Abstract
Background
Clinical trials and national performance measures increasingly mandate reporting patients’ perspectives of their health status: their symptoms, function and quality of life. While the Seattle Angina Questionnaire (SAQ) is a validated disease-specific health status instrument for coronary artery disease (CAD) with high test-retest reliability, predictive power, and responsiveness, its use in routine clinical practice has been limited, in part, by its length (19 items).
Methods and Results
Using data from 10,408 patients with CAD from 5 multi-center registries, we derived and validated a shortened version of the SAQ (SAQ-7) among patients presenting with stable CAD, undergoing percutaneous coronary intervention (PCI), and after acute myocardial infarction (AMI). We examined the psychometric properties of the SAQ-7, as compared with the full SAQ. Seven items from the Physical Limitations, Angina Frequency, and Quality of Life domains were identified for the SAQ-7, with high levels of concordance (0.88–1.00) with each original SAQ domain. The SAQ-7 demonstrated good construct validity (compared with Canadian Cardiovascular Society class for angina), with an correlation of 0.62 and 0.38 for patients with stable CAD and undergoing PCI, respectively. It was highly reproducible in patients with stable CAD (intra-class correlation of ≥0.78) and exhibited excellent responsiveness in patients after PCI (≥18 points in each SAQ domain). Finally, the SAQ-7 was predictive of 1-year mortality and readmission.
Conclusion
To increase the feasibility of measuring patient-reported outcomes in patients with CAD, we developed and validated a shortened 7-item SAQ instrument for use in clinical trials and routine care.
Keywords: Health status, Health-related quality of life, Health services research, Coronary artery disease, Health status instrument
INTRODUCTION
Accurately quantifying patients’ perspectives about the impact of their coronary artery disease (CAD) on their health status (i.e., symptoms, function and quality of life) has become increasingly important as an outcome in clinical trials, as well as in quality assessment and clinical care.1 The Seattle Angina Questionnaire (SAQ), a commonly-used instrument for measuring health status in patients with CAD, has been frequently used as an outcome in clinical trials, and has been endorsed as a performance measure for assessing the quality of CAD care.2 The use of the SAQ in quality assessment and clinical care, however, has been limited because of its length (19 questions) and the absence of a single summary score that facilitates an overall assessment of patients’ health status.3
Given the importance of being able to accurately and objectively assess patients’ health status and prognosis using a low-cost, non-invasive strategy, we sought to develop a shorter version of the SAQ that preserves the full instrument’s psychometric and prognostic properties. Shortening the SAQ directly addresses recently articulated challenges that hinder the routine use of patient-reported outcomes in clinical care.4 This report describes the development and validation of the SAQ-7 and its summary score, including its psychometric (validity, reliability, and responsiveness to change) and prognostic properties.
METHODS
The Seattle Angina Questionnaire
This SAQ quantifies 5 domains measuring the impact of angina on patients’ health status: Physical Limitation (9 items), Angina Stability (1 item), Angina Frequency (2 items), Treatment Satisfaction (4 items), and Quality of Life (3 items). Item responses are coded sequentially from worst to best status and range from 1 to 6 for Physical Limitation, Angina Stability and Angina Frequency items; 1 to 5/6 for Treatment Satisfaction items; and 1 to 5 for Quality of Life items. Scores are generated for each domain and are scaled 0–100, with 0 denoting the worst and 100 the best possible status. The SAQ has been shown to be valid, reproducible, and sensitive to clinical change.5 Moreover, patients’ SAQ scores have been found to be independently prognostic of subsequent mortality, hospitalization, and resource use.6–8
Data Sources
We used data from five longitudinal cohort studies of CAD patients for the development and validation of a shortened SAQ, all of which underwent institutional review board review and approval at each participating site: (1) the 24-center Translational Research Investigating Underlying disparities in acute Myocardial infarction (MI) Patients’ Health status (TRIUMPH) study9; (2) the 19-center Prospective Registry Evaluating outcomes after Myocardial Infarctions: Events and Recovery (PREMIER) study10; (3) the 6-center Patient Risk Information Services Manager (PRISM) study of patients undergoing percutaneous coronary intervention (PCI); (4) the 3-center Outcomes of PCI Study (OPS); and (5) the single-center PRESS study of patients undergoing revascularization.11 Descriptions of the studies and the timing of SAQ assessments are provided in Table 1. Using these studies, we derived and validated the short SAQ within three distinct clinical settings: (1) stable CAD, (2) elective PCI and (3) acute MI. Within each setting, derivation and validation analyses were performed in separate independent samples and the performance of a new summary score was assessed.
Table 1.
