Abstract
Rationale:
Serotonin 5-HT2A and 5-HT2C receptors are thought to be the primary pharmacological mechanisms for serotonin-mediated hallucinogenic drugs, but recently there has been interest in metabotropic glutamate (mGluR2) receptors as contributors to the mechanism of hallucinogens.
Objective:
The present study assesses the role of these 5-HT and glutamate receptors as molecular targets for two tryptamine hallucinogens, N,N-dimethyltryptamine (DMT) and N,N-diisopropyltryptamine (DiPT).
Methods:
Drug discrimination, head twitch and radioligand binding assays were used. A 5-HT2AR inverse agonist (MDL100907), 5-HT2CR antagonist (SB242084) and mGluR2/3 agonist (LY379268) were tested for their ability to attenuate the discriminative stimulus effects of DMT and DiPT; an mGluR2/3 antagonist (LY341495) was tested for potentiation. MDL100907 was used to attenuate head twitches induced by DMT and DiPT. Radioligand binding studies and inosital-1-phosphate (IP-1) accumulation were performed at the 5-HT2CR for DiPT.
Results:
MDL100907 fully blocked the discriminative stimulus effects of DMT, but only partially blocked DiPT. SB242084 partially attenuated the discriminative stimulus effects of DiPT, but produced minimal attenuation of DMT’s effects. LY379268 produced potent, but only partial blockade of the discriminative stimulus effects of DMT. LY341495 facilitated DMT- and DiPT-like effects. Both compounds elicited head twitches (DiPT>DMT) which were blocked by MDL1000907. DiPT was a low potency full agonist at 5-HT2CR in vitro.
Conclusions:
The 5-HT2AR likely plays a major role in mediating the effects of both compounds. 5-HT2C and mGluR2 receptors likely modulate the discriminative stimulus effects of both compounds to some degree.
Keywords: N,N-Diisopropyltryptamine; N,N-Dimethyltryptamine; hallucinogens; drug discrimination; 5-HT2A; 5-HT2C; mGluR2; rat
Introduction
N,N-Dimethyltryptamine (DMT) and N,N-diisopropyltryptamine (DiPT) are structurally similar hallucinogens, but produce somewhat different effects. DMT is an endogenous compound that is also found in a variety of plants, and causes brief, episodic visual hallucinations at high concentrations (Stoff et al. 1977; Strassman and Qualls 1994; Strassman et al. 1994). DiPT, a synthetic analog of DMT, produces auditory effects (primarily tone distortion) in people, according to anecdotal reports on user experience websites such as erowid.org and in published accounts (Shulgin 1997; Shulgin and Carter, 1980; Strassman et al. 1996).
Because the subjective effects of hallucinogens seem to drive their use rather than effects on the reward/reinforcement areas of the brain, drug discrimination is often used as an animal model. Overall, the discriminative stimulus effects of these compounds are similar to those of other hallucinogens, although there are asymmetries in cross-substitution. DMT fully substituted in DOM-trained rats (Glennon et al., 1983; Glennon 1986), produced mixed results in LSD-trained rats and pigeons (Appel et al. 1999; Helsley et al. 1998; Jarbe 1980), but only around 50% drug-appropriate responding (DAR) in MDMA-trained rats (Gatch et al., 2009); whereas DOM, LSD and MDMA all fully substituted in DMT-trained rats (Gatch et al., 2009). Conversely, DiPT fully substituted in DOM-trained rats, produced around 70% DAR in LSD-trained rats and failed to substitute in MDMA-trained rats (Gatch et al., 2011). In DiPT-trained rats, LSD, DOM and MDMA produced full substitution (Carbonaro et al. 2013). However, DMT and DiPT did not fully cross-substitute for each other. DiPT fully substituted in DMT-trained rats, but DMT only produced 65% DAR in DiPT-trained rats (Gatch et al. 2011; Gatch et al. 2009). Further, DMT is unusual as it produces little or no head twitch response (Fantegrossi et al. 2006), which is thought to be a 5-HT2A receptor-mediated behavior produced primarily by hallucinogens. Taken together, the differences in cross-substitution, the putative differences in subjective effects in humans, and the lack of a head twitch response to DMT may indicate that these compounds have different mechanisms of action.
