelain M, Bellini C. Combined networks regulating seed maturation. Trends Plant Sci. 2007;12:294–300. doi: 10.1016/j.tplants.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Santos-Mendoza M, Dubreucq B, Baud S, Parcy F, Caboche M, Lepiniec L. Deciphering gene regulatory networks that control seed development and maturation in Arabidopsis. Plant J. 2008;54:608–620. doi: 10.1111/j.1365-313X.2008.03461.x. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, McCarty DR. Functional symmetry of the B3 network controlling seed development. Curr Opin Plant Biol. 2008;11:548–553. doi: 10.1016/j.pbi.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M, Wang HH, McCarty DR. Repression of the LEAFY COTYLEDON1/B3 regulatory network in plant embryo development by VP1/ABSCISIC ACID INSENSITIVE3-LIKE B3 genes. Plant Physiol. 2007;143:902–911. doi: 10.1104/pp.106.092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukagoshi H, Morikami A, Nakamura K. Two B3 domain transcriptional repressors prevent sugar-inducible expression of seed maturation genes in Arabidopsis seedlings. Proc Natl Acad Sci U S A. 2007;104:2543–2547. doi: 10.1073/pnas.0607940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagaya Y, Toyoshima R, Okuda R, Usui H, Yamamoto A, Hattori T. LEAFY COTYLEDON1 controls seed storage protein genes through its regulation of FUSCA3 and ABSCISIC ACID INSENSITIVE3. Plant Cell Physiol. 2005;46:399–406. doi: 10.1093/pcp/pci048. [DOI] [PubMed] [Google Scholar]
- 13.Stone SL, Braybrook SA, Paula SL, Kwong LW, Meuser J, Pelletier J, Hsieh TF, Fischer RL, Goldberg RB, Harada JJ. Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: implications for somatic embryogenesis. Proc Natl Acad Sci U S A. 2008;105:3151–3156. doi: 10.1073/pnas.0712364105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.To A, Valon C, Savino G, Guilleminot J, Devic M, Giraudat J, Parcy F. A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell. 2006;18:1642–1651. doi: 10.1105/tpc.105.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto A, Kagaya Y, Usui H, Hobo T, Takeda S, Hattori T. Diverse roles and mechanisms of gene regulation by the Arabidopsis seed maturation master regulator FUS3 revealed by microarray analysis. Plant Cell Physiol. 2010;51:2031–2046. doi: 10.1093/pcp/pcq162. [DOI] [PubMed] [Google Scholar]
- 16.Kagaya Y, Okuda R, Ban A, Toyoshima R, Tsutsumida K, Usui H, Yamamoto A, Hattori T. Indirect ABA-dependent regulation of seed storage protein genes by FUSCA3 transcription factor in Arabidopsis. Plant Cell Physiol. 2005;46:300–311. doi: 10.1093/pcp/pci031. [DOI] [PubMed] [Google Scholar]
- 17.Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J. Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell. 1994;6:1567–1582. doi: 10.1105/tpc.6.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto A, Kagaya Y, Toyoshima R, Kagaya M, Takeda S, Hattori T. Arabidopsis NF-YB subunits LEC1 and LEC1-LIKE activate transcription by interacting with seed-specific ABRE-binding factors. Plant J. 2009;58:843–856. doi: 10.1111/j.1365-313X.2009.03817.x. [DOI] [PubMed] [Google Scholar]
- 19.Alonso R, Oñate-Sánchez L, Weltmeier F, Ehlert A, Diaz I, Dietrich K, Vicente-Carbajosa J, Dröge-Laser W. A pivotal role of the basic leucine zipper transcription factor bZIP53 in the regulation of Arabidopsis seed maturation gene expression based on heterodimerization and protein complex formation. Plant Cell. 2009;21:1747–1761. doi: 10.1105/tpc.108.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura S, Lynch TJ, Finkelstein RR. Physical interactions between ABA response loci of Arabidopsis. Plant J. 2001;26:627–635. doi: 10.1046/j.1365-313x.