Data Sources
| Study | Patient Population | Enrollment Time Frame | Sample Size | Time of SAQ Assessments |
|---|---|---|---|---|
| OPS | 3-center prospective registry of PCI | 2009–2011 | 1,901 | Baseline, 6, 12 months |
| PRISM | 6-center prospective registry of PCI | 2010–2011 | 1,398 | Baseline, 1, 6, 12 months |
| PRESS | Single center prospective registry of PCI | 1999 | 255 | Baseline, 1, 2, 3, 4, 5, 6 months |
| PREMIER | 19-center prospective registry of Acute MI | 2003–2004 | 2,498 | Baseline, 1, 6, 12 months |
| TRIUMPH | 23-center prospective registry of Acute MI | 2005–2008 | 4,340 | Baseline, 1, 6, 12 months |
Abbreviations: MI, Myocardial Infarction; OPS, Outcomes of PCI regiStry; PCI, Percutaneous Coronary Intervention; PREMIER, Prospective Registry Evaluating outcomes after Myocardial Infarction: Events and Recovery; PRESS, Post-Revascularization rEcovery and Survival Study; PRISM, Patient Risk Information Services Manager; SAQ, Seattle Angina Questionnaire; TRIUMPH, Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status
Derivation
In deriving the short SAQ, we restricted consideration to the three domains that directly measure patients’ current health status: Physical Limitation, Angina Frequency, and Quality of Life. Within each of these domains, we examined how closely each item tracked with its respective score. An explicit goal of this analysis was to maximize comparability between short- and full-version SAQ scores. Since the scores are essentially equivalent to unweighted averages of the item responses, we examined the concordance between each item and its domain score rather than the simple correlation. To accomplish this, we first rescaled the item responses to 0–100, to match the scale of the score. We then calculated Lin’s concordance correlation coefficient, which measures the agreement between two variables.12 Values range from −1 (perfect negative agreement) to 1 (perfect positive agreement), with 0 denoting no agreement. Items with higher concordance coefficients were preferred. In cases where items demonstrated similar concordance rates, the clinical importance, response variability, and non-response rates of an item question were also considered in determining which item questions were retained in the shortened SAQ. For the Physical Limitation scale, which covers low, moderate and high intensity activities (3 items in each level), item selection was performed separately within each level, in order to preserve the range of activities covered in the full scale. Analyses were repeated for each of the three clinical settings described above (stable CAD, elective PCI, acute MI), and the items identified within each setting were combined to arrive at a final short version of the SAQ.
Once the final set of items was identified, scores for each of the three domains were calculated using methodology analogous to that of the full SAQ, so that scores ranged from 0 to 100 for each domain. In addition, an overall summary SAQ score was derived as the average of the three domain scores. A summary score was also derived for the full SAQ using the same three domains, and the psychometric properties of the new summary score were calculated as for each scale of the short SAQ.
Validation
Within each of the three clinical settings, we conducted a series of analyses in independent samples to evaluate construct validity, reproducibility, responsiveness, and predictive validity of the short SAQ and summary scores. Parallel analyses were conducted for the full SAQ, which served as the gold standard for comparison. The specific clinical settings, studies and assessments used for each analysis are described in Table 2.
Table 2.
Analyses, Settings and Cohorts Used for Deriving and Validating the SAQ-7
| Objective | Analyses Performed | Clinical Settings Analyzed (Study, Assessment) |
|---|---|---|
| Item selection | Mean ± SD | Stable CAD (PRISM, Month 12) |
| Percent missing | PCI (PRISM, Baseline) | |
| Item-score concordance | Acute MI (TRIUMPH, Baseline) | |
|
| ||
| Score validation | ||
|
| ||
| Descriptive statistics | Mean ± SD | Stable CAD (OPS, Month 12) |
| Percent missing | PCI (OPS, Baseline) | |
| Acute MI (PREMIER, Baseline) | ||
|
| ||
| Construct validity | Concordance with full SAQ (concordance correlation coefficient; percent concordance; kappa statistic) | Stable CAD (PREMIER, Month 12) |
| PCI (OPS, Baseline) | ||
| Acute MI (PREMIER, Baseline) | ||
|
| ||
| Association of SAQ summary score with CCS class (mean ± SD; R2) | Stable CAD (PREMIER, Month 12) | |
| PCI (OPS, Baseline) | ||
|
| ||
| Predictive validity | 6-month mortality, 6-month ACS hospitalization (c-statistics) | Post-MI (TRIUMPH, Month 1) |
|
| ||
| Reproducibility | 1-month change in stable patients (mean ± SD; intra-class correlation) | Stable CAD (PRESS, Months 5–6) |
|
| ||
| Responsiveness | 1-month change post-PCI (mean ± SD; standardized response mean) | PCI (PRISM, Baseline-Month 1) |
Abbreviations: ACS, Acute Coronary Syndrome; CAD, Coronary Artery Disease; CCS, Canadian Cardiovascular Society; MI, Myocardial Infarction; OPS, Outcomes of PCI regiStry; PCI, Percutaneous Coronary Intervention; PREMIER, Prospective Registry Evaluating outcomes after Myocardial Infarction: Events and Recovery; PRESS, Post-Revascularization rEcovery and Survival Study; PRISM, Patient Risk Information Services Manager; SAQ, Seattle Angina Questionnaire; SD, Standard Deviation; TRIUMPH, Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status
Construct Validity
To evaluate construct validity, we first compared each of the short SAQ scores with their respective score from the full SAQ. Means and standard deviations of scores, mean and standard deviation of differences and concordance coefficients, as described above, are reported. In addition, among stable CAD and PCI patients, we calculated mean SAQ summary scores by Canadian Cardiovascular Society (CCS) angina class 0 through IV and estimated the association between SAQ score and CCS class using Kendall’s tau-b rank correlation coefficient.