Currently, the precise mechanism of action for hallucinogens is still unknown. The 5-HT2A receptor is thought to be necessary, but not sufficient for hallucinogenic effects, and 5-HT2C and 5-HT1A receptors may play important roles as well (see review by Nichols 2004). DMT was reported to bind to 5-HT1A (Pierce and Peroutka 1989), 5-HT2A (Lyon et al. 1988; Pierce and Peroutka 1989, Smith et al. 1998) and 5-HT2C receptors (Smith et al. 1998), whereas DiPT was found to be an agonist at 5-HT2A receptors, a weak partial agonist at 5-HT1A receptors, and an inhibitor of the serotonin transporter and VMAT2 (Nagai et al. 2007; Cozzi et al. 2009; Gatch et al. 2011). DiPT has not yet been tested at 5-HT2C receptors. Although it is likely that the 5-HT1A receptor plays a role in mediating the effects of tryptamine hallucinogens, the role of the 5-HT1A receptor was not investigated due to DiPT having low potency and low efficacy at this receptor (Gatch et al. 2011). However, recent work has suggested that Group II glutamate receptors (mGluR2/3) may be potential target sites for mediating hallucinogenic effects (Gonzales-Maeso et al 2007; 2008; Delille et al. 2012, Moreno et al. 2011; Winter et al. 2004).
mGlu2/3 receptor agonists act presynaptically to suppress glutamate release, whereas antagonists increase the amount of glutamate in the synapse, creating a potentiation of hallucinogenic effects (Cartmell et al. 1999; Forsythe and Barnes-Davies 1997; Ohishi et al. 1994; Shigemoto et al. 1997). A mGluR2 agonist blocked the discriminative stimulus effects of LSD, whereas a mGluR2 antagonist facilitated the discriminative stimulus effects of LSD (Winter et al., 2004). Further, mGluR2 knock-out mice showed little or no head twitch following DOI, and some signaling was disrupted, which may mean that mGlu2 receptors are necessary for hallucinogenic activity (Moreno et al. 2011). A possible explanation for these effects is that mGlu2 receptors co-localize with 5-HT2A receptors to form hetero-receptor complexes (Delille et al. 2012; Gonzalez-Maeso et al. 2008; Gonzalez-Maeso et al. 2007). It has been suggested that the hetero-receptors induce a hallucinogen-specific second messenger cascade (Gonzalez-Maeso et al. 2008; Gonzalez-Maeso et al. 2007), although this has not been definitely established (Delille et al. 2012).
The goal of the present study was to identify receptor mechanisms that account for differences in the behavioral effects produced by DMT and DiPT. Based on data from previous studies, we hypothesized that the 5-HT2A and mGlu2 receptors would both play an important role in the discriminative stimulus of both DMT and DiPT, and that the 5-HT2CR would contribute to the effects of DiPT, but to a lesser degree than either the 5-HT2A or mGlu2 receptors. MDL100907 (5-HT2A inverse agonist), SB242084 (5-HT2CR antagonist) and LY379268 (mGlu2/3 receptor agonist) were used to block the discriminative stimulus effects of DMT and DiPT and LY341495 (mGlu2/3 receptor antagonist) was used to facilitate the discriminative stimulus effects. We tested whether DiPT, like most hallucinogens, produces a head twitch response, and MDL100907 was used to block the head-twitch response. Finally, the ability of DiPT to bind to and activate the 5-HT2C receptor (inosital-1-phosphate (IP-1) accumulation) was assessed.
Methods
Animals
Male Sprague–Dawley rats and male C57BL/6 mice were obtained from Harlan Sprague–Dawley (Indianapolis, IN). All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00AM). Body weights were maintained at 320–350 g by limiting food to 20 g/day, which included the food received during training sessions. Water was freely available in the home cages. Three-month-old C57BL/6 mice weighing approximately 25-30 g were used. Mice had ad libitum food and water access and were group housed (2-4 per cage). All housing and procedures were in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003), and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Drug discrimination procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via LVB interfaces (MedAssociates, East Fairfield, VT). The computers were programmed in MED-PC IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data.
A group of 16 rats were trained to discriminate N,N-dimethyltryptamine (DMT, 5 mg/kg, 5 min) from saline by using a two-lever choice methodology. Another group of 16 rats was trained to discriminate N,N-diisopropyltryptamine (DiPT, 5 mg/kg, 15 min) from saline. Food (45 mg sucrose pellets; Bio-Serve, Frenchtown, NJ) was available under a fixed-ratio 10 schedule of reinforcement when responding occurred on the injection-appropriate lever. There were no consequences scheduled for incorrect responses. Half of the rats were trained with drug as the cue on the right lever; half were trained with drug on the left lever. Training sessions occurred in a double alternating fashion (D-D-S-S-D, etc.), and tests were conducted between pairs of identical training sessions (i.e., between either two saline or two drug-training sessions).
During each training session, the rats received an intraperitoneal injection of either saline or the appropriate training drug. The training dose was 5 mg/kg for both DMT and DiPT; pretreatment time was 5- or 15-min, respectively. After the pretreatment time, the rats were placed in the experimental chamber. During the session, the rats could earn up to 20 food pellets by responding under an FR10 schedule of food presentation. If all 20 food pellets were delivered before the end of the 10-min response period, the house lights were turned off and responding had no scheduled consequences for the remainder of that response period. Animals received approximately 60 training sessions in total before use in any behavioral experiment. Animals were selected for use in experiments when they had achieved 85% injection-appropriate responding for both the first reinforcer and for the total session during nine of their last ten training sessions.