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 21.Mönke G, Seifert M, Keilwagen J, Mohr M, Grosse I, Hähnel U, Junker A, Weisshaar B, Conrad U, Bäumlein H, et al. Toward the identification and regulation of the Arabidopsis thaliana ABI3 regulon. Nucleic Acids Res. 2012;40:8240–8254. doi: 10.1093/nar/gks594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Perry SE. Identification of direct targets of FUSCA3, a key regulator of Arabidopsis seed development. Plant Physiol. 2013;161:1251–1264. doi: 10.1104/pp.112.212282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baud S, Mendoza MS, To A, Harscoët E, Lepiniec L, Dubreucq B. WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J. 2007;50:825–838. doi: 10.1111/j.1365-313X.2007.03092.x. [DOI] [PubMed] [Google Scholar]
- 24.Mu J, Tan H, Zheng Q, Fu F, Liang Y, Zhang J, Yang X, Wang T, Chong K, Wang XJ, et al. LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol. 2008;148:1042–1054. doi: 10.1104/pp.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng W, Ying H, Helliwell CA, Taylor JM, Peacock WJ, Dennis ES. FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc Natl Acad Sci U S A. 2011;126:6680–6685. doi: 10.1073/pnas.1103175108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braybrook SA, Stone SL, Park S, Bui AQ, Le BH, Fischer RL, Goldberg RB, Harada JJ. Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc Natl Acad Sci U S A. 2006;103:3468–3473. doi: 10.1073/pnas.0511331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curaba J, Moritz T, Blervaque R, Parcy F, Raz V, Herzog M, Vachon G. AtGA3ox2, a key gene responsible for bioactive gibberellin biosynthesis, is regulated during embryogenesis by LEAFY COTYLEDON2 and FUSCA3 in Arabidopsis. Plant Physiol. 2004;136:3660–3669. doi: 10.1104/pp.104.047266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junker A, Mönke G, Rutten T, Keilwagen J, Seifert M, Thi TM, Renou JP, Balzergue S, Viehöver P, Hähnel U, et al. Elongation-related functions of LEAFY COTYLEDON1 during the development of Arabidopsis thaliana. Plant J. 2012;71:427–442. doi: 10.1111/j.1365-313X.2012.04999.x. [DOI] [PubMed] [Google Scholar]
- 29.Lumba S, Tsuchiya Y, Delmas F, Hezky J, Provart NJ, Lu Q, McCourt P, Gazzarrini S. The embryonic leaf identity gene FUSCA3 regulates vegetative phase transitions by negatively modulating ethylene-regulated gene expression in Arabidopsis. BMC Biol. 2012;10:8. doi: 10.1186/1741-7007-10-8. 10.1186/1741-7007-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE. Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15. Plant Physiol. 2003;133:653–663. doi: 10.1104/pp.103.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y, Ren N, Wang H, Stromberg AJ, Perry SE. Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-Like15. Plant Cell. 2009;21:2563–2577. doi: 10.1105/tpc.109.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang X, Bian S, Tang M, Lu Q, Li S, Liu X, Tian G, Nguyen V, Tsang EW, Wang A, et al. MicroRNA-mediated repression of the seed maturation program during vegetative development in Arabidopsis. PLoS Genet. 2012;8:e1003091. doi: 10.1371/journal.pgen.1003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Niu Q, Teng C, Li C, Mu J, Chua NH, Zuo J. Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Res. 2009;19:224–235. doi: 10.1038/cr.2008.276. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, Kikuchi A, Kamada H. The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination. Plant Physiol. 