Reproducibility
Reproducibility of the short SAQ was assessed by comparing serial scores in stable patients. For this analysis, we compared scores at 5 and 6 months post-PCI among PRESS study patients who had stable CAD, a period where patients’ health status is presumed to be stable. To further confirm stability of patients’ clinical status, we also required that patients in this analysis had had no intervening coronary revascularization events and reported (by the SAQ Angina Stability scale, which is not part of the SAQ-7 or the Summary Score and asks patients about recent changes in their angina, at 6 months) that they had no change in angina symptoms over the past 4 weeks. In this cohort, we calculated the mean and standard deviation of change scores, and intraclass correlations (ICCs). The ICC denotes the proportion of variability in scores due to between-patient (vs. within-patient) differences. ICCs greater than 0.4, 0.6 and 0.8 indicate moderate, substantial and excellent reproducibility.13
Responsiveness
The responsiveness of the short SAQ to clinical change was quantified by the change from baseline to 1 month following PCI in the PRISM study, a period when substantial improvements in patients’ health status is anticipated. We calculated the mean and standard deviation of change as well as the standardized response mean (SRM), which is defined as the mean change divided by the standard deviation of change. SRMs above 0.5 and 0.8 indicate moderate and strong responsiveness, respectively.13
Predictive Validity
Predictive validity was assessed by comparing 12-month outcomes of mortality and acute coronary syndrome (ACS) hospitalization among post-acute MI patients within the TRIUMPH study. Mortality was assessed via query of the Social Security Administration Death Master File, and ACS hospitalizations were determined via physician panel adjudication of patient-reported hospital visits. In these analyses, we used patients’ 1-month assessment following MI hospitalization as “time zero”, to measure prognosis after a patient’s health status has stabilized after the acute event. Cumulative 12-month incidence was calculated using Kaplan-Meier methods within predefined score categories of 0 to <50 (poor to fair), 50 to <75 (good) and 75 to 100 (excellent) for the Physical Limitation, Quality of Life, and Summary scores; and categories of 0 to 60 (daily to weekly angina), >60 to <100 (monthly angina) and 100 (no angina) for the Angina Frequency score.8 Score discrimination was evaluated by c-statistics from proportional hazards regression models.
RESULTS
Across the 5 registries represented in these analyses, SAQ data was available on 10,408 patients. Descriptions of the studies, patient populations, and timing of SAQ assessments are listed in Table 1, and a summary of patient characteristics for the derivation and validation cohorts is provided in Supplementary Appendix Table 1.
Derivation
Item selection was conducted within independent samples representing each of the three clinical settings: stable CAD (PRISM 12-month assessment, N=975), elective PCI (PRISM baseline assessment, N=1,116) and acute MI (TRIUMPH baseline assessment, N=4,340). Item response means and standard deviations, missing rates, and item-score concordance coefficients within each of these settings are outlined in Table 3. In general, stable CAD patients had few limitations, minimal symptoms and good quality of life; in comparison, acute MI patients had slightly more symptoms and worse quality of life, and PCI patients had the worst health status across the three domains. Non-response rates were minimal for all scales across all settings.
Table 3.
Item Descriptive Statistics and Concordance with Domain Scores
| Stable CAD (N=975) |
PCI (N=1,116) |
Acute MI (N=4,340) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean ± SD* |
Non- Response |
Concordance with Score |
Mean ± SD* |
Non- Response |
Concordance with Score |
Mean ± SD* |
Non- Response |
Concordance with Score |
|
| Physical Limitation | |||||||||
| Low intensity | |||||||||
| 1a. Dressing | 5.0 ± 0.3 | 0.1% | 0.35 | 4.7 ± 0.8 | 0.4% | 0.44 | 4.8 ± 0.6 | 0.6% | 0.48 |
| 1b. Walking indoors | 4.9 ± 0.5 | 0.1% | 0.67 | 4.4 ± 1.0 | 0.4% | 0.64 | 4.7 ± 0.8 | 0.2% | 0.68 |
| 1c. Showering | 5.0 ± 0.4 | 0.1% | 0.45 | 4.7 ± 0.8 | 0.4% | 0.44 | 4.8 ± 0.6 | 0.4% | 0.51 |
| Moderate intensity | |||||||||
| 1d. Climbing | 4.8 ± 0.7 | 0.1% | 0.84 | 3.7 ± 1.4 | 0.7% | 0.78 | 4.2 ± 1.2 | 0.4% | 0.85 |
| 1e. Daily tasks | 4.9 ± 0.5 | 0.4% | 0.89 | 4.0 ± 1.3 | 0.5% | 0.84 | 4.5 ± 1.1 | 0.6% | 0.87 |
| 1f. Walking briskly | 4.8 ± 0.8 | 0.3% | 0.75 | 3.6 ± 1.4 | 0.3% | 0.76 | 4.2 ± 1.3 | 0.6% | 0.84 |
| High intensity | |||||||||
| 1g. Running or jogging | 4.6 ± 1.0 | 0.0% | 0.79 | 3.3 ± 1.7 | 0.4% | 0.70 | 3.8 ± 1.6 | 0.5% | 0.78 |
| 1h. Lifting or moving | 4.8 ± 0.7 | 0.3% | 0.78 | 3.7 ± 1.5 | 0.6% | 0.80 | 4.2 ± 1.3 | 0.4% | 0.85 |
| 1i. Sports | 4.8 ± 0.8 | 0.1% | 0.80 | 3.5 ± 1.6 | 0.5% | 0.72 | 3.9 ± 1.6 | 0.3% | 0.79 |
| Angina Frequency | |||||||||
| 2. Symptom frequency | 5.6 ± 1.1 | 0.2% | 0.89 | 3.7 ± 1.7 | 1.3% | 0.72 | 4.9 ± 1.5 | 0.9% | 0.82 |
| 3. Nitroglycerin frequency | 5.8 ± 0.6 | 0.2% | 0.81 | 5.3 ± 1.3 | 0.4% | 0.63 | 5.7 ± 0.9 | 0.8% | 0.68 |
| Quality of Life | |||||||||
| 9. Enjoyment of life | 4.5 ± 0.9 | 1.0% | 0.70 | 3.3 ± 1.4 | 0.5% | 0.74 | 4.2 ± 1.2 | 1.3% | 0.54 |
| 10. Rest of life as is now | 4.3 ± 1.0 | 0.7% | 0.79 | 2.7 ± 1.7 | 1.6% | 0.70 | 2.6 ± 1.7 | 2.1% | 0.52 |
| 11. Worry about MI/death | 3.8 ± 1.1 | 0.8% | 0.65 | 3.6 ± 1.1 | 0.4% | 0.58 | 3.8 ± 1.2 | 0.8% | 0.58 |
Abbreviations: CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention
Response range is 1–5 for Physical Limitation items, 1–6 for Angina Frequency items and 1–5 for Quality of Life items. Physical Limitation items responses of 6 (“Limited for other reasons or did not do the activity”) are treated as missing.