The ability of MDL100907, SB242084, LY341495 and LY379268 were tested in groups of seven rats trained to discriminate DMT and DiPT from saline. A repeated-measures design was used, such that each rat was tested at all doses of the test compounds. Test sessions lasted for a maximum of 20 min to allow for potentially delayed effects of test compounds. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until 20 food pellets were obtained, or for a maximum of 20 min. Rats were tested only if they had achieved 85% injection-appropriate responding for both first reinforcer and total session during the two prior training sessions. At least 3 days elapsed between test sessions.
Head Twitch Response
Groups consisted of at least 7 mice. Test sessions included a vehicle control, a positive hallucinogen control (2,5-dimethoxy-4-iodo-amphetamine, DOI; 1 mg/kg) and doses of either DMT or DiPT (2.5, 5 and 10 mg/kg). Mice were kept in their home cages until testing began and weighed immediately before test sessions. Mice were given the 5-HT2A inverse agonist (MDL100907; 0.003 or 0.03 mg/kg) or vehicle (0.9% saline) 10 min prior to injection of the test compound (DMT, DiPT), vehicle or positive control. Subsequently, mice were placed into a separate holding cage, the same size as their home cage (8”×10”) containing clean bedding, where their behavior was recorded for 10 min after the last injection of DMT or DiPT or vehicle. When DOI was tested, the animals were recorded 10 min after administration, based on data suggesting maximal head twitch occurrence. Multiple observers later viewed the recordings and the number of head twitches were counted. A head twitch was defined as a rapid rotational jerk that was not contiguous with any grooming behavior.
[125I]DOI Binding
Binding to 5-HT2C receptors was tested in human embryonic kidney cells expressing the human 5-HT2C receptor (HEK-h5HT2C) adapting methods described previously for 5-HT2A receptors (Knight et al., 2004, Gatch et al., 2011). The cDNA for the h5-HT2C receptor was purchased from Missouri S&T cDNA Resource Center. The cells were grown until confluent on 15-cm plates. Medium was removed, and cells were washed with phosphate-buffered saline, scraped into 2 ml of phosphate-buffered saline, and frozen at −20°C until needed. Cell suspension was thawed, 10 ml of assay buffer (50 mM Tris, pH 7.4 at 37°C, with 0.1% ascorbic acid and 5 mM CaCl2) was added per plate of cells and polytronned at setting 6 for 5 s. The homogenate was centrifuged at 30,000 g for 20 min. To minimize the residual serotonin concentration, the pellet was resuspended in buffer, polytronned, and centrifuged twice as above. The final pellet was resuspended in 2 ml of buffer/plate of cells.
The binding assay included test compound, serotonin or buffer, cell homogenate, [125I]DOI (~0.1 nM), and buffer in a final volume of 250 μl. Specific binding was defined as the difference between total binding and binding in the presence of 10 μM serotonin. The reaction was incubated for 1 h at 37°C and terminated by filtration through Wallac A filtermats presoaked in 0.05% polyethylenimine using a Tomtec cell harvester. Radioactivity was determined by liquid scintillation counting.
Inosital-1-Phosphate Accumulation
Activation of 5-HT2C receptors was tested by measuring the accumulation of inosital monophosphate by using an IP-1 Elisa kit (Cisbio, Bedford, MA) as previously described (Eshleman et al., 2013b). Cells were plated at a density of 400,000 cells per well in 24-well plates in DMEM supplemented with 10% charcoal-stripped FetalClone and 450 μg G418/ml.. The next day, medium was removed, cells were rinsed and then pre-incubated with DMEM and 450 μg G418/ml.for 1 h. After removal of medium, stimulation buffer was added. After 10-min incubation, agonists were added, and the plates incubated for 60 min. Cells were lysed for 30 min, and 50-μl aliquots of the lysates were added to the IP-1 plate. The assay was conducted according to kit instructions. Stimulated IP-1 formation was normalized to the maximal effect of serotonin, which was determined in each assay.
Drugs
N,N,-dimethyltryptamine fumarate (DMT), and N,N-diisopropyltryptamine HCl (DiPT) were provided by the National Institute on Drug Abuse. LY379268, LY341495, SB242084 were received from Tocris. DOI was purchased from Sigma-Aldrich. MDL100907 was synthesized by Drs. Kejun Cheng and Kenner Rice at NIH. DMT, DiPT and DOI were dissolved in 0.9% saline. LY379268 (0.01-2.5 mg/kg) and LY341495 (0.5-2.5 mg/kg) were dissolved in deionized water with sodium hydroxide. SB242084 (0.5-5 mg/kg) was dissolved in Tween80 and deionized water and MDL100907 (0.01-1 mg/kg) was dissolved in saline and HCl. All drugs were administered i.p. in a volume of 1 ml/kg.