2008;146:149–161. doi: 10.1104/pp.107.111674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Tan B, Luo M, Li Y, Liu C, Chen C, Yu C, Yang S, Dong S, Ruan J, et al. HISTONE DEACETYLASE19 interacts with HSL1 and participates in the repression of seed maturation genes in Arabidopsis seedlings. Plant Cell. 2013;25:134–148. doi: 10.1105/tpc.112.096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowen AJ, Gonzalez D, Mullins JG, Bhatt AM, Martinez A, Conlan RS. PAH-domain-specific interactions of the Arabidopsis transcription coregulator SIN3-like1 (SNL1) with telomere-binding protein 1 and always early2 Myb-DNA binding factors. J Mol Biol. 2010;395:937–949. doi: 10.1016/j.jmb.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 37.Kim SY, Lee J, Eshed-Williams L, Zilberman D, Sung ZR. EMF1 and PRC2 cooperate to repress key regulators of Arabidopsis development. PLoS Genet. 2012;8:e1002512. doi: 10.1371/journal.pgen.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Clarenz O, Cokus S, Bernatavichute YV, Pellegrini M, Goodrich J, Jacobsen SE. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. Plos Biol. 2007;5:1026–1035. doi: 10.1371/journal.pbio.0050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen D, Molitor A, Liu C, Shen WH. The Arabidopsis PRC1-like ring-finger proteins are necessary for repression of embryonic traits during vegetative growth. Cell Res. 2010;20:1332–1344. doi: 10.1038/cr.2010.151. [DOI] [PubMed] [Google Scholar]
- 40.Holec S, Berger F. Polycomb group complexes mediate developmental transitions in plants. Plant Physiol. 2012;158:35–43. doi: 10.1104/pp.111.186445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aichinger E, Villar CB, Farrona S, Reyes JC, Hennig L, Köhler C. CHD3 proteins and polycomb group proteins antagonistically determine cell identity in Arabidopsis. PLoS Genet. 2009;5:e1000605. doi: 10.1371/journal.pgen.1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Bishop B, Ringenberg W, Muir WM, Ogas J. The CHD3 remodeler PICKLE associates with genes enriched for trimethylation of histone H3 lysine 27. Plant Physiol. 2012;159:418–432. doi: 10.1104/pp.112.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutzat R, Borghi L, Fütterer J, Bischof S, Laizet Y, Hennig L, Feil R, Lunn J, Gruissem W. Retinoblastoma-related protein controls the transition to autotrophic plant development. Development. 2011;138:2977–2986. doi: 10.1242/dev.060830. [DOI] [PubMed] [Google Scholar]
- 44.Jullien PE, Mosquna A, Ingouff M, Sakata T, Ohad N, Berger F. Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PloS Biol. 2008;6:1693–1705. doi: 10.1371/journal.pbio.0060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han SK, Sang Y, Rodrigues A BIOL425 F2010. Wu MF, Rodriguez PL, Wagner D. The SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA Represses Abscisic Acid Responses in the Absence of the Stress Stimulus in Arabidopsis. Plant Cell. 2012;24:4892–4906. doi: 10.1105/tpc.112.105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang X, Hou A, Babu M, Nguyen V, Hurtado L, Lu Q, Reyes JC, Wang A, Keller WA, Harada JJ, et al. The Arabidopsis BRAHMA chromatin-remodeling ATPase is involved in repression of seed maturation genes in leaves. Plant Physiol. 2008;147:1143–1157. doi: 10.1104/pp.108.121996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao MJ, Lydiate DJ, Li X, Lui H, Gjetvaj B, Hegedus DD, Rozwadowski K. Repression of seed maturation genes by a trihelix transcriptional repressor in Arabidopsis seedlings. Plant Cell. 2009;21:54–71. doi: 10.1105/tpc.108.061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogas J, Cheng JC, Sung ZR, Somerville C. Cellular differentiation regulated by gibberellin in the Arabidopsis thaliana pickle mutant. Science. 1997;277:91–94. doi: 10.1126/science.277.5322.91. [DOI] [PubMed] [Google Scholar]