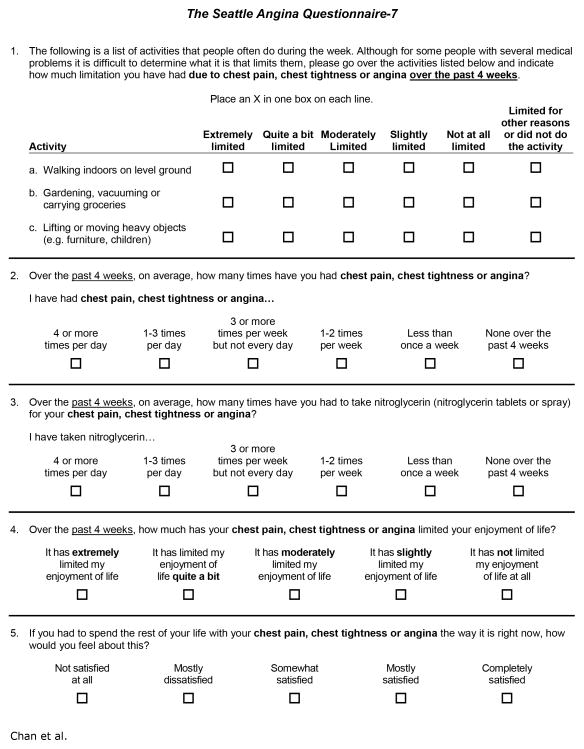
From the 9-item Physical Limitation scale, we selected one item from each of the 3 intensity levels: 1b (limitation walking indoors on level ground), 1e (limitation with gardening, vacuuming and carrying groceries) and 1h (limiting lifting or moving heavy objects). These items had the strongest concordance with the Physical Limitation score in all but one clinical setting (concordance was nominally higher for the other 2 high-intensity items among stable CAD patients, but the rates of selecting “limited for other reasons or did not do the activity” were also substantially higher). From the 2-item Angina Frequency scale, while concordance and variability were both greater for symptom frequency, we opted to retain both items for consistency with the full SAQ, given the wide use of this scale and the relatively minimal burden of a single additional item. From the 3-item Quality of Life scale, we selected items 9 (limitation of enjoyment of life) and 10 (feelings about spending the rest of your life with symptoms as they are now), which had superior concordance among both stable CAD patients and PCI patients. Item 11 (how often do you worry about having a heart attack or dying suddenly) was only slightly more concordant with the Quality of Life score among patients experiencing an acute MI. In summary, we identified 7 items (3 Physical Limitation, 2 Angina Frequency and 2 Quality of Life items) to retain in the final short version of the SAQ (Figure 1).
Figure 1. The Shortened SAQ-7 Instrument.
Although the original SAQ instrument was designed to independently assess patients’ symptoms, function and quality of life, the 19 items of the SAQ made interpretation more complex and less feasible to administer. In this figure, we present the shortened SAQ-7 instrument, with similar construct validity, predictiveness, reliability, and responsiveness as the original SAQ instrument.
Validation
Construct Validity
Agreement between the SAQ-7 and full SAQ scores was excellent in all clinical settings, with concordances ≥0.92 for Physical Limitation scores, ≥0.85 for Quality of Life scores and ≥0.96 for Summary scores (Table 4). Concordance was perfect for the Angina Frequency domain because the same items/scale are used in both instruments. As with the full SAQ, missing data occurred primarily for Physical Limitation scores but was slightly less frequent in the SAQ-7. The SAQ-7 also demonstrated a strong association with CCS class, comparable to that of the full SAQ, with mean ± SD summary scores ranging from 51 ± 21 for Class IV to 98 ± 7 for Class 0 patients with stable CAD (correlation of 0.62), and from 54 ± 23 for Class IV to 84 ± 18 for Class 0 patients prior to PCI (correlation of 0.38; Table 5). Finally, we confirmed that the performance of the SAQ-7 was concordant across patient demographic, educational, insurance, and comorbidity subgroups (Supplementary Appendix Table 2).
Table 4.