Data analysis
Drug discrimination data were expressed as the mean percentage of responses on the drug-appropriate lever in each test period. Response rates were expressed as a function of the number of responses made divided by the total session time. Graphs for the percentage of drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Error bars show standard error of the mean. The percentage of drug-appropriate responding was shown only if at least three rats completed the first fixed ratio. Full substitution was defined as ≥80% drug-appropriate responding (DAR) and the difference between the test data and the drug control was not statistically significant, and full antagonism was defined as ≤20% DAR and not statistically different from the vehicle control.
The potencies of test compounds that fully substituted were calculated by fitting straight lines to the individual dose–response data for each compound by means of TableCurve 2D (Jandel Scientific, San Rafael, CA). Straight lines were fitted to the linear portion of dose–effect curves, defined by doses producing 20% to 80% of the maximal effect, including not more than one dose producing <20% of the maximal effect and not more than one dose producing >80% of the maximal effect. Other doses were excluded from the analyses. The ED50 values are expressed as the mean of seven rats ± standard error of the means. Response rates were expressed as a function of the number of responses made divided by the total session time. Response rate data were analyzed by one-way, repeated measures analysis of variance. Effects of individual doses were compared to the appropriate control value by using a priori contrasts. Head twitch data were analyzed by using a three-way analysis of variance (antagonist × agonist × dose) and post hoc pair-wise multiple comparisons on significant effects and interactions by using Tukey’s honestly significant difference test. The effects of vehicle, DOI, DMT and DiPT alone were analyzed by one-way analysis of variance. Head twitch data are presented as mean ± standard error of the means. Criterion for significance was set a priori at p<0.05.
Results
Radioligand Binding and Functional Assay
DiPT bound with moderate affinity to the 5-HT2C receptor (Ki= 290 ± 110 nM; Hill coefficient = −0.72 ± 0.05) and was a full agonist in the IP-1 formation assay (EC50 = 2380 ± 340 nM), producing 107.4 ± 2.5% of the maximal 5-HT effect.
Drug Discrimination
5-HT2A receptor
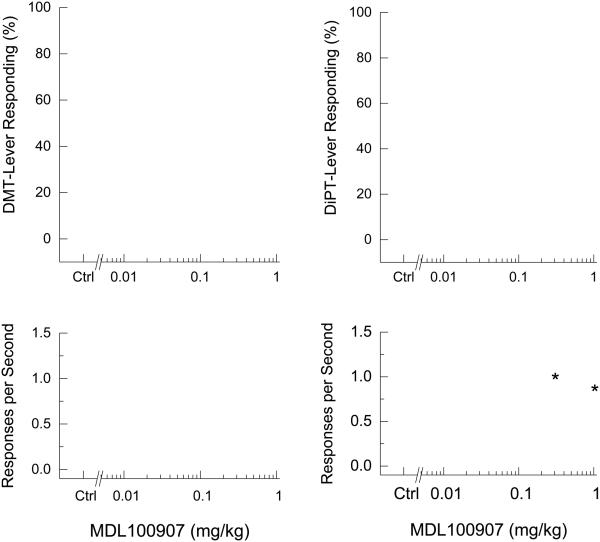
As shown in Figure 1, the 5-HT2A receptor inverse agonist MDL100907 dose-dependently blocked the effects of DMT (ED50 = 0.02± 0.14 mg/kg), reducing DMT-appropriate lever responding to 14.4 ± 14.3% at 0.1 mg/kg (p<0.05). Response rate was unchanged. MDL100907 reduced DiPT-lever responding to 28.9 ± 18.4 and 33.2 ± 17.3% following 0.3 and 1.0 mg/kg, respectively (p<0.05). Response rate was decreased following 0.3 and 1 mg/kg [F(5,30)=2.93, p=0.028].
Fig. 1.
Effects of the 5-HT2A receptor inverse agonist, MDL100907, on the discriminative stimulus effects of DMT (left panels) and DiPT (right panels). Points represent the mean and error bars represent the standard error of the mean. Top panel shows drug-appropriate responding of the test compounds. The x-axis represents dose of MDL100907. The y-axis represents percentage of drug-lever appropriate responding. Bottom panel shows rate of responding as responses per second as a function of dose. Open circles- saline control; Open diamond- drug control. N=7. Asterisk indicates points that are different from the drug control.