- 49.Gazzarrini S, Tsuchiya Y, Lumba S, Okamoto M, McCourt P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev Cell. 2004;7:373–385. doi: 10.1016/j.devcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 50.Parcy F, Giraudat J. Interactions between the ABI1 and the ectopically expressed ABI3 genes in controlling abscisic acid responses in Arabidopsis vegetative tissues. Plant J. 1997;11:693–702. doi: 10.1046/j.1365-313x.1997.11040693.x. [DOI] [PubMed] [Google Scholar]
- 51.Raz V, Bergervoet JH, Koornneef M. Sequential steps for developmental arrest in Arabidopsis seeds. Development. 2001;128:243–252. doi: 10.1242/dev.128.2.243. [DOI] [PubMed] [Google Scholar]
- 52.Kroj T, Savino G, Valon C, Giraudat J, Parcy F. Regulation of storage protein gene expression in Arabidopsis. Development. 2003;130:6065–6073. doi: 10.1242/dev.00814. [DOI] [PubMed] [Google Scholar]
- 53.Mönke G, Altschmied L, Tewes A, Reidt W, Mock HP, Bäumlein H, Conrad U. Seed-specific transcription factors ABI3 and FUS3: molecular interaction with DNA. Planta. 2004;219:158–166. doi: 10.1007/s00425-004-1206-9. [DOI] [PubMed] [Google Scholar]
- 54.Reidt W, Wohlfarth T, Ellerström M, Czihal A, Tewes A, Ezcurra I, Rask L, Bäumlein H. Gene regulation during late embryogenesis: the RY motif of maturation-specific gene promoters is a direct target of the FUS3 gene product. Plant J. 2000;21:401–408. doi: 10.1046/j.1365-313x.2000.00686.x. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki M, Kao CY, McCarty DR. The conserved B3 domain of VIVIPAROUS1 has a cooperative DNA binding activity. Plant Cell. 1997;9:799–807. doi: 10.1105/tpc.9.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ng DW, Chandrasekharan MB, Hall TC. Ordered histone modifications are associated with transcriptional poising and activation of the phaseolin promoter. Plant Cell. 2006;18:119–132. doi: 10.1105/tpc.105.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rider SD, Henderson JT, Jerome RE, Edenberg HJ, Romero-Severson J, Ogas J. Coordinate repression of regulators of embryonic identity by PICKLE during germination in Arabidopsis. Plant J. 2003;35:33–43. doi: 10.1046/j.1365-313x.2003.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoppmann V, Thorstensen T, Kristiansen PE, Veiseth SV, Rahman MA, Finne K, Aalen RB, Aasland R. The CW domain, a new histone recognition module in chromatin proteins. EMBO J. 2011;30:1939–1952. doi: 10.1038/emboj.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H, Ilin S, Wang W, Duncan EM, Wysocka J, Allis CD, Patel DJ. Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature. 2006;442:91–95. doi: 10.1038/nature04802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veerappan V, Wang J, Kang M, Lee J, Tang Y, Jha AK, Shi H, Palanivelu R, Allen RD. A novel HSI2 mutation in Arabidopsis affects the PHD-like domain and leads to derepression of seed-specific gene expression. Planta. 2012;236:1–17. doi: 10.1007/s00425-012-1630-1. [DOI] [PubMed] [Google Scholar]
- 61.Kagale S, Rozwadowski K. EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics. 2011;6:141–146. doi: 10.4161/epi.6.2.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makarevich G, Leroy O, Akinci U, Schubert D, Clarenz O, Goodrich J, Grossniklaus U, Köhler C. Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep. 2006;7:947–952. doi: 10.1038/sj.embor.7400760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berger N, Dubreucq B, Roudier F, Dubos C, Lepiniec L. Transcriptional regulation of Arabidopsis LEAFY COTYLEDON2 involves RLE, a cis-element that regulates trimethylation of histone H3 at lysine-27. Plant Cell. 2011;23:4065–4078. doi: 10.1105/tpc.111.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]