Score Descriptive Statistics and Concordance with Full SAQ
| SAQ-7 | Full SAQ | Correlation | Concordance | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean ± SD | Percent Missing | Mean ± SD | Percent Missing | |||
| Stable CAD (N=1,221) | ||||||
| Physical Limitation | 95 ± 14 | 17% | 95 ± 14 | 21% | 0.95 | 0.95 |
| Angina Frequency | 93 ± 15 | 0% | 93 ± 15 | 0% | 1.00 | 1.00 |
| Quality of Life | 87 ± 20 | 0% | 81 ± 19 | 0% | 0.89 | 0.85 |
| Summary Score | 91 ± 15 | 0% | 89 ± 14 | 0% | 0.96 | 0.95 |
| PCI (N=1,455) | ||||||
| Physical Limitation | 79 ± 25 | 10% | 75 ± 24 | 12% | 0.93 | 0.92 |
| Angina Frequency | 70 ± 25 | 0% | 70 ± 25 | 0% | 1.00 | 1.00 |
| Quality of Life | 52 ± 31 | 0% | 56 ± 26 | 0% | 0.94 | 0.91 |
| Summary Score | 66 ± 22 | 0% | 67 ± 21 | 0% | 0.97 | 0.97 |
| Acute MI (N=2,487) | ||||||
| Physical Limitation | 85 ± 26 | 15% | 83 ± 25 | 17% | 0.96 | 0.96 |
| Angina Frequency | 84 ± 22 | 0% | 84 ± 22 | 0% | 1.00 | 1.00 |
| Quality of Life | 57 ± 29 | 0% | 62 ± 24 | 0% | 0.91 | 0.88 |
| Summary Score | 74 ± 21 | 0% | 76 ± 19 | 0% | 0.98 | 0.96 |
Abbreviations: CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; SAQ, Seattle Angina Questionnaire
Table 5. Construct Validity of the SAQ-7.
In patients with stable CAD and after PCI, the SAQ-7 differentiated varying levels of angina as well as the full SAQ instrument.
| Stable CAD | PCI | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | SAQ-7 | Full SAQ | N | SAQ-7 | Full SAQ | |
| CCS Class | ||||||
| 0 | 1,602 | 98 ± 7 | 95 ± 7 | 298 | 84 ± 18 | 83 ± 16 |
| I | 81 | 76 ± 17 | 75 ± 15 | 102 | 71 ± 17 | 71 ± 16 |
| II | 131 | 72 ± 19 | 70 ± 17 | 308 | 65 ± 18 | 65 ± 17 |
| III | 39 | 56 ± 18 | 56 ± 17 | 260 | 55 ± 21 | 56 ± 18 |
| V | 25 | 51 ± 21 | 52 ± 21 | 258 | 54 ± 23 | 56 ± 21 |
|
| ||||||
| Correlation | −0.62 | −0.47 | −0.38 | −0.38 | ||
Abbreviations: CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; PCI, percutaneous coronary intervention; SAQ, Seattle Angina Questionnaire
Reproducibility
Among clinically stable patients assessed between 5 and 6 months after elective PCI, the SAQ-7 showed excellent reproducibility, with mean changes of <1 point for all scores and all intra-class correlations being ≥0.78 (Table 6). The Summary score had the highest reproducibility with an ICC of 0.86.
Table 6. Reliability of the SAQ-7.
Measured at 2 time points 1 month apart in patients with documented stable CAD, both the SAQ-7 and the full SAQ showed high reliability, with a mean difference of < 1 point over time for each domain (N=169).
| SAQ-7 | Full SAQ | |||
|---|---|---|---|---|
|
| ||||
| Mean ± SD | Intra-Class Correlation | Mean ± SD | Intra-Class Correlation | |
| Physical Limitation | 0.6 ± 15.2 | 0.80 | −0.1 ± 11.9 | 0.87 |
| Angina Frequency | 0.8 ± 13.4 | 0.78 | 0.8 ± 13.4 | 0.78 |
| Quality of Life | −0.1 ± 15.2 | 0.81 | 0.3 ± 11.9 | 0.86 |
| Summary Score | 0.5 ± 10.5 | 0.86 | 0.5 ± 9.8 | 0.86 |
Abbreviations: CAD, coronary artery disease; SAQ, Seattle Angina Questionnaire
Responsiveness
One month after PCI, mean SAQ-7 scores increased by ≥18 points for all domains (suggesting good responsiveness to clinical change), with the greatest increase in the Quality of Life score (Table 7). Standardized response means were large, ranging from 0.70 for Physical Limitation to 0.95 for the Summary score.
Table 7. Responsiveness of the SAQ-7.
Measured prior to and at 1 month after PCI, both the SAQ-7 and the full SAQ showed similar large improvements in SAQ scores, with mean gains of >18 points in each domain (N=1,045).
| SAQ-7 | Full SAQ | |||
|---|---|---|---|---|
|
| ||||
| Mean ± SD | Standardized Response Mean | Mean ± SD | Standardized Response Mean | |
| Physical Limitation | 18.3 ± 26.0 | 0.70 | 19.0 ± 24.2 | 0.78 |
| Angina Frequency | 21.2 ± 25.2 | 0.84 | 21.2 ± 25.2 | 0.84 |
| Quality of Life | 29.9 ± 35.6 | 0.84 | 21.1 ± 27.6 | 0.76 |
| Summary Score | 22.9 ± 23.4 | 0.95 | 20.0 ± 21.0 | 0.95 |
Predictive Validity
All SAQ-7 scores demonstrated a graded inverse relationship with 12-month outcomes, comparable to those of the full SAQ (Table 8). Predictive power for 12-month mortality was strongest for Physical Limitation scores, ranging from 2% for patients with scores of 75–100 to 10% for patients with scores <50 (c=0.64). Twelve-month ACS hospitalization was most strongly associated with Angina Frequency scores, ranging from 5% for patients with no angina to 16% for patients with daily to weekly angina (c=0.63).
Table 8. Predictive Ability of the SAQ-7.