5-HT2C receptor
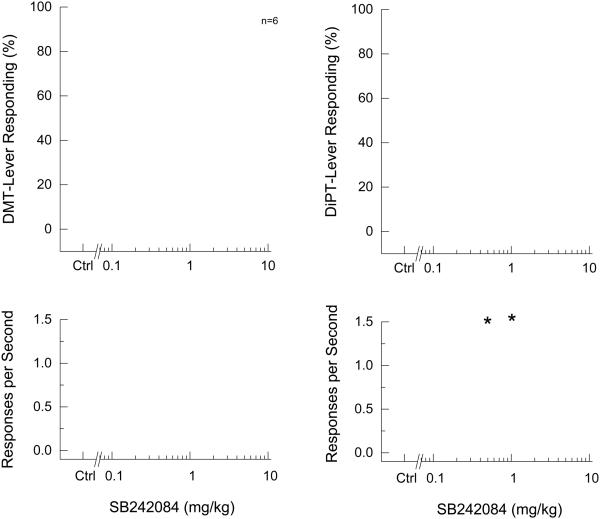
In DMT-trained rats, SB242084 reduced drug-lever appropriate responding to 63.2 ± 17.2% at 2.5 mg/kg, but this effect was not statistically different from drug control, and higher doses produced progressively less effect (Fig. 2). SB242084 failed to alter response rates. In DiPT-trained rats, SB242084 reduced drug-lever appropriate responding to 28.9 ± 18.4% at 1 mg/kg (p<0.05), but higher doses produced progressively less effect. Response rates were increased following 0.5 and 1 mg/kg [F(4,24)=2.901, p=0.043].
Fig. 2.
Effects of the 5-HT2C receptor antagonist (SB242084) on the discriminative stimulus effects of DMT (left panels) and DiPT (right panels). Points represent the mean and error bars represent the standard error of the mean. Top panel shows drug-appropriate responding of the test compounds. The x-axis represents dose of SB242084. The y-axis represents percentage of drug-lever appropriate responding. Bottom panel shows rate of responding in responses per second (y-axis) as a function of dose. Open circles- saline control; Open diamond- drug control. N=7, except where shown. Asterisk indicates points that are different from the drug control.
mGlu2/3 receptors
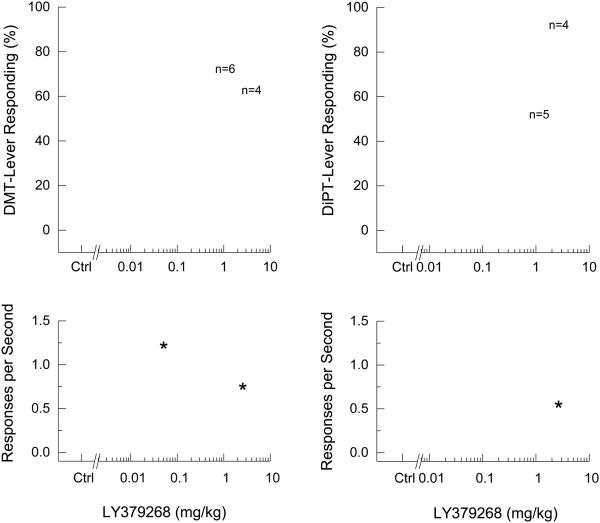
LY379268, a mGlu2/3 receptor agonist reduced DMT-appropriate responding to a plateau of approximately 60% drug-appropriate responding (Fig. 3). DMT-appropriate responding ranged from 44.3 ± 19.7% to 71.5 ± 18.3% in the dose range of 0.025 −2.5 mg/kg, but these effects were not significantly different from drug control. LY379268 altered response rates [F(8,48)=2.5, p=0.023], and suppressed responding in 4 of 7 rats following 2.5 mg/kg. Over the same dose range, LY379268 failed to decrease DiPT-appropriate responding, but decreased response rates [F(8,48)=8.551, p<0.001], and suppressed responding following 1 and 2.5 mg/kg.
Fig. 3.
Effects of the mGlu2/3 receptor agonist (LY379268) on the discriminative stimulus effects of DMT (left panels) and DiPT (right panels). Points represent the mean and error bars represent the standard error of the mean. Top panel shows drug-appropriate responding of the test compounds in DMT/DiPT-trained rats. The x-axis represents dose of LY379268. The y-axis represents percentage of drug-lever appropriate responding. Bottom panel shows rate of responding in responses per second (y-axis) as a function of dose. Open circles- saline control; Open diamond- drug control. N=7, except where shown. Asterisk indicates points that are different from the drug control.