The SAQ-7 was able to predict 1-year mortality and rehospitalization for an ACS as well as the full SAQ instrument. (N=2,941)
| 12-Month Mortality | 12-Month ACS Rehospitalization | |||
|---|---|---|---|---|
|
| ||||
| SAQ-7 | Full SAQ | SAQ-7 | Full SAQ | |
| Physical Limitation | ||||
| Poor-Fair (0–<50) | 10 ± 2% | 9 ± 2% | 15 ± 3% | 13 ± 3% |
| Good (50–<75) | 2 ± 1% | 3 ± 1% | 7 ± 2% | 8 ± 2% |
| Excellent (75–100) | 2 ± 0.3% | 2 ± 0.3% | 6 ± 1% | 5 ± 1% |
| C-statistic | 0.64 | 0.67 | 0.59 | 0.61 |
|
| ||||
| Angina Frequency | ||||
| Daily-Weekly (0–60) | 6 ± 1% | 6 ± 1% | 16 ± 3% | 16 ± 3% |
| Monthly (>60–<100) | 4 ± 1% | 4 ± 1% | 10 ± 2% | 10 ± 2% |
| None (100) | 4 ± 0.4% | 4 ± 0.4% | 5 ± 1% | 5 ± 1% |
| C-statistic | 0.53 | 0.53 | 0.63 | 0.63 |
|
| ||||
| Quality of Life | ||||
| Poor-Fair (0–<50) | 6 ± 1% | 5 ± 1% | 13 ± 2% | 12 ± 2% |
| Good (50–<75) | 4 ± 1% | 4 ± 1% | 6 ± 2% | 9 ± 2% |
| Excellent (75–100) | 4 ± 0.4% | 4 ± 0.4% | 6 ± 1% | 5 ± 1% |
| C-statistic | 0.54 | 0.54 | 0.58 | 0.61 |
|
| ||||
| Summary Score | ||||
| Poor-Fair (0–<50) | 8 ± 2% | 8 ± 2% | 14 ± 3% | 15 ± 3% |
| Good (50–<75) | 5 ± 1% | 5 ± 1% | 11 ± 2% | 10 ± 2% |
| Excellent (75–100) | 4 ± 0.4% | 4 ± 0.4% | 5 ± 1% | 5 ± 1% |
| C-statistic | 0.56 | 0.58 | 0.61 | 0.63 |
Abbreviations: SAQ, Seattle Angina Questionnaire
DISCUSSION
To achieve a more patient-centered healthcare system, a strategy to accurately document patients’ perspectives of their health status and track their health trajectories is a priority. Unfortunately, despite its importance, this goal often remains an unfulfilled need. Among those with CAD, the SAQ systematically quantifies patients’ angina symptoms, functional limitations due to angina, and the impact that angina has on perceptions of their quality of life. The SAQ has now been used for over 20 years in clinical trials and observational research studies and has been a sensitive measure for describing the relative benefits of coronary revascularization14–19, medical management of stable CAD20, and disparities in healthcare delivery.21–23 Yet, despite a long-standing call to incorporate measures of patients’ health status, such as the SAQ, into routine clinical care2, 24, this has seldom been done. One of the critical barriers to the routine use of the SAQ as a patient-reported outcome in clinical care is the length of the instrument.
To improve the feasibility of routinely using the SAQ, we developed a shortened version of the instrument (the SAQ-7) that can more easily be completed by patients at the time of a clinic visit or prior to a revascularization procedure. Importantly, we were able to demonstrate that the SAQ-7 generates substantially similar scores to the original SAQ instrument and preserves its high test-retest reliability, responsiveness, and prognostic ability. By minimizing the response burden for patients and preserving the psychometric properties of the original SAQ, we have developed a shortened disease-specific health status instrument to support measurement of patient-reported outcomes in future research studies in patients with CAD.
While shortening patient-reported outcome instruments to improve their ‘user-friendliness’ was recently cited as a research priority in supporting their adoption into clinical care, a second key priority has been improving the interpretability and efficiency of the measures.4 Accordingly, we have also created a single SAQ summary score that combines the 3 domains of symptoms, function and quality of life. As with the CCS Classification, which integrates clinicians’ interpretation of patients’ symptoms and function into a single entity, a single summary score may be potentially easier to interpret than the multiple SAQ domains and allow clinicians to quickly screen patients for a significant change in their health status.
While further testing is needed, we believe that the SAQ-7 has the potential to improve the efficiency of clinical care by enabling patients to complete the 7-item instrument prior to an office visit and for clinicians to instantly compare the overall summary score with a previous score to know whether, and how much, patients’ CAD health status has changed. Such a measurement of angina and health status from the patients’ perspective can more accurately describe patient-reported outcomes than one assigned by physicians, such as the CCS class for angina. In fact, a recent study found substantial discordance between patients’ and physicians’ assessments of angina control.25 By systematically asking the same questions in the same way over time, the SAQ Summary Score offers substantial advantages over CCS class in assessing angina from the patients’ perspective, as it uses a reproducible and sensitive standard for quantifying their health status. In fact, it is possible that the SAQ-7 may facilitate measurement of CAD patients’ health status as a ‘vital sign’ in routine clinical care, prompting physicians when a significant change in patients’ symptoms and quality of life has occurred. Whether the summary score can improve population management, shared decision-making or individual patients’ clinical outcomes needs to be formally tested in prospective studies.
The use of a shorter health status instrument and an overall summary score may also have applications in quality assessment. Recently, appropriate use criteria have been developed for coronary revascularization to better highlight the judicious use of procedures such as PCI in patients with obstructive CAD.26 In a subsequent study involving more than 500,000 patients undergoing PCI since the dissemination of these criteria, approximately 12% of procedures performed in patients with stable CAD were categorized as ‘inappropriate’, wherein a Technical Panel determined that the benefits of the procedure were not felt to outweigh the risks.27 Importantly, a key determinant of the appropriateness of the PCI procedure is the patient’s symptoms, as measured by CCS angina class. However, CCS class has been shown to be variably reported by physicians28 and potentially can be ‘gamed’ (i.e., reporting more severe angina than is truly present) to artificially reduce rates of inappropriate PCI at one’s institution. With an ever-growing focus on procedural appropriateness by national quality organizations, insurers, and emerging accountable care organizations, there may be significant financial pressures on physicians and hospitals to reduce rates of ‘inappropriate’ procedures. Because of concerns that the use of physician-assessed CCS class may be gamed, we believe the use of a patient-centered shortened SAQ could ensure a more accurate, systematic, and objective reporting of patients’ symptoms in future assessments of procedural appropriateness with greater consistency across hospitals.
The development of a shortened version of the SAQ and its overall summary score should be interpreted in the context of several potential limitations. First, while we demonstrated excellent psychometric performance of the SAQ-7 and Summary Score, as compared with the original SAQ, all of the limitations of the original instrument likely apply to the shortened version. For example, shortness of breath was not included in the original SAQ because of a desire to focus on symptoms more uniquely associated with CAD, as opposed to chronic lung disease—a common comorbidity in angina patients. We have previously shown that shortness of breath, as quantified by the Rose Dyspnea Scale, can add to the prognostic ability of the SAQ in terms of both mortality and quality of life.29 As such, future applications of patient-reported outcomes may choose to supplement the SAQ-7 with the Rose Dyspnea Questionnaire. A second potential limitation is that many of our cohorts were assessed during a period of relative stability and few had severe symptoms or markedly diminished health status, requiring us to collapse the lowest 2 categories of each domain into single categories. Given prior reports showing the worst prognosis in patients with the worst health status, the prognostic ability of the SAQ-7 may be even greater than that reported in this study. Finally, while we included over 10,000 patients in our analyses within a variety of clinical settings, all patients had confirmed CAD; therefore, the applicability of the SAQ in populations with angina but without known CAD remains unknown.
In conclusion, we have developed and validated the SAQ-7 instrument and a SAQ Summary Score to facilitate the measurement of health status in patients with CAD. This shortened version of the SAQ preserves the high test-retest reliability, responsiveness and prognostic ability of the original SAQ while reducing the response burden from 19 to 7 items. By overcoming the implementation challenges of the full SAQ and the limitations of physician assessments of patients’ health status, the SAQ-7 and SAQ Summary Score have the potential to transform care by facilitating the routine measurement of patient-reported outcomes and, in so doing, improving the quality and efficiency of care.
Supplementary Material
Acknowledgments
Funding:
Dr. Chan is supported by a Career Development Grant Award (K23HL102224) from the NHLBI to examine the appropriateness of PCI in the U.S.
Footnotes
Authorship: Dr. Chan had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and design: Chan, Spertus
Acquisition of Data: Spertus
Statistical Analysis: Jones
Analysis and interpretation of data: Chan, Jones, Arnold, Spertus
Drafting of the manuscript: Chan, Spertus
Critical revision of the manuscript for important intellectual content: Chan, Jones, Arnold, Spertus
Study Supervision: Chan, Spertus
- Dr. Spertus owns patent rights for the Seattle Angina Questionnaire.
- None of the other authors had any conflicts of interest to disclose.
References
- 1.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118:2103–2110. doi: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 2.Drozda J, Jr, Messer JV, Spertus J, Abramowitz B, Alexander K, Beam CT, Bonow RO, Burkiewicz JS, Crouch M, Goff DC, Jr, Hellman R, James T, 3rd, King ML, Machado EA, Jr, Ortiz E, O’Toole M, Persell SD, Pines JM, Rybicki FJ, Sadwin LB, Sikkema JD, Smith PK, Torcson PJ, Wong JB. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation. 2011;124:248–270. doi: 10.1161/CIR.0b013e31821d9ef2. [DOI] [PubMed] [Google Scholar]
- 3.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 4.Spertus J. Barriers to the use of patient-reported outcomes in clinical care. Circ Cardiovasc Qual Outcomes. 2014;7:2–4. doi: 10.1161/CIRCOUTCOMES.113.000829. [DOI] [PubMed] [Google Scholar]
- 5.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 6.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–1022. doi: 10.1016/S0002-8703(03)00436-8. [DOI] [PubMed] [Google Scholar]