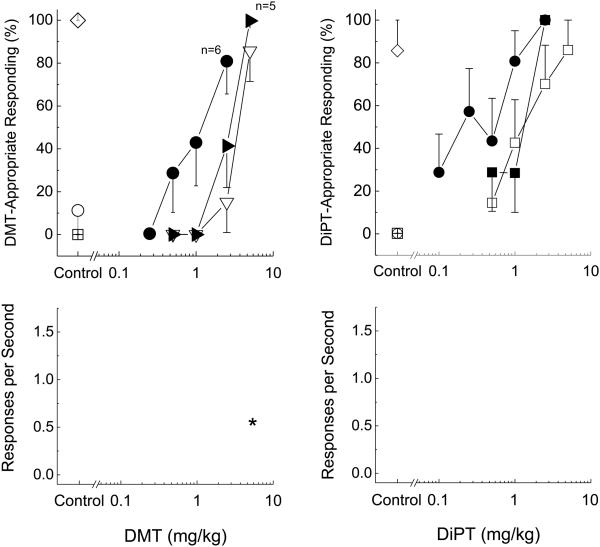
LY341495 produced a dose-dependent leftward shift in both the DMT and DiPT dose-effect curves (Fig 4). In DMT-trained rats, one-way analysis of variance of the ED50 values revealed a significant difference [F(2,28)=37.61, p<.001]. DMT alone produced a dose-dependent increase in DMT-appropriate responding (ED50=3.19 ± 0.30 mg/kg). LY341495 (0.5 mg/kg) did not alter the DMT dose effect curve (ED50=2.47 ± 0.24 mg/kg), but 2.5 mg/kg produced a leftward shift in the DMT dose curve (ED50=1.24 ± 0.06 mg/kg, p=0.016). DMT alone or in combination with 2.5 mg/kg LY341495 did not significantly alter response rates, whereas DMT in combination with 0.5 mg/kg LY341495 dose-dependently decreased response rates [F(5,30)=3.102, p=0.017]. Similarly, one-way analysis of variance of the ED50 values revealed a significant difference in the DiPT-trained rats [F(2,18)=5.286, p=0.016]. Increasing doses of DiPT produced a dose-dependent increase in DiPT-appropriate responding (ED50=1.42 ± 0.631 mg/kg). The 1.0 mg/kg dose of LY341495 failed to shift the DiPT dose-response curve (ED50=1.02 ± 0.09 mg/kg), but 2.5 mg/kg produced a leftward shift (ED50=0.28 ± 0.88 mg/kg, p=0.016). Response rates were not altered at any dose.
Fig. 4.
Effects of the mGlu2/3 receptor antagonist (LY341495) at facilitating the effects of DMT (left panels) and DiPT (right panels). Points represent the mean and error bars represent the standard error of the mean. Top panel shows drug-appropriate responding of the test compounds in DMT/DiPT-trained rats. The x-axis represents dose of DMT/DiPT. The y-axis represents percentage of drug-lever appropriate responding. Bottom panel shows rate of responding in responses per second (y-axis) as a function of dose. Open triangles- Dose-effect curve of DMT and DiPT; Closed triangles- 0.5 mg/kg of LY341495; Closed circles- 2.5 mg/kg LY341495. Open circles- saline control; Open diamond- drug control; open squares- LY341495 alone. N=7, except where shown. Asterisk indicates points that are different from the saline control.
Head Twitch Assay
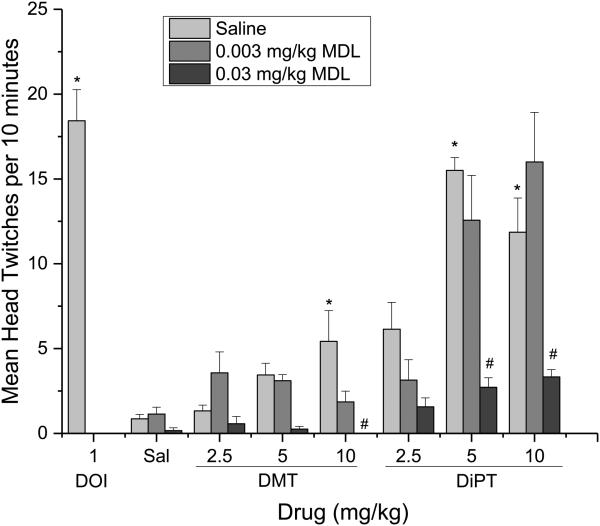
Head twitch data is shown in Figure 5. Little or no head twitches were observed in saline-treated animals or in those receiving MDL100907 alone, whereas DOI (1 mg/kg) produced an average of 18.4 ± 1.8 head twitches [F(3,24)=21.755, p<0.001]. There was an overall effect of hallucinogen (DMT vs. DiPT) [F(1,115)=73.989, p<.001], hallucinogen dose [F(2,115)=188.727, p<.001], and of antagonist dose [F(2,115)=27.765, p<.001]. There was an interaction between antagonist dose and hallucinogen [F(2,115)=6.958, p=.001], and interaction between hallucinogen and hallucinogen dose [F(2,115)=9.37, p<.001], and a three-way interaction [F(4,115)=3.753, p=.007]. DMT and DiPT each dose-dependently increased the number of head twitches (p<0.05), but DiPT produced two-fold more head twitches at 10 mg/kg (11.9±2.0) than did DMT (5.4±1.8). The higher dose of MDL100907 (0.03 mg/kg) reduced head twitches elicited by 10 mg/kg of DMT and DiPT (p<0.05). There was no difference in body weight of any of the treatment groups.
Fig. 5.
Effects of the 5-HT2A receptor inverse agonist, MDL100907 on head twitches induced by hallucinogens in C57Bl/6 mice. The x-axis represents vehicle controls or dose of DOI, DMT, or DiPT alone in combination with MDL100907. The y-axis represents mean head twitches in a 10-minute period. Error bars show the standard error of the mean. MDL indicates MDL100907. Sal indicates saline control. # indicates points different from hallucinogen alone (light gray bars); Asterisk indicates points different from the saline controls (Sal).
Discussion
The present study assessed the roles of 5-HT2A, 5-HT2C and mGlu2/3 receptors in mediating the discriminative stimulus effects of DiPT. To clarify whether DMT and DiPT have different effects at 5-HT2A receptors, the effects of DMT and DiPT on head twitch were assessed. Because molecular interactions of DiPT at the 5-HT2C receptor had not been tested, 5-HT2C receptor binding and function were examined. Finally, because mGlu2/3 receptors have been implicated in the effects of hallucinogens, and because the differences in effects of the serotonergic antagonists could not fully account for the differences in the behavioral effects of DMT and DiPT, the effects of mGlu2/3 receptor compounds on discriminative stimulus effects of DMT and DiPT were also tested.
5-HT2A receptors
The 5-HT2A receptor inverse agonist MDL100907 fully blocked the discriminative stimulus effects of DMT, but did not completely block those of DiPT (29-33% DAR). Further, higher doses of MDL100907 (0.3 and 1 mg/kg) were required to attenuate DiPT-appropriate responding. These results are unexpected, as 5-HT2A receptors are generally thought to be the primary receptor mediating the effects of hallucinogens (Nichols, 2004). Because the head-twitch response is a hallmark sign of hallucinogens and is thought to be primarily mediated through 5-HT2A receptors (e.g., Gonzalez-Maeso et al, 2007), the effects of DiPT and DMT on head twitch were assessed. DiPT produced a similar amount of head twitches as did the classic hallucinogen DOI. However, DMT produced markedly fewer head twitches in the present study than did DiPT. In a previous study, DMT failed to produce head twitches in Swiss Webster mice (Fantegrossi et al. 2006). However, the present study used C57BL/6 mice, which generally show more head twitches than most strains, including Swiss Webster (Canal et al. 2010), which may account for the disparate results.
In contrast to the drug discrimination data, MDL100907 dose-dependently attenuated head twitches produced by both DMT and DiPT, which suggests that 5-HT2A receptors are important in mediating the behavioral effects of both DMT and DiPT. The larger effect of MDL100907 on DiPT-induced head twitch than on drug discrimination may be due to the fact that DMT had a lower efficacy at the 5-HT2A receptor, as evidenced by DMT producing half as much IP-1 release relative to serotonin as did DiPT (Eshleman et al. 2013; Gatch et al. 2011). This might account for a 5-HT2A inverse agonist more fully blocking the effects of DMT, as well as the lower number of head twitches elicited by DMT. Conversely, the discriminative stimulus effects of DiPT may involve activation of several receptors, such that attenuation of the effects would require blockade of multiple receptors. It is also possible that the difference in the ability of MDL100907 to block the discriminative stimulus effects of DMT and DiPT is because the doses of the two compounds are not pharmacologically equivalent. Arguments against this possibility include: 1) the doses of DMT and DiPT used for training were the peak doses in cross-substitution studies with other hallucinogens (Gatch et al., 2009; 2011; Carbonaro et al., 2013) and produced similar amounts of rate suppression, and 2) MDL100907 attenuated DMT- and DiPT–induced head twitches proportionally. Finally, it is also possible that the difference is due to mice being used for head twitch and rats for drug discrimination.
5-HT2C receptors
The 5-HT2C receptor antagonist SB242084 produced a U-shaped dose-effect curve when tested in combination with both DMT and DiPT, with one dose producing maximal attenuation of drug-appropriate responding, and higher doses producing progressively less attenuation. The maximal attenuation was statistically significant only for DiPT. In contrast, both DMT (Smith et al. 1998) and DiPT (present study) bound to the 5-HT2C receptor and were full agonists in stimulating IP-1 formation. It is not clear what caused the upturn in the SB242084 dose-effect curve, but SB242084 was unable to fully block the discriminative stimulus effects of either DMT or DiPT. These findings suggest that 5-HT2C receptors are not necessary for the discriminative stimulus effects of DMT and DiPT, but may play a modulatory role in the discriminative stimulus effects of DiPT and DMT.
mGlu2/3 receptors
In the present study, a mGluR2/3 agonist (LY379268) had no effect in DiPT-trained rats, but produced a small, but statistically non-significant attenuation of the discriminative stimulus effects of DMT. LY379268 produced a rather more robust blockade of the discriminative stimulus effects of LSD (Winter et al., 2004). To further investigate the possibility that mGlu2/3 receptors may indeed modulate the stimulus effects of DMT and/or DiPT, a different approach was used. The mGluR2/3 antagonist (LY341495) was found to facilitate the stimulus effects of both DMT and DiPT. Similarly, LY341495 facilitated the discriminative stimulus effects of LSD (Winter et al., 2004), although to a larger degree than observed for DMT and DiPT in the present study.