- 8.Arnold S, Morrow D, Lei Y, Cohen DJ, Mahoney E, Braunwald E, Chan PS. Economic Impact of Angina after an Acute Coronary Syndrome: Insights from the MERLIN-TIMI 36 Trial. Circulation: Cardiovascular Quality and Outcomes. 2009;2:344–352. doi: 10.1161/CIRCOUTCOMES.108.829523. [DOI] [PubMed] [Google Scholar]
- 9.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): Design and Rationale of a Prospective Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)--evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–597. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Spertus JA, Bliven BD, Farner M, Gillen A, Hewitt T, Jones P, McCallister BD. Integrating baseline health status data collection into the process of care. Jt Comm J Qual Improv. 2001;27:369–380. doi: 10.1016/s1070-3241(01)27032-1. [DOI] [PubMed] [Google Scholar]
- 12.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 13.McDowell I. Measuring Health: A Guide to Rating Scales and Questionnaires. New York: Oxford University Press; 2006. [Google Scholar]
- 14.Weintraub WS, Barnett P, Chen S, Hartigan P, Casperson P, O’Rourke R, Boden WE, Lewis C, Veledar E, Becker E, Culler S, Kolm P, Mahoney EM, Dunbar SB, Deaton C, O’Brien B, Goeree R, Blackhouse G, Nease R, Spertus J, Kaufman S, Teo K. Economics methods in the Clinical Outcomes Utilizing percutaneous coronary Revascularization and Aggressive Guideline-driven drug Evaluation (COURAGE) trial. Am Heart J. 2006;151:1180–1185. doi: 10.1016/j.ahj.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Borkon AM, Muehlebach GF, House J, Marso SP, Spertus JA. A comparison of the recovery of health status after percutaneous coronary intervention and coronary artery bypass. The Annals of Thoracic Surgery. 2002;74:1526–1530. doi: 10.1016/s0003-4975(02)04063-8. [DOI] [PubMed] [Google Scholar]
- 16.Conaway DG, House J, Bandt K, Hayden L, Borkon AM, Spertus JA. The elderly: health status benefits and recovery of function one year after coronary artery bypass surgery. J Am Coll Cardiol. 2003;42:1421–1426. doi: 10.1016/s0735-1097(03)01052-0. [DOI] [PubMed] [Google Scholar]
- 17.Spertus JA, Nerella R, Kettlekamp R, House J, Marso S, Borkon AM, Rumsfeld JS. Risk of restenosis and health status outcomes for patients undergoing percutaneous coronary intervention versus coronary artery bypass graft surgery. Circulation. 2005;111:768–773. doi: 10.1161/01.CIR.0000155242.70417.60. [DOI] [PubMed] [Google Scholar]
- 18.Rumsfeld JS, Magid DJ, Plomondon ME, Sacks J, Henderson W, Hlatky M, Sethi G, Morrison DA. Health-related quality of life after percutaneous coronary intervention versus coronary bypass surgery in high-risk patients with medically refractory ischemia. J Am Coll Cardiol. 2003;41:1732–1738. doi: 10.1016/s0735-1097(03)00330-9. [DOI] [PubMed] [Google Scholar]
- 19.Abdallah MS, Wang K, Magnuson EA, Spertus JA, Farkouh ME, Fuster V, Cohen DJ. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. 2013;310:1581–1590. doi: 10.1001/jama.2013.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosiborod M, Arnold SV, Spertus JA, McGuire DK, Li Y, Yue P, Ben-Yehuda O, Katz A, Jones PG, Olmsted A, Belardinelli L, Chaitman BR. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) J Am Coll Cardiol. 2013;61:2038–2045. doi: 10.1016/j.jacc.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Longmore RB, Spertus JA, Alexander KP, Gosch K, Reid KJ, Masoudi FA, Krumholz HM, Rich MW. Angina frequency after myocardial infarction and quality of life in older versus younger adults: the Prospective Registry Evaluating Myocardial Infarction: Event and Recovery study. Am Heart J. 2011;161:631–638. doi: 10.1016/j.ahj.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Maddox TM, Reid KJ, Spertus JA, Mittleman M, Krumholz HM, Parashar S, Ho PM, Rumsfeld JS. Angina at 1 year after myocardial infarction: prevalence and associated findings. Arch Intern Med. 2008;168:1310–1316. doi: 10.1001/archinte.168.12.1310. [DOI] [PubMed] [Google Scholar]
- 23.Spertus J, Safley D, Garg M, Jones P, Peterson ED. The influence of race on health status outcomes one year after an acute coronary syndrome. J Am Coll Cardiol. 2005;46:1838–1844. doi: 10.1016/j.jacc.2005.05.092. [DOI] [PubMed] [Google Scholar]
- 24.Rumsfeld JS. Health status and clinical practice: when will they meet? Circulation. 2002;106:5–7. doi: 10.1161/01.cir.0000020805.31531.48. [DOI] [PubMed] [Google Scholar]
- 25.Beltrame JF, Weekes AJ, Morgan C, Tavella R, Spertus JA. The prevalence of weekly angina among patients with chronic stable angina in primary care practices: The Coronary Artery Disease in General Practice (CADENCE) Study. Arch Intern Med. 2009;169:1491–1499. doi: 10.1001/archinternmed.2009.295. [DOI] [PubMed] [Google Scholar]
- 26.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–553. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA, Rumsfeld JS, Brindis RG, Spertus JA. Appropriateness of percutaneous coronary intervention. JAMA. 2011;306:53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkhoff D, Schmidt S, Schulman SP, Myers J, Resar J, Becker LC, Weiss J, Jones JW. Transmyocardial laser revascularisation compared with continued medical therapy for treatment of refractory angina pectoris: a prospective randomised trial. ATLANTIC Investigators. Angina Treatments-Lasers and Normal Therapies in Comparison. Lancet. 1999;354:885–890. doi: 10.1016/s0140-6736(99)08113-1. [DOI] [PubMed] [Google Scholar]
- 29.Arnold SV, Spertus JA, Jones PG, Xiao L, Cohen DJ. The impact of dyspnea on health-related quality of life in patients with coronary artery disease: results from the PREMIER registry. Am Heart J. 2009;157:1042–1049. doi: 10.1016/j.ahj.2009.03.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.