Although there are two receptors in the group II metabotropic glutamate receptors (mGluR2/3), it is likely that mGluR2 is more important for the present results, based on co-localization and genetic knockout studies (Gonzalez-Maeso et al. 2007) which show knock out of the mGluR2, but not mGluR3 block the production of hallucinogen-induced head twitch response and mGluR2 co-localize with the 5-HT2A in cortical regions. Taken together, these findings suggest that mGlu2 receptors differentially modulate the stimulus effects of hallucinogens, having the largest contribution to the effects of LSD and the least to those of DiPT.
In conclusion, the discriminative stimulus effects of DMT and DiPT are mediated through similar mechanisms, but the degree to which each of these mechanisms contribute appear to be different. None of the targets (5-HT2A, 5-HT2C and mGlu2 receptors) were able to fully block the discriminative stimulus effects of DiPT, whereas 5-HT2A antagonism was sufficient to block the discriminative stimulus effects of DMT. 5-HT2A receptors did mediate DiPT-induced head twitch, and may contribute to other behavioral effects on DiPT. The role of 5-HT1A receptors in mediating the discriminative stimulus effects of DMT and DiPT were not tested in the present study because an earlier study indicated that DiPT interacts with low affinity and efficacy with the 5-HT1A receptor (Gatch et al., 2011). It is quite possible that at least some of the differences in the discriminative stimulus effects of DMT and DiPT are due to DMT being a full agonist at 5-HT1A receptors whereas DiPT is only a partial agonist. Taken together, it appears that the 5-HT2A receptor is most important for the discriminative stimulus effects of DMT, whereas the 5-HT2C and mGlu2 receptors may play modulatory roles. In contrast, none of the three sites may be sufficient for the production of the effects of DiPT. It is possible that several receptors may contribute to the discriminative stimulus effects of DiPT, so that combinations of targets should be studied in future research to isolate which receptors are necessary and sufficient for the discriminative stimulus effects of DiPT.
Acknowledgments
Funding was provided by the Addiction Treatment Discovery Program of the National Institute on Drug Abuse (NIH N01DA-7-8872) and by T32 AG020494. A portion of this work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism.
Footnotes
There are no conflicts of interest.
References
- Appel JB, West WB, Rolandi WG, Alici T, Pechersky K. Increasing the selectivity of drug discrimination procedures. Pharmacology Biochemistry and Behavior. 1999;64:353–358. doi: 10.1016/s0091-3057(99)00089-1. [DOI] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 2010;209:163–74. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro T, Forster MJ, Gatch MB. Discriminative stimulus effects of N,N-diisopropyltryptamine. Psychopharmacology (Berl) 2013;226:241–246. doi: 10.1007/s00213-012-2891-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–70. [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology. 2012;62:2184–91. doi: 10.1016/j.neuropharm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB. Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: Mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology (Berl) 2014;231:875–888. doi: 10.1007/s00213-013-3303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–9. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Barnes-Davies M. Synaptic transmission: well-placed modulators. Curr Biol. 1997;7:R362–5. doi: 10.1016/s0960-9822(06)00175-8. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther. 2011;338:280–9. doi: 10.1124/jpet.111.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Carbonaro T, Forster MJ. Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacology (Berl) 2009;204:715–24. doi: 10.1007/s00213-009-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–7. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Ogawa-Meguro R, Shigemoto R, Kaneko T, Nakanishi S, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron. 1994;13:55–66. doi: 10.1016/0896-6273(94)90459-6. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–22. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin AT, Shulgin A. TiHKAL. Transform Press; Berkeley, CA: 1997. [Google Scholar]
- Shulgin AT, Carter MF. N, N-Diisopropyltryptamine (DIPT) and 5-methoxy-N, N-diisopropyltryptamine (5-MeO-DIPT). Two orally active tryptamine analogs with CNS activity. Commun Psychopharmacol. 1980;4:363–369. [PubMed] [Google Scholar]
- Smith RL, Canton H, Barrett RJ, Sanders-Bush E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav. 1998;61:323–30. doi: 10.1016/s0091-3057(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Stoff DM, Moja EA, Gillin JC, Wyatt RJ. Dose response and time course effects of N,N-dimethyltryptamine on disruption of rat shuttlebox avoidance. Biol Psychiatry. 1977;12:339–46. [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51:85–97. doi: 10.1001/archpsyc.1994.03950020009001. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39:784–795. doi: 10.1016/0006-3223(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology (Berl) 2004;172:233–40. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]